Open Access Article
Open Access ArticleSynthesis, physicochemical characterization and aquatic toxicity studies of anionic surfactants derived from amino and α-hydroxy acids†
Demian
Kalebic
 a,
Koen
Binnemans
a,
Koen
Binnemans
 a,
Peter A. M.
de Witte
a,
Peter A. M.
de Witte
 b and
Wim
Dehaen
b and
Wim
Dehaen
 *a
*a
aKU Leuven, Department of Chemistry, Celestijnenlaan 200F, P. O. Box 2404, B-3001 Leuven, Belgium. E-mail: wim.dehaen@kuleuven.be
bKU Leuven, Laboratory for Molecular Biodiscovery, Department of Pharmaceutical and Pharmacological Sciences, Herestraat 49 Box 824, B-3000 Leuven, Belgium
First published on 13th September 2023
Abstract
Surfactants are extremely versatile ubiquitous compounds with a wide range of applications. Traditional surfactants are based on non-renewable sources, while the alternative surfactants from natural feedstock remain underexplored. In our work, we synthesized and characterized a library of bioderived compounds with different structural properties. Namely, amide and ester derivatives of C10–C16 fatty acids and amino or α-hydroxy acids, including methionine, aspartic, glutamic, malic and citric acid. To further elucidate the structure–property relationship, we also included iminodiacetic acid, a non-natural acid. The surfactant molecular structures varied in the number of carboxylic groups (one to three), the length of the hydrophobic chain and the type of linkage between the two parts of the molecule (ester, secondary or tertiary amide). The structural differences had a pronounced impact on their foaming properties, critical micelle concentration (CMC), and maximum tolerable concentration (MTC) in aquatic life, studied using zebrafish as model animals. The compounds exhibited a broad range of foaming properties across the whole pH range. Their respective CMC values spanned several orders of magnitude, and a linear relationship between the logarithm of CMC and the hydrophobic chain length was observed. Several compounds showed very high MTC values. The obtained results provide a basis for further development of bioderived surfactants and their use in different domains.
Sustainability spotlightTraditional fossil-fuel-derived surfactants generally bear inherent negative environmental impacts. As a more biocompatible alternative, recent research efforts have shown increasing interest in surfactants derived from renewable feedstock that could improve the overall environmental performance. Nonetheless, the bioderived surfactants should exhibit comparable surfactant properties to the state-of-the-art surfactants. Herein, we investigated the synthesis, foaming properties, critical micelle concentration and potential aquatic toxicity of surfactants derived from natural products: amino, α-hydroxy and fatty acids. We have elucidated their structure–property relationship to aid with the future design of bioderived surfactants. This work provides a basis for future research into the use of these compounds in different industries (consumer products, pharmaceuticals, cosmetics). Moreover, the presence of chelating groups as the polar head group of the surfactants enables research into their application in metal removal and recovery. Therefore, this work highlights the significance of UN Sustainable Development Goals (SDGs): industry, innovation and infrastructure (SDG 9), responsible consumption and production (SDG 12), climate action (SDG 13). |
Introduction
Due to the inherent negative environmental impact of traditional, fossil-fuel-derived anionic surfactants on the environment1–3 and the increasingly stricter environmental regulations, interest in surfactants derived from natural, renewable feedstock has been growing in recent years.4–6 Such surfactants can be based on amino acids,7–11 peptides,12 fatty acids,13 α-hydroxy acids,14 itaconic acid,15 furfural,16 isosorbide,17 saccharides,18–20 taurine,21,22 among many others. However, the full potential of these surfactants remains underutilized for reasons most probably associated with the lack of elaboration on their physicochemical and toxicological properties.Amino acid-based surfactants (AAS) are a growing research field that uses amino acids as the hydrophilic, polar head of the surfactant structure. Amino acids are natural products, building blocks of proteins, that have a fundamental role in many biological processes. They consist of a carboxylic group (COOH), an amino group (NH2) and a characteristic side chain bearing different functional groups (alkyl, carboxylic, amine, amide, thiol, thioether, alcohol, aromatic etc.). Their structural diversity allows for the synthesis of various surfactants, as different moieties can be introduced at different molecular sites via acylation, esterification, reductive amination or alkylation reactions.7–9,23 The resulting derivatives can therefore be anionic,24–27 cationic,28–30 zwitterionic31 or even non-ionic32–34 surfactants of wide structural variety and diverse physicochemical properties. This, in turn, leads to the potential for applications of AAS in different fields, including biomedicine,35–37 pharmaceuticals,38–42 gelators,42–46 micellar catalysis,47,48 cosmetics,49 consumer products,50 food additives and supplements.51,52
On the other hand, a similar group of surfactants derived from natural products, namely α-hydroxy-acid-based surfactants (AHAS), have not been studied as extensively for their surfactant properties. Tian et al. investigated amphiphilic polymers, derivatives of mucic acid, for drug encapsulation and transport,53 while Jin et al. studied alkyl glucoside citrate monoesters in terms of their surface activity, wetting and foaming ability.14
In addition to the properties and applications mentioned above, amino and α-hydroxy acids act as chelating agents with different binding modes.54–58 This property makes AAS and AHAS suitable for metal chelation and extraction, especially when employed in hydrometallurgical processes such as ion flotation. Ion flotation is a metal extraction technique based on the adsorption of metal ions at the air–water interface of solutions that contain a ligand and a surfactant. Common practice is that diluted aqueous solutions of metal ions get treated with a surfactant and a ligand as two separate entities.59 Synthetic and natural AAS have replaced this system acting as chelating surfactants, where both the ligand and the surfactant are combined in a single molecule.22,60–62 This not only reduces the number of equilibria and the complexity of the metal-extraction mechanism but also opens the door to the design of more environmentally friendly ion flotation systems. These processes have real-life applications such as valuable-metal recovery from treated waste or removal of heavy metal pollutants from drinking water.63–66
Surfactant biodegradability and biotoxicity (primarily aquatic toxicity) are among the most significant driving forces for the design of novel surfactants in recent years.23,67 Using surfactants from natural building blocks is a valid premise for their benign environmental impact. Even though there are relatively few reports on the biodegradation of AAS and AHAS,68 the presence of amide or ester bonds as a linkage between the polar surfactant head and the hydrophobic tail allows for biodegradability and biocompatibility.4,5 Conventional fossil fuel-derived, non-renewable surfactants still prevail over biobased surfactants due to their low cost and established performance in different areas. For a paradigm shift to occur, it is paramount to further investigate the structure–property relationship of surfactants derived from natural sources.
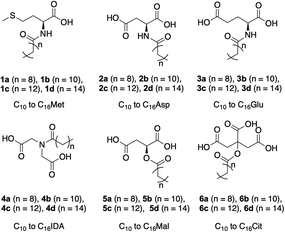
In the present paper, we report the synthesis of a library of N- and O-acyl surfactants (Fig. 1), conjugates of C10–C16 fatty acids (capric, lauric, myristic and palmitic acid) and amino (methionine, glutamic and aspartic acid) or α-hydroxy acids (malic and citric acid). Their structural diversity is manifested in the number of carboxylic groups (one to three), the overall structure of their hydrophilic head group, the length of the hydrophobic chain and the type of bond between the two moieties (ester, secondary or tertiary amide). We investigated the effect of the different structural features of the prepared compounds on their foaming properties, critical micelle concentration (CMC) and potential toxicity towards aquatic life using zebrafish larvae. For a better elucidation of the effect of the polar group structure on the surfactant properties, we included a non-natural amino acid (iminodiacetic acid, IDA) in the study as well.
