Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogenation of biodiesel catalysed by pyrazolyl nickel(II) and palladium(II) complexes†
Oluwasegun Emmanuel
Olaoye
 *a,
Olayinka
Oyetunji
*a,
Olayinka
Oyetunji
 *a,
Banothile C. E.
Makhubela
*a,
Banothile C. E.
Makhubela
 b,
Gopendra
Kumar
b,
Gopendra
Kumar
 a and
James
Darkwa
a and
James
Darkwa
 *bc
*bc
aDepartment of Chemistry, University of Botswana, Private Bag UB 00704, Gaborone, Botswana. E-mail: 201605304@ub.ac.bw; yoyetunji@gmail.com; jdarkwa@gmail.com
bDepartment of Chemical Sciences, University of Johannesburg, Kingsway Campus, Auckland Park, 2006, South Africa
cBotswana Institute for Technology Research and Innovation, Machel Drive, Gaborone, Botswana
First published on 4th September 2023
Abstract
Biodiesel from renewable sources offers an attractive alternative to conventional diesel fuel and partial hydrogenation of free-fatty acid methyl esters (FAME) is one way to improve this renewable fuel. We have used the mononuclear pyrazolyl nickel(II) and palladium(II) complexes, [NiBr2(L1)] (1), [NiBr2(L2)] (2), [NiBr2(L3)] (3), [PdCl2(L1)] (4), [PdCl2(L2)] (5) and [PdCl2(L3)] (6) (where L1 = 3,5-dimethyl-1H-pyrazole, L2 = 3,5-di-tert-butyl-1H-pyr azole and L3 = 5-ferrocenyl-1H-pyrazole), as hydrogenation catalysts to improve the fuel properties of selected plant biodiesel. These nickel and palladium complexes exhibit significant catalytic activities in the selective and partial hydrogenation of biodiesel produced from Jatropha curcas, chinaberry (Melia azedarach), and tsamma melon (Citrullus ecirrhosus) seed oils. Depending on the catalyst and reaction time, a blend of un-hydrogenated, partially hydrogenated, and fully hydrogenated biodiesel was produced whose fuel properties meet the requirements of EN 14214 and ASTM D 6751 standards as fuels.
Sustainability spotlightWith the rapid growth in fossil fuel consumption and global warming emissions, there is an urgent need to explore green and renewable energy resources. Biodiesel presents a compelling alternative to conventional petroleum/diesel fuel; because of its well-known advantages, such as non-toxicity, the derivation from renewable feedstocks, and biodegradability. However, the properties of biodiesel are negatively affected by a high degree of unsaturation as well as full saturation, hence the need for selective and/or partial hydrogenation to improve the properties. We report the catalytic activities of pyrazolyl Ni(II) and Pd(II) complexes for the selective/partial hydrogenation of biodiesel produced from nonedible seed oils. This work addresses the following UN SDGs: Responsible Consumption and Production (SDG 12) and Climate Action (SDG 13). |
1 Introduction
Hydrogenation of various chemical conversions of feedstocks to targeted products has been studied by many research groups using various transition metal compounds as either homogeneous or heterogeneous catalysts.1–5 Amongst the most used metals in the homogeneous catalytic hydrogenations of unsaturated compounds are ruthenium,6,7 osmium,8 cobalt,9 rhodium,10,11 iridium,2 and platinum;12 but nickel3–15 and palladium16 complexes stand out as metals of choice when it comes to hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Examples of nickel-catalysed homogeneous hydrogenation of C
C bonds. Examples of nickel-catalysed homogeneous hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are provided by work done by Angulo et al.17 Augulo and Bouwman,18 Angulo et al.19 and Shevlin et al.20 in the asymmetric hydrogenation of α,β-unsaturated esters with high enantio-selective products.
C bonds are provided by work done by Angulo et al.17 Augulo and Bouwman,18 Angulo et al.19 and Shevlin et al.20 in the asymmetric hydrogenation of α,β-unsaturated esters with high enantio-selective products.
Nickel catalysts offer good catalytic activities and selectivities at a lower cost as hydrogenation catalysts. Palladium catalysts, on the other hand, though more expensive, have superior catalytic activities and selectivity in the hydrogenation of unsaturated compounds; and also provide a NMR spectroscopic handle that allows in situ monitoring of the hydrogenation reaction.21,22 Examples of palladium-catalysed reactions are provided by Pelagatti et al.23 and Bacci et al.24 for hydrazonic-phosphine (P^N^O) palladium(II) complexes and Ding et al.25 have reported P^C^P palladium complexes in the transfer hydrogenation of α,β-unsaturated ketones where reactions were occurring under mild conditions and were chemo-selective.
However, because of the air and moisture sensitivity of phosphines, metal complexes with nitrogen-donor ligands are emerging as alternatives to phosphorus-donor palladium complexes as hydrogenation catalysts.26 For example, {bis(arylimino)acenaphthene}palladium(0) complexes have been found to chemo-selectively hydrogenate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in α,β-unsaturated aldehydes.27 But despite numerous applications of nitrogen-donor nickel(II) and palladium(II) in catalysis, little work has been reported on pyrazolyl metal complexes as catalysts for the hydrogenation of unsaturated esters, especially the long-chain free-fatty acid methyl esters (FAME).
C bonds in α,β-unsaturated aldehydes.27 But despite numerous applications of nitrogen-donor nickel(II) and palladium(II) in catalysis, little work has been reported on pyrazolyl metal complexes as catalysts for the hydrogenation of unsaturated esters, especially the long-chain free-fatty acid methyl esters (FAME).
