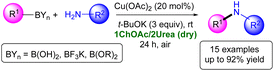Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu-catalysed Chan–Evans–Lam reaction meets deep eutectic solvents: efficient and selective C–N bond formation under aerobic conditions at room temperature†‡
Luciana
Cicco
 a,
Paola
Vitale
a,
Paola
Vitale
 a,
Filippo Maria
Perna
a,
Filippo Maria
Perna
 a,
Vito
Capriati
a,
Vito
Capriati
 *ab and
Joaquín
García-Álvarez
*ab and
Joaquín
García-Álvarez
 *c
*c
aDipartimento di Farmacia–Scienze del Farmaco, Università di Bari “Aldo Moro”, Consorzio C.I.N.M.P.I.S., Via E. Orabona 4, I-70125 Bari, Italy. E-mail: vito.capriati@uniba.it
bIstituto di Chimica dei Composti Organometallici (CNR-ICCOM), Via Madonna del Piano 10, Sesto Fiorentino, 50019, Italy
cLaboratorio de Química Sintética Sostenible (QuimSinSos), Departamento de Química Orgánica e Inorgánica, (IUQOEM), Centro de Innovación en Química Avanzada (ORFEO-CINQA), Facultad de Química, Universidad de Oviedo, E-33071 Oviedo, Spain. E-mail: garciajoaquin@uniovi.es
First published on 30th May 2023
Abstract
An unprecedented, simple and general protocol for the selective formation of C–N bonds was developed by using cheap and easily available Cu(OAc)2 as catalyst for the Cu(II)-catalysed Chan–Evans–Lam reaction in Deep Eutectic Solvents (DESs) as sustainable reaction media. The meticulous selection of both components of DES for this transformation (1ChOAc/2Urea) allows C–N coupling reactions under bench-type conditions (room temperature/under air), and in the absence of external ligands. The use of DES also permits to: (i) recycle the catalytic system for up to four consecutive runs; (ii) scale-up the C–N coupling reaction (without erosion of the yield); and (iii) apply this methodology to the synthesis of the anti-inflammatory drug Flufenamic acid (E-factor: 10).
Sustainability spotlightThe use of renewable feedstocks and of more environmentally responsible solvents represents one of the strategies in addressing public concerns related to the environmental effects of products during use and as waste, thereby driving the field of synthetic chemistry towards more green practices. The Chan–Evans–Lam amination is an important coupling reaction between a boronic acid and a N–H containing compound, which is still carried out in volatile organic compounds. We propose a scalable Cu-catalysed protocol for the synthesis of secondary amines taking place with a wide substrate scope in Deep Eutectic Solvents as sustainable and recyclable media. This work is aligned with the UN sustainable development goals of: responsible consumption and production (SDG 12) and climate action (SDG 13). |
Introduction
The use of transition metal catalysts1 is nowadays considered as one of the cornerstone concepts for the design of more sustainable chemical protocols both at academic and industrial levels.2 Moreover, the implementation of transition-metal catalysis in the synthesis of high-value organic compounds usually permits to improve the selectivity of the process under study, which indirectly affects its efficiency, by increasing yields and reducing energy/raw materials consumption,3 in agreement with the principles of Green Chemistry.4 However, in most cases, these transition-metal-based catalytic systems are constructed from toxic, non-abundant and expensive metals (e.g., Ir, Rh, Pd, Ru, Au). Thus, the search for new catalytic routes to access fine chemicals and pharmaceuticals using non-precious metals is a “hot topic” in modern synthetic organic chemistry.5 Among the most commonly used first raw, cheap and abundant transition metals in catalysis (e.g., Cr, Mn, Fe, Co, Ni, Zn), copper is one of the best candidates as: (i) is capable to promote both two-electron or radical bond-formation processes; (ii) presents a rich and diverse redox chemistry [usually ranging from Cu(0) to Cu(I)/Cu(II), and even to Cu(III) species]; and (iii) is involved in both σ- or π-interactions with alkenes or alkynes.6 Needless to say, the recent Nobel Prize in Chemistry (2022) has been awarded for research on copper-catalysed azide–alkyne click chemistry.7Together with the choice of a safe, cheap and abundant transition metal as catalyst, another crucial point to take into consideration when designing a more sustainable catalytic chemical process is related to the choice of the solvent employed as the reaction medium. Solvents are, indeed, responsible for the vast majority of the waste generated in chemical synthetic processes, both at academic and industrial levels.8 In addition, a large part of the most commonly used organic solvents in organic synthesis, usually denoted as Volatile Organic Compounds (VOCs), are toxic (e.g., n-hexane, toluene, N,N-dimethylformamide, methanol) and/or flammable (e.g., Et2O, n-hexane, toluene, N,N-dimethylformamide, methanol, ethyl acetate, ethanol, t-butyl methyl ether), and, in some cases, also carcinogenic (e.g., dichloromethane, carbon tetrachloride).9
