Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fast and simple preparation of microparticles of KHCO3 by a freeze-dissolving method with single solvent or additional antisolvent†
Jiaqi
Luo
 a,
Qifan
Su
a,
Qiushuo
Yu
*a,
Xinyue
Zhai
a,
Yuan
Zou
a and
Huaiyu
Yang
a,
Qifan
Su
a,
Qiushuo
Yu
*a,
Xinyue
Zhai
a,
Yuan
Zou
a and
Huaiyu
Yang
 *b
*b
aSchool of Chemical Engineering, Northwest University, Taibaibei Road 229, Xi'an, 710069, Shaanxi, China. E-mail: yuqiushuo@nwu.edu.cn
bDepartment of Chemical Engineering, Loughborough University, Loughborough LE11 3TU, UK. E-mail: H.yang3@lboro.ac.uk
First published on 7th September 2023
Abstract
Microparticles featuring specific attributes are essential for the chemical industries. Microparticles of KHCO3 were prepared by a freeze-dissolving method, with one solvent or with additional antisolvent. Firstly, KHCO3 aqueous solution was dripped into liquid nitrogen to prepare ice spherical particles, and additional antisolvent, ethanol, was used to dissolve the ice scaffolding to isolate the microparticles of KHCO3. In this work we have developed a new freeze-dissolving method with only one solvent, water. After formation of ice particles, a low-temperature saturated aqueous solution of KHCO3 was used to dissolve the ice in frozen spherical particles at low temperature to isolate the microparticles. Both freeze-dissolving methods were 100 times faster and more energy-efficient than the traditional freeze-drying method. The microparticles of KHCO3 obtained by the freeze-drying method and freeze-dissolving with antisolvent and with saturated solution were characterised with SEM and the particle size distributions were compared.
Sustainability spotlightThis work demonstrates a fast, efficient and energy-saving technology for the production of microparticles. The freeze-dissolving methods can produce KHCO3 microparticles of similar size distribution for only 1% of the time period and 1% of energy consumption compared with the traditional freeze-drying method. The freeze-dissolving in saturated solution method, with only one solvent, allows fast application for not only salts but other chemicals, organics and pharmaceuticals. The new technology can ensure sustainable consumption and production patterns, which align with UN SDG 12. |
Introduction
Particle production technology accounts for a significant value for the chemical industry.1–5 Microparticles have been reported to have advantages over normal powder6 in terms of storage and transportation. Microparticles have a series of special properties because of their ultra-high specific surface area,7–9 leading to fast chemical reaction speed, strong surface adsorption capacity and large solubility. Microparticles can be produced by mechanical crushing of normal powder10–12 or be directly manufactured by physical and chemical methods.13–15 As the traditional process for preparing microparticles still has challenges on cost, efficiency and product properties such as size distribution, innovative methods are required16–19 to manufacture microparticles. One widely used method is freeze-drying (FDry).20,21 Firstly, an aqueous solution with certain concentration of target product is frozen into ice particles, with a structure of ice scaffolding mixed with microparticles formed during the freezing. Then, the ice scaffolding is sublimated under vacuum condition at low temperature, and the microparticles remain.In previous work, we demonstrated the freeze-dissolving method.22 With the same first step to form ice particles, the second step is to dissolve the ice particles in an antisolvent at temperature below 283.15 K, and then the microparticles are easily isolated after filtration. Compared with the traditional FDry method, the freeze-dissolving in antisolvent (FDas) method is much faster, with much smaller facility footprint and much less energy consumption. Moreover, the products of the FDas method were similar to or even better than the products of the FDry method as demonstrated in previous works.22,23 However, for the FDas method, it is essential to find an antisolvent, in which the target compound has much lower solubility (ideally close to zero), because for an antisolvent with moderately low solubility it could still be possible to dissolve some of the microparticles, especially tiny particles, during the second step. For inorganic salt systems, it is not difficult to find an antisolvent such as organic solvents, like ethanol or acetone. However, for organic compounds or organic salts, it can be challenging to find a perfect antisolvent, and binary solvents can induce liquid–liquid phase separation, hindering nucleation and crystallization.24,25 In summary, it is challenging to identify a suitable second solvent as antisolvent for some systems, especially for complex molecules, such as organics and proteins,26,27 and it requires more experiments and time to determine the solubility in the solvent mixture to design and control the freeze-dissolving process with the FDas method.