Results and discussion
Synthesis of surfactants
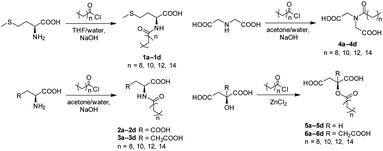
Synthesis of amide derivatives of amino acids is well established. It ranges from simple classical conditions, such as the Schotten–Baumann reaction (using acid chlorides), to the use of peptide coupling reagents and protecting groups and even enzyme-catalyzed reactions.5,73 Although enzymatic synthesis is potentially more sustainable and may see more of an implementation in the future, currently, the classical chemical synthesis routes offer a much more versatility and robustness, albeit generating more waste. Nevertheless, even for simple reactions such as the Schotten–Baumann reaction, conditions must be tailored to the type of amino acid and the hydrophobic group used. Factors such as temperature control, pH control by the addition of a base and a suitable ratio of water and an organic solvent are paramount for reaction success.74 For example, in our study, when comparing the synthesis of dicarboxylic surfactants versus methionine (Scheme 1), using acetone as a solvent provided better yields for the dicarboxylic surfactants like aspartate, glutamate and IDA derivatives. At the same time, the yield of methionine-derived surfactants using acetone was inferior to THF. In some cases, the extraction step during the work-up can be avoided by direct filtration of the formed precipitate after acidification.75 However, based on our experience, it can be rather cumbersome, with problems like gel formation and pore-clogging hindering the process and leading to products of lesser purity. The main side product of the reaction – the fatty acid resulting from the hydrolysis of the acid chloride is easily removed from the crude mixture by recrystallization using either a EtOAc/petroleum ether or a diethyl ether/petroleum ether system.For the synthesis of AHAS, a solventless zinc(II) chloride-catalyzed esterification has been described in the literature, where ZnCl2 acts as a Lewis-acid catalyst and an excess of acid chloride is used instead of a solvent (Scheme 1).53,76 In the subsequent aqueous work-up, the unreacted acid chloride and the main side product of the reaction (acid anhydride)77 are hydrolyzed, while the final recrystallization step is used to purify the surfactant from the residual fatty acid impurity.
Structural variation amongst surfactants
Several research groups have investigated the effect of different AAS polar head group structures and the hydrophobic chain length on their surfactant, antimicrobial and biological properties.24–26,41,75,78,79 Therefore, it has already been established that the difference in the structure of a surfactant polar head group leads to different adsorption and aggregation properties as well as biological activity. Since carboxylic groups are highly polar and acidic, they substantially increase the hydrophilicity of the surfactant polar head and, in turn, have a significant effect on the solubility and surface activity of carboxylate surfactants. The overall hydrophilicity of surfactants is also affected by the type of linkage between the hydrophobic and hydrophilic moiety (e.g. secondary amide, tertiary amide or ester bond). The amide bond dipole moment is higher than that of an ester bond, owing to the greater electronegativity difference between the bonded atoms, so it contributes more to the hydrophilicity of surfactants.Additionally, planarity of the amide bond prevents its rotation leading to more rigid structures and different mechanical properties, which can affect foam formation and stability. The difference between secondary and tertiary amides stems from the fact that tertiary amides are not hydrogen bond (H-bond) donors and can be more sterically hindered. That manifests in lower solubility, compared to secondary amides, in water and protic solvents. The formation of intermolecular H-bonds of secondary amides can also affect self-assembly. Even though self-assembly processes are mainly based on entropic contributions, van der Waals interactions between the hydrophobic moieties, the repulsion between the polar head groups, and the type of amide bond can have a considerable effect as well.80
Within the scope of our work, these effects were evident concerning aspartic and malic acid derivatives (Fig. 1). The compounds are nearly isostructural, bar the difference in the type of linkage to the hydrophobic group (ester versus amide). Glutamic acid and aspartic acid differ only in the methylene spacer on the amino-acid side chain. Iminodiacetic acid, the only non-natural product in the series and a widely used chelating agent, can be regarded as an isomer of aspartic acid with the side chain transposed from the α-carbon to the nitrogen, making it the only tertiary amide in the series. Reports so far mainly focused on zwitterionic N-alkyl-IDA derivatives, which have been extensively studied.81–85 As regards its biocompatibility, IDA and related imino- and amino acids were shown to be biodegradable in river water.86 The influence of the number of carboxylic groups on the surfactant properties was warranted by including methionine and citric acid as a polar head group for the monocarboxylic and tricarboxylic derivatives, respectively.
Foaming properties
The foaming properties of surfactants are essential when it comes to their application in flotation or consumer products and can be determined by multiple means.87 Since the prepared surfactants are acidic and hence form different surface-active species in solution depending on its pH,75,88 the foaming properties were investigated using the Bartsch shaking test in a volumetric cylinder at multiple values over the whole pH range (Fig. 2). We used SDS, one of the most used petroleum-derived anionic surfactants, for comparison. As expected, SDS formed ample stable foam across the investigated pH range, with no sign of precipitate.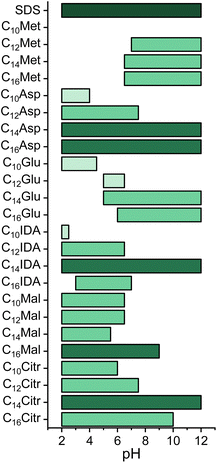 | ||
| Fig. 2 Overall foaming properties of the studied surfactants (40 mL solutions, 0.1% w/v) across the studied pH range. Bars represent the pH range in which compounds formed stable foam (>5 mL after 1 minute) and the intensity of the green color represents their overall rating relative to SDS. Determined by the Bartsch method at 21 °C. Further details regarding the foaming properties are presented in Fig. S1 in the ESI.† | ||
With C10Met derivatives, no stable foam formation was observed as the foam would dissipate right away. Conversely, for C12-, C14- and C16-derivatives, strong foaming power was observed above pH = 7 as ample amounts of dense, stable foam formed (Fig. S1a†). In all cases, acidifying the solution to pH < 7 resulted in the formation of a silky precipitate at first, which would aggregate into gel-like globules.
In the series of dicarboxylic surfactants, aspartic acid derivatives exhibited the best foaming properties, in line with what has already been reported on N-dodecanoyl (C12) surfactant properties.12 With the increase of the hydrophobic group from C10 to C16, the pH range in which the surfactants formed stable foam progressively extended into the basic pH range. Stable foam formation of C10Asp was observed in pH = 2–4 range. C12Asp formed ample stable foam from pH = 2 to pH = 7, while C16Asp showed great foaming properties in the entire studied pH range (Fig. S1b†).
An interesting effect was observed with the C14Asp derivative springing from the fact that anionic surfactants are not only pH-sensitive but also affected by changes in the ionic strength of the solution. When C14Asp was directly dissolved in a slightly basic solution (pH = 8), transient foam formation was observed. However, after increasing the pH to 11, the formed foam was stable. Then, after progressively acidifying the solution back to pH = 8, stable foam formation was observed. The same effect was noticed with C14-derivatives of Glu, IDA and Cit. The reason for this could lie in the fact that the surface charge of the surfactant head group can be stabilized or neutralized by counterions leading to decreased repulsion between the polar head groups. This reinforces attractive interactions, increases the surface rigidity and stabilizes the formed foam.89,90 After repeating the experiment at constant ionic strength using 0.1 M NaCl, stable foam formation was again observed throughout the whole basic pH range. This further corroborated that the observed foamability and foam stability in the mildly basic pH range of C14-derivatives resulted from surface-charge stabilization of deprotonated carboxylic groups by Na+ ions. Interestingly, the effect was not observed with malic acid derivatives as they had rather poor foaming properties in basic conditions overall (see below). It is important to note that alkali metals do not form coordination complexes with carboxylic groups and that the interactions are purely electrostatic.91
Glutamic acid derivatives showed diverse foaming properties. C10Glu formed stable foam in the pH = 2–4 range, similar to C10Asp (Fig. S1c†). At higher pH values, though, only a small amount of transient foam formation was observed. In contrast, longer alkyl chain derivatives did not foam in the low pH range but formed a gel-like precipitate until completely precipitating at very low pH values. The foaming properties of C12Glu were confined to the pH = 5–7 range. However, with C14Glu, the same foam stabilizing ionic-strength effect was observed as with C14Asp, virtually extending the foaming range to the entire basic pH range. C16Glu showed good foaming properties at pH = 6–12, with the most stable foam observed at neutral pH.
