Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Base metal iron catalyzed sustainable oxidation of vanillyl alcohol to vanillic acid in deep eutectic solvents and implementation of vanillic acid for fine-chemical synthesis†
Rahul
Ghosh
,
Surajit
Panda
 ,
Ashutosh
MahaKhuda
,
Ratnakar
Saha
and
Bidraha
Bagh
,
Ashutosh
MahaKhuda
,
Ratnakar
Saha
and
Bidraha
Bagh
 *
*
School of Chemical Sciences, National Institute of Science Education and Research (NISER), An OCC of Homi Bhabha National Institute, Bhubaneswar, Jatni, Khurda, Odisha 752050, India. E-mail: bidraha@niser.ac.in
First published on 25th May 2023
Abstract
In the modern era, sustainable development for the production of fine chemicals from abundant biomass by utilizing various chemical transformations has become a strong trend of research in the scientific community. This may provide a sustainable alternative to petrochemicals as the major source of fine chemicals. Lignin is an alternative major source of monomeric phenolic compounds, and the syntheses of fine chemicals by oxidising lignin-based monomeric phenolics are gaining serious attention. For instance, biomass derived vanillin or vanillyl alcohol can be oxidized to vanillic acid which has been employed as a new building block for the syntheses of various value-added products. In this context, an air-stable iron(II) complex has been synthesized and utilized as an excellent base-metal catalyst for the selective oxidation of vanillyl alcohol to vanillic acid. We used hydrogen peroxide and tert-butyl hydroperoxide as green oxidants. These peroxidative oxidations of vanillyl alcohol to vanillic acid were performed in metal-free type-III deep eutectic solvents as green and sustainable reaction media. After the first set of oxidation reactions, the catalyst and the reaction medium were recycled five times without any noticeable change in catalytic performance. The CHEM21 green metrics toolkit was also used to examine the sustainable and green features of the optimized oxidation protocol for the conversion of vanillyl alcohol to vanillic acid. Low E-factors (4.65) suggest waste minimized sustainable oxidations of vanillyl alcohol to vanillic acid. Finally, vanillic acid was used as a starting material for the syntheses of several fine chemicals with various (potential) applications such as flavorants, odorants, surfactants and bio-based plasticizers.
Sustainability spotlightLignocellulosic biomass waste is an encouraging carbon feedstock for the sustainable production of fine chemicals. Particularly, various value-added aromatic phenolic chemicals can be obtained from lignin, and vanillyl alcohol is a prominent example. Vanillyl alcohol can be oxidized to vanillic acid which is an interesting building block for the production of various fine chemicals. In this context, we have developed an efficient iron catalyst for the selective peroxidative oxidation of vanillyl alcohol to vanillic acid in deep eutectic solvents as reusable reaction media. Vanillic acid was utilized as a starting material for the syntheses of several fine chemicals with practical applications. The present work emphasizes the following UN sustainable development goals: industry, innovation, and infrastructure (SDG 9) and responsible consumption and production (SDG 12). |
Introduction
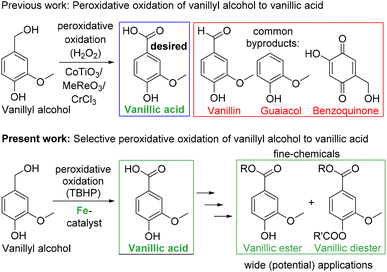
Fossil fuels are undoubtedly the primary source of the majority of useful organic chemicals and their needs are ever growing to satisfy the demand of the rising human population with better quality of living standards. But petro-based resources have limited reserves and they are non-renewable. Hence, their exponentially growing demand will certainly cause a shortage in the foreseeable future. In addition, the extensive use of fossil fuels has generated various serious environmental concerns. Therefore, searching for alternatives to non-renewable petroleum resources has become a strong trend in research in the present era. In this context, the proper use of biomass feedstock, which is renewable, can certainly provide a vital alternative to fossil fuels. Thus, bio-refineries may play an important role in biomass valorization and the use of a biorefinery to produce a wide range of fine chemicals has attracted serious attention.1,2 Hemicellulose, cellulose and lignin are the three primary components of lignocellulosic biomass. A majority of the bio-originated fine chemicals are aliphatic in nature, and cellulose is considered as their major source.3 In contrast, lignin contains a wide variety of aromatic compounds, and thus, the effective valorization of lignin in a biorefinery is an essential task. Already, lignin, as the second largest resource of biomass, has been established as a major source of aromatic fine chemicals including fuel-grade compounds, and several synthetic protocols of lignin-valorization are presently operational.4,5 Lignin produced from wood and cashew nutshell liquid is an important source of aromatic phenolic compounds such as p-coumaryl, p-sinapyl, coniferyl, veratryl and vanillyl alcohol.6 In this context, oxidation of lignin-based aromatic phenolic compounds to value-added products has attracted significant research interest. Vanillyl alcohol is considered as one of the most studied lignin-derived monomeric phenolic compounds, which is further oxidized to vanillin, an aroma molecule with wide application in industry.7–9 In addition, reactive groups in vanillin can be functionalized easily, and thus, vanillin is also an interesting building block.10,11 Various groups have developed synthetic catalytic protocols for the oxidation of vanillyl alcohol to vanillin. Recently, we reported a sustainable copper-catalyzed aerobic oxidation protocol for the selective oxidation of vanillyl alcohol to vanillin.12 So far, the research community has paid little attention to the selective oxidation of vanillyl alcohol to vanillic acid which is often found as an over-oxidation product (along with other by-products) during the oxidation of vanillyl alcohol to vanillin. However, vanillic acid has huge potential as a building block for the syntheses of various functional materials. For example, several simple esters of vanillic acids such as methyl, ethyl and butyl vanillate are effective perfuming agents in various commodities such as deodorants, room deodorizers, skin- and haircare products, cosmetics and toiletries. Ito and co-workers systematically evaluated the antioxidative properties of methyl, ethyl and butyl vanillate in multiple antioxidant assays and compared them with those of well-known antioxidants.13 Ma and co-workers utilized vanillic acid for the synthesis of thermoplastic polyesters with improved mechanical properties.14 Gauthier and co-workers used vanillic acid for the synthesis of polyesters as a bio-based alternative to commonly used polyethylene terephthalate (PET).15 These vanillic acid-based polyesters exhibited good thermal stability. D'Arrigo and Griffini and coworkers utilized vanillic acid for the development of biobased polyurethane coatings with high biomass content, enhanced thermal stability and good adhesion performance.16 Recently, Yang and coworkers synthesized vanillic diesters with different alkyl chains, which were used as effective plasticizers for poly(vinyl chloride) (PVC).17 These bio-based vanillic diesters have provided a good alternative to traditional phthalate plasticizers, and the resulting PVC materials showed similar or better flexibility and stretchability as compared to a PVC blend of dioctyl phthalate. Therefore, it is of high importance to develop an efficient catalytic protocol for the selective oxidation of vanillyl alcohol to vanillic acid with high potential applicability.Selective oxidation of vanillyl alcohol to vanillic acid has been very much ignored in contrast to the selective oxidation of vanillyl alcohol to vanillin. In a process of targeted oxidation of vanillyl alcohol to vanillin, vanillic acid has often been found as an over-oxidation product.18–25 There are few reports for the selective oxidation of vanillyl alcohol or vanillin to vanillic acid. In a very old report, a stoichiometric amount of silver oxide (in situ generated from AgNO3 and NaOH) was used as an oxidant to convert vanillin to vanillic acid.26 The reaction was performed in basic aqueous solution at an elevated temperature. Recently, Repo and coworkers reported heterogeneous gold nanoparticles supported on titania and alumina as a catalyst for the selective oxidation of vanillin to vanillic acid.27 The oxidation was performed in an aqueous alkaline medium at an elevated temperature (80 °C) using pressurized oxygen as the oxidant. Very recently, Jiang and Wei and coworkers reported selective oxidation of vanillyl alcohol to vanillic acid (95% conversion and 86% isolated yield) using an inorganic ligand-supported FeMo6 system as a heterogeneous catalyst.28 The oxidation was performed in water at 70 °C using O2 as the oxidant and KCl as the additive. In addition, a few reports described enzymatic oxidation of vanillin to vanillic acid using unheated milk,29 soil bacteria30 and freshly prepared liver slices of guinea-pig.31 Compared to the oxidation of vanillin to vanillic acid, the relatively difficult oxidation of vanillyl alcohol to vanillic acid is very rare and often accompanied by the formation of a significant amount of byproducts (Scheme 1). Ali and Hamid and coworkers used heterogeneous mixed metal oxide (CoTiO3) for the peroxidative oxidation of vanillyl alcohol in various organic solvents, and high selectivity to vanillic acid could be obtained.32 However, a significant amount of byproducts such as vanillin and guaiacol were also noted. Immobilized methyltrioxo rhenium has also been used as a heterogeneous catalyst for the same purpose.33 The peroxidative oxidation was performed in acetic acid. Microwave assisted peroxidative oxidation of vanillyl alcohol to vanillic acid by using metal salts (particularly CrCl3 and MnCl2) is also reported in a mixture of water and an organic solvent.34 In both cases, selectivity to either vanillic acid (10–12%) or vanillin (50–60%) was not good. In contrast to these reports, we have developed a highly selective catalytic protocol for the peroxidative oxidation of vanillyl alcohol to vanillic acid at r.t. (Scheme 1). Utilization of a base metal iron catalyst (in low loading) is also advantageous. In contrast to the use of organic solvents, the use of environmentally benign deep eutectic solvents is beneficial.