In this paper, we propose a new freeze-dissolving method using a saturated solution to dissolve the ice particles, which can be easily applied to more systems. The freeze-dissolving in saturated solution (FDss) method was demonstrated by producing KHCO3 microparticles with advantages of using only one solvent to save effort and time to identify a suitable second solvent as antisolvent and to design the freeze-dissolving process without extra solubility data of solvent mixtures. Similar to FDry and FDas, ice particles of KHCO3 were produced in the first step of FDss. In the second step, the ice particles of KHCO3 were dissolved in a low-temperature saturated solution of KHCO3, and then the microparticles of KHCO3 were easily isolated after filtration. Four concentrations, 0.3–0.5 g KHCO3 per g water, and different sizes of ice particles, 0.01 and 0.04 cm3, were used to compare three methods, FDry, FDas and FDss. The size distribution and PXRD patterns of all the microparticle products were determined and the particles were observed by SEM. The mechanisms of particle formation are discussed, and sustainabilities of these methods were analyzed.
Materials and methods
Materials
Ethanol was purchased from Tianjin Damao Chemical Reagent Factory (purity >99.7%). Potassium bicarbonate, KHCO3, was purchased from Tianjin Baishi Chemical Co. Ltd (purity >99.5%). Liquid nitrogen (purity >99.9%) was purchased from Xi'an Aier Industrial Gas Co. Ltd. All chemicals were used without further purification. Distilled deionized water (conductivity <0.5 μS cm−1) was used.Method
Potassium bicarbonate aqueous solutions were prepared at 328.15 K, with different weights of 3, 4, 4.5, and 5 g of KHCO3 in 10 g water. Then each solution was transferred by a pipette into an insulated pan with 10 mL of liquid nitrogen to form ice spherical particles. Two droplets with average masses of 0.0135 g and 0.0425 g were selected and dropped into the liquid nitrogen. All droplets quickly sank into the liquid nitrogen to form frozen spherical particles with mainly two different volumes. The average mass was calculated based on 300 frozen spherical particles of each size, the uncertainty was less than 10%, and the corresponding average volumes were 0.01 and 0.04 cm3, respectively.Three techniques, FDry (freeze-drying), FDas (freeze-dissolving in antisolvent) and FDss (freeze-dissolving in saturated solution), were used to obtain microparticles from the ice spherical particles, shown in Fig. 1. With FDry method, frozen spherical particles were put into a freeze dryer (FD-1A-50, Beijing Boyikang Experimental Instrument Co. Ltd, China) for 24 h for sublimation of ice, with microparticles remaining. With FDas method, the frozen ice particles were poured into ethanol (mass 7 times more than that of the ice frozen particles, Rice/as=1:7,) at 253.15 K, and stirred continuously. With FDss method, the frozen particles were dissolved in saturated KHCO3 solution at 275.15 K, and stirred continuously. For both FDas and FDss methods, the ice scaffolding in the frozen ice particles were dissolved for less than 10 min, and the KHCO3 particles were filtered with PTFE (Whatman) with a 1 μm pore size during 5 min.
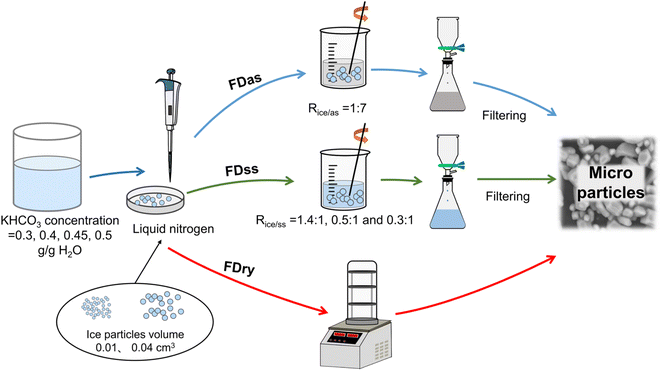 | ||
| Fig. 1 Schematic diagram of experimental setup for three methods of freeze-drying, FDry, freeze-dissolving in antisolvent, FDas, and freeze-dissolving in saturated solution, FDss. | ||
For the antisolvent crystallization method,24,26 KHCO3 solution of 0.5 g g−1 was poured into pure ethanol at room temperature under stirring of 400 rpm. The solid particles were obtained after filtration.
