Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The critical role of H2 reduction roasting for enhancing the recycling of spent Li-ion battery cathodes in the subsequent neutral water electrolysis†
Jiayin
Zhou
 ,
Jihong
Ni
,
Jihong
Ni
 and
Xiaofei
Guan
and
Xiaofei
Guan
 *
*
School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China. E-mail: guanxf@shanghaitech.edu.cn
First published on 26th September 2023
Abstract
Effective and sustainable recycling of lithium-ion batteries is critically important for comprehensive resource utilization and environmental protection. Herein, we propose a novel recycling process that combines H2 reduction roasting and neutral water electrolysis to recover valuable metal elements from the waste cathodes of spent lithium-ion batteries. Firstly, the waste cathode materials were calcined in H2 to form oxides with lower valence transition metal ions. Then, the low-valence transition metal ions were leached from the reduced materials in the low-pH chamber and precipitated as hydroxides in the high-pH chamber of the neutral water electrolyzer. Three common LIB cathodes (i.e., LiCoO2, LiMn2O4, LiNi0.5Co0.2Mn0.3O2) were processed with this new combined method, and the leaching efficiencies of the Li ion and the transition metal ions significantly improved versus not using the reductive H2 roasting. The leaching kinetics of LiCoO2, LiMn2O4, LiNi0.5Co0.2Mn0.3O2, CoO, MnO, and NiO were carefully analyzed and compared to further understand the advantages of the combined method. The kinetics study supports the experimental finding that the transition metal elements are more easily leached from the roasted products than from the pristine cathode materials. Moreover, the H2 produced at the cathode chamber of the neutral water electrolyzer can be recycled to the first step of reduction roasting, realizing the closed-loop utilization of H2. This work highlights the critical role of H2 reduction roasting in improving the recycling of the waste cathodes in the subsequent neutral water electrolysis.
Sustainability spotlightThe recycling of Li-ion batteries is becoming a matter of growing urgency because of the skyrocketing demand for new Li-ion batteries and the accumulation of enormous quantities of spent ones. The lack of feasible and sustainable recycling technologies will not only cause wastage of resources and environmental pollution, but also subdue the further penetration of Li-ion batteries in electric vehicles and grid-scale energy storage applications. Here, we develop a novel and effective solution that combines H2 reduction roasting and neutral water electrolysis to recover valuable metals from the waste cathodes of spent Li-ion batteries. This work makes contributions to enable the UN Sustainable Development Goals 9 (industry, innovation and infrastructure), 12 (responsible consumption and production), and 13 (climate action). |
1. Introduction
Lithium-ion batteries (LIBs) have become an indispensable part of the modern society since their commercialization. They have wide applications ranging from electric and hybrid vehicles, portable electronic devices, to stationary energy storage systems.1–3 The rapid growth of the LIB market will lead to the accumulation of enormous amounts of spent LIBs. By the year 2035, 6.76 million spent LIBs will be generated per year.4 Nevertheless, the percentage of LIBs recycled worldwide is still relatively low.5,6 This gap motivates the research and development of novel technologies for recycling spent LIBs, which will not only alleviate the possible resource shortage in the future but also bring the benefits of economic growth and environmental protection.7,8At present, there are four major methods to recover valuable metals from waste LIB cathode materials: hydrometallurgy, biometallurgy, pyrometallurgy, and direct recycling process. Among them, hydrometallurgical processes have received significant research attention because they consume less energy and enable a more efficient and comprehensive recovery of the valuable elements. Reducing agents are often used along with the acid leachant to lower the valence state of the transition metals (e.g., Co, Mn) to +2 and thereby improve the leaching efficiency.9,10 However, the conventional hydrometallurgical processes with convoluted chemistry often involve large consumption of chemicals, leading to the issues of high cost, waste generation and environmental pollution.11
Recently, the electrochemical pH gradient produced by neutral water electrolysis in an H-shaped electrolysis cell (H-cell) has been systematically investigated as an environmentally benign method to recover valuable metals from waste LIB cathodes (e.g., LiCoO2 (ref. 12) and LiMn2O4 (ref. 13)) in addition to decarbonating CaCO3 (ref. 14 and 15) and direct air capture of CO2.16 This method shows promise in reducing the chemical consumption and simplifying the process. However, it is incompatible with the use of water-soluble reducing agents (e.g., H2O2, NaHSO3, Na2S2O5, ethanol, or glucose)4,12 in the anode chamber, because a portion of the reducing agent added would be inevitably consumed through oxidization at the anode. When not adding any external reducing agents, only the oxygen ions in the lattice of waste cathodes would serve as the reducing agent,17 and the leaching kinetics would be slow. To circumvent the abovementioned issues and improve the leaching efficiencies of transition metals in the anode chamber of the neutral water electrolyser, the waste cathode materials of LIBs can be pretreated by reduction roasting. The previous studies on other different methods of recycling LIBs have reported various solid reductants for reduction roasting, such as graphite,18–21 coal (e.g., lignite),22 and Al foil.23 Although satisfactory leaching efficiencies in the subsequent water leaching are obtained, these solid reductants resulted in low product purity and required further treatment steps to remove residual reducing agents.24 Gaseous reductants including NH3,25 CH4,26 and H2 (ref. 27 and 28) have also been explored, and the results were promising in reducing the waste LIB cathodes.