The oxidation of alcohols to the corresponding carbonyl compounds or their complete oxidation to acids are among the central reactions in organic chemistry and are of interest for the development of environmentally benign processes, and production of new materials and energy sources.35,36 Various hypervalent iodine compounds,37 chromium salts,38 activated DMSO compounds39 and manganese dioxide40 have been used as stoichiometric oxidants for alcohol oxidation. Stoichiometric oxidations are still in common use, despite the formation of a large amount of undesirable by-products. Therefore, there is a need for oxidants which are environmentally benign. Recent environmental compatibility and sustainable approaches lead to two types of oxidation which are aerobic oxidation and peroxidative oxidation, and various metal-catalysts have been developed. Mostly, hydrogen peroxide41–43 and tert-butyl hydrogen peroxide44–48 have been used as green oxidants and the by-products are non-hazardous. However, aerobic oxidation utilizes air as the cheapest, most sustainable and green oxidant. In addition, the by-products are water and hydrogen peroxide, which are environmentally benign. However, pressurized oxygen cylinders are often used. Although various metal-catalysts (both homogeneous and heterogeneous) have been developed,49–59 copper is a heavily used metal in aerobic oxidation of alcohols.60–64 In the context of aerobic oxidation of lignin-model compound vanillyl alcohol, the research community has particularly focused on the transformation of vanillyl alcohol selectively to vanillin with wide applications in commodity products. Various heterogeneous catalysts, particularly a wide range of metal oxides and mixed metal oxides, have been utilized for the selective aerobic oxidation of vanillyl alcohol to vanillin.65–76 However, high temperature and high oxygen pressure have been used in most of the reports. In this context, the use of homogeneous catalysts is often beneficial; however, the number of reports is limited for the selective aerobic oxidation of vanillyl alcohol to vanillin.77–79 We have also contributed in this field by developing air-stable and recyclable copper catalysts. Similar to aerobic oxidation, there are only a few examples of a homogeneous system for peroxidative oxidation of vanillyl alcohol to vanillin.80–82 Although selective aerobic oxidation of vanillyl alcohol to vanillin as an important aroma chemical has been studied extensively, selective oxidation of vanillyl alcohol to vanillic acid has been ignored. In the present report, we describe the development of a base-metal catalyzed peroxidative oxidation protocol for selective oxidation of vanillyl alcohol to vanillic acid (Scheme 1). An air-stable iron-complex has proved to be an excellent catalyst in various deep eutectic solvents at r.t. in the absence of added additives. We also demonstrated the syntheses of various vanillic esters and diesters as (potential) value-added chemicals.
Results and discussion
Pincer ligands are considered as one of the most well-known, widely used and important class of ligands, and coordination chemists have successfully utilized these tridentate ligands for the syntheses of a wide variety of transition metal complexes.83,84 A large majority of these complexes have found applications in varieties of catalytic transformations in the last twenty years. Often one or more phosphine donor arms are found in a large number of pincer ligands such as PNP, PNN and PCP, and these phosphine-containing pincer ligands generally form air-sensitive metal complexes. In addition, alkylidene and carbanions are central in various other pincer ligands which also result in air-sensitive metal complexes. Therefore, phosphine-free (and carbene-free) ligand synthesis is important for the development of air-stable complexes and gaining serious attention in homogeneous catalysis.85–89 Particularly, air-sensitive metal complexes are of no use in the field of oxidation of alcohols. Therefore, we wanted to use a tridentate NNN pincer ligand which is expected to give good stability of the resulting metal complex by tridentate coordination and might give an air-stable metal complex. We selected a known NNN pincer ligand L1 which can be readily synthesized from cheap commercially available starting materials (Scheme 2). The condensation of 2-pyridinecarboxaldehyde with 2-morpholino ethylamine in ethanol under reflux yielded the Schiff base imine intermediate which was further reduced with sodium borohydride to get the desired amine ligand L1 (Scheme 2). Ligand L1 was characterized by standard characterization techniques such as 1H and 13C NMR spectroscopy. Thereafter, we focused on the synthesis of the metal complex. We selected iron as it is non-toxic, cheap and earth-abundant (most abundant transition metal and the second most abundant metal). Facile coordination of L1 with cheap iron(II) acetate at r.t. in air resulted in the formation of complex 1 as an air-stable solid (Scheme 2).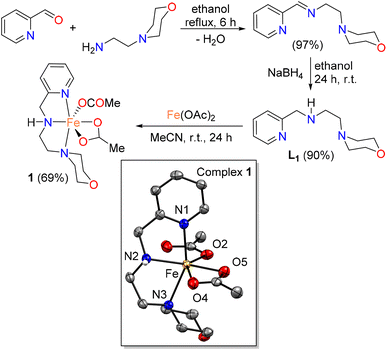 | ||
| Scheme 2 Synthesis of complex 1 (the molecular structure of complex 1 showing 70% ellipsoids, and all hydrogen atoms except N–H are omitted for clarity). | ||
Complex 1 was NMR silent and was characterized by FT-IR, mass and elemental analyses. In the mass spectrum, a peak was observed at 395.0230 which corresponds to [M–H]+. C(sp3)–H symmetric and asymmetric stretching bands of ligand L1 were noticed at 2900–2750 cm−1. The corresponding C–H stretching bands were shifted to 3000–2900 cm−1 for the coordinated ligand in complex 1. Similar shifting of the band from 1658 cm−1 to 1632 cm−1 was also observed for pyridyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching. The broad N–H stretching appeared at around 3400 cm−1 in the IR spectrum of the free ligand L1, but a sharp peak at 3200 cm−1 indicates the presence of a characteristic Fe–NH moiety in complex 1.90–92 Furthermore, the geometrical identity of complex 1 was confirmed by single crystal X-ray crystallography. Complex 1 crystallized in an orthorhombic system with space group Pna21. X-ray analysis reveals that the asymmetric unit has one [Fe(L1)(OAc)2] unit and the geometry around the iron center is distorted octahedral with the acetate groups occupying the equatorial sites. The pyridyl nitrogen (N1) and morpholine nitrogen (N3) coordinated axially to the metal center. The Fe–Npyridyl bond distance is 2.207(2) Å while the Fe–Nmorpholine distance is 2.378(2) Å. The rest of the bond distances and angles are in good agreement with those of previously reported similar iron(II) complexes with octahedral geometry.93–95 It is worth mentioning that the distance between amine nitrogen (N2) and iron is 2.179(19) Å which is similar to the previously reported Fe–Namine distances.96–98 Extensive N–H⋯O and C–H⋯O hydrogen bonding interactions as well as C–H⋯π interactions are also observed in the solid-state structure of complex 1.