The KHCO3 ice particles produced from the aqueous solutions (4 g in 10 g water) were dissolved in different amounts of saturated solution to investigate its influence. The mass of KHCO3 saturated solution used for dissolving frozen particles was 10 g, 30 g and 50 g with different ratios, Rice/ss, of the mass of ice spherical particles to the mass of KHCO3 saturated solution: 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.3
1 and 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
Characterization
The morphologies of the particle products were characterized with a scanning electron microscope (TM3000, Hitachi High Technologies Co. Ltd, Japan). The particles were analyzed using the powder X-ray diffraction at room temperature, with 40 kV and 40 mA. The scan rate was 8° min−1. About 0.5 g of particles was added to an ethanol solution, which was then dispersed in an ultrasonic disperser for 5–10 min, and no influence was observed with a longer dispersion period (ESI, Table S1†). Each sample was measured three times using a laser particle size analyzer (Master Sizer 2000).Density test
The particles prepared by FDss, FDas and FDry were filled into a measuring cup until it was full. The mouth of the cup was scraped with a straightedge. The bulk density of particles was calculated using the following equation: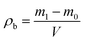 | (1) |
Then, the measuring cup with particles was filled with ethanol slowly until it was full. The granule density of particles was calculated by:
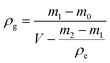 | (2) |
Angle of repose measurement
The angle of repose was calculated using the fixed diameter method with the following equation: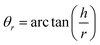 | (3) |
Results and discussion
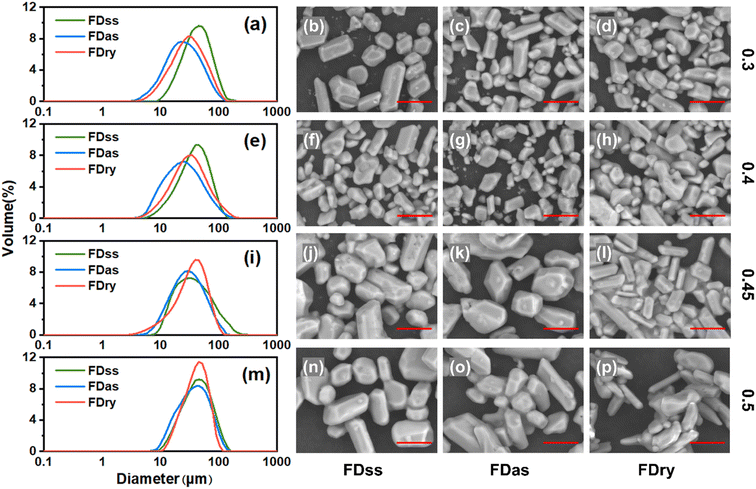
Fig. 2 shows that with low-concentration solutions of 0.3 and 0.4 g KHCO3 per g water, the size distributions of particles obtained by FDss were narrowest, and the size distributions of those obtained by FDry method were narrower than those obtained by FDas method. The product average sizes obtained from these three methods were similar and products obtained by FDss were slightly bigger than those obtained by the other two methods, and the products from FDas had overall the smallest average size.For the products of the same method, with an increase of the concentration, the size of microparticles tended to increase, as shown in the size distributions and SEM images in Fig. 2. For products from solutions with equal concentrations, fewer agglomerations were observed in the microparticles obtained by the FDss and FDas. With high-concentration solutions of 0.45 and 0.5 g KHCO3 per g water, the size distributions of particles obtained by FDry were narrowest, and size distributions of those obtained by FDss and FDas were similar (Fig. 2(j–l)). There was no obvious change in the crystal morphology. With a solution of 0.5 g KHCO3 per g water, the average sizes of products of the three methods were very similar.