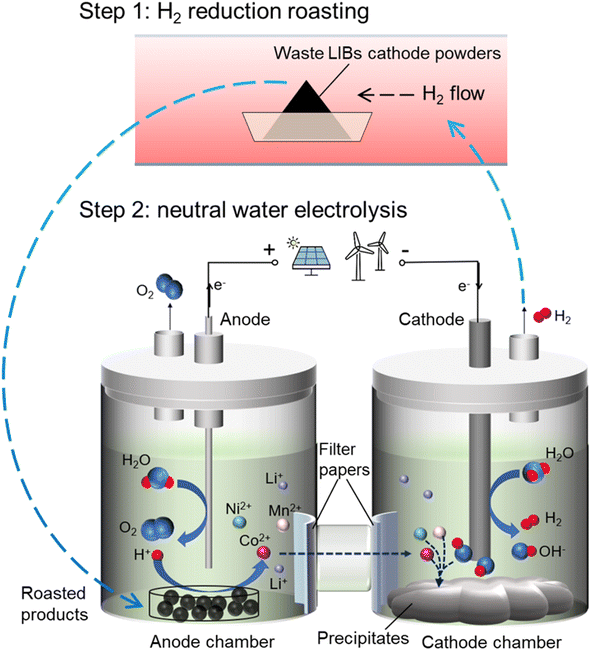
In this work, we, for the first time, propose a novel combined process of H2 reduction roasting and neutral water electrolysis to enable the effective and sustainable recovery of cathode materials from spent LIBs (Fig. 1). Firstly, the cathode material is reduced into low-valence metal oxides (MO; M: Co, Mn, or Ni) which are easy to dissolve in acid. The ideal chemical reactions between H2 and LiCoO2, LiMn2O4, or LiNi0.5Co0.2Mn0.3O2 are expressed by eqn (1)–(3). Then, the low-valence transition metal elements in the reduced cathode materials are efficiently leached and recovered using the pH gradient generated by neutral water electrolysis. During the neutral water electrolysis, the oxygen evolution reaction (OER, eqn (4)) occurs at the anode, which can provide H+ for the dissolution of the reduced cathode materials, as described by eqn (5). The hydrogen evolution reduction (HER, eqn (6)) takes place at the cathode. The transition metal ions are transported from the anode chamber to the cathode chamber, where they are precipitated into hydroxides as represented by eqn (7). The mixed hydroxide precipitates can be readily separated from the electrolysis cell, after which the ratio of transition metals can be adjusted. The Li+ that are leached out can be precipitated in the form of Li2CO3 (eqn (8)). The new layered ternary LiNixCoyMnzO2 (NCM) can be synthesized from the Li2CO3 and the transition metal hydroxides recovered, closing the loop. In addition, some of the H2 generated by water electrolysis can be recycled to the first step of reduction roasting. Compared with traditional hydrometallurgical methods, this new combined process minimizes the use of chemicals (e.g., external acids, bases and reducing agents), generates no hazardous chemical waste, and also enables the closed-loop utilization of H2. Therefore, this novel combined method is effective and environmentally friendly, and has industrial potential for large-scale recycling of spent LIBs.
| 2LiCoO2 + H2(g) = 2LiOH + 2CoO | (1) |
| 2LiMn2O4 + 3H2(g) = 2LiOH + 4MnO + 2H2O(g) | (2) |
| LiNi0.5Co0.2Mn0.3O2 + 1/2H2(g) = LiOH + 0.5NiO + 0.2CoO + 0.3MnO | (3) |
| H2O = 1/2O2 + 2H+ + 2e− | (4) |
| MO + 2H+ = M2+ + H2O (M2+: Co2+, Ni2+, Mn2+) | (5) |
| 2H2O + 2e− = 2OH− + H2 | (6) |
| M2+ + 2OH− = M(OH)2 (M2+: Co2+, Ni2+, Mn2+) | (7) |
| 2Li+ + CO32− = Li2CO3 | (8) |
2. Experimental
2.1. Pretreatment
The spent LIBs with the three common cathode chemistries (LiCoO2, LiMn2O4, and LiNi0.5Co0.2Mn0.3O2) were purchased from recycling merchants located in Guangdong province, China. The recycling of LIBs with two other important cathode chemistries (i.e., LiFePO4, LiNixCoyAl1−x−yO2) was not investigated in this work. The spent LIBs were fully discharged by immersing in a NaCl aqueous solution (1 mol L−1) for 24 hours, and then manually disassembled inside a fume hood after washing and drying at room temperature. To remove the electrolyte and the binder, the cathode scraps consisting of Al foil and the cathode powders were transferred to a quartz-tube furnace and heated at 400 °C under vacuum for 5 hours.29 Then, the cathode powders were easily peeled off. To decrease the particle size, the cathode powders were ground in a planetary ball mill at 500 rpm for two hours. The milling medium and the volume ratio used were consistent with the previous work.12 Afterwards, DI water was added to the cathode powders to remove some graphite by flotation. Eventually, the cathode powders were dried and used in the electrolysis experiments. For convenience, these dried cathode powders were referred to as the pristine waste cathode powders. The composition was characterized by inductively coupled plasma optical emission spectroscopy (ICP-OES). For example, the molar ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn was measured to be 5.02
Mn was measured to be 5.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.88 in a batch of pristine waste ternary cathode materials. Considering the experimental error introduced in the process of sample solution preparation, the waste cathode material was inferred to be LiNi0.5Co0.2Mn0.3O2, hereinafter referred to as NCM523 (or NMC532).
2.88 in a batch of pristine waste ternary cathode materials. Considering the experimental error introduced in the process of sample solution preparation, the waste cathode material was inferred to be LiNi0.5Co0.2Mn0.3O2, hereinafter referred to as NCM523 (or NMC532).
2.2. H2 reduction roasting
A certain quality of cathode material powders was added to an alumina boat, and reduction roasting was performed inside a quartz tube in an electric furnace. Ar gas was used to ensure an inert atmosphere during the heating process at 5 °C min−1. When the furnace reached the target temperature, Ar was turned off and 5% H2–95% Ar gas was flowed to the reactor at 150 mL min−1. After the predetermined time elapsed, the 5% H2–95% Ar was switched off and the roasted products were cooled to room temperature at 5 °C min−1 under Ar protection.2.3. Electrochemical cells
The H-shaped electrochemical cells (H-cells) for water electrolysis were assembled following the process as described in Note S1.† Neutral water electrolysis experiments were carried out under different applied voltages, temperatures, durations, and solid-to-liquid ratios for recycling the waste cathode materials. The solid-to-liquid ratio is defined as the ratio of the mass of the pristine waste cathode powders to the volume of the aqueous electrolyte in the anode chamber (10 ml). Gamry™ Interface 1000 potentiostat (Gamry, PA, USA) was used for all electrochemical measurements. The pH values of the aqueous electrolyte in the anode and cathode chambers were measured by using a double-junction pH probe (Leici Instrument, Shanghai, China).2.4. Regeneration of NCM523
A high-temperature solid-state reaction was used to resynthesize NCM523. In brief, the transition metal hydroxide precursor of the desired molar ratio (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 5
Mn = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and the Li2CO3 powders recovered were mixed with a Li
3) and the Li2CO3 powders recovered were mixed with a Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Ni + Co + Mn) molar ratio of 1.05
(Ni + Co + Mn) molar ratio of 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ground with an agate mortar and pestle. The mixture was pressed into a pellet and then sintered at 950 °C for 10 h in air in a muffle furnace.