N stretching. The broad N–H stretching appeared at around 3400 cm−1 in the IR spectrum of the free ligand L1, but a sharp peak at 3200 cm−1 indicates the presence of a characteristic Fe–NH moiety in complex 1.90–92 Furthermore, the geometrical identity of complex 1 was confirmed by single crystal X-ray crystallography. Complex 1 crystallized in an orthorhombic system with space group Pna21. X-ray analysis reveals that the asymmetric unit has one [Fe(L1)(OAc)2] unit and the geometry around the iron center is distorted octahedral with the acetate groups occupying the equatorial sites. The pyridyl nitrogen (N1) and morpholine nitrogen (N3) coordinated axially to the metal center. The Fe–Npyridyl bond distance is 2.207(2) Å while the Fe–Nmorpholine distance is 2.378(2) Å. The rest of the bond distances and angles are in good agreement with those of previously reported similar iron(II) complexes with octahedral geometry.93–95 It is worth mentioning that the distance between amine nitrogen (N2) and iron is 2.179(19) Å which is similar to the previously reported Fe–Namine distances.96–98 Extensive N–H⋯O and C–H⋯O hydrogen bonding interactions as well as C–H⋯π interactions are also observed in the solid-state structure of complex 1.
With the air-stable iron complex (1) in hand, we explored the catalytic activity of complex 1 for the peroxidative oxidation of vanillyl alcohol by using tert-butyl hydroperoxide (TBHP) as a standard oxidant (Table 1). Most homogeneous catalytic systems for peroxidative/aerobic oxidation operate in hazardous organic solvents such as acetonitrile, toluene and dichloromethane. However, we wanted to perform the oxidation of vanillyl alcohol in a sustainable reaction medium. Previously, we utilized mixtures of water and green organic solvents such as acetone, methanol and ethanol for the selective aerobic oxidation of vanillyl alcohol to vanillin. Finding new green reaction media for sustainable chemical transformations is important, and we continued our search for other potential sustainable reaction media as an alternative to water or other non-hazardous organic solvents. We turned our attention to deep eutectic solvents (DESs). In 2003, Abbott and coworkers reported DESs for the first time as eutectic mixtures of urea and various quaternary ammonium salts.99 DESs, eutectic mixtures of a hydrogen bond acceptor and a hydrogen bond donor, are considered as an emerging type of sustainable and green reaction media because of their minor economic and environmental impact. DESs come with several advantages over ionic liquids which are associated with several serious drawbacks such as high cost, high toxicity, complex synthesis and purification processes, and nonbiodegradability.100–106 So far, DESs have limited use as solvents in organic reactions. With our goal of finding alternative sustainable reaction media, we decided to use a common DES, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (molar ratio) mixture of choline chloride and glycerol, as the reaction medium for the peroxidative oxidation of vanillyl alcohol (Table 1). We started using 2 eq. of TBHP as the oxidant. Using 5 mol% of catalyst loading, we obtained complete conversion (with a near quantitative yield) of vanillyl alcohol to vanillic acid in 3 h at r.t. (entry 1). Reducing the catalyst loading to 3 (entry 2) and 2 mol% (entry 3) also gave full conversion to vanillic acid in 3 h at r.t. If the reaction time was shortened to just 1 h, complete conversion of vanillyl alcohol to vanillic acid was observed in the presence of 2 mol% of catalyst loading (entry 3). If the catalyst loading was further reduced to just 1 mol%, vanillyl alcohol could not be completely oxidised to vanillic acid in 1 h at r.t. (entry 4). The obvious trend of decreasing catalytic activity with decreasing catalyst loading was observed from entry 3 to entry 4. 2 mol% catalyst loading is enough for full conversion of vanillyl alcohol (0.25 mmol) in 1 h, but 1 mol% catalyst loading is not capable of performing complete oxidation of 0.25 mmol vanillyl alcohol in 1 h. Vanillin as an intermediate oxidation product was also observed if the peroxidative oxidation of vanillyl alcohol was run for just half an hour in the presence of 2 mol% catalyst loading at r.t. (entry 5). However, vanillyl alcohol was completely oxidized to vanillic acid in just half an hour if the reaction temperature was increased to 70 °C (entry 6). Thereafter, we used hydrogen peroxide instead of TBHP and a much reduced yield of vanillic acid was obtained in 1 h along with a decent amount of vanillin as an intermediate oxidation product (entry 7). Thereafter, we increased the reaction time to 3 h in the presence of hydrogen peroxide as the oxidant and complete conversion of vanillyl alcohol to vanillin was noted (entry 8). If the reaction was performed in air without adding any other peroxide oxidant, we did not observe any oxidation of vanillyl alcohol to vanillic acid; less than 10% vanillin formation was noted (entry 9). So, TBHP was the best oxidant in the present system. Although slightly inferior than TBHP, hydrogen peroxide also acted as a good oxidant in the present transformation. We also carried out the peroxidative oxidation of vanillyl alcohol with TBHP in commonly used organic solvents such as acetonitrile (entry 10), toluene (entry 11) and dichloromethane (entry 12); however, poor conversion to vanillic acid was noted and presence of vanillin as well as unreacted vanillyl alcohol was also detected. Very poor conversion (<10%) was noted in the absence of any iron catalyst (entry 13). In the presence of iron salts such as Fe(NO3)3 and Fe(OAc)2, a small amount of vanillic acid (<30%) was formed (entries 14 and 15). Peroxidative oxidation of vanillyl alcohol with TBHP was also performed under inert conditions (in the absence of dioxygen) using optimized reaction conditions and complete conversion of vanillic acid was noted.
2 (molar ratio) mixture of choline chloride and glycerol, as the reaction medium for the peroxidative oxidation of vanillyl alcohol (Table 1). We started using 2 eq. of TBHP as the oxidant. Using 5 mol% of catalyst loading, we obtained complete conversion (with a near quantitative yield) of vanillyl alcohol to vanillic acid in 3 h at r.t. (entry 1). Reducing the catalyst loading to 3 (entry 2) and 2 mol% (entry 3) also gave full conversion to vanillic acid in 3 h at r.t. If the reaction time was shortened to just 1 h, complete conversion of vanillyl alcohol to vanillic acid was observed in the presence of 2 mol% of catalyst loading (entry 3). If the catalyst loading was further reduced to just 1 mol%, vanillyl alcohol could not be completely oxidised to vanillic acid in 1 h at r.t. (entry 4). The obvious trend of decreasing catalytic activity with decreasing catalyst loading was observed from entry 3 to entry 4. 2 mol% catalyst loading is enough for full conversion of vanillyl alcohol (0.25 mmol) in 1 h, but 1 mol% catalyst loading is not capable of performing complete oxidation of 0.25 mmol vanillyl alcohol in 1 h. Vanillin as an intermediate oxidation product was also observed if the peroxidative oxidation of vanillyl alcohol was run for just half an hour in the presence of 2 mol% catalyst loading at r.t. (entry 5). However, vanillyl alcohol was completely oxidized to vanillic acid in just half an hour if the reaction temperature was increased to 70 °C (entry 6). Thereafter, we used hydrogen peroxide instead of TBHP and a much reduced yield of vanillic acid was obtained in 1 h along with a decent amount of vanillin as an intermediate oxidation product (entry 7). Thereafter, we increased the reaction time to 3 h in the presence of hydrogen peroxide as the oxidant and complete conversion of vanillyl alcohol to vanillin was noted (entry 8). If the reaction was performed in air without adding any other peroxide oxidant, we did not observe any oxidation of vanillyl alcohol to vanillic acid; less than 10% vanillin formation was noted (entry 9). So, TBHP was the best oxidant in the present system. Although slightly inferior than TBHP, hydrogen peroxide also acted as a good oxidant in the present transformation. We also carried out the peroxidative oxidation of vanillyl alcohol with TBHP in commonly used organic solvents such as acetonitrile (entry 10), toluene (entry 11) and dichloromethane (entry 12); however, poor conversion to vanillic acid was noted and presence of vanillin as well as unreacted vanillyl alcohol was also detected. Very poor conversion (<10%) was noted in the absence of any iron catalyst (entry 13). In the presence of iron salts such as Fe(NO3)3 and Fe(OAc)2, a small amount of vanillic acid (<30%) was formed (entries 14 and 15). Peroxidative oxidation of vanillyl alcohol with TBHP was also performed under inert conditions (in the absence of dioxygen) using optimized reaction conditions and complete conversion of vanillic acid was noted.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio)a
2 molar ratio)a
| Entry | 1 (mol%) | Oxidant (eq.) | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
a Reactions conducted in a vial (10 mL) with 0.25 mmol of vanillyl alcohol, 0.50 mmol of oxidant, and 5/3/2/1 mol% of complex 1 in 2 mL of solvent at r.t./100 °C. DES: choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
b Yields were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene (0.25 mmol) as the standard.