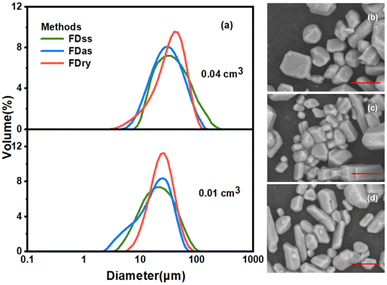
Fig. 3 shows that with equal sizes of frozen spherical particles, the KHCO3 microparticles obtained by the FDas and FDss methods were overall smaller than those obtained by the Fdry method. With a decrease in the sizes of the frozen spherical particles, the sizes of microparticles all decreased with the same method. As shown in Table 1, when the average size of frozen spherical particles was 0.01 cm3, the average sizes of microparticles obtained by the three methods were 24.9 μm, 20.7 μm and 26.2 μm. These values were about half of those obtained with the average particle size of 0.04 cm3 at the same concentration. The outcomes were consistent with NaHCO3 system,22 in which smaller ice particles resulted in smaller particles of NaHCO3, as the microparticle formation was completed during the ice frozen steps, and for smaller droplets freezing rate was faster to form smaller ice particles.
| Freezing condition | Average particle size | ||||
|---|---|---|---|---|---|
| Concentration (g g−1) | V (cm3)a | FDss (μm)b | FDas (μm)c | FDry (μm) | AS (μm)d |
a The average sizes of the frozen spherical particles.
b Mass ratio between ice and saturated solution, Rice/ss = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
c Mass ratio between ice and antisolvent solvent, Rice/as = 1 2.
c Mass ratio between ice and antisolvent solvent, Rice/as = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.
d Antisolvent crystallization. 7.
d Antisolvent crystallization.
|
|||||
| 0.3 | 0.04 | 47.7 | 30.2 | 35.4 | |
| 0.4 | 0.04 | 42.9 | 31.4 | 39.1 | |
| 0.45 | 0.04 | 46.6 | 35.4 | 38.3 | |
| 0.01 | 24.9 | 20.7 | 26.2 | ||
| 0.5 | 0.04 | 48.4 | 43.1 | 44.2 | |
| 0.5 | — | 154.1 | |||
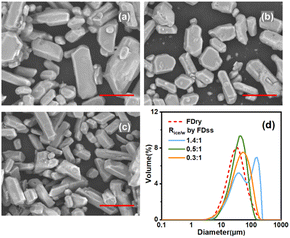
Fig. 4(d) shows that two particle size peaks appeared when the value of Rice/ss was equal to 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was in agreement with Fig. 4(a) where both large crystals and small crystals were observed. With very high Rice/ss, the water from melting of the ice particles would dissolve some of the microparticles. Despite the dissolution of microparticles, the temperature of saturated solution decreased, which generated supersaturation to produce some crystals by cooling crystallization, and the crystal particles from cooling crystallization were always much bigger. The first peak at about 40 μm shows the diameter of the microparticles inside the ice particles and the second park at about 158 μm shows the crystals formed due to cooling crystallization of the saturated solution. When Rice/ss was decreased to 0.5
1, which was in agreement with Fig. 4(a) where both large crystals and small crystals were observed. With very high Rice/ss, the water from melting of the ice particles would dissolve some of the microparticles. Despite the dissolution of microparticles, the temperature of saturated solution decreased, which generated supersaturation to produce some crystals by cooling crystallization, and the crystal particles from cooling crystallization were always much bigger. The first peak at about 40 μm shows the diameter of the microparticles inside the ice particles and the second park at about 158 μm shows the crystals formed due to cooling crystallization of the saturated solution. When Rice/ss was decreased to 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 0.3
1 or 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the size of the microparticles by FDas and FDss was in a similar range to those obtained by FDry. At Rice/ss of 0.5
1, the size of the microparticles by FDas and FDss was in a similar range to those obtained by FDry. At Rice/ss of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the average particle size of microparticles was 42.9 μm, which was slightly smaller than those obtained at Rice/ss of 0.3
1, the average particle size of microparticles was 42.9 μm, which was slightly smaller than those obtained at Rice/ss of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (average particle size was 52.6 μm), due to dissolution of more microparticles, especially in the small size range. As less ice was melted and the influence of the temperature change was limited, i.e. solubility remained the same, the melting ice increased the mass of the solvent, leading to dissolution of some of the microparticles. It is noted that based on the solubility of different systems, the optimised Rice/ss can be different. In sum, due to the influence of temperature change and quantity of water due to melting of ice, the optimised condition to produce microparticles of KHCO3 was at Rice/ss of 0.5