With the depletion of fossil fuel consumption, there is an urgent need to explore green and renewable energy sources as a substitute for fossil fuels in devices where batteries are either cumbersome or impractical.28 One way to solve these problems is to increase the use of bio-based fuels.29 Biodiesel (Scheme 1) offers attractive alternatives to conventional petroleum diesel fuel; because of its well-known advantages, such as non-toxicity, derivation from renewable feedstocks, and biodegradability.30–32
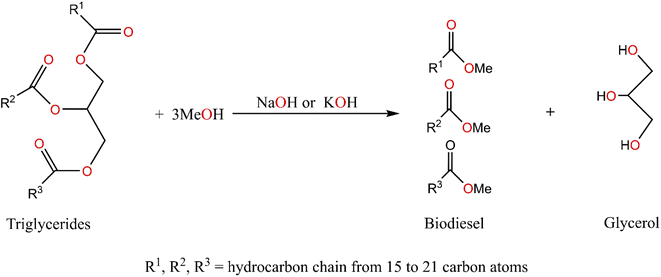 | ||
| Scheme 1 Transesterification of triglycerides with low molecular weight alcohol to produce free-fatty methyl esters (biodiesel).31 | ||
We report the partial and/or selective hydrogenation of biodiesels produced from Jatropha curcas, chinaberry (Media azedarach) and tsamma melon (Citrullus ecirrhosus) seed oils that leads to mixtures of the original un-hydrogenated biodiesel, partially hydrogenated biodiesel, and fully hydrogenated biodiesel as biodiesel blends.
2 Experimental
2.1 General information
All air and moisture-sensitive compounds were manipulated using standard Schlenk and vacuum line techniques under an argon atmosphere. Argon HP/zero-grade, and hydrogen gas HP/zero-grade (>99%) were purchased from Afrox (South Africa). Reagent grade ethyl formate (97%), acetic anhydride (99%), ferrocene (98%), hydrazine monohydrate (98%), hydrazine dihydrochloride (98%), (ethyleneglycoldimethylether)nickel(II) bromide (98%), 1,4-dioxane (99%), formic acid (95%), linoleic acid (99%) and 3,5-dimethyl-1H-pyrazole (L1) (99%) were procured from Sigma-Aldrich; while potassium hydroxide (85%) and sodium hydrogen carbonate were purchased from Promark (South Africa) and Rochelle chemicals (South Africa) respectively. Compounds 3,5-di-tert-butyl-1H-pyrazole (L2)33 and 5-ferrocenyl-1H-pyrazole (L3)34 and [PdCl2(NCMe)2],35,36 and nickel(II) and palladium(II) complexes (1–6)37,38 were synthesized according to the literature procedures.NMR spectra were recorded on a Bruker-400 ultra-shield MHz NMR spectrometer (1H at 400 MHz). Spectrometer chemical shifts were reported relative to the internal standard tetramethylsilane (δ 0.00 ppm) and referenced to the residual proton and carbon signals at 7.24 ppm and 77.0 ppm respectively of CDCl3.
All hydrogenation reactions were performed in PPV-CTR01-CE high-pressure autoclave reactor coupled with a stirring pact, heating and cooling system.3 The hydrogenation reactions were followed by 1H NMR spectroscopy and gas chromatography, using dioxane and methyl heptadecanoate as internal standards for hydrogenations of methyl linoleate (ML) and biodiesels respectively, which were used to determine the percentage conversions.
2.2 Synthesis of methyl linoleate (ML)
Linoleic acid (5 g, 17.83 mmol) was dissolved in methanol (20 mL) and allowed to stir for 30 min. About 7 drops of concentrated H2SO4 were added, and the reaction was allowed to reflux at 60–65 °C for 6 h. After the completion of the reaction, Na2CO3 (pH ∼12) was added dropwise forming two layers of the solution. The organic layer was separated from the aqueous layer, concentrated, and dried over MgSO4 to afford a pale-yellow oily compound. Yield: 4.7 g (90%).2.3 Typical transesterification reaction of jatropha, chinaberry and melon seed oils
Biodiesels from three plants were produced by an alkali-catalysed transesterification reaction between the appropriate plant oil and methanol. A typical Jatropha curcas seed oil (JASO) transesterification reaction was performed with 45 g (52 mmol) of JASO in a 250 mL three-necked round bottom flask and heated to 60 °C. Methanol (15 g, 468 mmol) and KOH (0.45 g; 1 wt% concerning JASO) were mixed vigorously in a different flask, to obtain a homogeneous mixture before the mixture was added to the heated JASO (with approximately 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol
1 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) JASO molar ratio) and stirred for 2 h. The resulting solution was then transferred into a separating funnel and left overnight to afford two layers or phases (i.e., the methyl esters of JASO and glycerol). The methyl esters of JASO (biodiesel) were then washed twice with distilled water to ensure the removal of all traces of glycerol, and unreacted methanol was removed in vacuo before the final product was dried over anhydrous Na2SO4.
JASO molar ratio) and stirred for 2 h. The resulting solution was then transferred into a separating funnel and left overnight to afford two layers or phases (i.e., the methyl esters of JASO and glycerol). The methyl esters of JASO (biodiesel) were then washed twice with distilled water to ensure the removal of all traces of glycerol, and unreacted methanol was removed in vacuo before the final product was dried over anhydrous Na2SO4.
A similar procedure was used to prepare biodiesels from chinaberry (CHSO) and melon (MESO) seed oils. The compositions of free-fatty methyl esters of Jatropha curcas, chinaberry (Melia azedarach) and tsamma melon (Citrullus ecirrhosus) seed oils were determined by gas chromatography-mass spectrometry (using a Thermo Scientific TSQ series fitted GC-FID that is coupled to Triple Quadrupole Mass Spectrometer) and a TG-5MS (30 m × 0.25 mm × 0.25 μm) fused-silica capillary column with helium as the carrier gas at a flow rate of 1 mL min−1. About 1 μL of the sample was injected into the oven (150 °C) with an injector and detector of 250 °C, with a split ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oven temperature was kept at the initial 80 °C for 2 min, and then gradually increased from 80 °C to 300 °C at the rate of 8 °C min−1, the total run time was 32 min. The type and the amount of each free-fatty acid methyl esters (FAME) composition were identified by reference to the retention time and calculation from the fraction of area under the peak of the FAME, respectively (Fig. S23†).