c Isolated yields of vanillic acid. 2).
b Yields were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene (0.25 mmol) as the standard.
c Isolated yields of vanillic acid.
|
||||||
| 1 | 5 | TBHP (2) | DES | r.t. | 12/6/3 | >99 (96c) |
| 2 | 3 | TBHP (2) | DES | r.t. | 3 | >99 (97c) |
| 3 | 2 | TBHP (2) | DES | r.t. | 3/2/1 | >99 (97c) |
| 4 | 1 | TBHP (2) | DES | r.t. | 1 | 78 (75c) |
| 5 | 2 | TBHP (2) | DES | r.t. | 0.5 | 72 (68c) |
| 6 | 2 | TBHP (2) | DES | 70 | 0.5 | >99 (96c) |
| 7 | 2 | H2O2 (2) | DES | r.t. | 1 | 60 |
| 8 | 2 | H 2 O 2 (2) | DES | r.t. | 3 | >99 (97 ) |
| 9 | 2 | Air | DES | r.t. | 1 | 0 |
| 10 | 2 | TBHP (2) | MeCN | r.t. | 1 | 57 |
| 11 | 2 | TBHP (2) | Toluene | r.t. | 1 | 46 |
| 12 | 2 | TBHP (2) | DCM | r.t. | 1 | 53 |
| 13 | No | TBHP (2) | DES | r.t. | 1 | <10 |
| 14 | Fe(NO3)3 | TBHP (2) | DES | r.t. | 1 | 24 |
| 15 | Fe(OAc)2 | TBHP (2) | DES | r.t. | 1 | 28 |
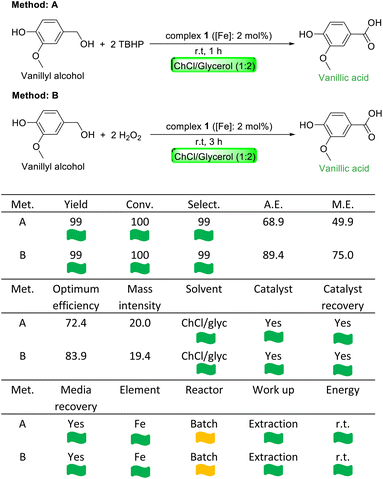
The best optimized reaction conditions for the peroxidative oxidation of vanillyl alcohol to vanillic acid are the following: (A) 2 mol% complex 1, 2 eq. TBHP, choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the reaction medium, 1 h and r.t. (entry 3 in Table 1, depicted in bold) and (B) 2 mol% complex 1, 2 eq. hydrogen peroxide, choline chloride/glycerol (1
2) as the reaction medium, 1 h and r.t. (entry 3 in Table 1, depicted in bold) and (B) 2 mol% complex 1, 2 eq. hydrogen peroxide, choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the reaction medium, 3 h and r.t. (entry 8 in Table 1, depicted in bold). So, we successfully used a common DES (choline chloride/glycerol (1
2) as the reaction medium, 3 h and r.t. (entry 8 in Table 1, depicted in bold). So, we successfully used a common DES (choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
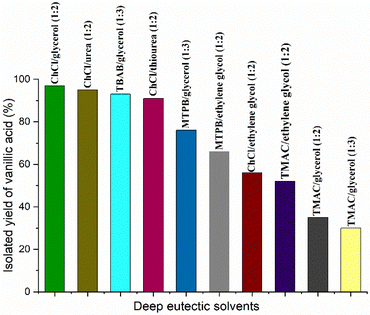
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) as a sustainable reaction medium. We also wanted to check the catalytic performance of complex 1 for the peroxidative oxidation of vanillyl alcohol to vanillic acid in various other DESs. Therefore, we prepared a series of DESs (in total ten) by combining various hydrogen-bond acceptors such as choline chloride (ChCl), tetramethyl ammonium chloride (TMAC), tetra butyl ammonium bromide (TBAB) and methyl triphenyl phosphonium bromide (MTPB) and various hydrogen bond donors such as glycerol, urea, thiourea and ethylene glycol. The catalytic oxidation of vanillyl alcohol was performed using TBHP as the oxidant in the above DESs using previously optimized reaction conditions (condition A, entry 3 in Table 1) and the results are summarized in Table 2 and Fig. 1. Complete oxidation of vanillyl alcohol to vanillic acid with almost quantitative isolated yields was observed in ChCl/glycerol (1
2)) as a sustainable reaction medium. We also wanted to check the catalytic performance of complex 1 for the peroxidative oxidation of vanillyl alcohol to vanillic acid in various other DESs. Therefore, we prepared a series of DESs (in total ten) by combining various hydrogen-bond acceptors such as choline chloride (ChCl), tetramethyl ammonium chloride (TMAC), tetra butyl ammonium bromide (TBAB) and methyl triphenyl phosphonium bromide (MTPB) and various hydrogen bond donors such as glycerol, urea, thiourea and ethylene glycol. The catalytic oxidation of vanillyl alcohol was performed using TBHP as the oxidant in the above DESs using previously optimized reaction conditions (condition A, entry 3 in Table 1) and the results are summarized in Table 2 and Fig. 1. Complete oxidation of vanillyl alcohol to vanillic acid with almost quantitative isolated yields was observed in ChCl/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl/urea (1
2) and ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (entries 1 and 2). Excellent yields of vanillic acid (92–93%) were also obtained in TBAB/glycerol (1
2) (entries 1 and 2). Excellent yields of vanillic acid (92–93%) were also obtained in TBAB/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and ChCl/thiourea (1
3) and ChCl/thiourea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (entries 3 and 4). Using MTPB/glycerol (1
2) (entries 3 and 4). Using MTPB/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and MTPB/ethylene glycol (1
3) and MTPB/ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as reaction media, we noted 76 and 65% yields of vanillic acid, respectively (entries 5 and 6). A bit more than 50% yield of vanillic acid was observed if the oxidations of vanillyl alcohol were carried out in ChCl/ethylene glycol (1
2) as reaction media, we noted 76 and 65% yields of vanillic acid, respectively (entries 5 and 6). A bit more than 50% yield of vanillic acid was observed if the oxidations of vanillyl alcohol were carried out in ChCl/ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and TMAC/ethylene glycol (1
2) and TMAC/ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (entries 7 and 8). Very poor yields (30–35%) of vanillic acid were noted using TMAC/glycerol (1
2) (entries 7 and 8). Very poor yields (30–35%) of vanillic acid were noted using TMAC/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and TMAC/glycerol (1
2) and TMAC/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the reaction media (entries 9 and 10). In case of incomplete oxidation to vanillic acid, we observed unreacted starting material vanillyl alcohol as well as vanillin as an intermediate oxidation product. Therefore, it can be concluded that ChCl/glycerol (1
3) as the reaction media (entries 9 and 10). In case of incomplete oxidation to vanillic acid, we observed unreacted starting material vanillyl alcohol as well as vanillin as an intermediate oxidation product. Therefore, it can be concluded that ChCl/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl/urea (1
2) and ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) are the best reaction media for the catalytic peroxidative oxidation of vanillyl alcohol to vanillic acid.