1 (average particle size was 52.6 μm), due to dissolution of more microparticles, especially in the small size range. As less ice was melted and the influence of the temperature change was limited, i.e. solubility remained the same, the melting ice increased the mass of the solvent, leading to dissolution of some of the microparticles. It is noted that based on the solubility of different systems, the optimised Rice/ss can be different. In sum, due to the influence of temperature change and quantity of water due to melting of ice, the optimised condition to produce microparticles of KHCO3 was at Rice/ss of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
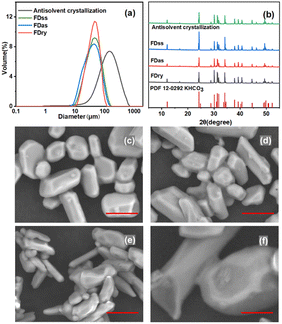
The microparticles were obtained with equal concentration condition, 0.5 g g−1, from the three methods, FDss, FDas, FDry, as well as the antisolvent crystallization method. The average particle size of microparticles obtained by FDss and FDas was 48.4 μm and 43.1 μm, respectively, and the microparticle size distributions were narrow, shown in Fig. 5. KHCO3 microparticles are widely used as dry powder fire extinguishing agent, and the sizes for applications are mainly in the range of 10–75 μm.28–30 The average particle sizes obtained by FDss and FDas were in the range of 30–50 μm. The bulk densities, by eqn (1), of products obtained by FDss, FDas and FDry were in a similar range, 0.93 g mL−1, 0.71 g mL−1 and 0.82 g mL−1, respectively. The granule densities, by eqn (2), were also similar, 2.16 g mL−1, 2.04 g mL−1 and 2.09 g mL−1, respectively. The products were all of good fluidity, with angles of repose, by eqn (3), of 26.5°, 31.3° and 28.7°, respectively.
The particles with average size of 150 μm obtained by antisolvent crystallization were more than 4 times larger than the particles obtained by FDss, FDas and FDry methods. The big size of particles from cooling crystallization also supported the hypothesis that the second peak in the size distribution in Fig. 4 was due to the cooling crystallization. When KHCO3 solution was added to ethanol at a higher rate, the supersaturation of the whole system became high, and then the accumulation of supersaturation could only be consumed rapidly by crystal growth. However, the supersaturation decreased with more KHCO3 solution added into ethanol. With FDas, FDss and FDry methods, the superstation in ice particles was very high, and the nucleation and crystal growth completed in seconds or less, with nearly no growth time for the crystals inside. The PXRD patterns in Fig. 5(b) showed that the particles produced from all four methods were all crystalline with the same polymorph.
After the frozen ice particles were produced with microparticles inside, FDss, FDas and FDry can all be used to separate the microparticles out of the ice scaffolding. With FDry, the method was to sublimate the ice into vapor phase, requiring about 1440 min. With FDas, it was to dissolve the ice scaffolding with the antisolvent, which was ethanol, at a temperature about 20 K lower than the ice point. With FDss, it was to dissolve the ice scaffolding with the saturated solution at low temperature, about 10 K above the ice point. The dissolving processes were both fast, and the whole process including filtration only needed about 15 min, which was only about 1% of the time period compared to the application of FDry. The energy consumption for production of 1 g microparticles was estimated to be 4.3 × 104 kJ for FDry, 3.0 × 102 kJ for FDas and 2.4 × 102 kJ for FDss, based on the lab operations (details in Table S2†). The energy consumption was much lower without the requirement of a vacuum, which was about 1% energy consumption for both FDas and FDss compared to the application of FDry.22 Due to the dissolution process occurring at higher temperature, the energy consumption of FDss was even less than that of FDas. Comparing the FDss, FDas and FDry methods, the freeze-dissolving methods (FDss and FDas) did not require a vacuum, providing a rapid method for preparing microparticles with low energy requirements, leading to the benefit of sustainability, shown in Fig. 6.