IDA derivatives, the only tertiary amides in the series, displayed a similar trend as the other dicarboxylic surfactants (Fig. S1d†). C10IDA showed foaming properties only in very low pH range (pH = 2–3). C12IDA performance was very much comparable to C12Asp and C12Mal in terms of the pH range and the amount of formed foam. At the same time, C14IDA showed similar performance to the C14Asp derivative, exhibiting the foam-stabilizing effect of increased ionic strength. However, C16IDA showed good foaming properties in the acidic and neutral range, while in the basic range, stable foam formed in a very low amount (<5 mL), unlike the other dicarboxylic C16-derivatives.
On the other hand, C10Mal formed stable foam in the broadest pH range (pH = 2–6) owing to the less polar ester bond, compared to the amide bond of the other dicarboxylic surfactants. Foam formation of almost all malic acid derivatives was confined to the pH range of 2–6, except for C16Mal which formed a minor amount of stable foam in the neutral and basic pH (Fig. S1e†). Additionally, with C16Mal an unusual decrease in foaming power at around pH = 5 was observed, while at the adjacent pH values (2, 3 and 7) the compound produced ample stable foam.
The tricarboxylic surfactants of the series, citric acid derivatives, showed similar properties to their dicarboxylic counterparts and are very much comparable to the aspartic acid derivatives, for the most part. C10- and C12Cit formed an ample amount of stable foam in the acidic pH range, with the foaming range of the C12-derivative reaching neutral pH as well (Fig. S1f†). C14Cit, much like the other C14 dicarboxylic surfactants (apart from C14Mal), foamed throughout the whole pH range for the same reason. However, C16Cit showed relatively poor foaming properties with low amounts of stable foam forming throughout the entire pH range, bar the very basic conditions, akin to C16IDA.
Interestingly, the solutions of C10-derivatives became turbid at lower pH values, compared to their counterparts with longer hydrophobic chains, forming gel-like lumps and/or fine silky precipitate in the process. The reason behind this most probably lies in the fact that the length of the alkyl chain affects the pKa of the acid. Longer hydrophobic chains increase in pKa values of the acidic groups due to premicellar aggregation and the resulting interactions.92 Another reason for that occurrence could be the lower solubility of the more hydrophobic compounds that causes their monoprotonated species to precipitate out of the solution already in mildly acidic conditions. Nevertheless, although the solutions were turbid, most of the compounds still formed ample stable foam (Fig. S1†), indicating that the monoprotonated derivatives are still surface-active even when present at very low concentrations in solution.
Overall, an improvement of the foaming properties in the basic range was observed with the increase of the hydrophobic group length in most cases. This is because the improved molecular interactions stabilize the surface film, increase its viscosity and decrease the liquid drainage rate, ultimately leading to overall foam stability. The only major discrepancies were observed with C16Cit and C16IDA, which showed poorer foaming properties than their respective derivatives with shorter hydrophobic chains. This is most probably caused by the relative size and structure of their polar head group and the hydrophobic moiety, which leads to low packing ability of surfactant molecules at the surface, affecting surface viscosity and elasticity. As a result, the probability of hole formation in the stretched lamellae increases and both foamability and foam stability decrease.89,93
It is worth noting that simply because surfactants foaming properties are not satisfactory does not mean that they cannot find their application in flotation processes. For chelating surfactants with sub-par foaming properties, adding an auxiliary surfactant as a foaming agent can aid in overcoming that, if they exhibit synergy. The extent of synergistic effects depends on the type of interactions between the chelating and auxiliary surfactants, springing from their charge type and factors such as the polar head group structure or hydrophobic chain length. The synergy between the two surfactants is also conditioned by their relative concentrations respective CMC. Several research papers deal with that topic in more detail.94–98
Critical micelle concentration and self assembly
CMC is a fundamental property of surfactant solutions that bears considerable practical significance, as surfactant solutions exhibit different properties at pre-micellar and post-micellar concentrations as well as concentrations around CMC. It can be determined by different techniques, depending on the surfactant's chemical structure. For surfactants bearing a net charge, electrical conductivity studies are often used since the change in electrical conductance of the surfactant solution is caused by different degrees of surfactant ionization below and above the CMC. Above the CMC, the conductivity usually decreases as the counterions get included within the micelles. In principle, any technique that can detect a marked change in the measured property in relation to the monomeric versus the micellar state of surfactant solutions can be used. For data processing and calculation of the actual CMC value, numerous approaches have been suggested in the literature.36,71,99–101In our work, the poor solubility of the mono-deprotonated species made their CMC determination very difficult. Nonetheless, significant foaming was still observed below neutral pH, indicating that these species are still surface active despite their low solubility. An attempt to measure the CMC of mono-deprotonated amino acids resulted in precipitate formation after dissolution by heating and letting it cool down to room temperature. In the case of disodium salts, precipitate formation was also observed upon standing. This was most probably caused by hydrolysis of the fully deprotonated surfactants and precipitation of their mono-deprotonated species, adding more to the fact that even mono-deprotonated species of dicarboxylic surfactants were not that well soluble in water. Such properties limit their application in some fields at higher concentrations and low pH. In reference literature, the CMC is almost exclusively described for the fully deprotonated sodium (or other alkali metal) salts for already investigated compounds.75,78
The CMC of ionic surfactants is highly affected by the pH and ionic strength of the solution. In an attempt to use buffers for pH control, the buffer capacity (borate, phosphate) was not strong enough to maintain the set pH at lower concentrations (10 mM). At higher concentrations, the effect of the added buffer contributing to the ionic strength of the solution would have introduced too much discrepancy between the measured and real CMC.102,103 Additionally, the dissolved surfactants form an intrinsic buffered solution themselves since they are derivatives of weak acids, so neutralization by base was not an option either. These issues were circumvented by converting the compounds into fully deprotonated sodium salts.
As expected, a decreasing trend in the CMC values of the studied surfactants was observed with the increase in hydrophobic group size. Interestingly, the secondary amide derivatives of the dicarboxylic surfactants (Asp, Glu, Mal) exhibited a similar trend with only a minor distinguishable difference between the more apolar malate derivatives versus glutamates and aspartates (Table 1). Conversely, IDA derivatives not only had a lower CMC compared to the other dicarboxylic surfactants but a more steadily decreasing trend with the increase in surfactant hydrophobicity. This indicates that the increase in the hydrophobic chain length has less of an effect on IDA than the other dicarboxylic surfactants. An increase in hydrophobicity followed by a decrease in CMC of tertiary vs. secondary amide surfactants has already been reported.80,104 The relative differences are more prominent when log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC is plotted against the number of carbon atoms in the hydrophobic moiety (Fig. S2†). A linear dependence is clearly observed for all surfactants. In the case of the monocarboxylic methionine derivatives, the CMC is lower by an order of magnitude compared to the rest of the studied surfactants. Likewise, the kink in the conductivity vs. concentration curve is much more pronounced (Fig. S3–S8†) owing to the relatively higher hydrophobicity and surfactant aggregation number in micelles.