In principle, the ideal synthetic chemical protocol would be the one in which no solvent is used (the so-called neat conditions).10 In real chemical practice, there are very few synthetic protocols that can take place in the absence of an externally-added solvent. Thus, synthetic chemists have focused their attention on finding and using neoteric solvents (as replacement for classical VOCs), which present a reduced environmental impact.11 Deep Eutectic Solvents (DESs) are nowadays increasingly utilized as sustainable reaction media for a variety of chemical transformations (ranging from metal-, bio- and organocatalysis to main group chemistry, even including emerging fields related to photosynthesis or crystallization) as they show the following properties: (i) negligible vapour pressures; (ii) thermal stability; (iii) non-flammability; and (iv) easy recycling.12,13 DES concept traces back to 2003 when Abbott and collaborators first described an eutectic mixture with a low melting point (mp, 12 °C) obtained by mixing two solid compounds: choline chloride (ChCl, mp = 302 °C) and urea (mp = 133 °C).13a DESs are binary or ternary mixtures comprising at least one hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), which are strongly associated with each other to form a three-dimensional network structured through hydrogen bonds.14 A wide variety of cheap, non-toxic, renewable and easy accessible HBAs [e.g., ChCl, choline acetate (ChOAc)]15 and HBDs [e.g., naturally occurring amides, alcohols or carboxylic acids (e.g., urea, glycerol (Gly), sugars, lactic/citric acid)] are available for the synthesis of DESs.16 In addition, DES preparation: (i) requires no purifications steps; (ii) takes place with total atom economy and under bench-type reaction conditions; and (iii) is also accessible through efficient continuous synthesis by a twin-screw extrusion.17
Some of us have recently demonstrated that the partnership formed between Cu-catalysed organic transformations and DESs is an easily accessible, efficient and selective synthetic tool capable of working under mild and bench-type reaction-conditions for: (i) Goldberg- or Ullmann-type C–N or C–O couplings;18 (ii) the synthesis of poly(methyl methacrylate) through ARGET-ATRP polymerization;19 (iii) the chemo-enzymatic preparation of enantiopure (R)-β-hydroxy-1,2,3-triazoles;6e and (iv) the selective aerobic oxidation of alcohols into carbonyl compounds.20
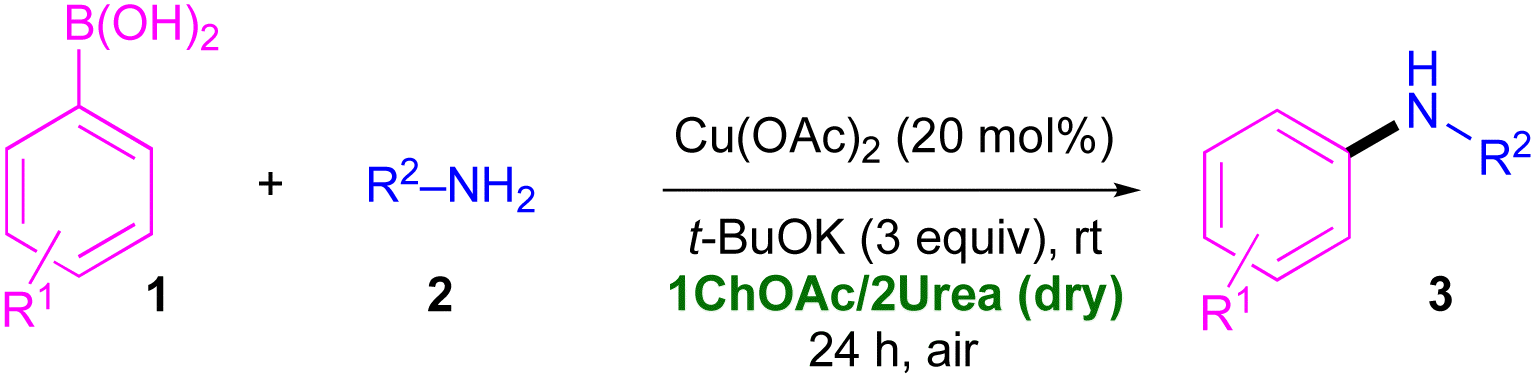
The Chan–Evans–Lam (CEL) amination is a coupling reaction between a boronic acid and a N–H containing compound induced by a stoichiometric or a catalytic amount of a Cu(II) salt. It has been traditionally carried out by using VOCs or VOC/alcohol/aqueous mixtures in air, with moderate to high heating, and in the presence of additives and/or additional ligands.21 To the best of our knowledge, CEL coupling has not yet been investigated in DESs.12c Thus, we decided to study the C–N bond formation in DES between a family of primary amines and different organoboron partners, like aromatic boronic acids, phenylboronic acid pinacole ester, or potassium phenyltrifluoroborate. After screening various conditions, the best conversions were obtained when using the eutectic mixture 1ChOAc/2Urea as the solvent (Scheme 1). Moreover, the following features are worth mentioning: (i) CEL takes place at room temperature (rt), under aerobic conditions and in the absence of ligands; (ii) a cheap Cu(OAc)2 salt is useful to promote the desired C–N coupling; (iii) the catalytic system could be recycled (up to 4 consecutive runs) and the process scaled-up; and (iv) the reported methodology has been applied to the synthesis of Flufenamic acid, which is a non-steroidal anti-inflammatory, selective COX-1 inhibitor.22
Results and discussion