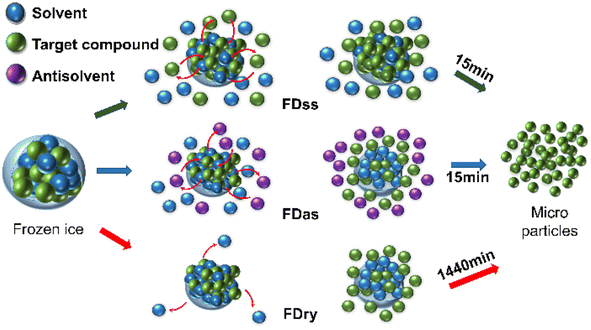 | ||
| Fig. 6 Schematic diagram of the FDss, FDas and FDry mechanisms for the formation and isolation of microparticles. | ||
For FDas, only one solvent (water) was used to obtain KHCO3 (or NaHCO3 (ref. 22)) aqueous solution. For the second solvent, it is easy to choose an organic solvent as antisolvent, as most salts do not dissolve in organic solvents. For applying this technology in producing target organic chemicals, drug micronization and food additives, if an organic solvent is chosen as the solvent (frozen particles are possible to form in liquid N2), antisolvent can be difficult to choose, with low solubility and low melting point (below that of the organic solvent used to form frozen particles). However, if a saturated solution with the current organic solvent can be directly used, it will be much easier to operate without efforts on screening another suitable solvent. Therefore, the FDss method has the advantages of simple equipment requirements, easy operation, low investment and low energy consumption, and a smaller amount of the saturated solution could be designed to recover the particles with high yield. Further research is needed for optimizing the saturated solution temperatures, freezing process, and ice particle sizes to produce smaller microparticles.
Conclusions
A new freeze-dissolving in saturated solution method was demonstrated in this work, using only one solvent to isolate microparticles of KHCO3. This method is simple and fast with energy- and cost-efficiency advantages. In the first step, frozen spherical particles, from KHCO3 aqueous solution, were obtained. Then the frozen spherical particles were dissolved by low-temperature saturated KHCO3 aqueous solution. The ice scaffolding was dissolved and microparticles of KHCO3 with size below 100 μm were collected.Comparing with the freeze-dissolving in antisolvent method, the new freeze-dissolving in saturated solution method only requires one solvent, which can be rapidly applied in many more systems without searching for compatible new antisolvent. Comparing with the freeze-drying method, both the freeze-dissolving methods can produce KHCO3 microparticles of similar size distribution without using specific facility of vacuum, and with 99% reduction in both time consumption and energy usage.
Author contributions
Jiaqi Luo: data curation, visualization, investigation, validation, writing – original draft. Qifan Su: writing – review & editing. Xinyue Zhai: formal analysis, investigation, methodology. Yuan Zou: writing – review & editing. Qiushuo Yu: conceptualization, methodology, writing – review & editing, funding acquisition. Huaiyu Yang: conceptualization, methodology, writing – review & editing, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the National Science Foundation (NSFC21978234) for financial assistance in this project.Notes and references
- L.-M. Xu, T.-T. Hu, Y. Pu, Y. Le, J.-F. Chen and J.-X. Wang, Chem. Eng. J., 2014, 252, 281–287 CrossRef CAS.
- Y. Bayat and V. Zeynali, J. Energ. Mater., 2011, 29, 281–291 CrossRef CAS.
- R. Kumar, P. F. Siril and P. Soni, Propellants, Explos., Pyrotech., 2014, 39, 383–389 CrossRef CAS.
- F. Nie, J. Zhang, Q. Guo, Z. Qiao and G. Zeng, J. Phys. Chem. Solids, 2010, 71, 109–113 CrossRef CAS.
- V. Stepanov, V. Anglade, W. A. B. Hummers, A. V. Bezmelnitsyn and L. N. Krasnoperov, Propellants, Explos., Pyrotech., 2011, 36, 240–246 CrossRef CAS.
- N. Mezzomo, S. R. Rosso Comim, C. E. M. Campos and S. R. S. Ferreira, Powder Technol., 2015, 270, 378–386 CrossRef CAS.
- K. Donaldson, D. Brown, A. Clouter, R. Duffin, W. MacNee, L. Renwick, L. Tran and V. Stone, J. Aerosol Med., 2002, 15, 213–220 CrossRef CAS PubMed.