1. The oven temperature was kept at the initial 80 °C for 2 min, and then gradually increased from 80 °C to 300 °C at the rate of 8 °C min−1, the total run time was 32 min. The type and the amount of each free-fatty acid methyl esters (FAME) composition were identified by reference to the retention time and calculation from the fraction of area under the peak of the FAME, respectively (Fig. S23†).
The conventional diesel fuel used for comparison in this study was purchased from a local Shell petrol depot/station and has properties including a boiling point of 149 °C, a density of 845 kg m−3, the viscosity of 2.7 mm2 s−1 at 40 °C, the acid value of 0.28 mg KOH g−1 and calorific value of 45.6 MJ kg−1.
2.4 General procedure for hydrogenation reactions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120. The solution mixture was purged twice with nitrogen gas, followed by the introduction of hydrogen gas (5 bar) and the mixture was stirred at 50 °C for 3 h. After the reaction period had elapsed, the reacting vessel was cooled, and excess pressure generated was vented off slowly. Aliquots of the products were drawn from the reaction mixture at different reaction times, filtered using MS® nylon syringe filter (0.22 μm, 13 mm), and the hydrogenation products' conversions were determined by 1H NMR spectroscopy. Due to the small scale of reactions performed, accurate isolated yields were not determined for all reactions explored. The 1HNMR spectrum of methyl linoleate is shown in Fig. S1 and S2.† The conversions and product distributions, using dioxane as an internal standard, were determined using the integration of dioxane from NMR spectra of the reaction mixture (Fig. S22†). The nature of the products was confirmed by GC-MS.
120. The solution mixture was purged twice with nitrogen gas, followed by the introduction of hydrogen gas (5 bar) and the mixture was stirred at 50 °C for 3 h. After the reaction period had elapsed, the reacting vessel was cooled, and excess pressure generated was vented off slowly. Aliquots of the products were drawn from the reaction mixture at different reaction times, filtered using MS® nylon syringe filter (0.22 μm, 13 mm), and the hydrogenation products' conversions were determined by 1H NMR spectroscopy. Due to the small scale of reactions performed, accurate isolated yields were not determined for all reactions explored. The 1HNMR spectrum of methyl linoleate is shown in Fig. S1 and S2.† The conversions and product distributions, using dioxane as an internal standard, were determined using the integration of dioxane from NMR spectra of the reaction mixture (Fig. S22†). The nature of the products was confirmed by GC-MS.
2.5 Fuel properties analyses
The fuel properties of biodiesels produced from the three seed oils and the partially hydrogenated biodiesels were analysed for relative density, cold properties (cloud and pour points), flash point, acid value and heat of combustion (calorific value). The cloud and pour points were determined using the ASTM standard test methods (ASTM D97 and D2500). The ASTM D93 standard test method for flash point by Pensky-Martens closed cup tester was used to determine flash points of the biodiesels. The heat of combustion of the biodiesels was determined using the CAL 3K-AP Calorimeter system with an automatic data acquisition through the CalWin Calorimeter software, which determines the calorific values of samples. The acidity values of the biodiesel were measured according to the EN14104 standard test method.383 Results and discussion
All the pyrazolyl nickel(II) and palladium(II) complexes that were used as catalysts in this study, as illustrated in Scheme 2, are known compounds. For example, complexes 1,372,374 (ref. 39–41) and 5 (ref. 39 and 40) have been used as ethylene37,39 or phenylacetylene40 polymerization catalysts and as anticancer agents41 respectively.42b–e There are also several examples of bis(pyrazolyl)- nickel(II) and palladium(II) complexes in which the N–H in complexes 1–4 has been derivatised, as in complexes 3 (ref. 42a) and 6,42a and used as catalysts. Some examples of bis(pyrazolyl)palladium complexes have been used as Heck and Suzuki coupling catalysts. The complexes in Scheme 2 that were used for this were fully characterised by NMR spectroscopy and in selected cases by single-crystal X-ray crystallography. Fig. S36–S43† display the 1H and 13C{1H} NMR spectra.42 Fig. S44 and S45† show the X-ray crystallographic parameters for 5 and 6.42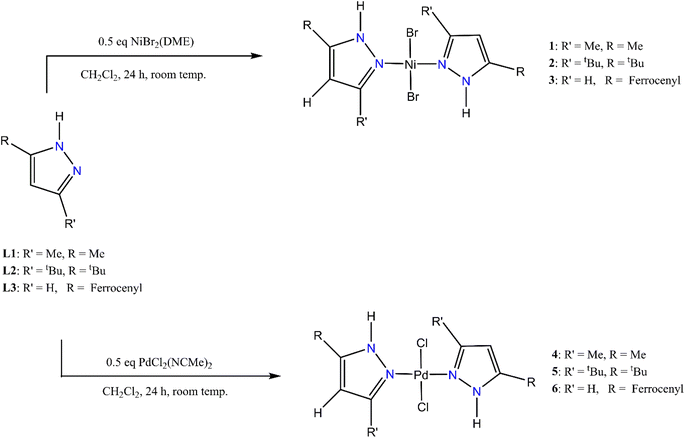 | ||
| Scheme 2 Schematic illustrations of monomeric pyrazolyl nickel(II) and palladium(II) complexes.42 | ||
3.1 Hydrogenation reactions
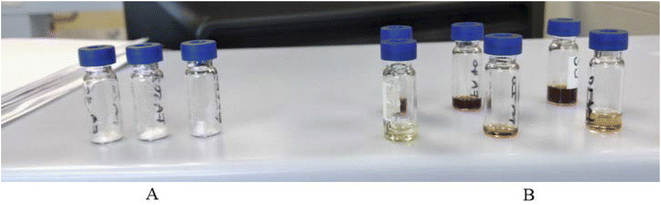
So, complexes 1–6 were evaluated as catalysts for the hydrogenation of methyl linoleate using molecular hydrogen at 0.5 h at 50 °C and 5 bar. Catalytic activities (in terms of the conversion of methyl linoleate) were established in the orders 1 > 3 > 2 for the nickel(II) complexes and 5 > 4 > 6 for the palladium(II) complexes (Table 1). At this temperature and pressure, five of the six complexes partially hydrogenated the model biodiesel, methyl linoleate (ML), to a mixture of un-hydrogenated ML, partially hydrogenated ML and fully hydrogenated ML, except complex 5 which completely hydrogenated ML to methyl stearate (MS), as ‘chunky’ white solids, in 30 min (Table 1, entry 4; Fig. 1A and S8†).