2) are the best reaction media for the catalytic peroxidative oxidation of vanillyl alcohol to vanillic acid.
| Entry | 1 (mol%) | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions conducted in a vial (10 mL) with 0.25 mmol of vanillyl alcohol, 0.50 mmol of TBHP, and 2 mol% of complex 1 in 2 mL of DES (molar ratio) as the solvent at r.t. b Isolated yields of vanillic acid. ChCl, TBAB, TMAC, MTPB, MTAC and EG stand for choline chloride, tetra butyl ammonium bromide, tetramethyl ammonium chloride, methyl triphenyl phosphonium bromide and ethylene glycol, respectively. | |||||
| 1 | 2 | ChCl/glycerol (1 : 2) | r.t. | 1 | 97 |
| 2 | 2 | ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
r.t. | 1 | 95 |
| 3 | 2 | TBAB/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
r.t. | 1 | 93 |
| 4 | 2 | ChCl/thiourea (1 : 2) | r.t. | 1 | 92 |
| 5 | 2 | MTPB/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
r.t. | 1 | 76 |
| 6 | 2 | MTPB/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
r.t. | 1 | 65 |
| 7 | 2 | ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
r.t. | 1 | 56 |
| 8 | 2 | TMAC/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
r.t. | 1 | 52 |
| 9 | 2 | TMAC/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
r.t. | 1 | 35 |
| 10 | 2 | TMAC/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
r.t. | 1 | 30 |
To establish the practical viability of the present catalytic protocol, we successfully performed two gram-scale reactions using two previously optimized reaction conditions and the product vanillic acid was isolated in almost quantitative yield (methods A and B in Table 3). We also used the CHEM21 green metrics toolkit to examine the green and sustainable features of the optimized catalytic protocol for the peroxidative oxidation of vanillyl alcohol to vanillic acid (see the ESI† for details).107–110 The results are summarized in Table 3 (green flag, amber flag and red flag stand for an acceptable process, acceptable process with concerns and undesirable process, respectively). An almost quantitative yield, conversion and selectivity were noted for both methods A and B and these metrics were assigned with green flags. Method B is better than method A in terms of atom economy (A.E.), reaction mass efficiency (M.E.) and optimum efficiency. This is due to the formation of a lighter byproduct, water, in method B as compared to the formation of a heavier byproduct, tert-butanol, in method A. An environmentally benign solvent was used for these catalytic oxidations and thus, both solvent and catalyst metrics got green flags. Base metal iron was used as the catalyst and element metrics of both methods received green flags. In addition to the use of a cheap and abundant base metal based catalyst, it is important to recycle and reuse a catalyst for sustainable catalytic development. The role of the solvent is equally important as solvents in a chemical reaction often produce the most amount of chemical waste. As a consequence, recycling the reaction media is even more important than catalyst recycling. Therefore, we set out to explore the possibility of catalyst and reaction media recovery and reuse. After performing the catalytic oxidation of vanillyl alcohol to vanillic acid using standard optimized reaction conditions, we extracted the product vanillic acid with ethyl acetate, another green solvent. For both methods A and B, the leftover mixture of reaction media and the catalyst was reused for the second set of peroxidative oxidation reactions of vanillyl alcohol. We did not observe any noticeable change in catalytic activity (isolated yields of vanillic acid: 97% in method A and 96% in method B). After the second set of reactions, the recovered reaction media and catalyst were reused another four times without any significant change in activity for both methods. Therefore, the solution of the iron catalyst in DES (ChCl/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) was effectively recycled five times. A TON of 49 was obtained for the first run of both methods. If all six consecutive oxidations are considered, a rough TON of 290 was calculated. We also calculated the E-factor, a reliable measure to estimate the quantity of waste generated for the production of one kilogram of the desired product.111,112 A favourable process should have an E-factor of 1 to 5. The calculated E-factor for both methods A and B is 4.65.
2)) was effectively recycled five times. A TON of 49 was obtained for the first run of both methods. If all six consecutive oxidations are considered, a rough TON of 290 was calculated. We also calculated the E-factor, a reliable measure to estimate the quantity of waste generated for the production of one kilogram of the desired product.111,112 A favourable process should have an E-factor of 1 to 5. The calculated E-factor for both methods A and B is 4.65.
In our catalytic oxidation protocol, vanillic acid was synthesized from vanillyl alcohol which is a renewable and abundant biomaterial derived from lignin biomass. Recently, vanillic acid has been utilized for the syntheses of various value-added derivatives with wide potential applications.13–17 Finally, we tested the practical applicability of the present catalytic protocol for the oxidation of vanillyl alcohol to vanillic acid and the scope of this optimized oxidation protocol was extended to real-life applications. The robustness of this catalytic process motivated us to test its applicability in synthesizing various esters with proven applications (Scheme 3). At first we targeted the carboxylic moiety of vanillic acid to synthesize various vanillic esters with different alkyl chains (Scheme 3a). Vanillic acid was reacted with various alcohols in the presence of p-toluenesulfonic acid and the corresponding vanillic esters E1a, E1b, E1c, E1d and E1e were isolated in excellent yields (E1a: 97%, E1b: 96%, E1c: 97%, E1d: 94% and E1e: 98%). Methyl, ethyl and butyl vanillate (E1a, E1b and E1c) are effective aroma chemicals used as perfuming agents in various commodities. Long chain fatty esters find possible application as surfactants. In the following step, we targeted the syntheses of several vanillic diesters which were previously used as effective plasticizers for poly(vinyl chloride). We used butyl vanillate as the starting material, and esterification of the hydroxyl moiety of butyl vanillate with acyl chloride in the presence of triethylamine resulted in the formation of various vanillic diesters (Scheme 3b). Four vanillic diesters E2a, E2b, E2c and E2d were isolated in excellent yields (E2a: 95%, E2b: 98%, E2c: 91% and E2d: 93%).