- C. Monteiller, L. Tran, W. MacNee, S. Faux, A. Jones, B. Miller and K. Donaldson, Occup. Environ. Med., 2007, 64, 609–615 CrossRef CAS PubMed.
- Q. S. Yu, W. Y. Jia, J. J. Pu, Y. C. Wang and H. Y. Yang, Chem. Eng. Sci., 2021, 229, 116082 CrossRef CAS.
- C. M. Keck and R. H. Muller, Eur. J. Pharm. Biopharm., 2006, 62, 3–16 CrossRef CAS PubMed.
- V. B. Patravale, A. A. Date and R. M. Kulkarni, J. Pharm. Pharmacol., 2010, 56, 827–840 CrossRef.
- M. Kakran, R. Shegokar, N. G. Sahoo, L. Al Shaal, L. Li and R. H. Müller, Eur. J. Pharm. Biopharm., 2012, 80, 113–121 CrossRef CAS PubMed.
- A. H. L. Chow, H. H. Y. Tong, P. Chattopadhyay and B. Y. Shekunov, Pharm. Res., 2007, 24, 411–437 CrossRef CAS PubMed.
- H. Mansour, X. Wu, D. Hayes, J. B. Zwischenberger and R. Kuhn, Drug Des., Dev. Ther., 2013, 7, 59–72 CrossRef PubMed.
- Y. H. Kim and K. S. Shing, Powder Technol., 2008, 182, 25–32 CrossRef CAS.
- A. Jaworek, Powder Technol., 2007, 176, 18–35 CrossRef CAS.
- I. Zbicinski, I. Smucerowicz, C. Strumillo and C. Crowe, Braz. J. Chem. Eng., 2000, 17, 441–450 CrossRef CAS.
- J. Thies and B. W. Müller, Eur. J. Pharm. Biopharm., 1998, 45, 67–74 CrossRef CAS PubMed.
- R. Ghaderi, P. Artursson and J. Carlfors, Eur. J. Pharm. Sci., 2000, 10, 1–9 CrossRef CAS PubMed.
- T. K. Maiti, P. Dixit, A. Suhag, S. Bhushan, A. Yadav, N. Talapatra and S. Chattopadhyay, RSC Sustain., 2023, 1, 665–697 RSC.
- M. Négrier, E. El Ahmar, R. Sescousse, M. Sauceau and T. Budtova, RSC Sustain., 2023, 1, 335–345 RSC.
- Q. S. Yu, Y. C. Wang, J. Q. Luo and H. Y. Yang, ACS Sustain. Chem. Eng., 2022, 10, 7825–7832 CrossRef CAS PubMed.
- J. Q. Luo, Q. F. Su, Q. S. Yu, X. Y. Zhai, Y. Zou and H. Y. Yang, Green Chem. Eng., 2023 DOI:10.1016/j.gce.2023.07.003.
- X. H. Zhang, Z. X. Wei, H. Choi, H. Hao and H. Y. Yang, Adv. Mater. Interfaces, 2021, 8, 2001200 CrossRef CAS.
- H. Y. Yang and Å. C. Rasmuson, Fluid Phase Equilib., 2014, 376, 69–75 CrossRef CAS.
- L. P. Wang, Y. Bao, Z. Sun, V. J. Pinfield, Q. X. Yin and H. Y. Yang, Ind. Eng. Chem. Res., 2021, 60, 4110–4119 CrossRef CAS.
- H. Y. Yang, B. D. Belviso, X. Y. Li, W. Q. Chen, T. F. Mastropietro, G. Di Profio, R. Caliandro and J. Y. Y. Heng, Crystals, 2019, 9, 230 CrossRef CAS.
- K. Q. Kuang, W. K. Chow, X. M. Ni, D. L. Yang, W. R. Zeng and G. X. Liao, Fire Mater., 2011, 35, 353–366 CrossRef CAS.
- J. H. Zhou, H. P. Jiang, Y. H. Zhou and W. Gao, Process Saf. Environ. Prot., 2019, 132, 303–312 CrossRef CAS.
- J. Z. Jia, X. Y. Tian and F. X. Wang, ACS Omega, 2022, 7, 31974–31982 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00234a. |
| This journal is © The Royal Society of Chemistry 2023 |