1 and ground with an agate mortar and pestle. The mixture was pressed into a pellet and then sintered at 950 °C for 10 h in air in a muffle furnace.
2.5. Materials characterization
The crystalline phases of the samples were characterized by using X-ray diffraction (XRD) with a Cu target and a θ–2θ geometry (Bruker™ D8). The XRD patterns were scanned with an increment of 0.02° and a scanning rate of 0.2° or 0.02° per second. The ICDD Powder Diffraction File (PDF)-4+ database was used for phase identification. Raman spectra (Andor Shamrock SR-500i) and the X-ray photoelectron spectroscopy (XPS; Thermo Fisher Scientific ESCALAB™ 250Xi) results of the samples were also collected. The surface morphologies and elemental distribution were observed using a scanning electron microscope equipped with an energy dispersive X-ray spectroscopy instrument (SEM-EDS, JEOL SEM IT-500HR).Inductive coupled plasma optical emission spectroscopy (ICP-OES) was employed to determine the content of metal ions in the samples (Thermo Scientific ICP-OES iCAP™ 7400). The sample solution was prepared by first dissolving the sample in concentrated HCl (Sinopharm, 36.5%) and then diluting with DI water by 10 times. The leaching efficiencies of various metal ions from the powders were calculated from γM = (cMm – c′Mm′)/cMm, where γM is the leaching efficiency of M (M: Li, Co, Ni and Mn); cM and c′M are the contents of M in the powders in the anode chamber before and after leaching, respectively; m and m′ are the masses of the powders in the anode chamber before and after leaching, respectively.
2.6. Leaching kinetics analysis
Leaching of the commercially purchased high-purity oxide powders (CoO, MnO, NiO, LiCoO2, LiMn2O4, and LiNi0.5Co0.2Mn0.3O2) was carried out in the standard sulfuric acid solutions (0.05 mol L−1, 200 ml) in single-chamber glass reactors. The acid concentration was selected for two reasons: first, the pH value was stable during the leaching; second, the pH value was relatively low but close to the actual pH in the anode chamber during the neutral water electrolysis. In each experiment, the oxide powders (0.05 g) inside a removable quartz crucible were placed into the acid solution after reaching the desired temperature. The solution was then agitated with a magnetic stir bar at 450 rpm. During the leaching process, 1 ml of the solution was sampled at certain intervals to measure the leaching efficiency of each transition metal cation for the kinetics analysis.The leaching process of the oxide powders in acid can be simplified into one of the three typical kinetic models: the liquid boundary layer mass transfer control model (eqn (9)), the surface chemical reaction control model (eqn (10)) and the residue layer diffusion control model (eqn (11)).30 In these equations, X is the leaching efficiency of a metal cation, t is the reaction time (min); k1, k2 and k3 are the specific reaction rate constants and also the slopes of the fitting lines for the three models, respectively.
| X = k1t | (9) |
| 1 − (1 − X)⅓ = k2t | (10) |
| 1 − 3(1 − X)⅔ + 2(1 − X) = k3t | (11) |
After obtaining the values of specific rate constant, k, at various temperatures, the data were fitted to the Arrhenius relationship as described by eqn (12),31 where A is the pre-exponential factor (min−1), R is the gas constant (8.31 J mol−1 K−1), Ea is the apparent activation energy for the leaching of a certain type of metal cation (J mol−1), and T is the absolute temperature (K). The value of Ea can be obtained as the slope of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. −1/RT curve. It is noted that the factor A varies with the experimental conditions including the acid concentration and the solid-to-liquid ratio.32
k vs. −1/RT curve. It is noted that the factor A varies with the experimental conditions including the acid concentration and the solid-to-liquid ratio.32
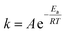 | (12) |
3. Results and discussion
3.1. Recycling LiCoO2
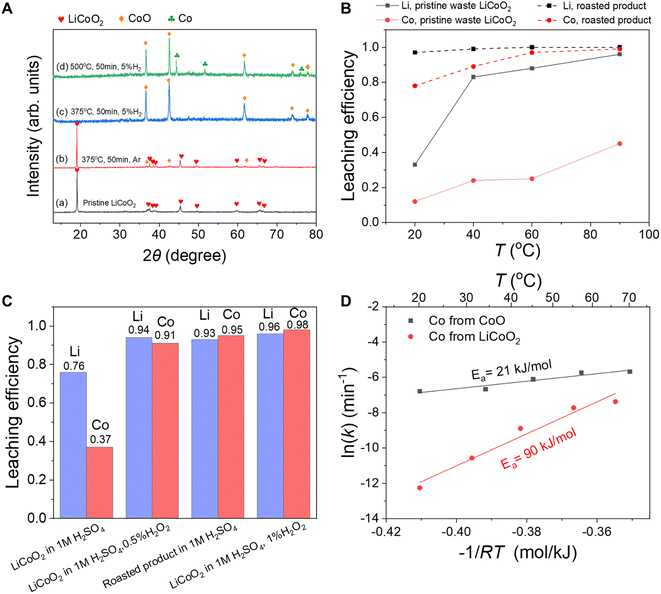
The roasted product treated with 5% H2 at 375 °C for 50 minutes was further characterized using XPS. Based on the core-level C 1s XPS spectrum (Fig. S2†), there were two kinds of carbon species on the surface of the roasted product. The major peak at ∼284.8 eV was attributed to the adventitious carbon, while the other major peak at ∼290 eV was assigned to the carbonate.35 As shown in the core-level Co 2p XPS spectra (Fig. S3†), the binding energy of Co 2p3/2 in the pristine waste LiCoO2 was ∼780.1 eV, and the separation of the binding energies of Co 2p3/2 and Co 2p1/2 was ∼15 eV. In comparison, the separation of the binding energy of Co 2p1/2 and Co 2p3/2 of the roasted product was ∼15.8 eV, confirming the change of the Co valence state from +3 to +2 after the H2 roasting.12
As shown in Fig. 2C, the leaching efficiencies of Li and Co of pristine waste LiCoO2 in 1 M H2SO4 without H2O2 were 0.76 and 0.37, respectively. When the roasted product directly reacted with 1 M H2SO4, the leaching efficiencies of Li and Co reached as high as 0.93 and 0.95, respectively. The results were comparable to the leaching efficiencies of LiCoO2 in 1 M H2SO4 containing 0.5% H2O2 or 1% H2O2. In other words, the reduction effect of H2 roasting pretreatment was comparable to that of adding 0.5–1% H2O2 to acid. As an environmentally benign reducing agent, H2O2 is widely adopted as a reducing agent in scientific research and industrial processes; however, it is relatively expensive and decomposes readily upon exposure to light or heat, leading to cost increase and waste of resources.4 In comparison, the use of H2 reduction roasting might be more advantageous especially considering that the excess H2 can be recirculated.