CMC is plotted against the number of carbon atoms in the hydrophobic moiety (Fig. S2†). A linear dependence is clearly observed for all surfactants. In the case of the monocarboxylic methionine derivatives, the CMC is lower by an order of magnitude compared to the rest of the studied surfactants. Likewise, the kink in the conductivity vs. concentration curve is much more pronounced (Fig. S3–S8†) owing to the relatively higher hydrophobicity and surfactant aggregation number in micelles.
| Polar head group | Hydrophobic group size | |||
|---|---|---|---|---|
| C10 | C12 | C14 | C16 | |
a Determined by the method of Carpena et al.; errors expressed as the standard deviation of the fit; n.d. – not determined.
b Values extrapolated from a linear fit of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC dependence on the number of carbon atoms in the hydrophobic moiety (Fig. S2). CMC dependence on the number of carbon atoms in the hydrophobic moiety (Fig. S2).
|
||||
| MetNa | 20.7 ± 0.8 | 6.15 ± 0.06 | 1.71 ± 0.05 | 0.4 ± 0.1 |
| AspNa2 | 225b | 82.4 ± 0.4 | 28.9 ± 0.3 | 10.8 ± 0.1 |
| GluNa2 | 209b | 71.9 ± 0.9 | 26.3 ± 0.3 | 8.79 ± 0.08 |
| IDANa2 | 51b | 34 ± 9 | 20 ± 3 | 14.21 ± 0.08 |
| MalNa2 | 161b | 66.6 ± 0.9 | 25 ± 3 | 10.8 ± 0.1 |
| CitNa3 | n.d. | n.d. | n.d. | n.d. |
The CMC of the tricarboxylic citrate derivatives could not be determined in the case of C10- and C12-derivatives due to their inherent hydrophilicity and relative sizes of the polar head group and hydrophobic moiety, leading to a low aggregation number and a negligible effect of the inclusion of counterions within micelles. Experimentally, that is evident from the absence of a prominent kink in the concentration vs. conductance curve (Fig. S8†).36 In the case of the C14- and C16CitNa3, precipitation was observed shortly after the salt had been dissolved and was allowed to cool down to room temperature, rendering the determination of CMC of those derivatives impossible. This observation is in line with the fact that the citrate ion (Citr3−) is a stronger conjugate base than glutamate (Glu2−) or aspartate (Asp2−) and, therefore, hydrolyzes more easily.
The same effect as with C10- and C12Cit was observed with the dicarboxylic C10 derivatives. Their CMC could not be determined due to their low aggregation number and low curvature in the conductivity vs. concentration plot. Nonetheless, we have extrapolated a theoretical value based on the linear relation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC and the size of the hydrophobic group (Fig. S2†). These values could potentially be measured by other techniques less dependent on the surfactant aggregation number, such as spectrophotometrically by dye solubilization or fluorescence spectroscopy. Surface tension experiments, however, might prove cumbersome as they require a large amount of material per measurement since large solutions are usually handled.
CMC and the size of the hydrophobic group (Fig. S2†). These values could potentially be measured by other techniques less dependent on the surfactant aggregation number, such as spectrophotometrically by dye solubilization or fluorescence spectroscopy. Surface tension experiments, however, might prove cumbersome as they require a large amount of material per measurement since large solutions are usually handled.
Biological properties
Since surfactants find numerous applications in different fields, they may be discharged in wastewater and end up in surface waters, soil or sediment after use.105,106 Therefore, aquatic toxicity and biodegradability are important factors that must be addressed. Even though there are relatively few reports on biodegradation and aquatic toxicity of surfactants based on amino acids, fatty acids and α-hydroxy acids, the available literature highly suggests low aquatic toxicity and a high rate of biodegradation owing to the presence of amide or ester bonds as a linker between the polar head group and the hydrophobic moiety.68 Such bonds are highly susceptible to enzymatic cleavage. In our work, we investigated a worst-case scenario where no biodegradation would occur in order to gain further insights into their environmental compatibility.Surfactant toxicity springs from their affinity for interfaces. Consequently, they accumulate at the cell membrane/water interface, disrupting the membrane integrity through hydrophobic/ionic adsorption phenomena. Even though the responses of different organisms to surfactants depend on various factors, in general, anionic surfactants are less toxic than cationic surfactants due to the net negative charge of most biosolids in the aquatic environment.107,108 Arginine-based cationic AAS, however, have been investigated in recent years for their antibacterial properties and aquatic toxicity, reporting lower acute toxicity compared to conventional cationic surfactants.109
Based on the research of Perinelli et al., N-decanoyl (C10) derivatives of different monocarboxylic amino acids show favorable toxicological profiles regarding cytotoxicity to different human cell lines.41 In similar, when comparing C10 to C16N-acyl derivatives of alanine and serine, it was reported that the hydrophobic chain length had a pronounced effect both on surface properties and cytotoxicity, while the polar head of the respective amino acids affected only the latter.24 EC50 value decreased with the increase in hydrocarbon chain length and was dependent on the concentration of the respective surfactant relative to its CMC. Nevertheless, the cytotoxicity of these compounds was still lower than what was reported for SDS.110 The reports of Infante et al. pointed out that the increased hydrophobicity was a negative parameter in terms of biodegradability and toxicity in the case of N-acylated arginine methyl-ester cationic surfactants.111 When it comes to anionic surfactants, however, the increase in hydrophobic chain length has less impact on their biodegradability. Sivasamy et al. investigated stearoyl (C18) derivatives of numerous amino acids, including glutamic and aspartic acid, and deemed them readily biodegradable, in addition to their good antimicrobial activity.68
Previous studies have investigated the safety and toxicity of various surfactants on different organisms.112,113 However, few studies have examined the effects of anionic surfactants on aquatic animals.114 In our study, we have used zebrafish as they are a commonly used model organism due to their homology with the human genome, high fecundity and short development cycle. Zebrafish larvae develop different behavioral characteristics already at 4–5 days post-fertilization (dpf).115,116
A select number of surfactants were investigated based on their foaming properties, CMC values and degree of novelty. Even though C14Cit exhibited better foaming properties than C12Cit, due to the issues with precipitation of C14Cit observed during the CMC-determination experiments, C12Cit was selected for the toxicity studies instead. SDS was included in the study for comparison. Our toxicological evaluation experiments yielded different concentrations of surfactant tolerance in zebrafish, affected both by the hydrophobic-group size and the type of the polar head group (Table 2).