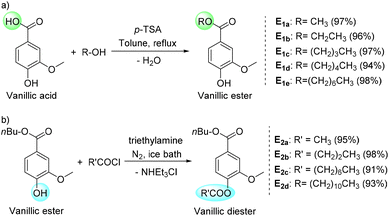 | ||
| Scheme 3 Application of vanillic acid in the syntheses of (a) vanillic esters and (b) diesters with (potential) applications. | ||
Conclusions
An air-stable phosphine-free NNN pincer ligand L1 was readily synthesized from cheap commercially available reagents. Facile coordination of the tridentate pincer ligand L1 with cheap iron(II) acetate resulted in the formation of a mononuclear octahedral complex 1 which was characterized by IR spectroscopy, mass spectrometry, elemental analysis and single crystal X-ray diffraction study. Complex 1 was proved to be an efficient catalyst for the selective peroxidative oxidation of vanillyl alcohol to vanillic acid (via vanillin as an intermediate oxidation product). Selective oxidation of vanillyl alcohol to vanillin as an important aroma molecule is well studied. Although vanillic acid as an abundant renewable biomaterial has been proven to be an interesting building block in recent studies, selective oxidation of vanillyl alcohol to vanillic acid is ignored by the research community. Developing a sustainable catalytic protocol for selective oxidation of vanillyl alcohol to vanillic acid is an important development. Previously demonstrated methods utilized heterogeneous metal oxides or metal salts based on non-abundant metals in organic solvents. A green solvent such as water was used very rarely; however, a large amount of salt-additive was used. In contrast, using a base metal iron catalyst is undoubtedly advantageous as iron is cheap and non-toxic and the second most abundant metal in the earth’s crust. Instead of using commonly used hazardous organic solvents for alcohol oxidations such as acetonitrile, toluene and dichloromethane, the use of environmentally friendly DESs as reaction media is also sustainable. A major setback of the previously reported processes is the generation of a significant or large amount of by-products. In contrast, our catalytic oxidation method is extremely selective to the desired product vanillic acid. Purification of the final product was carried out by a simple work-up process (extraction with ethyl acetate). Although catalyst recycling was performed in a previous report, the recovery and reuse of the reaction medium was not performed for the oxidation of vanillyl alcohol to vanillic acid. In the present work, the catalyst and reaction media were recovered and recycled effectively without any noticeable change in catalytic activity. This helps to minimize the waste generated in the present process and it is reflected in low E-factors (4.65). As solvents produce a huge amount of waste in most of the chemical processes, the recycling of the reaction medium in the present catalytic oxidation method is beneficial for the environment. In addition, the optimized condition used a metal-free type-III DES and the components glycerol and choline chloride are not harmful (as reflected by green H-codes). Gram-scale syntheses of vanillic acid show the robust nature of this catalytic oxidation protocol. In previous reports, catalytic methods showed the oxidation of vanillyl alcohol or vanillin to vanillic acid; however, further utilization of vanillic acid was not demonstrated. In the present report, the potential applicability of this oxidation process is illustrated by synthesizing various vanillic esters and diesters with (potential) applications in real-life. This base metal catalyzed oxidation of vanillyl alcohol to vanillic acid is realistic for possible future use in industry. However, we performed the catalytic oxidation in batches. Oxidation in a continuous flow reactor would be more attractive. In the future, we will explore the possibility of performing this selective oxidation of vanillyl alcohol to vanillic acid in a continuous flow reactor. Our peroxidative oxidation protocol requires H2O2 and TBHP as oxidants and produces water and tert-butanol (though green) as by-product waste. Aerobic oxidation using air as the most sustainable oxidant is more attractive, and we will focus on developing a base metal catalyst for aerobic oxidation of vanillyl alcohol to vanillic acid.Experimental
Detailed experimental processes are included in the ESI.†Synthesis of 1
A mixture of L1 (0.221 g, 1.00 mmol) and Fe(OAc)2 (0.173 g, 1.00 mmol) in acetonitrile (25 mL) under an inert atmosphere was stirred at r.t. for 24 h resulting in a dark yellow precipitate. All volatiles were removed under high vacuum to give a dark yellow solid which was extracted with dichloromethane (15 mL). Then the solvent was removed under high vacuum to give a yellow solid as pure complex 1 (0.272 g, 69%). X-ray quality single crystals were obtained by slow diffusion of diethyl ether into a solution of 1 in dichloromethane. HRMS (ESI-TOF) m/z: [M–H]+ calcd for C16H25FeN3O5 395.1029; found 395.0230. Anal. calcd for C16H26FeN3O5 (396.54): C, 48.50; H, 6.61; N, 10.60. Found: C, 49.21; H, 6.87; N, 10.01.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank SERB New Delhi (ECR/2018/000003), DAE, and NISER for financial support. R. G., S. P. and R. S. acknowledge NISER for fellowships.References
- B. Kamm, P. R. Gruber and M. Kamm, in Biorefineries–Industrial Processes and Products, Status Quo and Future Directions, Wiley-VCH Verlag GmbH, Weinheim, 2006, vol. 1 Search PubMed.
- J. H. Clark, R. Luque and A. Matharu, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 183–207 CrossRef CAS PubMed.
- M. N. Belgacem and A. Gandini, Monomers, Polymers and Composites from Renewable Resources, Elsevier, 2011 Search PubMed.
- P. Sudarsanam, R. Zhong, S. Van den Bosch, S. M. Coman, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2018, 47, 8349–8402 RSC.
- W. Deng, H. Zhang, X. Wu, R. Li, Q. Zhang and Y. Wang, Green Chem., 2015, 17, 5009–5018 RSC.
- R. Behling, S. Valange and G. Chatel, Green Chem., 2016, 18, 1839–1854 RSC.
- D. J. Fitzgerald, M. Stratford and A. Narbad, Int. J. Food Microbiol., 2003, 86, 113–122 CrossRef CAS PubMed.
- N. J. Walton, M. J. Mayer and A. Narbad, Phytochemistry, 2003, 63, 505–515 CrossRef CAS PubMed.
- L. Esposito, K. Formanek, G. Kientz, F. Mauger, V. Maureaux, G. Robert and F. Truchet, Kirk-Othmer Encyclopedia of Chemical Technology, 4th edn, 1997, vol. 24, pp. 812–825 Search PubMed.
- H.-R. Bjørsvik and F. Minisci, Org. Process Res. Dev., 1999, 3, 330–340 CrossRef.
- M. Fache, B. Boutevin and S. Caillol, ACS Sustainable Chem. Eng., 2016, 4, 35–46 CrossRef CAS.
- N. C. Jana, S. Sethi, R. Saha and B. Bagh, Green Chem., 2022, 24, 2542–2556 RSC.
- A. Tai, T. Sawano and H. Ito, Biosci., Biotechnol., Biochem., 2012, 76, 314–318 CrossRef CAS PubMed.
- C. Pang, J. Zhang, G. Wu, Y. Wang, H. Gao and J. Ma, Polym. Chem., 2014, 5, 2843–2853 RSC.
- S. Zhang, Z. Cheng, S. Zeng, G. Li, J. Xiong, L. Ding and M. Gauthier, J. Appl. Polym. Sci., 2020, 137, 49189–49198 CrossRef CAS.
- J. C. de Haro, C. Allegretti, A. T. Smit, S. Turri, P. D'Arrigo and G. Griffini, ACS Sustainable Chem. Eng., 2019, 7, 11700–11711 CrossRef CAS.
- H. Zhu, J. Yang, M. Wu, Q. Wu, J. Liu and J. Zhang, ACS Sustainable Chem. Eng., 2021, 9, 15322–15330 CrossRef CAS.
- P. Elamathi, M. K. Kolli and G. Chandrasekar, Int. J. Nanosci., 2017, 17, 1760010 CrossRef.
- Y. Zhou, W. Xiong, Y. Jin, P. Wang, W. Wei, J. Ma and X. Zhang, Green Chem., 2023, 25, 1179–1190 RSC.
- A. Kumar and R. Srivastava, ACS Appl. Nano Mater., 2021, 4, 9080–9093 CrossRef CAS.
- M.-W. Zheng, K.-Y. A. Lin and C.-H. Lin, Waste Biomass Valorization, 2019, 11, 6917–6928 CrossRef.
- W. Sun, P. Lin, Q. Tang, F. Jing, Q. Cao and W. Fang, Catal. Sci. Technol., 2021, 11, 7268–7277 RSC.
- M.-W. Zheng, H.-K. Lai and K.-Y. A. Lin, Waste Biomass Valorization, 2018, 10, 2933–2942 CrossRef.
- Z. L. Yuan, S. H. Chen and B. Liu, J. Mater. Sci., 2017, 52, 164–172 CrossRef CAS.
- K.-Y. A. Lin, H.-K. Lai and Z.-Y. Chen, J. Taiwan Inst. Chem. Eng., 2017, 78, 337–343 CrossRef CAS.