| Entry | Complex | Time (h) | Conversion (%) | TON | TOF | Amount of MO detectedb (%) | Amount of MS detectedb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 0.3 mmol of methyl linoleate; 2.5 μmol (0.83 mol%) of complex; 5 mL of methanol; 50 °C; 5 bar. b Conversions were estimated by 1H NMR spectroscopy, using dioxane as an internal standard. Each run was performed in duplicates. TOF in molsubstrate molcatalyst−1 h−1 | |||||||
| 1 | 4 | 0.5 | 29 | 35 | 70 | 71 | 3 |
| 2 | 4 | 1 | 52 | 62 | 62 | 48 | 30 |
| 3 | 4 | 2 | 100 | 120 | 60 | Fully hydrogenated | |
| 4 | 5 | 0.5 | 100 | 120 | 240 | Fully hydrogenated | |
| 5 | 6 | 0.5 | 20 | 24 | 48 | 80 | 9 |
| 6 | 6 | 1 | 35 | 42 | 42 | 65 | 21 |
| 7 | 6 | 2 | 70 | 84 | 44 | 30 | 38 |
| 8 | 1 | 0.5 | 35 | 42 | 84 | 65 | 4 |
| 9 | 1 | 1 | 38 | 46 | 46 | 62 | 5 |
| 10 | 1 | 2 | 41 | 49 | 25 | 59 | 6 |
| 11 | 2 | 0.5 | 28 | 34 | 64 | 71 | 2 |
| 12 | 2 | 1 | 34 | 41 | 41 | 66 | 17 |
| 13 | 2 | 2 | 46 | 55 | 28 | 54 | 16 |
| 14 | 3 | 0.5 | 29 | 35 | 70 | 71 | 3 |
| 15 | 3 | 1 | 40 | 48 | 48 | 61 | 8 |
| 16 | 3 | 2 | 75 | 90 | 45 | 25 | 44 |
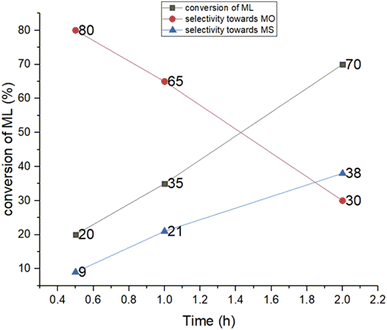
All six metal complexes were active as catalysts for the hydrogenation of ML as described in (Scheme 3, Table 1, and Fig. 2). Of the three palladium complexes (4–6), complex 5 completely hydrogenated ML to MS within 0.5 h (Table 1, entry 4 and Fig. S8†), whilst complexes 4 (71%) and 6 (80%) selectively produced methyl oleate (MO) in comparable conversions of 29% and 20% respectively (Table 1, entries 1 and 5; Fig. S3†). While the selectivity towards MO dropped from 71% at a reaction time of 0.5 h to 48% at 1 h for complex 4 (Table 1, entries 1 and 2), at reaction time 2 h the fully hydrogenated product MS was formed by this complex. Selectivity for complex 6 dropped from 80% at 0.5 h to 65% at 1 h and 30% at 2 h (Table 1, entries 6 and 7) (Fig. S4, S5, and S11†). It is noteworthy that the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the ML were fully hydrogenated to selectively produce MS after 3 h (Fig. S12†). The nickel complexes (1–3), on the other hand, had products that were not fully hydrogenated even after 2 h. For example, complex 1 had only a slight drop in conversion from 65% to 62% from 0.5 h to 1 h reaction time (Table 1, entries 8 and 9) (Fig. S6 and S7†). It is not clear why complex 5 is highly active, although we suspect it could be due to the high solubility of this compound since it has a tertiary-butyl substituent on the pyrazolyl ligand. However, the nickel analogue (complex 2), has only 28% conversion of ML to MO (71% selectivity) and MS (2% selectivity) when the reaction is run for 0.5 h (Table 1, entry 11). There is an increase in the conversion of ML from 34% (Fig. S9†) up to 46% (Fig. S10†) when the reaction is run from 1 h to 2 h at 5 bar (Table 1, entries 12 and 13). Similar trends were observed with complex 3 as a catalyst, interestingly with higher % conversions of methyl linoleate and much lower selectivity towards MO at 2 h (Table 1, entries 15, 16; Fig. S14 and S15†). It must be noted that Liu et al. have reported Pd(0)-PEG nanoparticles that partially hydrogenate methyl linoleate selectively to MO.44 The mass spectra for the ML, partially hydrogenation product (MO) and fully hydrogenated product (MS) are shown in Fig. S16–S18† respectively.