We started our investigations by employing the archetypical DES formed by ChCl and urea (1ChCl/2Urea), taking as a model reaction the Cu(OAc)2-catalysed CEL C–N coupling between phenylboronic acid (1a) and 4-methoxyaniline (anisidine, 2a) (Table 1). We selected as catalyst Cu(OAc)2 as good efficiency and selectivity have been reported with this Cu(II)-source in the presence of different ligands and in polar solvents like water,23a,b MeOH23c or DMF.23d After 24 h, working at rt, under air and using t-BuOK as a base, the desired secondary amine 3a formed in poor yield (10%; entry 1, Table 1). We screened the nature of the HBD to include: (i) natural polyols [e.g., glycerol (Gly) forming the eutectic mixture 1ChCl/2Gly] (entry 2, Table 1); (ii) biorenewable carboxylic acids [e.g., lactic acid (LA), forming the eutectic mixture 1ChCl/2LA] (entry 3, Table 1); or (iii) water, forming the eutectic mixture 1ChCl/2H2O (entry 4, Table 1). However, in all cases, a complete shutdown of the CEL C–N coupling took place. We envisaged that a possible in situ scramble of anions between the acetate (AcO−) and the Cl− of the ChCl-based eutectic mixtures could convert the starting Cu(OAc)2 into CuCl2, the latter being previously described as a barely non-active catalyst for this transformation.23a| Entry | Catalyst | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a General conditions: reactions performed under air, at room temperature (rt) using 0.50 mmol of 1a and 0.25 mmol of 2a in 1 g of solvent. b Isolated yield. c Yield determined by 1H NMR analysis of the crude reaction mixture in the presence of the internal standard CH2Br2. d Reaction run under argon. e Conversion: 97%, by GC analysis. | ||||
| 1 | Cu(OAc)2 | 1ChCl/2Urea | t-BuOK | 10c |
| 2 | Cu(OAc)2 | 1ChCl/2Gly | t-BuOK | — |
| 3 | Cu(OAc)2 | 1ChCl/2LA | t-BuOK | — |
| 4 | Cu(OAc)2 | 1ChCl/2H2O | t-BuOK | <5c |
| 5 | Cu(OAc)2 | 1ChOAc/2Urea | t-BuOK | 70 |
| 6 | Cu(OAc)2 | 1ChOAc/2Urea | t-BuOK | 8c,d |
| 7 | Cu(OAc)2 | 1ChOAc/2Gly | t-BuOK | — |
| 8 | Cu(OAc)2 | Gly | t-BuOK | — |
| 9 | Cu(OAc)2 | EG | t-BuOK | — |
| 10 | Cu(OAc)2 | 1Lys/4.5Gly | t-BuOK | 36 |
| 11 | Cu(OAc)2 | 1Bet/3Gly | t-BuOK | 5c |
| 12 | Cu(OAc)2 | 1Arg/4.5Gly | t-BuOK | 16c |
| 13 | Cu(OAc)2 | 1Prol/3Gly | t-BuOK | — |
| 14 | Cu(OAc)2 | 1ChOAc/2Urea | — | — |
| 15 | — | 1ChOAc/2Urea | t-BuOK | — |
| 16 | Cu(OAc)2 | 1ChOAc/2Urea | K2CO3 | 12c |
| 17 | Cu(OAc)2 | 1ChOAc/2Urea | KOH | 5c |
| 18 | Cu(OAc)2 | 1ChOAc/2Urea | Cs2CO3 | 10c |
| 19 | Cu(OAc)2 | 1ChOAc/2Urea | HCOOK | 2c |
| 20 | Cu(OAc)2 | 1ChOAc/2Urea | CH3CO2K | — |
| 21 | Cu(NO3)2 | 1ChOAc/2Urea | t-BuOK | 20 |
| 22 | Cu(SO4) | 1ChOAc/2Urea | t-BuOK | 11c |
| 23 | CuCl2 | 1ChOAc/2Urea | t-BuOK | 7c |
| 24 | Cu(OAc)2·H2O | 1ChOAc/2Urea | t-BuOK | 30 |
| 25 | Cu(OAc)2 | 1ChOAc/2Urea (dry) | t-BuOK | 92 (97)e |
| 26 | CuI | 1ChOAc/2Urea | t-BuOK | — |
Thus, next experiments fulfilled the following requirements: (i) absence of chlorinated HBAs; and (ii) the presence of urea as HBD (compare entries 1–3, Table 1). Based on this premise, the eutectic mixture 1ChOAc/2Urea was selected and used, under the previously reaction conditions, with a considerable increase of the yield of 3a up to 70% (entry 5, Table 1).18c
On the other hand, upon running the reaction under Ar, formation of 3a was suppressed dramatically (8%) (entry 6, Table 1). This result is also in agreement with previous studies in this field,23 and with the proposed mechanism for this reaction.21
Either the replacement of urea with Gly as HBD in the above eutectic mixture, or with pure Gly or ethylene glycol (EG), was found to erode completely the catalytic activity of Cu(OAc)2 (entries 7–9, Table 1). Next, we tested the Cu(OAc)2-catalysed CEL C–N coupling protocol in the following eutectic mixtures, which are not based on ChCl: (i) 1Lys/4.5Gly (Lys = L-lysine; entry 10, Table 1); (ii) 1Bet/3Gly (Bet = betaine; entry 11, Table 1); (iii) 1Arg/4.5Gly (Arg = arginine; entry 12, Table 1); and (iv) 1Prol/3Gly (Prol = L-proline; entry 13, Table 1). However, previous results obtained with 1ChOAc/2Urea could not be improved (yields up to 36%). Thus, the proper selection of both components of the eutectic mixture is important for designing an effective transition-metal-catalysed protocol. As control experiments, we found that the reaction did not take place in the absence of the base (entry 14, Table 1) or the Cu(II)-catalyst (entry 15, Table 1). At this point, we decided to deepen the effect of different bases on the outcome of this catalytic reaction.