| Surfactant | MTC | |
|---|---|---|
| c/μM | γ/mg L−1 | |
| a 4 dpf larvae, incubated for 18 h at 28 °C. | ||
| SDS | 100 | 28.8 |
| C14AspNa2 | 50 | 18.0 |
| C16AspNa2 | 7.5 | 2.9 |
| C14GluNa2 | 100 | 40.1 |
| C14IDANa2 | 100 | 35.9 |
| C14MetNa | 500 | 191 |
| C16MalNa2 | 500 | 208 |
| C12CitNa3 | 500 | 220 |
We have noticed the impact of different hydrophobic chain lengths on aquatic toxicity when C14Asp and C16Asp were concerned. No changes in the zebrafish were observed at concentrations below the MTC of C14Asp (50 μM). However, a sudden spike in toxic effects occurred in a relatively narrow concentration range, already at 75 μM. The MTC of C16Asp was significantly lower (7.5 μM) depicting the negative effect of increased hydrophobicity on the aquatic toxicity of the aspartate derivatives. On the other hand, the differences in the surfactant polar head group were evident from the fact that C16Mal was one of the least toxic surfactants, together with C12Cit and C14Met, with an MTC of 500 μM. C14IDA and C14Glu were placed in the medium toxicity bracket, relative to the others studied herein, with an MTC of 100 μM. Based on this, it can be expected that less hydrophobic derivatives of these compounds would be even less toxic. We have also observed that all surfactants, except C14- and C16Asp, have shown equal or lower toxicity than SDS, which had an MTC of 100 μM. However, it should be stressed that these concentrations are not found in the environment even in the case of the commonly used consumer surfactants such as linear alkylbenzenesulfonates (LAS), alkyl ethoxysulfates (AES) or polyethoxylates (PEO).105,106
The different toxicity of the studied compounds is related to their structural features that affect their ability to penetrate the cell membrane. Apart from the hydrophobic-chain size previously discussed, the overall charge of the molecule and the presence of different functional groups (i.e. sulfate, carboxylate, amide, ester, thioether) influence the extent of their adsorption at the cell surface.24 Likewise, they contribute to various electrostatic interactions with the constituents of cell membranes, affecting the ease of membrane penetration for each compound.41 It has already been reported that SDS can form specific electrostatic interactions (e.g. H-bonds) with membrane proteins via the sulfate head group.117,118 In the case of the studied bioderived surfactants, distinctly larger, more complex and more hydrophilic head groups might prevent a simple insertion into the cell membrane. However, the aspartate derivatives deviate from this rationale. Still, as observed from their foaming properties, they exhibited better surface-adsorption ability than the rest of the compounds, which would justify their increased toxicity. The lower toxicity of the AHAS (C16Mal and C12Cit) might also be supported by the fact that the ester linkage is more easily cleaved enzymatically, as already reported for lysine derivatives and carbohydrate-based surfactants.111,119,120 This would also warrant better biodegradability of AHAS.
According to the Organization for Economic Co-operation and Development (OECD) environmentally benign surfactants should exhibit an LC50 value of 10 mg L−1 toxicity for fish.121 It should be noted that, in practice, the LC50 value is almost always higher than the MTC. From the surfactants that have been tested, we can conclude that none would cause significant damage to aquatic life if they ended up in the wastewater, apart from C16Asp. They can therefore be classified as environmentally benign. The same could be assumed for other derivatives bearing smaller hydrophobic groups (C10 and C12) and some C14-derivatives such as C14Mal. It is important to reiterate that this concerns a worst-case scenario without prior biodegradation. Nonetheless, more detailed studies are advised and required if the surfactants are to reach a higher level of industrial application.
Experimental
Synthesis of the bioderived surfactants
Synthesis of the bioderived surfactants is reported below. The full characterization data can be found in the ESI.†Foaming properties
Foamability and foam stability were determined by the Bartsch method at 21 °C.69,70 40 mL aqueous solutions (0.1% w/v) of the prepared surfactants were prepared and poured carefully into a 100 mL measuring cylinder with a ground glass joint to avoid foam formation. The cylinder was capped and turned upside down at a rate of 10 times in 20 seconds. The volume of the formed foam (in mL) was measured immediately after turning the cylinder and after 1 minute. These two values indicated the foamability and foam stability, respectively. pH adjustment was done by adding 1 M aqueous solutions of NaOH or HCl. Sodium dodecyl sulfate (SDS) was used for comparison.Critical micelle concentration determination
The conductivity of surfactant solutions was measured using a Mettler Toledo SevenCompact conductivity meter and InLab® 731-ISM conductivity probe. 12.88 mS cm−1 and 1413 μS cm−1 standard solutions (Mettler Toledo) were used for calibration for high-conductivity and low-conductivity studies, respectively. 9 mL of surfactant solution in Milli-Q water was transferred into a glass vial equipped with a stirring bar. The solution was diluted by the addition of water until 15 mL after which the dilution-extraction method was used.72 Conductivity was measured under constant stirring (500 rpm) at 21 °C and the solutions were allowed to equilibrate after each addition. Due to solubility issues with protonated species of surfactants, the compounds were dissolved in EtOH, after which the exact number of equivalents of ethanolic solution of NaOH (c = 2 M), depending on the number of surfactant's carboxylic groups, was added. The precipitate was filtered, washed with EtOH twice and dried in vacuo. Measured datasets were processed and analyzed using Origin 2018 software package (OriginLab, US), where the CMC values of the compounds were determined as a parameter of the regression fitting according to the method of Carpena and co-workers.71pH measurements
The pH of the solutions was measured at 21 °C using a Mettler-Toledo SevenCompact S220 pH meter. Standard buffer solutions of pH 1, 4, 7, and 12 were used for calibration.Experimental animals
Embryos were obtained via natural spawning and were kept in Petri dishes (92 × 16 mm, Sarstedt, Nümbrecht, Germany) at 28 °C in Danieau's medium in a Peltier-cooled incubator (IPP 260, Memmert, Schwabach, Germany). The larvae were used for studies at 4 days post fertilization (dpf). Zebrafish embryos and larvae in the EU are legally not considered an animal during the first 5 dpf.Aquatic toxicity studies
A stock solution of each compound (in a sodium-salt form) was prepared in Milli-Q water (c = 4 mM) which was diluted to specific concentrations using Danieau's solution. The toxicity of compounds was evaluated by determining the maximum tolerable concentration (MTC) in zebrafish, i.e. the highest concentration at which no signs of locomotor impairment, loss of posture, body malformation, weak response upon a light touch of the tail with a fine needle, or death occurred after an 18 h incubation period. To that end, five zebrafish larvae (4 dpf) per well in 6-well plates were incubated with different concentrations of the surfactants at 28 °C and, after incubation, the larvae were individually investigated for signs of toxicity under the microscope. Danieau's solution was used for the control group.Conclusions
In this work, we have successfully prepared a library of surfactants derived from natural products, namely N- and O-acyl derivatives of amino acids and α-hydroxy acids using fatty acids as the hydrophobic moiety. For better elucidation of their physicochemical properties, we have prepared derivatives of a non-natural amino acid – iminodiacetic acid (IDA). The structural diversity of the prepared compounds is exhibited in different features in their molecular structure, such as the number of carboxylic groups (one to three), type of bond between the polar head group and the hydrophobic moiety (secondary or tertiary amide, ester) and the size of the hydrophobic group (C10–C16). A diverse range of foaming properties was observed, from compounds whose foamability and foam stability was confined to acidic, basic or even neutral pH, to compounds whose foaming ability spanned throughout the investigated pH range (2–12). In general, the increase in length of the hydrophobic chain led to an extension of the foaming properties from acidic to basic pH. However, this was not the case with C16IDA and C16Cit which displayed worse foaming properties than the other derivatives of the same hydrophobic-group size. This was most probably caused by their low packing ability at the surface due to the repulsion of their polar heads relative to the size and interactions of the hydrophobic groups. A pronounced foam stabilizing effect by increased ionic strength was observed with di- and tricarboxylic C14-derivatives, virtually extending their foaming properties from only acidic and neutral pH to the whole pH range. The self-assembly properties in bulk showed an increasing trend with the increase in the number of carboxylic groups and a decreasing trend with the increase in hydrophobic chain size. Due to a small aggregation number, the CMC of most C10-derivatives and C12Cit could not be determined. The prepared compounds are expected to be fully biodegradable due to the ease of enzymatic cleavage of amide or ester bonds and the fact that all compounds, but the IDA derivatives, are fully bioderived. Still, a select number of compounds were investigated on their biocompatibility. Their potential aquatic toxicity using zebrafish larvae as experimental animals yielded different MTC values, with the effect of increased hydrophobicity manifesting in different toxicity of C14Asp vs. C16Asp. Among the studied compounds, C12Cit, C14Met and C16Mal exhibited very high MTCs of 500 μM. Apart from C14- and C16Asp, all compounds had the same or higher MTC than SDS, showing that they were equally or less toxic. Additionally, all compounds subjected to toxicity studies, besides C16Asp, can be classified as environmentally benign. The results reported herein serve as a basis for further, more detailed studies of surfactants derived from natural products and their employment in different fields.Author contributions
Demian Kalebic: conceptualization, investigation, analysis, writing – original draft, review and editing, resources. Koen Binnemans: conceptualization, supervision, writing – review and editing, resources, funding acquisition. Peter A. M. de Witte: analysis, writing – review and editing, resources. Wim Dehaen: conceptualization, supervision, writing – review and editing, resources, funding acquisition.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The research that led to the results published in this paper was funded from the European Community's Horizon 2020 Program under Grant Agreement No. 812580 (MSCA-ETN SULTAN). This publication reflects only the authors' view, exempting the Community from any liability. Mass spectrometry was made possible by the support of the Hercules Foundation of the Flemish Government (grant 20100225-7). The authors would like to thank Prof. Jef Rozenski for the HRMS measurements, Jan Maes for the help with zebrafish experiments and Tamara Rinkovec for helping with data analysis.References
- S. Rebello, A. K. Asok, S. Mundayoor and M. S. Jisha, Environ. Chem. Lett., 2014, 12, 275–287 CrossRef CAS.