C bonds in the ML were fully hydrogenated to selectively produce MS after 3 h (Fig. S12†). The nickel complexes (1–3), on the other hand, had products that were not fully hydrogenated even after 2 h. For example, complex 1 had only a slight drop in conversion from 65% to 62% from 0.5 h to 1 h reaction time (Table 1, entries 8 and 9) (Fig. S6 and S7†). It is not clear why complex 5 is highly active, although we suspect it could be due to the high solubility of this compound since it has a tertiary-butyl substituent on the pyrazolyl ligand. However, the nickel analogue (complex 2), has only 28% conversion of ML to MO (71% selectivity) and MS (2% selectivity) when the reaction is run for 0.5 h (Table 1, entry 11). There is an increase in the conversion of ML from 34% (Fig. S9†) up to 46% (Fig. S10†) when the reaction is run from 1 h to 2 h at 5 bar (Table 1, entries 12 and 13). Similar trends were observed with complex 3 as a catalyst, interestingly with higher % conversions of methyl linoleate and much lower selectivity towards MO at 2 h (Table 1, entries 15, 16; Fig. S14 and S15†). It must be noted that Liu et al. have reported Pd(0)-PEG nanoparticles that partially hydrogenate methyl linoleate selectively to MO.44 The mass spectra for the ML, partially hydrogenation product (MO) and fully hydrogenated product (MS) are shown in Fig. S16–S18† respectively.
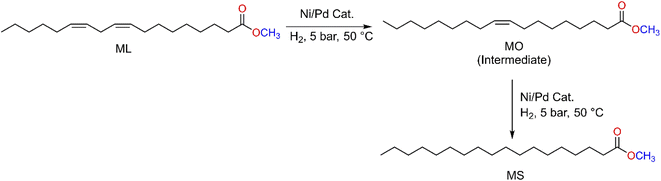 | ||
| Scheme 3 Hydrogenation of methyl linoleate catalysed by pyrazolyl nickel(II) and palladium(II) complexes; showing partially hydrogenated and fully hydrogenated products. | ||
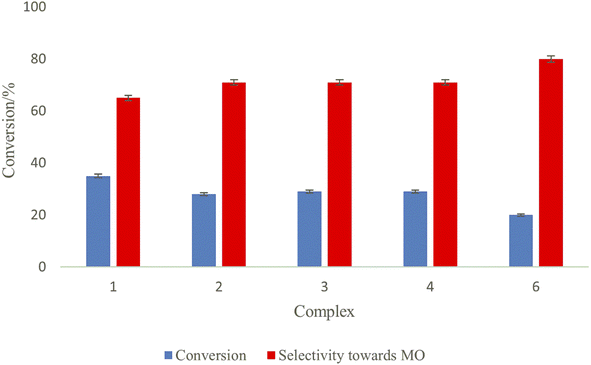 | ||
| Fig. 2 Hydrogenation of methyl linoleate using molecular hydrogen. ML, 0.3 mmol; complex, 2.5 μmol (0.83 mol%); methanol, 5 mL; 50 °C; 5 bar; 0.5 h. Conversions were estimated by 1H NMR spectroscopy. | ||
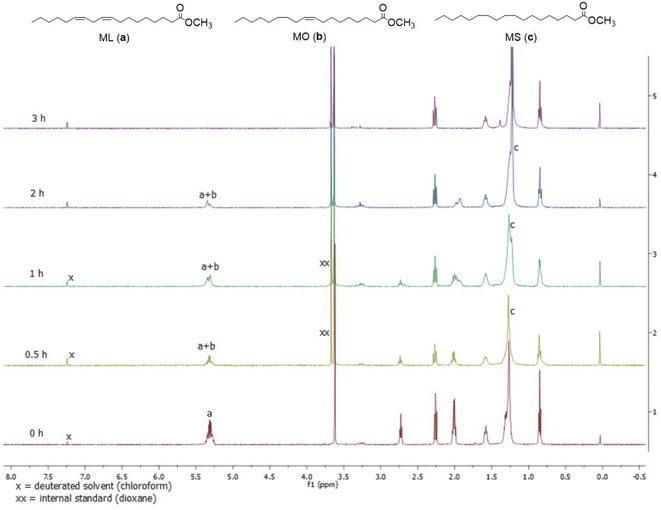
To determine the optimal conditions for the hydrogenation of ML, we investigated the effect of temperature and pressure on the hydrogenation of ML with complex 6 from 40–70 °C and from 5–15 bar and reaction time kept at 1 h (Table S1†). With temperature variation, conversion peaked at 41% when temperature was 60 °C (Table S1,† entries 1–4); however, selectivity for MO dropped from 65% at 50 °C to 59% at 60 °C, and at 70 °C, there was no significant change in the conversion (with 36%) of methyl linoleate and the selectivity (with 64%) towards MO (Table S1, entry 4; Fig. S19†). With pressure variation, conversion continued to increase until it was 100% at 15 bar (Table S1,† entries 2, 5 and 6), but at the expense of MO selectivity which dropped from 65% at 5 bar to 49% at 10 bar and 0% at 15 bar. Therefore, to obtain a mixture of ML, MO and MS, hydrogenation had to be run at 50 °C, and 5 bar to maximise the amount of MO produced. Indeed time-dependent NMR experiments (Fig. 3–5, and S12†) using complex 6 as catalyst provided further support for when the maximum amount of MO was formed during the hydrogenation of ML. The representative of the gas chromatogram showing the distribution of the products of MO and MS for the hydrogenation of methyl linoleate is shown in Fig. S13.†
We were able to establish that the hydrogenation of ML was catalysed homogenously by performing the mercury drop experiment using complex 6.45 Table S1† (entries 2 and 7) and Fig. S20† provide evidence for this conclusion and that if there were Pd(0) nanoparticles formed during the hydrogenation the amount was minimal.
3.2 Hydrogenation of biodiesel
We were able to use the hydrogenation of ML to determine which catalysts and reaction conditions to use for the hydrogenation of plant biodiesel we prepared. Since complex 5 gave the highest catalytic activity with regards to the hydrogenation of methyl linoleate (Table 1, entry 4), complex 5 and its nickel analogue (complex 2) were evaluated for the partial and/or selective hydrogenation of jatropha, chinaberry and tsamma melon seed oil methyl esters. The hydrogenation reactions with complexes 2 and 5 were first screened for 0.5 h at 50 °C and 10 bar. Scheme 4 illustrates the number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in biodiesel. This C
C bonds in biodiesel. This C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond nomenclature is further used in Tables 2–5 to indicate intermediate compounds that are partially or fully hydrogenated in each plant biodiesel. Methyl ester compositions of biodiesel produced from the three seed oil are shown in (Fig. S23–S25†).