The replacement of either t-BuOK with various organic or inorganic bases (K2CO3, KOH, Cs2CO3, HCOOK, CH3CO2K) (entries 16–20, Table 1) or the acetate in Cu(OAc)2 with other anions (nitrate, sulphate, chloride) (entries 21–23, Table 1), as well as the use of Cu(OAc)·H2O (entry 24, Table 1), all resulted in a dramatic decrease in 3a yield. A careful analysis of the crude reaction mixtures by GC-MS, where yields of 3a were low or null (Table 1), revealed the formation of phenol and diphenyl ether as the main side products. According to Evans and co-workers,24 these products can originate from O-arylation of water (adventitious or deliberate addition), and the subsequent Cu-promoted arylation of phenol with the starting arylboronic acid, the latter process taking place competitively with the desired C–N cross-coupling reaction. Based on these and previous results related to the direct use of hygroscopic eutectic mixtures,18c,25 we run a new experiment in which the dry DES 1ChOAc/2Urea was used as the reaction medium. Satisfactorily, under these new conditions, the yield of the secondary amine 3a increased up to 92% (entry 25, Table 1). Finally, we confirmed that a anhydrous Cu(II) salt is crucial for the success of the reaction as a Cu(I) precursor (CuI) was ineffective (entry 26, Table 1). This result is in good agreement with previous reported examples in the field of Cu-catalysed CEL couplings in polar solvents.23 It is worth noting that, in order to catalytically generate the new C–N bond under copper catalysis, compound 3a has been alternatively isolated in 89% yield by reacting aniline (0.2 mmol) with the corresponding aryl boronic acid (2 equiv.) in MeOH, in the presence of K2CO3 (1 equiv.) and a tetradentate copper(II) N-heterocyclic carbene complex (8 mol%).26
With these satisfactory conditions in place, we explored the scope of this reaction with a series of anilines (2a–e, Table 2). The presence of electron-donating groups in the aromatic ring of the starting anilines [e.g., MeO (2a,b; entries 1, 2, Table 2)] afforded the secondary aromatic amines 3a,b in good to almost quantitative yields (79–92%) when using Ph-B(OH)2 (1a) as the coupling partner. The presence of electron-withdrawing substituents in the aromatic ring of the starting aniline [e.g., Cl– (2c), C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (2d), CF3 (2e)] produced similar or lower yields of the corresponding secondary anilines 3c–e (52–81%; entries 3–5, Table 2). Aniline 2f proved to be a competent reaction partner as well, yielding the corresponding diphenylamine (3f) in a good yield (80%, entry 6, Table 2). The employed catalytic system also tolerates the use of other aromatic/aliphatic primary amines like: (i) benzylamine (2g; entry 7, Table 2); (ii) 2-phenylethan-1-amine (2h; entry 8, Table 2); or (iii) aliphatic cyclohexanamine (2i; entry 9, Table 2), providing moderate to good yields of secondary amines 3g–i (40–70%). Aromatic boronic acids containing either electron-donating [e.g., Me (1b, entry 10, Table 2), Et (1c, entry 11, Table 2)] or electron-withdrawing groups [e.g., C
N (2d), CF3 (2e)] produced similar or lower yields of the corresponding secondary anilines 3c–e (52–81%; entries 3–5, Table 2). Aniline 2f proved to be a competent reaction partner as well, yielding the corresponding diphenylamine (3f) in a good yield (80%, entry 6, Table 2). The employed catalytic system also tolerates the use of other aromatic/aliphatic primary amines like: (i) benzylamine (2g; entry 7, Table 2); (ii) 2-phenylethan-1-amine (2h; entry 8, Table 2); or (iii) aliphatic cyclohexanamine (2i; entry 9, Table 2), providing moderate to good yields of secondary amines 3g–i (40–70%). Aromatic boronic acids containing either electron-donating [e.g., Me (1b, entry 10, Table 2), Et (1c, entry 11, Table 2)] or electron-withdrawing groups [e.g., C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (1d, entry 12, Table 2), CF3 (1e, entry 13, Table 2)] are also well tolerated, furnishing the anticipated non-symmetric secondary amines 3j–m in reasonable yields (52–67%).