3.2 Recycling LiMn2O4
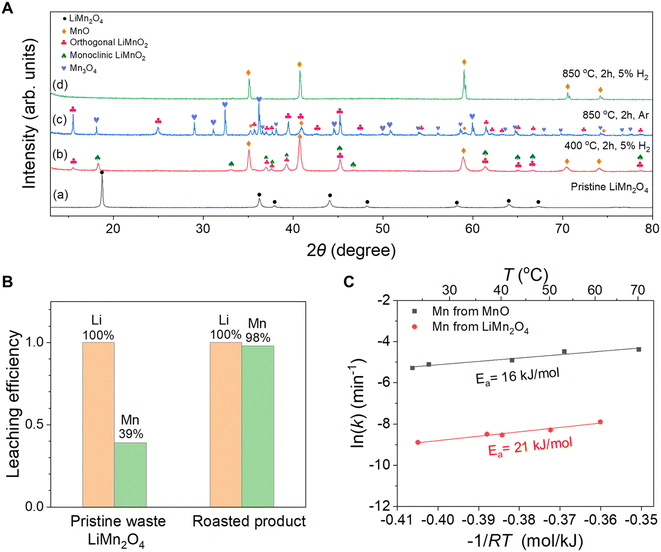
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and −1/RT, the activation energies for the Mn leaching from MnO and LiMn2O4 were determined to be 16 kJ mol−1 and 21 kJ mol−1, respectively (Fig. 3C). For LiMn2O4, the dissolution of Mn was initially facilitated by the Jahn–Teller effect;37,39 however, further leaching of the Mn was limited by the highly covalent Mn(IV)–O bonds.13,39 In comparison, for the Mn leaching from MnO, the value of Ea was slightly smaller and the value of ln(k) was larger, confirming that the MnO was more easily soluble in acid than LiMn2O4.
k and −1/RT, the activation energies for the Mn leaching from MnO and LiMn2O4 were determined to be 16 kJ mol−1 and 21 kJ mol−1, respectively (Fig. 3C). For LiMn2O4, the dissolution of Mn was initially facilitated by the Jahn–Teller effect;37,39 however, further leaching of the Mn was limited by the highly covalent Mn(IV)–O bonds.13,39 In comparison, for the Mn leaching from MnO, the value of Ea was slightly smaller and the value of ln(k) was larger, confirming that the MnO was more easily soluble in acid than LiMn2O4.
3.3. Recycling NCM523
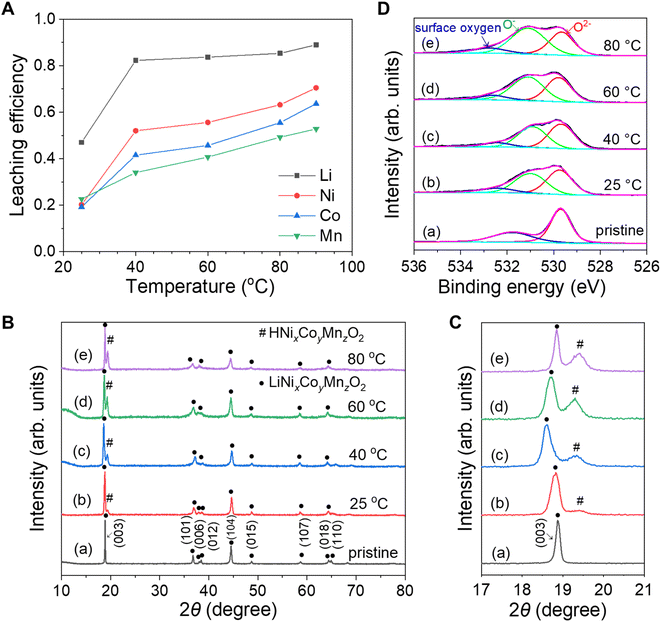
Fig. 4A shows the leaching efficiencies of Li, Ni, Co and Mn from waste NCM523 powders at different temperatures (25, 40, 60, 80, and 90 °C). In this series of experiments, the solid-to-liquid ratio was 10 g L−1, the voltage was 3.5 V, and the leaching time was 24 hours. The leaching efficiency of the four metal elements all increased with temperature. This was mainly because the temperature increase caused the electrolyte ohmic resistance to decrease, and sped up the kinetics of the leaching reaction, resulting in a higher concentration of H+ in the anode chamber. The leaching behavior of Li in NCM523 was similar to that in LiCoO2 reported in a previous study.12 The leaching efficiency of Li increased from 47% to 82.3% as the temperature increased from 25 °C to 40 °C and gradually increased to 89% at 90 °C. The leaching behavior of Ni and Co increased rapidly from 20.2% and 19.2% at 25 °C to 52% and 41.5% at 40 °C, and then gradually increased to 70.5% and 63.7% at 90 °C, respectively. Although the bond dissociation energies of Ni–O and Co–O were close,42 the leaching efficiency of Ni was slightly higher than that of Co, because a significant portion of the Ni in NCM523 was in the divalent state43 and was relatively easy to leach without the reduction.37 The leaching efficiency of Mn gradually increased with temperature, but reached only 52.8% at 90 °C. This was because the Mn(IV)–O bond was more stable than the Ni–O bond and the Co–O bond, rendering the leaching of Mn more difficult. The results were consistent with the previous reports that Mn played a role of stabilizing the structure of the ternary NCM materials.44,45
The residue powders after leaching at different temperatures were characterized by XRD. It is noted that the amount of residue powders after leaching at 90 °C was too little to characterize. Fig. 4B and C show the XRD patterns for the waste NCM523 powders before leaching and after leaching at different temperatures (25, 40, 60, and 80 °C) for 24 hours. The powders maintained the α-NaFeO2 structure after leaching. The peak splitting of (006)/(012) and (018)/(110) was unclear and amalgamated into one peak, indicating a disordered lamellar phase, with a mixing of lithium and metal ions. The (003) diffraction peak implied that all the residue powders retained a layer structure in the c-axis direction. Compared with the XRD pattern of the pristine sample as shown in Fig. 4C(a), the (003) diffraction peak for the residual powders shifted toward lower 2θ, which was caused by the increased lattice parameters in the c-axis direction. Due to the decreasing lithium content, the electrostatic repulsion between the oxygen anions increased and the distance between the metal–oxygen octahedral layers increased. It was noted that the (003) diffraction peak shifted toward higher 2θ after leaching at 60 °C and 80 °C compared with that after leaching at 25 °C and 40 °C, likely due to the migration of Ni2+ to the Li+ layer. Ni2+ (0.069 nm)46 and Li+ (0.076 nm)47 have similar radii. Since Ni2+ has one more positive charge than Li+, when the Ni2+ occupied the vacancies created by the removal of Li+, the electrostatic attraction between Ni–O reduced the distance between metal–oxygen