C double bond nomenclature is further used in Tables 2–5 to indicate intermediate compounds that are partially or fully hydrogenated in each plant biodiesel. Methyl ester compositions of biodiesel produced from the three seed oil are shown in (Fig. S23–S25†).
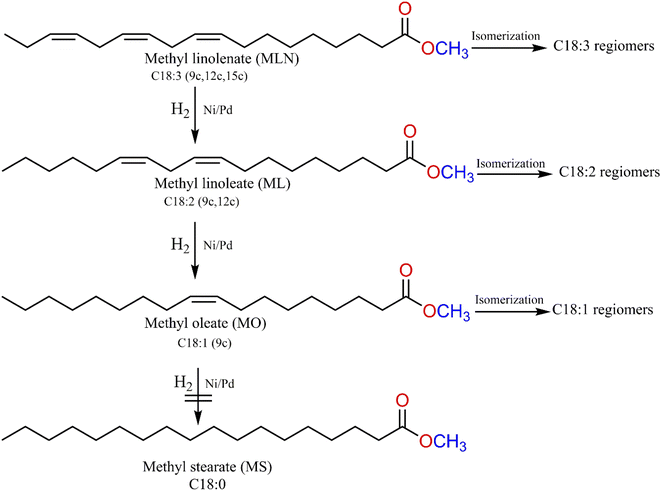 | ||
| Scheme 4 Partial/selective hydrogenation of free-fatty acid methyl esters with pyrazolyl nickel(II) and palladium(II) complexes. | ||
| FAME/FFA | Jatropha (%) | Chinaberry (%) | Tsamma melon (%) |
|---|---|---|---|
| a n.d – not detected. | |||
| (9c,12c,15c)-C18:3 | 5.3 | 7.8 | 4.3 |
| (9c,12c)-C18:2 | 31.4 | 76.9 | 47.7 |
| (9c)-C18:1 | 36.7 | 0.2 | 22.6 |
| C18:0 | 5.8 | 3.0 | 11.2 |
| C16:0 | 14.1 | 9.3 | 13 |
| Stearic acid | 1.1 | n.d | n.d |
| Palmitic acid | 0.5 | n.d | n.d |
| Oleic acid | 3.7 | n.d | n.d |
It is also important to note that partial and/or selective hydrogenation of polyunsaturated FAME improves the oxidative stability of hydrogenated biodiesel that meets desired fuel properties.46 This hydrogenation should lead to minimal C18:3 FAME content in biodiesel since a high amount of C18:3 FAME is not suitable for biodiesel formulations. Hence, desirable plant biodiesel products would be products with minimal C18:3 FAME content. This is because the presence of free fatty acid methyl esters containing three or more C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds impacts oxidative stability since the bis-allylic position is always prone to oxidative attack.47
C double bonds impacts oxidative stability since the bis-allylic position is always prone to oxidative attack.47
Generally, the hydrogenation of the three plant biodiesels resulted in products with low C18:3 content, while maintaining or improving C18:2 and C18:1 content (Tables 3–5). For example, the hydrogenation of biodiesel produced from jatropha seed oil (JCO) with complex 2 produced MLN (1.5%), ML (23.3%), MO (37.4%), MS (5.6%), with no selectivity towards C18:2 (9c,11t) when the reaction was monitored for 1 h at 50 °C and 10 bar (Table 3, entry 2) (JCO). It is important to note that the hydrogenation of jatropha methyl esters with complex 2 produced no significant changes in the distribution of the products of polyunsaturated FAME when the reaction was run from 1 h to 3 h at 50 °C and 10 bar (Table 3) (Fig. S26–S28†). These experimental conditions are crucial as they determine when the polyunsaturated FAME preserve a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond per molecule for biodiesel formulation. After 4 h, the jatropha methyl ester FAME was fully saturated (Table 3, entry 5; Fig. S29†). All the polyunsaturated FAME were converted to monosaturated FAME when the hydrogenation reaction was monitored with complex 5 for 0.5 h at 50 °C and 10 bar, producing mainly 73.4% MS and 1% MO with no selectivity towards C18:2 (9c,11t) (Fig. S30†). In contrast, hydrogenation of the jatropha methyl ester FAME at 25 °C and 10 bar with complex 5 after 0.5 h produced a mixture of MLN (0.5%), ML (26.3%), MO (41.0%) and MS (10.3%) (Fig. S31†); whilst at the same temperature and pressure the hydrogenation products after 1 h gave a mixture of MLN (0.2%), ML (2.3%), MO (58.8%) and MS (16.3%) (Table 3, entries 7 and 8). The effect of the reaction temperature on the partial hydrogenation of biodiesel which affords a significant change in the composition of MLN, ML, and MO offers similar observations as reported by Thunyaratchatanon et al.48 for the partial hydrogenation of soybean oil-derived biodiesel using palladium silica as a catalyst.
C double bond per molecule for biodiesel formulation. After 4 h, the jatropha methyl ester FAME was fully saturated (Table 3, entry 5; Fig. S29†). All the polyunsaturated FAME were converted to monosaturated FAME when the hydrogenation reaction was monitored with complex 5 for 0.5 h at 50 °C and 10 bar, producing mainly 73.4% MS and 1% MO with no selectivity towards C18:2 (9c,11t) (Fig. S30†). In contrast, hydrogenation of the jatropha methyl ester FAME at 25 °C and 10 bar with complex 5 after 0.5 h produced a mixture of MLN (0.5%), ML (26.3%), MO (41.0%) and MS (10.3%) (Fig. S31†); whilst at the same temperature and pressure the hydrogenation products after 1 h gave a mixture of MLN (0.2%), ML (2.3%), MO (58.8%) and MS (16.3%) (Table 3, entries 7 and 8). The effect of the reaction temperature on the partial hydrogenation of biodiesel which affords a significant change in the composition of MLN, ML, and MO offers similar observations as reported by Thunyaratchatanon et al.48 for the partial hydrogenation of soybean oil-derived biodiesel using palladium silica as a catalyst.