N (1d, entry 12, Table 2), CF3 (1e, entry 13, Table 2)] are also well tolerated, furnishing the anticipated non-symmetric secondary amines 3j–m in reasonable yields (52–67%).
| Entry | R 1 (1a–e) | R 2 (2a–k) | Product 3 | Yield(%) b |
|---|---|---|---|---|
| a General conditions: reactions performed under air, at room temperature using 0.50 mmol of 1a–e and 0.25 mmol of 2a–k in 1 g of dry eutectic mixture 1ChOAc/2Urea. b Isolated yield. Cy = cyclohexyl. | ||||
| 1 | H (1a) | 4-MeOC6H4 (2a) | 3a | 92 |
| 2 | H (1a) | 2-MeOC6H4 (2b) | 3b | 79 |
| 3 | H (1a) | 4-ClC6H4 (2c) | 3c | 81 |
| 4 | H (1a) | 3-CN-4-tolyl (2d) | 3d | 62 |
| 5 | H (1a) | 3-CF3C6H4 (2e) | 3e | 52 |
| 6 | H (1a) | Ph (2f) | 3f | 80 |
| 7 | H (1a) | Benzyl (2g) | 3g | 67 |
| 8 | H (1a) | Ph(CH2)2 (2h) | 3h | 70 |
| 9 | H (1a) | Cy (2i) | 3i | 40 |
| 10 | 2-Me (1b) | 4-FC6H4 (2j) | 3j | 60 |
| 11 | 4-Et (1c) | 4-MeOC6H4 (2a) | 3k | 67 |
| 12 | 2-CN (1d) | 3-CF3C6H4 (2k) | 3l | 52 |
| 13 | 4-CF3 (1e) | 4-MeOC6H4 (2a) | 3m | 62 |
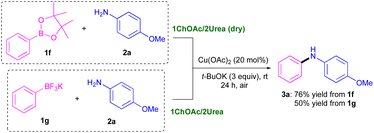
The described methodology could also be successfully applied to other boron-containing organometallic coupling partners, like acid pinacole ester (1f) or potassium phenyltrifluoroborate (1g),27 jointly with 2a, (Scheme 2), giving rise to the secondary aromatic amine 3a in moderate to good yields (50–76%).
In order to prove the robustness of our approach, we scaled-up this protocol up to 1 g of 1a (8.2 mmol) in 10 g of 1ChOAc/2Urea, (Scheme 3). Under these conditions, secondary amine 3a was isolated in similar yield (89%) after 24 h reaction time. One of the advantages associated with the use of biorenewable eutectic mixtures as solvents in transition-metal-catalysed organic transformations is the possibility to recycle the catalytic system/DES.12,13 To this end, both the study of the lifetime of a catalytic system and its level of reusability are crucial.28 The recyclability of Cu(OAc)2, the eutectic mixture and the base was assessed in the CEL C–N coupling between 1a and 2a in 1ChOAc/2Urea, when using t-BuOK as base, and working under aerobic conditions at rt (Scheme 3). Extraction of the reaction crude with cyclopentyl methyl ether (CPME),29 followed by the addition of new fresh reagents (1a and 2a), allowed the recycle of the catalyst, the 1ChOAc/2Urea mixture and the base for three consecutive runs with no reduction of the catalytic activity (Scheme 3). After 4 cycles, DES mass loss was within 5%. To better quantify the green credentials of the synthetic process developed, we calculated the Sheldon's environmental factor (E-factor; total mass of waste/mass of product),30 finding a value of 10,31 which is in good agreement with that suggested for fine chemicals (between 5 and 50).30
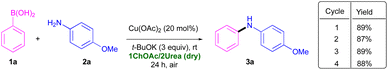 | ||
| Scheme 3 Cu(OAc)2-catalysed CEL C–N coupling between phenylboronic acid (1a) and 4-methoxyaniline (2a) in the eutectic mixture 1ChOAc/2Urea for the scaled-up synthesis of 3a, and recycling studies. | ||
Finally, we targeted the synthesis of Flufenamic acid (4), which is known to be a COX-1 inhibitor.22 This drug could be smoothly synthesized after hydrolysis of the nitrile moiety present in adduct 3j (98% yield), the latter in turn being obtained via a CEL C–N coupling between 1d and 2k (entry 12, Table 2) (Scheme 4). Flufenamic acid (4) was isolated with an overall yield of 51%.