octahedral layers, resulting in the decrease of the lattice parameter in the c-axis direction,48 so the (003) diffraction peak shifted toward higher 2θ. It's noted that the claim for Ni2+ might also be applicable to Co3+ (radius: 0.0545 nm)49 in the lattice which has two more positive charges than Li+. Another revealing finding of this study was the observation of a small diffraction peak next to the (003) diffraction peak at higher 2θ value in all patterns after leaching, indicating the ion exchange between H+ and Li+ during leaching.12 With the increase of temperature, the intensity of this peak gradually increased, indicating that higher temperature accelerated the exchange of H+ and Li+, and promoted the formation of the HNixCoyMnzO2 phase.
The residue powders after leaching at different temperatures were also characterized by XPS. The high-resolution XPS spectra of Co 2p, Ni 2p, Mn 2p and Mn 3s did not show obvious changes (Fig. S9†). The dramatic change of high-resolution XPS spectra of O 1s confirmed that lattice oxygen played a vital role during leaching (Fig. 4D).12,17 The O 1s spectrum of the pristine waste NCM523 powders displayed two main peaks. A well-defined peak observed at 529.7 eV was characteristic of O2− anions of the crystalline network, whereas the peak at 531.8 eV was attributed to the adsorbed oxygen surface species. Interestingly, a new peak attributed to O− at 531 eV appeared after leaching and the intensity of this peak gradually increased with increased leaching efficiency. The O−/O2− intensity ratio increased from 0.81 at 25 °C to 0.86 at 40 °C, 1 at 60 °C and 1.11 at 80 °C (Table S1†), indicating that the electrons of lattice oxygen were transferred to transition metals during leaching.
In addition to temperature, other parameters on the leaching efficiency of Li, Ni, Co and Mn from waste NCM523 powders were also carefully investigated. The effect of applied voltage and duration is discussed in Note S2 and Fig. S10.† The effect of solid-to-liquid ratio is discussed in Note S3, Fig. S11 and S12 in the ESI.† The optimal leaching conditions were determined to be an electrolysis voltage of 3.5 V, a solid-to-liquid ratio of 10 g L−1, and a temperature of 90 °C. Overall, the leaching rates of the metals from NCM523 were still relatively slow due to the lack of external reducing agents.
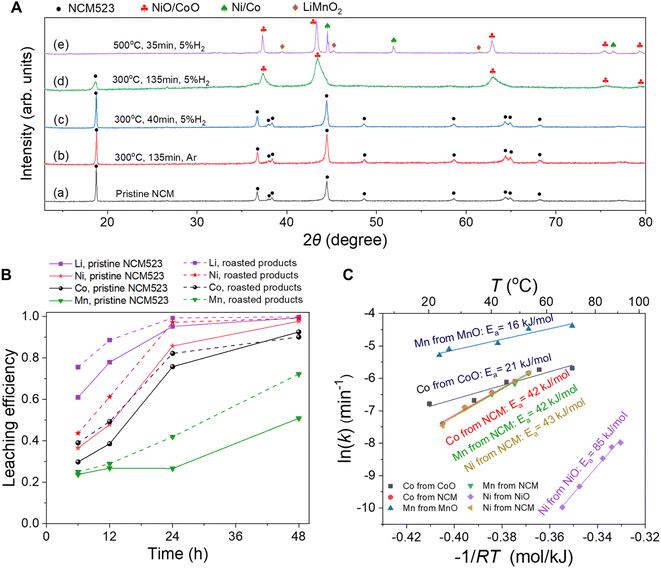 | ||
| Fig. 5 (A) The XRD patterns of (a) the pristine waste NCM523 and the products roasted at (b) 300 °C for 135 min in Ar, (c) 300 °C for 40 min in 5% H2, (d) 300 °C for 135 min in 5% H2, (e) 500 °C for 35 min in 5% H2. Scanning rate: 0.02° s−1. The data from the reference on LiNixCoyMnzO2 (ref. 16) and the standard cards of NiO (PDF No. 00-004-0835), Ni (PDF No. 00-001-1260), CoO (PDF No. 00-43-1004), Co (PDF No. 00-15-0806), and orthogonal LiMnO2 (No. 00-035-0749) are used to index the diffraction peaks. The diffraction peaks of NiO and CoO are too close to differentiate. NiO/CoO refers to a mixture or solid solution of NiO and CoO. Similarly, the diffraction peaks of Ni and Co are also very close, and Ni/Co refers to a mixture or alloy of Ni and Co. (B) The leaching efficiencies of Li, Ni, Co, and Mn from the pristine waste NCM523 and the roasted products in H-cells with an applied voltage of 3.5 V and a solid-to-liquid ratio of 10 g L−1 at 90 °C for varying periods of time (6, 12, 24, and 48 h). (C) Kinetics analysis showing the Arrhenius plots for the Co, Mn and Ni leaching from NCM523, the Co leaching from CoO, the Mn leaching from MnO, and the Ni leaching from NiO in 0.05 mol L−1 H2SO4 solutions. All these oxides were commercially purchased. | ||
Fig. 5B shows the leaching efficiencies of Li, Ni, Co, and Mn from the roasted products and the pristine waste NCM523 powders after electrolysis at 3.5 V for varying periods of time (6, 12, 24, and 48 h) with the same solid-to-liquid ratio 10 g L−1 at 90 °C. Remarkably, the leaching efficiencies of Li, Ni, Co and Mn from the roasted products were generally greater. After the 24 h electrolysis of the pristine NCM523, the leaching efficiencies of Li, Ni, Co, and Mn were 95%, 86%, 76% and 27%, respectively. In comparison, the leaching efficiencies of Li, Ni, Co and Mn from the roasted products were 99%, 97%, 82% and 42%, respectively. After the 48 h electrolysis experiment, the leaching efficiencies of Li, Ni, Co and Mn from the roasted products increased to 100%, 99%, 90%, and 72%, respectively.