- T. Cserháti, E. Forgács and G. Oros, Environ. Int., 2002, 28, 337–348 CrossRef PubMed.
- S. O. Badmus, H. K. Amusa, T. A. Oyehan and T. A. Saleh, Environ. Sci. Pollut. Res., 2021, 28, 62085–62104 CrossRef CAS PubMed.
- D. G. Hayes and G. A. Smith, in Biobased Surfactants, Elsevier, 2019, pp. 3–38 Search PubMed.
- R. Bordes and K. Holmberg, in Encyclopedia of Surface and Colloid Science, 3rd edn, CRC Press, 2016, pp. 362–379 Search PubMed.
- A. Bhadani, A. Kafle, T. Ogura, M. Akamatsu, K. Sakai, H. Sakai and M. Abe, Curr. Opin. Colloid Interface Sci., 2020, 45, 124–135 CrossRef CAS.
- A. Pinazo, R. Pons, L. Pérez and M. R. Infante, Ind. Eng. Chem. Res., 2011, 50, 4805–4817 CrossRef CAS.
- R. Bordes and K. Holmberg, Adv. Colloid Interface Sci., 2015, 222, 79–91 CrossRef CAS PubMed.
- M. C. Morán, A. Pinazo, L. Pérez, P. Clapés, M. Angelet, M. T. García, M. P. Vinardell and M. R. Infante, Green Chem., 2004, 6, 233–240 RSC.
- N. Ferlin, D. Grassi, C. Ojeda, M. J. L. Castro, E. Grand, A. Fernández and J. Kovensky, Carbohydr. Res., 2010, 345, 598–606 CrossRef CAS PubMed.
- G. O. Reznik, P. Vishwanath, M. A. Pynn, J. M. Sitnik, J. J. Todd, J. Wu, Y. Jiang, B. G. Keenan, A. B. Castle, R. F. Haskell, T. F. Smith, P. Somasundaran and K. A. Jarrell, Appl. Microbiol. Biotechnol., 2010, 86, 1387–1397 CrossRef CAS PubMed.
- J. Xia, Protein-Based Surfactants, CRC Press, New York, 2001 Search PubMed.
- D. G. Hayes, in Fatty Acids, Elsevier, 2017, pp. 355–384 Search PubMed.
- Z. Jin, J. Zhang, X. Yang and Y. Zhou, J. Surfactants Deterg., 2016, 19, 885–891 CrossRef CAS.
- D. Malferrari, N. Armenise, S. Decesari, P. Galletti and E. Tagliavini, ACS Sustain. Chem. Eng., 2015, 3, 1579–1588 CrossRef CAS.
- A. Gassama, C. Ernenwein and N. Hoffmann, Green Chem., 2010, 12, 859 RSC.
- A. Lavergne, Y. Zhu, A. Pizzino, V. Molinier and J.-M. Aubry, J. Colloid Interface Sci., 2011, 360, 645–653 CrossRef CAS PubMed.
- S. Aury, P. Rubini, C. Gérardin and C. Selve, Eur. J. Org. Chem., 2004, 2004, 2057–2066 CrossRef.
- N. Ferlin, D. Grassi, C. Ojeda, M. J. L. Castro, E. Grand, A. Fernández Cirelli and J. Kovensky, Carbohydr. Res., 2008, 343, 839–847 CrossRef CAS PubMed.
- S. Consola, M. Blanzat, E. Perez, J.-C. Garrigues, P. Bordat and I. Rico-Lattes, Eurasian J. Chem., 2007, 13, 3039–3047 CAS.
- V. S. Balachandran, S. R. Jadhav, P. Pradhan, S. De Carlo and G. John, Angew. Chem., Int. Ed., 2010, 49, 9509–9512 CrossRef CAS PubMed.
- M. Taseidifar, M. Ziaee, R. M. Pashley and B. W. Ninham, J. Environ. Chem. Eng., 2019, 7, 103263 CrossRef CAS.
- N. Joondan, S. Jhaumeer Laulloo and P. Caumul, J. Dispersion Sci. Technol., 2018, 39, 1550–1564 CrossRef CAS.
- D. R. Perinelli, M. Cespi, L. Casettari, D. Vllasaliu, M. Cangiotti, M. F. Ottaviani, G. Giorgioni, G. Bonacucina and G. F. Palmieri, Eur. J. Pharm. Biopharm., 2016, 109, 93–102 CrossRef CAS PubMed.
- R. Bordes and K. Holmberg, Colloids Surf., A, 2011, 391, 32–41 CrossRef CAS.
- R. O. Brito, S. G. Silva, R. M. F. Fernandes, E. F. Marques, J. Enrique-Borges and M. L. C. do Vale, Colloids Surf., B, 2011, 86, 65–70 CrossRef CAS PubMed.
- S. Yada, M. Wakizaka, H. Shimosegawa, H. Fujita, M. Yamada, Y. Matsue and T. Yoshimura, Colloids Surf., A, 2021, 611, 125757 CrossRef CAS.
- F. Goursaud, M. Berchel, J. Guilbot, N. Legros, L. Lemiègre, J. Marcilloux, D. Plusquellec and T. Benvegnu, Green Chem., 2008, 10, 310 RSC.
- N. Joondan, P. Caumul, M. Akerman and S. Jhaumeer-Laulloo, Bioorg. Chem., 2015, 58, 117–129 CrossRef CAS PubMed.
- A. Mezei, L. Pérez, A. Pinazo, F. Comelles, M. R. Infante and R. Pons, Langmuir, 2012, 28, 16761–16771 CrossRef CAS PubMed.
- Y. Li, K. Holmberg and R. Bordes, J. Colloid Interface Sci., 2013, 411, 47–52 CrossRef CAS PubMed.
- R. da C. Duarte, R. Ongaratto, L. A. Piovesan, V. R. de Lima, V. Soldi, A. A. Merlo and M. G. M. D'Oca, Tetrahedron Lett., 2012, 53, 2454–2460 CrossRef CAS.
- W. Wang, D. An and Z. Ye, J. Dispersion Sci. Technol., 2017, 39, 292–297 CrossRef.