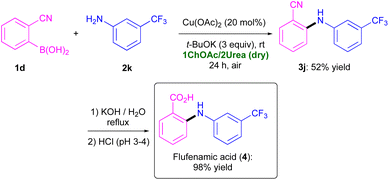 | ||
| Scheme 4 Synthesis of COX-1 inhibitor Flufenamic acid (4) through a Cu(OAc)2-catalysed CEL C–N coupling between 1d and 2k in the eutectic mixture 1ChOAc/2Urea, followed by hydrolysis. | ||
Conclusions
In summary, we have reported the first example of Cu(II)-catalysed Chan–Evans–Lam C–N coupling between a family of organoboron reagents and different primary amines in Deep Eutectic Solvents (DESs) as sustainable reaction media. The screening of the eutectic mixture's components identified 1ChOAc/2Urea as the best DES for carrying out the above coupling reaction, which took place: (i) under bench-type reaction conditions (rt/under air); (ii) in the absence of external ligands; and (iii) with high chemoselectivity, with electron-withdrawing and electron-donating groups being well tolerated.Additional benefits include: (i) the possibility to scale-up the process; (ii) the effective recycling of the catalytic system and DES (up to four consecutive runs), without erosion of the catalytic activity; (iii) a low E-factor value (up to 10); and (iv) the successful synthesis of Flufenamic acid, a COX-1 inhibitor. Overall, this methodology represents a reliable, sustainable, affordable and chemoselective synthetic tool for the formation of C–N bonds, which is considered one of the most important transformations in transition-metal-catalysed organic transformations.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
L. C. and J. G. A. thank MCIN/AEI/10.13039/501100011033 (projects number RED2018-102387-T and PID2020-113473GB-I00) for financial support. This work was carried out within the framework of the PRIN project “Unlocking Sustainable Technologies Through Nature-Inspired Solvents” (NATUREChem) (Grant No. 2017A5HXFC_002) financially supported by the Ministero dell’Università e della Ricerca (MUR-PRIN), the Interuniversity Consortium C.I.N.M.P.I.S., and the University of Bari Aldo Moro. L. C. also thanks the Erasmus+ Staff Mobility for a Training (SST) Programme 2020–2021.Notes and references
- (a) Homogeneous Catalysis: Understanding the Art, ed. P. W. N. M. van Leeuwen, Kluwer Academic Publishers, Amsterdam, 2004 Search PubMed; (b) J. F. Hartwig, Organotransition Metal Chemistry: from Bonding to Catalysis, University Science Books, Sausalito, 2010 Search PubMed; (c) Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective, ed. M. L. Crawley and B. M. Trost, John Wiley & Sons, Hoboken, 1st edn, 2012 Search PubMed; (d) H. Yorimitsu, M. Kotora and N. T. Patil, Chem. Rec., 2021, 21, 3335 CrossRef CAS PubMed.
- As G. Rothenberg stated in his book: “catalysis is the key to sustainability”: G. Rothenberg, Catalysis, Wiley-VCH, Weinheim, 2008 Search PubMed.
- P. Ball, Natl. Sci. Rev., 2015, 2, 202 CrossRef.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (b) A. S. Matlack, Introduction to Green Chemistry, Marcel Dekker, New York, 2001 Search PubMed; (c) M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807 CrossRef CAS PubMed; (d) M. Lancaster, Green Chemistry: An Introductory Text, RSC Publishing, Cambridge, 2002 Search PubMed; (e) I. T. Horváth, Chem. Rev., 2018, 118, 369 CrossRef.
- (a) J. R. Dunetz, D. Fandrick and H.-J. Federsel, Org. Process Res. Dev., 2015, 19, 1325 CrossRef CAS; (b) F. Buono, T. Nguyen, B. Qu, H. Wu and N. Haddad, Org. Process Res. Dev., 2021, 25, 1471 CrossRef CAS.
- (a) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed; (b) S. R. Chemler, Beilstein J. Org. Chem., 2015, 11, 2252 CrossRef CAS PubMed; (c) M. B. Gawande, A. Goswami, F.-X. Felpi, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722 CrossRef CAS PubMed; (d) Copper Catalysis in Organic Synthesis, ed. G. Anilkumar and S. Saranya, John Wiley & Sons, Hoboken, 2020 Search PubMed; (e) P. Vitale, F. Lavolpe, F. Valerio, M. Di Biase, F. M. Perna, E. Messina, G. Agrimi, I. Pisano and V. Capriati, React. Chem. Eng., 2020, 5, 859 RSC.
- The Royal Swedish Academy of Sciences, https://www.nobelprize.org/uploads/2022/10/advanced-chemistryprize2022-2.pdf, (accessed on December 2022).
- D. J. C. Constable, C. Jimenez-Gonzalez and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133 CrossRef CAS.
- (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2009, 39, 301 RSC; (b) R. Heinrich-Ramm, M. Jakubowski, B. Heinzow, J. M. Christensen, E. Olsen and O. Hertel, Pure Appl. Chem., 2000, 72, 385 CrossRef CAS.
- J. A. Gladysz, Chem, 2018, 4, 2007 CAS.
- P. T. Anastas, Handbook of Green Chemistry; Green Solvents, Wiley-VCH, Weinheim, 2010, vol 4–6 Search PubMed.