Fig. S15† shows the XRD patterns for the roasted products and the residues after leaching for various periods of time (6,12, and 24 h) in the anode chamber of the H-cell. The amount of residues after leaching for 48 h was too little to characterize with XRD. The diffraction peaks for NiO and CoO gradually became weaker with time and disappeared after 24 h due to the dissolution. The LiNixCoyMnzO2 phase was present in all these scans. The γ-MnO2 and the birnessite-type phase were formed in the residues after 24 hours of leaching; such phases were also observed after the leaching of the pristine waste NCM523 in neutral water electrolysis for 48 hours (Fig. S12A†).
The relatively low leaching efficiency of Mn from the roasted products was attributed to the following factors. First, the acid-soluble MnO was not formed after the reduction roasting at 300 °C for 135 min in 5% H2. Second, a small amount of NCM phase was still present in the roasted products (Fig. 5A(d)). NCM exhibited a relatively slow leaching kinetics. Nevertheless, the leaching efficiency of Mn would in principle be improved significantly if a pathway could be found to convert all the Mn in NCM523 to the low-valence MnO by H2 reduction roasting. For example, the roasted product (calcined at 300 °C for 135 min in 5% H2) could be first subjected to the electrolysis in an H-cell to leach out almost all the Li, Ni, and Co. Afterwards, the residues containing mainly Mn could be collected and subjected to H2 reduction roasting at a greater temperature (e.g., 850 °C) to produce MnO, similar to the H2 reduction of the pristine waste LiMn2O4 as described in Section 3.2.1. Then, the MnO product obtained could be subjected to the electrolysis again in an H-cell to leach out all the Mn ions.
To obtain a high-quality precursor, the universal co-precipitation method reported in a previous study was adopted.54 In brief, the mixed hydroxides obtained from the electrolysis experiments were first dissolved in dilute H2SO4. Additional NiSO4, CoSO4 and MnSO4 were then added to adjust the ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn to 5
Mn to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Finally, excessive NaOH solution was added to fully co-precipitate the transition metal ions and produce a high-quality hydroxide precursor with a 5
3. Finally, excessive NaOH solution was added to fully co-precipitate the transition metal ions and produce a high-quality hydroxide precursor with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 Ni
3 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio. Besides, the Li+ that were leached out were precipitated in the form of Li2CO3 following the procedures reported previously.12 New NCM was resynthesized by calcining the Li2CO3 recovered and the hydroxide precursor co-precipitated. In the XRD pattern for the resynthesized powders, the diffraction peaks were well indexed with the hexagonal α-NaFeO2-type structure of the space group R
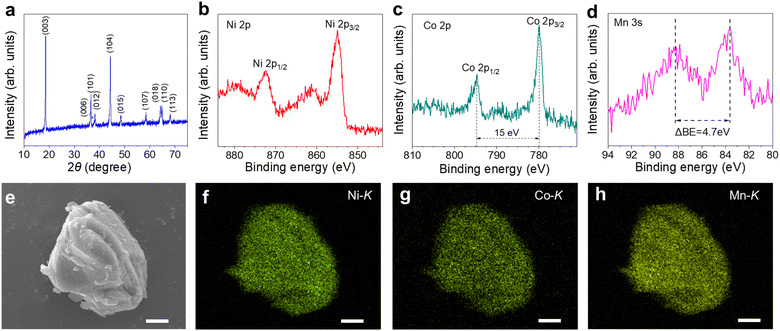
Mn ratio. Besides, the Li+ that were leached out were precipitated in the form of Li2CO3 following the procedures reported previously.12 New NCM was resynthesized by calcining the Li2CO3 recovered and the hydroxide precursor co-precipitated. In the XRD pattern for the resynthesized powders, the diffraction peaks were well indexed with the hexagonal α-NaFeO2-type structure of the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (Fig. 6a). The obvious splitting of peaks (006)/(012) and (018)/(110) indicated that the degree of cation mixing was small. The XPS spectra confirmed the existence of Ni, Co and Mn in the synthesized powder (Fig. 6b–d). The binding energy of Ni 2p3/2 was 854.9 eV, consistent with the previous literature on NCM523.43 The separation of Co 2p3/2 and Co 2p1/2 was approximately 15 eV, indicating the valence state of Co as +3.12 The magnitude of the Mn 3s peak splitting was approximately 4.7 eV, suggesting the valence state of Mn as +4.41 The SEM-EDS analysis of the resynthesized powders showed that Ni, Co and Mn were homogeneously distributed throughout the particle (Fig. 6e–h). Detailed evaluation of the electrochemical performance of the regenerated NCM523 as new cathode materials for LIBs is beyond the scope of the current work and will be performed in the future work.