- I. A. Pearl, Org. Synth., 1963, 4, 972–976 Search PubMed.
- S. Rautiainen, J. Chen, M. Vehkamäki and T. Repo, Top. Catal., 2016, 59, 1138–1142 CrossRef CAS.
- H. Yu, J. Ren, Y. Xie, X. Su, A. Wang, L. Yan, F. Jiang and Y. Wei, Green Chem., 2022, 24, 6511–6516 RSC.
- S. Kuramoto, R. Jenness and S. T. Coulter, J. Dairy Sci., 1957, 40, 187–191 CrossRef CAS.
- G. W. J. Robbins and E. C. Lathrop, Soil Sci., 1919, 7, 475–485 CrossRef.
- G. Panoutsopoulos and C. I. Beedham, Cell. Physiol. Biochem., 2005, 15, 89–98 CrossRef CAS PubMed.
- M. Shilpy, M. A. Ehsan, T. H. Ali, S. B. Abd Hamid and M. E. Ali, RSC Adv., 2015, 5, 79644–79653 RSC.
- C. Crestini, M. C. Caponi, D. S. Argyropoulos and R. Saladino, Bioorg. Med. Chem., 2006, 14, 5292–5302 CrossRef CAS PubMed.
- J. Pan, J. Fu and X. Lu, Energy Fuels, 2015, 29, 4503–4509 CrossRef CAS.
- T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037–3058 CrossRef CAS PubMed.
- E. B. da Silva, M. Zabkova, J. Araújo, C. Cateto, M. Barreiro, M. Belgacem and A. Rodrigues, Chem. Eng. Res. Des., 2009, 87, 1276–1292 CrossRef.
- Q.-G. Ren, S.-Y. Chen, X.-T. Zhou and H.-B. Ji, Bioorg. Med. Chem., 2010, 18, 8144–8149 CrossRef CAS PubMed.
- G. Cainelli and G. Cardillo, in Chromium Oxidations in Organic Chemistry, Springer, 1984, pp. 118–216 Search PubMed.
- T. T. Tidwell, Synthesis, 1990, 1990, 857–870 CrossRef.
- L. Blackburn and R. J. Taylor, Org. Lett., 2001, 3, 1637–1639 CrossRef CAS.
- H. Ünver and I. Kani, Polyhedron, 2017, 134, 257–262 CrossRef.
- H. Ünver and I. Kani, J. Chem. Sci., 2018, 130, 33–41 CrossRef.
- C. Wu, B. Liu, X. Geng, Z. Zhang, S. Liu and Q. Hu, Polyhedron, 2019, 158, 334–341 CrossRef CAS.
- A. N. Kulakova, A. N. Bilyachenko, M. M. Levitsky, V. N. Khrustalev, A. A. Korlyukov, Y. V. Zubavichus, P. V. Dorovatovskii, F. Lamaty, X. Bantreil, B. Villemejeanne, J. Martinez, L. S. Shul’pina, E. S. Shubina, E. I. Gutsul, I. A. Mikhailov, N. S. Ikonnikov, U. y. S. Tsareva and G. B. Shul’pin, Inorg. Chem., 2017, 56, 15026–15040 CrossRef CAS PubMed.
- S. Hazra, L. M. D. R. S. Martins, M. F. C. Guedes da Silva and A. J. L. Pombeiro, RSC Adv., 2015, 5, 90079–90088 RSC.
- L. M. T. Frija, E. C. B. A. Alegria, M. Sutradhar, M. L. S. Cristiano, A. Ismael, M. N. Kopylovich and A. J. L. Pombeiro, J. Mol. Catal. A: Chem., 2016, 425, 283–290 CrossRef CAS.
- M. Sutradhar, T. Roy Barman, A. J. L. Pombeiro and L. M. D. R. S. Martins, Catalysts, 2019, 9, 1053–1067 CrossRef CAS.
- Z. Ma, Q. Wang, E. C. B. A. Alegria, M. F. C. Guedes da Silva, L. M. D. R. S. Martins, J. P. Telo, I. Correia and A. J. L. Pombeiro, Catalysts, 2019, 9, 424–446 CrossRef CAS.
- S. Zavahir, Q. Xiao, S. Sarina, J. Zhao, S. Bottle, M. Wellard, J. Jia, L. Jing, Y. Huang and J. P. Blinco, ACS Catal., 2016, 6, 3580–3588 CrossRef CAS.
- Q. Gao, C. Giordano and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 11740–11744 CrossRef CAS PubMed.
- R. Ray, S. Chandra, D. Maiti and G. K. Lahiri, Chem.–Eur. J., 2016, 22, 8814–8822 CrossRef CAS PubMed.
- S. Devari, R. Deshidi, M. Kumar, A. Kumar, S. Sharma, M. Rizvi, M. Kushwaha, A. P. Gupta and B. A. Shah, Tetrahedron Lett., 2013, 54, 6407–6410 CrossRef CAS.
- H. Su, K.-X. Zhang, B. Zhang, H.-H. Wang, Q.-Y. Yu, X.-H. Li, M. Antonietti and J.-S. Chen, J. Am. Chem. Soc., 2017, 139, 811–818 CrossRef CAS PubMed.
- Z. Wu and K. L. Hull, Chem. Sci., 2016, 7, 969–975 RSC.
- C. J. Weiss, P. Das, D. L. Miller, M. L. Helm and A. M. Appel, ACS Catal., 2014, 4, 2951–2958 CrossRef CAS.
- J. N. Jaworski, S. D. McCann, I. A. Guzei and S. S. Stahl, Angew. Chem., 2017, 129, 3659–3664 CrossRef.
- X. Ning, Y. Li, H. Yu, F. Peng, H. Wang and Y. Yang, J. Catal., 2016, 335, 95–104 CrossRef CAS.
- H. Li, F. Qin, Z. Yang, X. Cui, J. Wang and L. Zhang, J. Am. Chem. Soc., 2017, 139, 3513–3521 CrossRef CAS PubMed.
- J. Zhu, Y. Zhao, D. Tang, Z. Zhao and S. A. Carabineiro, J. Catal., 2016, 340, 41–48 CrossRef CAS.
- G. Zhang, E. Liu, C. Yang, L. Li, J. A. Golen and A. L. Rheingold, Eur. J. Inorg. Chem., 2015, 2015, 939–947 CrossRef CAS.
- A. G. Mahmoud, M. F. C. Guedes da Silva, E. I. Śliwa, P. Smoleński, M. L. Kuznetsov and A. J. L. Pombeiro, Chem.–Asian J., 2018, 13, 2868–2880 CrossRef CAS PubMed.
- E. Lagerspets, K. Lagerblom, E. Heliövaara, O.-M. Hiltunen, K. Moslova, M. Nieger and T. Repo, J. Mol. Catal., 2019, 468, 75–79 CrossRef CAS.
- L. Marais, J. Burés, J. H. L. Jordaan, S. Mapolie and A. J. Swarts, Org. Biomol. Chem., 2017, 15, 6926–6933 RSC.
- A. Ochen, R. Whitten, H. E. Aylott, K. Ruffell, G. D. Williams, F. Slater, A. Roberts, P. Evans, J. E. Steves and M. J. Sanganee, Organometallics, 2019, 38, 176–184 CrossRef CAS.
- R. Ma, Y. Xu and X. Zhang, ChemSusChem, 2015, 8, 24–51 CrossRef CAS PubMed.
- A. Jha, K. R. Patil and C. V. Rode, ChemPlusChem, 2013, 78, 1384–1392 CrossRef CAS PubMed.
- A. Jha and C. V. Rode, New J. Chem., 2013, 37, 2669–2674 RSC.
- J. Zhang, F. Zhang, S. Guo and J. Zhang, New J. Chem., 2018, 42, 11117–11123 RSC.