- (a) J. García-Álvarez, Deep Eutectic Solvents and Their Applications as New Green and Biorenewable Reaction Media. Handbook of Solvents, Vol. 2: Use, Health, and Environment, ed. G. Wypych, ChemTec Publishing, Toronto, 3rd edn, 2019 Search PubMed; (b) B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232 CrossRef CAS PubMed; (c) X. Marset and G. Guillena, Molecules, 2022, 27, 8445 CrossRef CAS; (d) S. E. Hooshmand, S. KumarI, I. Bahadur, T. Singh and R. S. Varma, J. Mol. Liq., 2023, 371, 121013 CrossRef CAS; (e) F. Milano, L. Giotta, M. R. Guascito, A. Agostiano, S. Sblendorio, L. Valli, F. M. Perna, L. Cicco, M. Trotta and V. Capriati, ACS Sustainable Chem. Eng., 2017, 5, 7768 CrossRef CAS; (f) B. D. Belviso, F. M. Perna, B. Carrozzini, M. Trotta, V. Capriati and R. Caliandro, ACS Sustainable Chem. Eng., 2021, 9, 8435 CrossRef CAS.
- (a) A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70 RSC , For general reviews in DESs chemistry, see:; (b) A. P. Abbott, R. C. Harris, K. Ryder, C. d'Agostino, L. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82 RSC; (c) C. Ruß and B. König, Green Chem., 2012, 14, 2969 RSC; (d) D. Carriazo, M. C. Serrano, M. C. Gutiérrez, M. L. Ferrer and F. Del Monte, Chem. Soc. Rev., 2012, 41, 4996 RSC; (e) Q. Zhang, K. de Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108 RSC; (f) F. Del Monte, D. Carriazo, M. C. Serrano, M. C. Gutierrez and M. L. Ferrer, ChemSusChem, 2014, 7, 999 CrossRef CAS PubMed; (g) A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063 CrossRef CAS; (h) F. Pena-Pereira and J. Namieśnik, ChemSusChem, 2014, 7, 1784 CrossRef CAS PubMed; (i) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060 CrossRef CAS PubMed; (j) M. J. Rodríguez-Álvarez, S. E. García-Garrido, S. Perrone, J. García-Álvarez and V. Capriati, Curr. Opin. Green Sustainable Chem., 2023, 39, 100723 CrossRef; (k) L. Cicco, G. Dilauro, F. M. Perna, P. Vitale and V. Capriati, Org. Biomol. Chem., 2021, 19, 2558 RSC.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, J. Solution Chem., 2019, 48, 962 CrossRef CAS.
- J. K. Blusztajn, Choline chloride (ChCl, an essential micro- and human nutrient) is manufactured in the scale of millions of tons per year, Science, 1998, 284, 794 CrossRef PubMed.
- There is a recent controversy about DES toxicity and risks, especially when DES components (like amides of dialkanolamines) could form nitrosamine; see: (a) J. Torregrosa-Crespo, X. Marset, G. Guillena, D. J. Ramón and R. M. Martínez-Espinosa, Sci. Total Environ., 2020, 704, 135382 CrossRef CAS PubMed; (b) T. Shaikh, A. Gosar and H. Sayyed, J. Adv. Pharm. Res., 2020, 2, 48 Search PubMed; (c) R. N. Loeppky, W. Tomasik and T. G. Millard, IARC Sci. Publ., 1984, 57, 353 CAS.
- D. E. Crawford, L. A. Wright, S. L. James and A. P. Abbott, Chem. Commun., 2016, 52, 4215 RSC.
- (a) A. F. Quivelli, P. Vitale, F. M. Perna and V. Capriati, Front. Chem., 2019, 7, 723 CrossRef CAS PubMed; (b) L. Cicco, J. A. Hernández-Fernández, A. Salomone, P. Vitale, M. Ramos-Martín, J. González-Sabín, A. Presa Soto, F. M. Perna, V. Capriati and J. García-Álvarez, Org. Biomol. Chem., 2021, 19, 1773 RSC; (c) A. F. Quivelli, F. V. Rossi, P. Vitale, J. García-Álvarez, F. M. Perna and V. Capriati, ACS Sustainable Chem. Eng., 2022, 10, 4065 CrossRef CAS; (d) A. F. Quivelli, M. Marinò, P. Vitale, J. García-Álvarez, F. M. Perna and V. Capriati, ChemSusChem, 2022, 15, e202102211 CrossRef CAS PubMed.
- L. Quirós-Montes, G. A. Carriedo, J. García-Álvarez and A. Presa Soto, Green Chem., 2019, 21, 5865 RSC.
- L. Cicco, M. Roggio, M. López-Aguilar, M. Ramos-Martín, F. M. Perna, J. García-Álvarez, P. Vitale and V. Capriati, ChemistryOpen, 2022, 11, e202200160 CrossRef CAS PubMed.