m (Fig. 6a). The obvious splitting of peaks (006)/(012) and (018)/(110) indicated that the degree of cation mixing was small. The XPS spectra confirmed the existence of Ni, Co and Mn in the synthesized powder (Fig. 6b–d). The binding energy of Ni 2p3/2 was 854.9 eV, consistent with the previous literature on NCM523.43 The separation of Co 2p3/2 and Co 2p1/2 was approximately 15 eV, indicating the valence state of Co as +3.12 The magnitude of the Mn 3s peak splitting was approximately 4.7 eV, suggesting the valence state of Mn as +4.41 The SEM-EDS analysis of the resynthesized powders showed that Ni, Co and Mn were homogeneously distributed throughout the particle (Fig. 6e–h). Detailed evaluation of the electrochemical performance of the regenerated NCM523 as new cathode materials for LIBs is beyond the scope of the current work and will be performed in the future work.
4. Conclusions
In summary, we have demonstrated a sustainable and effective approach that combined H2 reduction roasting and neutral water electrolysis to recover valuable metals from spent LIBs. H2 served as a gas reductant to reduce the pristine waste cathode materials to low-valence transition metal oxides that easily dissolved in the subsequent electrolysis. We highlight that the step of the H2 reduction roasting circumvented a major obstacle to the neutral water electrolysis method (i.e., incompatibility with an external water-soluble reducing agent) and significantly increased the leaching efficiencies of the transition metal ions. For example, the leaching efficiency of Co from the roasted product was almost 4 times greater than that from the pristine waste LiCoO2 in neutral water electrolysers at 3.5 V for 24 h at 60 °C. In a neutral water electrolyser at 3.5 V for 24 h at 90 °C, the leaching efficiency of Mn from the roasted product reached almost 100%, which was approximately 2.5 times greater than that from the pristine waste LiMn2O4. The H2 roasting treatment was also effective in improving the leaching efficiencies of Ni, Co, and Mn in the case of recycling NCM523. Besides, the transition metal hydroxides and the Li2CO3 recovered were used to resynthesize new NCM523 materials. Moreover, a portion of the H2 generated by the water electrolysis can be used as the gaseous reducing agent in the first step of roasting, enabling the closed-loop utilization of H2.Although the scale up of a chemical process is known to be complex with multiple key factors to be considered,55 our combined method has great potential for industrial-scale applications. In the first step of H2 reduction roasting, the relatively mild temperature conditions, compared with those of the current pyrometallurgical methods,33 suggest a low energy consumption in a scaled-up plant. In addition, lessons and experiences on the scale up could be earned from the fast-developing hydrogen metallurgy industry due to the similarity.56 Besides, the use of H2 rather than carbon or carbon monoxide as the reducing agent also cuts down the CO2 emission and reduces the environmental impact. In the second step of the neutral water electrolysis, the energy consumption can be reduced by optimizing the cell design and the electrocatalytic electrodes to lower the overpotential losses. In the future, the heat required for H2 reduction roasting and the power for the neutral water electrolysis might be provided by the emerging affordable renewable electricity. Overall, this combined method represents a promising approach for recycling waste cathode materials from spent LIBs in an effective and environmentally friendly manner. It could also be of broad interest and motivate further research on recovering valuable elements from other solid wastes.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support from ShanghaiTech University. Part of the work was performed at the Analytical Instrumentation Center (Grant No. SPST-AIC10112914) and the Center for High-resolution Electron Microscopy (CħEM, Grant No. EM02161943) at ShanghaiTech University.References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- W. Yu, Y. Guo, Z. Shang, Y. Zhang and S. Xu, eTransportation, 2022, 11, 100155 CrossRef.
- X. Zhang, L. Li, E. Fan, Q. Xue, Y. Bian, F. Wu and R. Chen, Chem. Soc. Rev., 2018, 47, 7239–7302 RSC.
- M. Jacoby, https://cen.acs.org/materials/energy-storage/time-serious-recycling-lithium/97/i28, 2020, accessed October 19, 2020.
- S. Sun, C. Jin, W. He, G. Li, H. Zhu and J. Huang, Sci. Total Environ., 2021, 776, 145913 CrossRef CAS PubMed.
- A. Chagnes and B. Pospiech, J. Chem. Technol. Biotechnol., 2013, 88, 1191–1199 CrossRef CAS.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. S. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- A. Porvali, A. Chernyaev, S. Shukla and M. Lundström, Sep. Purif. Technol., 2020, 236 Search PubMed.
- P. Liu, L. Xiao, Y. Chen, Y. Tang, J. Wu and H. Chen, J. Alloys Compd., 2019, 783, 743–752 CrossRef CAS.
- C. Stinn and A. Allanore, Nature, 2022, 602, 78–83 CrossRef CAS PubMed.
- J. Ni, J. Zhou, J. Bing and X. Guan, Sep. Purif. Technol., 2021, 278, 119485 CrossRef.
- J. Zhou, J. Bing, J. Ni, X. Wang and X. Guan, Mater. Today Sustain., 2022, 20, 100205 CrossRef.
- L. D. Ellis, A. F. Badel, M. L. Chiang, R. J. Park and Y. M. Chiang, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12584–12591 CrossRef CAS PubMed.
- G. H. Rau, Environ. Sci. Technol., 2008, 42, 8935–8940 CrossRef CAS PubMed.
- C. Zhou, J. Ni, H. Chen and X. Guan, Sustain. Energy Fuels, 2021, 5, 4355–4367 RSC.
- E. Billy, M. Joulié, R. Laucournet, A. Boulineau, E. De Vito and D. Meyer, ACS Appl. Mater. Interfaces, 2018, 10, 16424–16435 CrossRef CAS PubMed.
- J. Li, G. Wang and Z. Xu, J. Hazard. Mater., 2016, 302, 97–104 CrossRef CAS PubMed.
- S. Pindar and N. Dhawan, JOM, 2019, 71, 4483–4491 CrossRef CAS.
- Y. Zhang, W. Wang, Q. Fang and S. Xu, Waste Manag., 2020, 102, 847–855 CrossRef CAS PubMed.