| Entry | Complex | t (h) | C18:3 (%) | C18:2 (total) (%) | C18:2 (9c,12c) (%) | C18:2 (9c,11t) (%) | C18:1 (%) | C18:0 (%) | TOFc (h−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 8.6 μmol of the complex (1 wt%); 0.5 g of JCO; 10 mL of methanol; 50 °C; 10 bar. b At 25 °C; 10 bar. Conversions were estimated by gas chromatography. The composition of the FAME was estimated with ∼90% match factor. c Defined as moles of hydrogenated polyunsaturated FAME units in the C18:3, C18:2 and C18:1 compounds in both the starting feed and other regiomers formed during the reaction per mole of the catalyst (complex) per h. | |||||||||
| 1 | Starting feed | 5.3 | 31.4 | 31.4 | — | 36.7 | 5.8 | — | |
| 2 | 2 | 1 | 1.5 | 23.3 | 23.3 | — | 37.4 | 5.6 | 123 |
| 3 | 2 | 2 | 1.0 | 21.3 | 21.3 | — | 33.6 | 5.4 | 55 |
| 4 | 2 | 3 | 0.5 | 20.1 | 20.1 | — | 30.3 | 6.9 | 34 |
| 5 | 2 | 4 | — | — | — | — | 0.1 | 75.2 | 0.2 |
| 6 | 5 | 0.5 | — | — | — | — | 1.0 | 73.4 | 4 |
| 7 | 5 | 0.5 | 0.5 | 26.3 | 26.2 | 0.1 | 41.0 | 10.3 | 268 |
| 8 | 5 | 1 | 0.2 | 2.3 | 2.2 | 0.1 | 58.8 | 16.1 | 120 |
Similar trends were observed for the catalytic hydrogenation of both chinaberry and tsamma melon seed oil methyl esters (CBO) as compared to the hydrogenation of jatropha methyl esters (JCO) with complexes 2 and 5 (Tables 4 and 5) (Fig. S32–S36†). For the chinaberry methyl ester FAME partial hydrogenation with complex 2 at 50 °C and 10 bar for 0.5 h gave a product mixture of MLN (4.4%), ML (41.0%), MO (40.4%) and MS (4.9%) (CBO) (Fig. S32†); while the most desired partial hydrogenation products mix with the same catalysts and reaction condition for the tsamma melon methyl ester was MLN (2.7%), ML (36.6%), MO (40.3%) and MS (14.0%) (MSO) (Fig. S33†). At a lower temperature, the product distributions of the polyunsaturated FAME were greatly improved using complex 5 when the reaction temperature was reduced to 25 °C at 10 bar, producing 0.8% MLN 30.9% ML, 23.5% MO for the chinaberry methyl ester hydrogenation (Table 4, entry 6) (Fig. S35†) and 0.9% MLN; 36.4% ML, and 20.2% MO for the hydrogenation of tsamma melon methyl ester (Table 5, entry 6) (Fig. S36†). Fig. S37† shows a representative gas chromatogram for the partial hydrogenation of biodiesels from the three seed oils.
| Entry | Complex | t (h) | C18:3 (%) | C18:2 (total) (%) | C18:2 (9c,12c) (%) | C18:2 (9c,11t) (%) | C18:1 | C18:0 | TOFc (h−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 8.6 μmol of the complex (1 wt%); 0.5 g of CBO; 10 mL of methanol; 50 °C; 10 bar. b At 25 °C; 10 bar. Conversions were estimated by gas chromatography. The composition of the FAME was estimated with ∼90% match factor. c Defined as moles of hydrogenated polyunsaturated FAME units in the C18:3, C18:2 and C18:1 compounds in both the starting feed and other regiomers formed during the reaction per mole of the catalyst (complex) per h. | |||||||||
| 1 | Starting feed | 7.8 | 76.9 | 76.9 | — | 0.2 | 3.02 | — | |
| 2 | 2 | 0.5 | 4.4 | 41.1 | 41.0 | 0.1 | 40.4 | 4.9 | 339 |
| 3 | 2 | 1 | 0.5 | 34.3 | 33.9 | 0.4 | 33.5 | 4.0 | 135 |
| 4 | 2 | 2 | 0.1 | 20.7 | 20.5 | 0.2 | 54.5 | 5.2 | 74 |
| 5 | 5 | 0.5 | — | — | — | — | 5.8 | 66.5 | 22.78 |
| 6 | 5 | 0.5 | 0.8 | 31.1 | 30.9 | 0.2 | 23.5 | 14.5 | 219 |
| 7 | 5 | 1 | 0.6 | 2.7 | 2.7 | 0.1 | 50.8 | 14.7 | 106 |
| Entry | Complex | t (h) | C18:3 (%) | C18:2 (total) (%) | C18:2 (9c,12c) (%) | C18:2 (9c,11t) (%) | C18:1 | C18:0 | TOFc (h−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 8.6 μmol of the complex (1 wt%); 0.5 g of MSO; 10 mL of methanol; 50 °C; 10 bar. b At 25 °C; 10 bar. Conversions were estimated by Gas chromatography. The composition of the FAME was estimated with ∼90% match factor. c Defined as moles of hydrogenated polyunsaturated FAME units in the C18:3, C18:2 and C18:1 compounds in both the starting feed and other regiomers formed during the reaction per mole of the catalyst (complex) per h. | |||||||||
| 1 | Starting feed | 4.3 | 47.7 | 47.7 | — | 22.6 | 11.2 | — | |
| 2 | 2 | 0.5 | 2.7 | 36.6 | 36.1 | 0.5 | 40.3 | 14.0 | 314 |
| 3 | 2 | 1 | 1.9 | 38.1 | 38.0 | 0.1 | 31.0 | 14.2 | 140 |
| 4 | 2 | 2 | 0.1 | 31.0 | 30.5 | 0.5 | 40.5 | 14.1 | 71 |
| 5 | 2 | 3 | — | 25.7 | 15.6 | 0.1 | 52.1 | 14.7 | 17 |
| 6 | 5 | 0.5 | 0.9 | 36.7 | 36.4 | 0.3 | 20.2 | 16.6 | 228 |
| 7 | 5 | 1 | 0.6 | 3.5 | 3.4 | 0.1 | 56.9 | 15.5 | 120 |
3.3 Properties of the biodiesels
We selected partially hydrogenated biodiesels produced with complex 2 catalysed reactions at 50 °C, 10 bar and 0.5 h, as comparable mixtures, to determine the fuel properties to compare them to the un-hydrogenated ones. The properties of the feed biodiesel (JCO, CBO and MSO) and the partially hydrogenated biodiesels (JBH, BCB and MSB); namely, relative density, cold flow properties (cloud and pour points), flash point, calorific value and acid value are presented in Table 6.| Biodiesel/diesel fuel | Cloud point (°C) | Pour point (°C) | Flash point (°C) | Calorific value (MJ kg−1) | Acid value (mg KOH g−1) | Density (at 20°C) (kg m−3) |