- S. Saha and S. B. Abd Hamid, RSC Adv., 2017, 7, 9914–9925 RSC.
- P. R. G. N. Reddy, B. G. Rao, T. V. Rao and B. M. Reddy, Catal. Lett., 2019, 149, 533–543 CrossRef CAS.
- C. S. Hinde, D. Ansovini, P. P. Wells, G. Collins, S. V. Aswegen, J. D. Holmes, T. S. A. Hor and R. Raja, ACS Catal., 2015, 5, 3807–3816 CrossRef CAS.
- I. B. Baguc, M. Celebi, K. Karakas, I. E. Ertas, M. N. Keles, M. Kaya and M. Zahmakiran, ChemistrySelect, 2017, 2, 10191–10198 CrossRef CAS.
- S. Ramana, B. G. Rao, P. Venkataswamy, A. Rangaswamy and B. M. Reddy, J. Mol. Catal. A: Chem., 2016, 415, 113–121 CrossRef CAS.
- L. Yang, R. Ma, H. Zeng, Z. Rui and Y. Li, Catal. Today, 2020, 355, 280–285 CrossRef CAS.
- W. Sun, S. Wu, Y. Lu, Y. Wang, Q. Cao and W. Fang, ACS Catal., 2020, 10, 7699–7709 CrossRef CAS.
- A. I. Martín-Perales, D. Rodríguez-Padrón, A. García Coleto, C. Len, G. de Miguel, M. J. Muñoz-Batista and R. Luque, Ind. Eng. Chem. Res., 2020, 59, 17085–17093 CrossRef.
- J. J. Bozell, B. R. Hames and D. R. Dimmel, J. Org. Chem., 1995, 60, 2398–2404 CrossRef CAS.
- P. Zucca, F. Sollai, A. Garau, A. Rescigno and E. Sanjust, J. Mol. Catal. A: Chem., 2009, 306, 89–96 CrossRef CAS.
- J. Hu, Y. Hu, J. Mao, J. Yao, Z. Chen and H. Li, Green Chem., 2012, 14, 2894–2898 RSC.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- C. Crestini, P. Pro, V. Neri and R. Saladino, Bioorg. Med. Chem., 2005, 13, 2569–2578 CrossRef CAS PubMed.
- H. Lange, S. Decina and C. Crestini, Eur. Polym. J., 2013, 49, 1151–1173 CrossRef CAS.
- M. A. W. Lawrence, K.-A. Green, P. N. Nelson and S. C. Lorraine, Polyhedron, 2018, 143, 11–27 CrossRef CAS.
- E. Peris and R. H. Crabtree, Chem. Soc. Rev., 2018, 47, 1959–1968 RSC.
- A. Jana, K. Das, A. Kundu, P. R. Thorve, D. Adhikari and B. Maji, ACS Catal., 2020, 10, 2615–2626 CrossRef CAS.
- X. J. Wu, H. J. Wang, Z. Q. Yang, X. S. Tang, Y. Yuan, W. Su, C. Chen and F. Verpoort, Org. Chem. Front., 2019, 6, 563–570 RSC.
- S. Panda, R. Saha, S. Sethi, R. Ghosh and B. Bagh, J. Org. Chem., 2020, 85, 15610–15621 CrossRef CAS PubMed.
- R. Ghosh, R. R. Behera, S. Panda, S. K. Behera, N. C. Jana and B. Bagh, ChemCatChem, 2022, e202201062 Search PubMed.
- R. Saha, S. Panda, A. Nanda and B. Bagh, J. Org. Chem., 2003 DOI:10.1021/acs.joc.2c02026.
- I. Koehne, T. J. Schmeier, E. A. Bielinski, C. J. Pan, P. O. Lagaditis, W. H. Bernskoetter, M. K. Takase, C. Würtele, N. Hazari and S. Schneider, Inorg. Chem., 2014, 53, 2133–2143 CrossRef CAS.
- S. Mao, S. Budweg, A. Spannenberg, X. Wen, Y. Yang, Y. W. Li, K. Junge and M. Beller, ChemCatChem, 2022, 14, e202101668 CrossRef CAS.
- P. O. Lagaditis, P. E. Sues, J. F. Sonnenberg, K. Y. Wan, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2014, 136, 1367–1380 CrossRef CAS PubMed.
- O. J. Driscoll, C. H. Hafford-Tear, P. McKeown, J. A. Stewart, G. Kociok-Köhn, M. F. Mahon and M. D. Jones, Dalton Trans., 2019, 48, 15049–15058 RSC.
- R. Surendhran, A. A. D'Arpino, B. Y. Sciscent, A. F. Cannella, A. E. Friedman, S. N. MacMillan, R. Guptad and D. C. Lacy, Chem. Sci., 2018, 9, 5773–5780 RSC.
- M. Darari, A. Francés-Monerris, B. Marekha, A. Doudouh, E. Wenger, A. Monari, S. Haacke and P. C. Gros, Molecules, 2020, 25, 5991 CrossRef CAS PubMed.
- S. Chakraborty, H. Dai, P. Bhattacharya, N. T. Fairweather, M. S. Gibson, J. A. Krause and H. Guan, J. Am. Chem. Soc., 2014, 136, 7869–7872 CrossRef CAS PubMed.
- J. B. Curley, N. E. Smith, W. H. Bernskoetter, M. Z. Ertem, N. Hazari, B. Q. Mercado, T. M. Townsend and X. Wang, ACS Catal., 2021, 11, 10631–10646 CrossRef CAS.
- S. Chakraborty, P. Bhattacharya, H. Dai and H. Guan, Acc. Chem. Res., 2015, 48, 1995–2003 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- N. Nebra and J. García-Álvarez, Molecules, 2020, 25, 2015–2032 CrossRef CAS PubMed.
- S. V. Giofrè, M. Tiecco, A. Ferlazzo, R. Romeo, G. Ciancaleoni, R. Germani and D. Iannazzo, Eur. J. Org. Chem., 2021, 2021, 4777–4789 CrossRef.
- A. Kafle and S. T. Handy, Tetrahedron, 2017, 73, 7024–7029 CrossRef CAS.
- L. Hladnik, F. A. Vicente, U. Novak, M. Grilc and B. Likozar, Ind. Crops Prod., 2021, 164, 113359 CrossRef CAS.
- A. Bjelić, B. Hočevar, M. Grilc, U. Novak and B. Likozar, Rev. Chem. Eng., 2022, 38, 243–272 CrossRef.
- T. Ročnik, B. Likozar, E. Jasiukaitytė-Grojzdek and M. Grilc, Chem. Eng. J., 2022, 448, 137309 CrossRef.
- R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- M. A. Droesbeke and F. E. Du Prez, ACS Sustainable Chem. Eng., 2019, 7, 11633–11639 CrossRef CAS.
- R. Ghosh, N. C. Jana, S. Panda and B. Bagh, ACS Sustainable Chem. Eng., 2021, 9, 4903–4914 CrossRef CAS.
- S. Sethi, N. C. Jana, S. Behera, R. R. Behera and B. Bagh, ACS Omega, 2023, 8, 868–878 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- C. H. Lam, V. Escande, K. E. Mellor, J. B. Zimmerman and P. T. Anastas, J. Chem. Educ., 2019, 96, 761–765 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of L1 and 1, general experimental procedure for the peroxidative oxidation of vanillyl alcohol to vanillic acid, gram-scale synthesis of vanillic acid, synthesis of vanillic esters and diesters as fine chemicals (E1a, E1b, E1c, E1d, E1e, E2a, E2b, E2c and E2d), copies of the NMR spectra of L1 and all products, single crystal X-ray analysis of 1, summary of the zero and first pass of the optimized method for the peroxidative oxidation of vanillyl alcohol to vanillic acid (PDF) and X-ray data (1). CCDC 2243929. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3su00114h |
| This journal is © The Royal Society of Chemistry 2023 |