- M. R. Infante, J. Seguer, A. Pinazo and M. P. Vinardell, J. Dispersion Sci. Technol., 1999, 20, 621–642 CrossRef CAS.
- A. Pinazo, M. A. Manresa, A. M. Marques, M. Bustelo, M. J. Espuny and L. Pérez, Adv. Colloid Interface Sci., 2016, 228, 17–39 CrossRef CAS PubMed.
- D. R. Perinelli, M. Cespi, N. Lorusso, G. F. Palmieri, G. Bonacucina and P. Blasi, Langmuir, 2020, 36, 5745–5753 CrossRef CAS PubMed.
- N. Joondan, S. Jhaumeer Laulloo, P. Caumul, D. E. P. Marie, P. Roy and E. Hosten, Colloids Surf., A, 2016, 511, 120–134 CrossRef CAS.
- R. Muzzalupo, L. Pérez, A. Pinazo and L. Tavano, Int. J. Pharm., 2017, 529, 245–252 CrossRef CAS PubMed.
- N. Ménard, N. Tsapis, C. Poirier, T. Arnauld, L. Moine, F. Lefoulon, J.-M. Péan and E. Fattal, Int. J. Pharm., 2012, 423, 312–320 CrossRef PubMed.
- N. Ménard, N. Tsapis, C. Poirier, T. Arnauld, L. Moine, C. Gignoux, F. Lefoulon, J.-M. Péan and E. Fattal, Pharm. Res., 2012, 29, 1882–1896 CrossRef PubMed.
- D. R. Perinelli, L. Casettari, M. Cespi, F. Fini, D. K. W. Man, G. Giorgioni, S. Canala, J. K. W. Lam, G. Bonacucina and G. F. Palmieri, Colloids Surf., A, 2016, 492, 38–46 CrossRef CAS.
- B. Hu, H. Yan, Y. Sun, X. Chen, Y. Sun, S. Li, Y. Jing and H. Li, Artif. Cells, Nanomed., Biotechnol., 2020, 48, 266–275 CrossRef CAS PubMed.
- N. Minakuchi, K. Hoe, D. Yamaki, S. Ten-no, K. Nakashima, M. Goto, M. Mizuhata and T. Maruyama, Langmuir, 2012, 28, 9259–9266 CrossRef CAS PubMed.
- C. Luo, B. Yang, Y. Zhou, J. Yang, F. Han and X. Baocai, Colloids Surf., A, 2020, 585, 124184 CrossRef CAS.
- Q. Yu, D. Li, M. Cai, F. Zhou and W. Liu, Tribol. Lett., 2016, 61, 16 CrossRef.
- D. Das, A. Dasgupta, S. Roy, R. N. Mitra, S. Debnath and P. K. Das, Eurasian J. Chem., 2006, 12, 5068–5074 CAS.
- N. Armenise, D. Malferrari, S. Ricciardulli, P. Galletti and E. Tagliavini, EurJOC, 2016, 2016, 3177–3185 CAS.
- S. Roy, D. Das, A. Dasgupta, R. N. Mitra and P. K. Das, Langmuir, 2005, 21, 10398–10404 CrossRef CAS PubMed.
- M. Lukic, I. Pantelic and S. Savic, Tenside, Surfactants, Deterg., 2016, 53, 7–19 CrossRef CAS.
- K. P. Ananthapadmanabhan, Tenside, Surfactants, Deterg., 2019, 56, 378–386 CrossRef CAS.
- A. Paquet, Can. J. Biochem., 1980, 58, 573–576 CrossRef CAS PubMed.
- R. Damico, J. Agric. Food Chem., 1975, 23, 30–33 CrossRef CAS PubMed.
- L. Tian, L. Yam, N. Zhou, H. Tat and K. E. Uhrich, Macromolecules, 2004, 37, 538–543 CrossRef CAS.
- K. Severin, R. Bergs and W. Beck, Angew. Chem., Int. Ed., 1998, 37, 1634–1654 CrossRef PubMed.
- A. J. Francis, C. J. Dodge and J. B. Gillow, Nature, 1992, 356, 140–142 CrossRef CAS.
- Y. Shimazaki, M. Takani and O. Yamauchi, Dalton Trans., 2009, 7854 RSC.
- K. Kabra, R. Chaudhary and R. L. Sawhney, J. Hazard. Mater., 2008, 155, 424–432 CrossRef CAS PubMed.
- N. Dolev, Z. Katz, Z. Ludmer, A. Ullmann, N. Brauner and R. Goikhman, Environ. Res., 2020, 183, 109140 CrossRef CAS PubMed.
- F. M. Doyle, Int. J. Miner. Process., 2003, 72, 387–399 CrossRef CAS.
- A. Eivazihollagh, I. Svanedal, H. Edlund and M. Norgren, J. Mol. Liq., 2019, 278, 688–705 CrossRef CAS.
- N. Ferlin, D. Grassi, C. Ojeda, M. J. L. Castro, E. Grand, A. Fernández Cirelli and J. Kovensky, Carbohydr. Res., 2010, 345, 598–606 CrossRef CAS PubMed.
- A. Patra, H. A. Taner, R. Bordes, K. Holmberg and A.-C. Larsson, J. Dispersion Sci. Technol., 2019, 40, 1205–1216 CrossRef CAS.
- A. Wan Nafi and M. Taseidifar, J. Environ. Manage., 2022, 319, 115666 CrossRef CAS PubMed.
- N. Ferlin, D. Grassi, C. Ojeda, M. J. L. Castro, A. F. Cirelli, J. Kovensky and E. Grand, Colloids Surf., A, 2015, 480, 439–448 CrossRef CAS.
- P. Xanthopoulos, D. Kalebić, N. Kamariah, J. Bussé, W. Dehaen, J. Spooren and K. Binnemans, J. Sustain. Metall., 2021, 7, 1552–1564 CrossRef.
- L. Chang, Y. Cao, G. Fan, C. Li and W. Peng, RSC Adv., 2019, 9, 20226–20239 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- A. Sivasamy, M. Krishnaveni and P. G. Rao, J. Am. Oil Chem. Soc., 2001, 78, 897–902 CrossRef CAS.
- J. J. Bikerman, Foams, Springer Berlin Heidelberg, Berlin, Heidelberg, 1973 Search PubMed.
- O. Bartsch, Kolloidchem. Beih., 1924, 20, 1–49 CrossRef CAS.
- P. Carpena, J. Aguiar, P. Bernaola-Galván and C. Carnero Ruiz, Langmuir, 2002, 18, 6054–6058 CrossRef CAS.
- A. Jover, F. Meijide, V. Mosquera and J. V. Tato, J. Chem. Educ., 1990, 67, 530 CrossRef CAS.
- P. Clapés and M. Rosa Infante, Biocatal. Biotransform., 2002, 20, 215–233 CrossRef.
- M. Takehara, I. Yoshimura, K. Takizawa and R. Yoshida, J. Am. Oil Chem. Soc., 1972, 49, 157 CrossRef CAS.
- R. Bordes, J. Tropsch and K. Holmberg, J. Colloid Interface Sci., 2009, 338, 529–536 CrossRef CAS PubMed.
- J. Guo, W. Wang, J. Hu, D. Xie, E. Gerhard, M. Nisic, D. Shan, G. Qian, S. Zheng and J. Yang, Biomaterials, 2016, 85, 204–217 CrossRef CAS PubMed.
- M. J. Milewska, Z Chem, 1988, 28, 204–211 CrossRef CAS.
- Q. Wang, Z. Song, F. Han, B. Xu and B. Xu, Colloids Surf., A, 2022, 640, 128474 CrossRef CAS.