- (a) D. M. T. Chan, K. L. Monaco, R.-P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933 CrossRef CAS; (b) D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937 CrossRef CAS; (c) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941 CrossRef CAS; (d) J. X. Qiao and P. Y. S. Lam, Recent Advances in Chan–Lam Coupling Reaction: Copper-Promoted C–Heteroatom Bond Cross Coupling Reactions with Boronic Acids and Derivatives, Boronic Acids, Wiley-VCH, Hoboken, 2011, pp. 315–361 Search PubMed; (e) P. Y. S. Lam, Chan-Lam Coupling Reaction: Copper-Promoted C-Element Bond Oxidative Coupling Reaction with Boronic Acids. Synthetic Methods in Drug Discovery, The Royal Society of Chemistry, Cambridge, 2016, ch. 7, vol 1, pp. 242–273 Search PubMed; (f) J. X. Qiao and P. Y. S. Lam, Synthesis, 2011, 6, 829 CrossRef; (g) S. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang and D. Ma, Angew. Chem., Int. Ed., 2017, 56, 16136 CrossRef CAS PubMed.
- (a) T. Daikoku, D. Wang, S. Tranguch, J. D. Morrow, S. Orsulic, R. N. DuBois and S. K. Dey, Cancer Res., 2005, 65, 3735 CrossRef CAS; (b) P. Vitale, A. Panella, A. Scilimati and M. G. Perrone, Med. Res. Rev., 2016, 36, 641 CrossRef CAS; (c) P. Malerba, B. C. Crews, K. Ghebreselasie, C. K. Daniel, E. Jashim, A. M. Aleem, R. A. Salam, L. J. Marnett and Md. J. Uddin, ACS Med. Chem. Lett., 2020, 11, 1837 CrossRef CAS PubMed.
- (a) A. Gogoi, G. Sarmah, A. Dewan and U. Bora, Tetrahedron Lett., 2014, 55, 31 CrossRef CAS; (b) N. Gogoi, G. Bora and P. K. Gogoi, Heteroat. Chem., 2018, 29, e21414 CrossRef; (c) N. Akatyev, M. Il', M. Il'in(Jr), S. Peregudova, A. Peregudov, A. Buyanovskaya, K. Kudryavtsev, A. Dubovik, V. Grinberg, V. Orlov, A. Pavlov, V. Novikov, I. Volkov and Y. Belokon, ChemCatChem, 2020, 12, 3010 CrossRef CAS; (d) S. P. Vibhute, P. M. Mhaldar, D. D. Gaikwad, R. V. Shejwal and D. M. Pore, Monatsh. Chem., 2020, 151, 87 CrossRef CAS . The Cu(OAc)2-catalysed N-arylation of primary and secondary amines with PhB(OH2) using a 20% aqueous solution of n-Bu4NOH has been also reported; see:; (e) H. Molaei and M. M. Ghanbari, Chin. Chem. Lett., 2012, 23, 301 CrossRef CAS . For a recent chapter which covers the Cu-catalysed CEL couplings in water, see:; (f) N. Nebra and J. García-Álvarez, Cu-Catalyzed Organic Reactions in Aqueous Media, Copper Catalysis in Organic Synthesis, ed. G. Anilkumar and S. Saranya, Wiley-VCH, Hoboken, 2020, pp. 73–102 Search PubMed.
- D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937 CrossRef CAS.
- L. Sapir and D. Harries, J. Chem. Theory Comput., 2020, 16, 3335 CrossRef CAS PubMed.
- J. D. Cope, P. E. Sheridan, C. J. Galloway, R. F. Awoyemi, S. L. Stokes and J. P. Emerson, Organometallics, 2020, 39, 4457 CrossRef CAS.
- The 1ChOAc/2Urea eutectic mixture was not anhydrified as it was found that the incorporation of water in a hydrophilic DES is key in promoting the hydrolysis of organotrifluoroborates to the corresponding boronic acids, which are required for the transmetalation step; see: (a) G. Dilauro, S. M. García, D. Tagarelli, P. Vitale, F. M. Perna and V. Capriati, ChemSusChem, 2018, 11, 3495 CrossRef CAS PubMed; (b) V. Pelliccioli, G. Dilauro, S. Grecchi, S. Arnaboldi, C. Graiff, F. M. Perna, P. Vitale, E. Licandro, A. Aliprandi, S. Cauteruccio and V. Capriati, Eur. J. Org. Chem., 2020, 6981 CrossRef CAS.
- (a) Catalyst Separation, Recovery and Recycling. Chemistry and Process Design, ed. D. Cole-Hamilton and R. Tooze, Springer, Dordrecht, 2006 Search PubMed; (b) Recoverable and Recyclable Catalyst, ed. M. Benaglia, Wiley, Chichester, UK, 2009 Search PubMed.
- Cyclopentyl methyl ether (CPME) has been proposed as a possible green ethereal solvent thank to its low peroxide formation, high hydrophobicity and high boiling point; see: U. Azzena, M. Carraro, L. Pisano, S. Monticelli, R. Bartolotta and V. Pace, ChemSusChem, 2019, 12, 40 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
- For more details on the E-factor calculation, see ESI.‡.
Footnotes |
| † Dedicated to Professor Ilan Marek on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00093a |
| This journal is © The Royal Society of Chemistry 2023 |