- J. Xiao, J. Li and Z. Xu, Environ. Sci. Technol., 2017, 51, 11960–11966 CrossRef CAS PubMed.
- J. Hu, J. Zhang, H. Li, Y. Chen and C. Wang, J. Power Sources, 2017, 351, 192–199 CrossRef CAS.
- W. Wang, Y. Zhang, X. Liu and S. Xu, ACS Sustainable Chem. Eng., 2019, 7(14), 12222–12230 CAS.
- Z. Huang, D. Yu, B. Makuza, Q. Tian, X. Guo and K. Zhang, Front. Chem., 2022, 10, 1019493 CrossRef CAS PubMed.
- J. Xiao, B. Niu and Z. Xu, J. Hazard. Mater., 2021, 418, 126319 CrossRef CAS PubMed.
- C. Yang, J. Zhang, B. Yu, H. Huang, Y. Chen and C. Wang, Sep. Purif. Technol., 2021, 267, 118609 CrossRef CAS.
- Z. Huang, F. Liu, B. Makuza, D. Yu, X. Guo and Q. Tian, ACS Sustainable Chem. Eng., 2022, 10, 756–765 CrossRef CAS.
- F. Liu, C. Peng, Q. Ma, J. Wang, S. Zhou, Z. Chen, B. P. Wilson and M. Lundström, Sep. Purif. Technol., 2021, 259, 118181 CrossRef CAS.
- J. Zhao, B. Zhang, H. Xie, J. Qu, X. Qu, P. Xing and H. Yin, Environ. Res., 2020, 181, 108803 CrossRef CAS PubMed.
- O. Levenspiel, Wiley, New York, 1972, p. 367.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Chem. Eng. J., 2015, 281, 418–427 CrossRef CAS.
- W. Gao, J. Song, H. Cao, X. Lin, X. Zhang, X. Zheng, Y. Zhang and Z. Sun, J. Clean. Prod., 2018, 178, 833–845 CrossRef CAS.
- B. Makuza, Q. Tian, X. Guo, K. Chattopadhyay and D. Yu, J. Power Sources, 2021, 491, 229622 CrossRef CAS.
- P. Xu, D. H. S. Tan, H. Gao, S. Rose and Z. Chen, in Encyclopedia of Energy Storage, ed. L. F. Cabeza, Elsevier, Oxford, 2022, pp. 98–107 Search PubMed.
- Q. Yang, J. Liu, C. Zhou, J. Ni, E. I. Vovk, Y. Yang, B. Yang and X. Guan, Mater. Today Chem., 2022, 25, 100949 CrossRef CAS.
- C. K. Lee and K.-I. Rhee, Hydrometallurgy, 2003, 68, 5–10 CrossRef CAS.
- L. Li, Y. Bian, X. Zhang, Y. Guan, E. Fan, F. Wu and R. Chen, Waste Manag., 2018, 71, 362–371 CrossRef CAS PubMed.
- X. Zhu, F. Meng, Q. Zhang, L. Xue, H. Zhu, S. Lan, Q. Liu, J. Zhao, Y. Zhuang, Q. Guo, B. Liu, L. Gu, X. Lu, Y. Ren and H. Xia, Nat. Sustainability, 2021, 4, 392–401 CrossRef.
- H. Y. Asl and A. Manthiram, Science, 2020, 369, 140–141 CrossRef CAS PubMed.
- P. He, J. Luo, J. He and Y. Xia, J. Electrochem. Soc., 2009, 156, A209 CrossRef CAS.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2002, 14, 3946–3952 CrossRef CAS.
- S. Chang, Y. Chen, Y. Li, J. Guo, Q. Su, J. Zhu, G. Cao and W. Li, J. Alloys Compd., 2019, 781, 496–503 CrossRef CAS.
- Z. Fu, J. Hu, W. Hu, S. Yang and Y. Luo, Appl. Surf. Sci., 2018, 441, 1048–1056 CrossRef CAS.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- K. M. Shaju and P. G. Bruce, Adv. Mater., 2006, 18, 2330–2334 CrossRef CAS.
- D. Wu, X. Liu, P. Gao, L. He and J. Li, Ceram. Int., 2022, 48, 11228–11237 CrossRef CAS.
- X. Wang, X. Zhang, Y. Sun, H. Zhang, C. Pei, M. Zhao, J. Zhou, Q. Tang, H. Chen, B. Xi, Y. Qi, Z. Liu, G. Li and X. Guan, Appl. Surf. Sci., 2023, 624, 157103 CrossRef CAS.
- M. Li and J. Lu, Science, 2020, 367, 979–980 CrossRef CAS PubMed.
- H. Darjazi, S. J. Rezvani, S. Brutti and F. Nobili, Electrochim. Acta, 2022, 404, 139577 CrossRef CAS.
- X. Zhang, H. Cao, Y. Xie, P. Ning, H. An, H. You and F. Nawaz, Sep. Purif. Technol., 2015, 150, 186–195 CrossRef CAS.
- Z. Tang, X. Meng, Y. Shi and X. Guan, ChemSusChem, 2021, 14, 4697–4707 CrossRef CAS PubMed.
- X. Guan, J. Jiang, J. Lattimer, M. Tsuchiya, C. M. Friend and S. Ramanathan, Energy Technol., 2016, 5, 616–622 CrossRef.
- Y. Zhou, X. Guan, H. Zhou, K. Ramadoss, S. Adam, H. Liu, S. Lee, J. Shi, M. Tsuchiya, D. D. Fong and S. Ramanathan, Nature, 2016, 534, 231–234 CrossRef CAS PubMed.
- H. Zou, E. Gratz, D. Apelian and Y. Wang, Green Chem., 2013, 15, 1183–1191 RSC.
- J. R. Hitchin, Nat. Rev. Methods Primers, 2022, 2, 28 CrossRef CAS.
- J. Tang, M.-s. Chu, F. Li, C. Feng, Z.-g. Liu and Y.-s. Zhou, Int. J. Miner. Metall. Mater., 2020, 27, 713–723 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00201b |
| This journal is © The Royal Society of Chemistry 2023 |