- D. Zhang, Y. Sun, Q. Deng, X. Qi, H. Sun and Y. Li, Colloids Surf., A, 2016, 504, 384–392 CrossRef CAS.
- R. Bordes, J. Tropsch and K. Holmberg, Langmuir, 2010, 26, 3077–3083 CrossRef CAS PubMed.
- L. Häggman, C. Lindblad, H. Oskarsson, A.-S. Ullström and I. Persson, J. Am. Chem. Soc., 2003, 125, 3631–3641 CrossRef PubMed.
- J. Liu, T. Zheng, J. Wang and G. Jia, Mater. Chem. Phys., 2022, 278, 125622 CrossRef CAS.
- U. Bazylińska, R. Skrzela, M. Piotrowski, K. Szczepanowicz, P. Warszyński and K. A. Wilk, Bioelectrochemistry, 2012, 87, 147–153 CrossRef PubMed.
- H. Fonge, L. Jin, J. Cleynhens, G. Bormans and A. Verbruggen, Bioorg. Med. Chem., 2010, 18, 396–402 CrossRef CAS PubMed.
- D. A. Jaeger, X. Zeng and Y. Wang, Colloids Surf., A, 2006, 289, 158–162 CrossRef CAS.
- C. B. Warren and E. J. Malec, Science, 1972, 176, 277–279 CrossRef CAS PubMed.
- D. Exerowa, G. Gochev, D. Platikanov, L. Liggieri and R. Miller, Foam Films and Foams, CRC Press, 2018 Search PubMed.
- I. Svanedal, G. Persson, M. Norgren and H. Edlund, Langmuir, 2013, 29, 13708–13716 CrossRef CAS PubMed.
- A. Eivazihollagh, J. Tejera, I. Svanedal, H. Edlund, A. Blanco and M. Norgren, Ind. Eng. Chem. Res., 2017, 56, 10605–10614 CrossRef CAS.
- C. Micheau, P. Bauduin, O. Diat and S. Faure, Langmuir, 2013, 29, 8472–8481 CrossRef CAS PubMed.
- I. Svanedal, F. Andersson, E. Hedenström, M. Norgren, H. Edlund, S. K. Satija, B. Lindman and A. R. Rennie, Langmuir, 2016, 32, 10936–10945 CrossRef CAS PubMed.
- J. R. Kanicky and D. O. Shah, Langmuir, 2003, 19, 2034–2038 CrossRef CAS.
- K. Holmberg, B. Jönsson, B. Kronberg and B. Lindman, in Surfactants and Polymers in Aqueous Solution, John Wiley & Sons, Ltd, Chichester, UK, 2002, pp. 437–450 Search PubMed.
- J. Ferreira, A. Mikhailovskaya, A. Chenneviere, F. Restagno, F. Cousin, F. Muller, J. Degrouard, A. Salonen and E. F. Marques, Soft Matter, 2017, 13, 7197–7206 RSC.
- I. Svanedal, G. Persson, M. Norgren and H. Edlund, Langmuir, 2014, 30, 1250–1256 CrossRef CAS PubMed.
- H. Jian, X. Liao, L. Zhu, W. Zhang and J. Jiang, J. Colloid Interface Sci., 2011, 359, 487–492 CrossRef CAS PubMed.
- K. Theander and R. J. Pugh, J. Colloid Interface Sci., 2003, 267, 9–17 CrossRef CAS PubMed.
- M. J. Rosen and Z. H. Zhu, J. Am. Oil Chem. Soc., 1988, 65, 663–668 CrossRef CAS.
- N. Scholz, T. Behnke and U. Resch-Genger, J. Fluoresc., 2018, 28, 465–476 CrossRef CAS PubMed.
- W. Al-Soufi and M. Novo, Molecules, 2021, 26, 5339 CrossRef CAS PubMed.
- K. Holmberg, B. Jönsson, B. Kronberg and B. Lindman, in Surfactants and Polymers in Aqueous Solution, John Wiley & Sons, Ltd, Chichester, UK, 2002, pp. 39–66 Search PubMed.
- E. Fuguet, C. Ràfols, M. Rosés and E. Bosch, Anal. Chim. Acta, 2005, 548, 95–100 CrossRef CAS.
- I. Svanedal, G. Persson, M. Norgren and H. Edlund, Langmuir, 2013, 29, 13708–13716 CrossRef CAS PubMed.
- Y. Zhang, L. S. Romsted, L. Zhuang and S. de Jong, Langmuir, 2013, 29, 534–544 CrossRef PubMed.
- J. M. Traverso-Soto, P. A. Lara-Martín, E. González-Mazo and V. M. León, Sci. Total Environ., 2015, 503–504, 87–96 CrossRef CAS PubMed.
- C. Corada-Fernández, J. Jiménez-Martínez, L. Candela, E. González-Mazo and P. A. Lara-Martín, Chemosphere, 2015, 119, S131–S137 CrossRef PubMed.
- K. Masakorala, A. Turner and M. T. Brown, Water, Air, Soil Pollut., 2011, 218, 283–291 CrossRef CAS.
- N. Joondan, S. Jhaumeer-Laulloo and P. Caumul, Microbiol. Res., 2014, 169, 675–685 CrossRef CAS PubMed.
- A. Colomer, A. Pinazo, M. T. García, M. Mitjans, M. P. Vinardell, M. R. Infante, V. Martínez and L. Pérez, Langmuir, 2012, 28, 5900–5912 CrossRef CAS PubMed.
- E. K. Anderberg and P. Artursson, J. Pharm. Sci., 1993, 82, 392–398 CrossRef CAS PubMed.
- C. Morán, P. Clapés, F. Comelles, T. García, L. Pérez, P. Vinardell, M. Mitjans and M. R. Infante, Langmuir, 2001, 17, 5071–5075 CrossRef.
- Ž. Pavlić, Ž. Vidaković-Cifrek and D. Puntarić, Chemosphere, 2005, 61, 1061–1068 CrossRef PubMed.
- D. Jin, X. Jiang, X. Jing and Z. Ou, J. Hazard. Mater., 2007, 144, 215–221 CrossRef CAS PubMed.
- M. Vaughan and R. van Egmond, Altern. Lab. Anim., 2010, 38, 231–238 CrossRef CAS PubMed.
- S. Guo, Genes, Brain Behav., 2004, 3, 63–74 CrossRef CAS PubMed.
- W. B. Barbazuk, I. Korf, C. Kadavi, J. Heyen, S. Tate, E. Wun, J. A. Bedell, J. D. McPherson and S. L. Johnson, Genome Res., 2000, 10, 1351–1358 CrossRef CAS PubMed.
- N. Zaidi, S. Nusrat, F. K. Zaidi and R. H. Khan, J. Phys. Chem. B, 2014, 118, 13025–13036 CrossRef CAS PubMed.
- M. F. Vitha, J. D. Weckwerth, K. Odland, V. Dema and P. W. Carr, J. Phys. Chem., 1996, 100, 18823–18828 CrossRef CAS.
- I. J. A. Baker, B. Matthews, H. Suares, I. Krodkiewska, D. N. Furlong, F. Grieser and C. I. Drummond, J. Surfactants Deterg., 2000, 3, 1–11 CrossRef CAS.
- O. Kirk, F. D. Pedersen and C. C. Fuglsang, J. Surfactants Deterg., 1998, 1, 37–40 CrossRef CAS.
- K. Holmberg, B. Jönsson, B. Kronberg and B. Lindman, in Surfactants and Polymers in Aqueous Solution, John Wiley & Sons, Ltd, Chichester, UK, 2002, pp. 1–37 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00189j |
| This journal is © The Royal Society of Chemistry 2023 |