|---|---|---|---|---|---|---|
| a CDF – Conventional diesel fuel. | ||||||
| JCO | 3.0 | 2.0 | 133 | 42.2 | 1.58 | 870 |
| CBO | 4.0 | 2.0 | 145 | 44.5 | 3.22 | 875 |
| MSO | 4.0 | 2.0 | 130 | 41.7 | 1.22 | 880 |
| JBH | 4.0 | 3.0 | 135 | 39.6 | 0.23 | 867 |
| BCB | 4.0 | 2.0 | 147 | 43.6 | 0.35 | 870 |
| MSB | 4.0 | 3.0 | 126 | 42.9 | 0.10 | 866 |
| CDF | 3.0 | −6.0 | 91 | 45.6 | 0.28 | 845 |
These properties were evaluated due to their correlation to improved biodiesel quality and biodiesel formulation via partial hydrogenation. The relative density is an important fuel property that directly affects the fuel performance, as some of the engine properties, such as heating value and viscosity, correlate with it. The densities of the partially hydrogenated biodiesel are in the range of 866–870 kg m−3 (Table 6), which meet the standard requirement by the European standard EN 14214 for biodiesel as heating oil.
The most significant difference in the properties of our biodiesels compared to the conventional diesel in our study is the cloud point (CP) and pour point (PP). The cloud point (CP) is the temperature at which a fuel becomes cloudy, forming a jelly-like crystal. It is a fuel property that describes the low-temperature operational characteristic of the fuel. At the same time, the pour point (PP) is the temperature at which the fuel contains so many agglomerated crystals that it is essentially a gel and will no longer flow. The cloud points (with an average of 4.0 °C) of the partially hydrogenated FAMEs (i.e., JBH, BCB and MSB) were higher than that for the conventional diesel fuel. These values are lower as compared to the cloud points (with an average of 9.5 °C) reported by Thunyaratchatanon et al. for the hydrogenation of soybean oil-based biodiesel using palladium-silica as catalysts.48 Similarly, all the partially hydrogenated FAMEs have pour points (with an average of 2.7 °C) higher than that of the conventional diesel fuel. However, no information on the pour points was reported by Thunyaratchatanon et al. for the hydrogenation of biodiesel produced from soybean oil.48 Although, in another work reported by the same authors for the hydrogenation of biodiesel produced from soybean oil, the cloud points (with an average of 4.7 °C) are slightly higher with a difference of 0.7 °C. However, the pour points (with an average of −1.2 °C) are lower as compared to our work.49 The high CP and PP values of the partially hydrogenated FAME may be due to the high content of the saturated FAME since higher contents of saturated FAME account for higher pour and cloud points.38,50 The use of these biodiesels in warm climate countries might not affect the engine, however, for the use of these biodiesels in cold climate countries, the CP and PP might need to be improved by blending with other diesel fuels or adding appropriate chemical additives known as depressants.51,52 It is noteworthy that the CP and PP of biodiesels depend on the feedstocks from which the biodiesel is produced.38,50
Flash point (FP) is another critical property that must be considered in assessing the overall flammability hazard of a fuel. It is used to classify fuels for transport, storage, and distribution according to the hazard level.53 The flash points of the partially hydrogenated FAMEs (with an average of 136 °C) are higher than the EN 14214 and ASTM D 6751/D 93 limits of 120 °C and 130 °C, respectively, for conventional diesel fuel. This implies that there is a lower fire hazard that is associated with the use of partially hydrogenated biodiesel as compared to conventional diesel fuel. These results are similar to those reported earlier for peanut oil biodiesel54 and jatropha seed oil biodiesel.55 The flash points for the un-hydrogenated and the partially hydrogenated biodiesels in our study are higher than the conventional diesel in this study, which is an advantage since these types of diesels are less likely to ignite at normal temperatures found in hot climates compared to conventional diesels.
The heat of combustions or calorific values of the partially hydrogenated FAME, having an average of 42.0 MJ kg−1, is higher than the EN 14214 minimum standard of 35 MJ kg−1 requirement for biodiesel, although lower than the conventional diesel fuel of 45.6 MJ kg−1 (Table 6). The lower calorific values could result from high oxygen content, as reported by Yamane et al. that oxygen in fuel improves the combustion properties and emissions but reduces the calorific value.56
Another significant property improvement for the partially hydrogenated biodiesel is its acid values, comparable to conventional diesel. Acid value describes the free fatty acids (FFAs) in the biodiesels. Higher acid values in biodiesel cause operational problems, such as corrosion and fuel clogging by deposit formation.38 The acid values (0.10–0.35 mg KOH/g) presented in Table 6 for the three partially unsaturated FAMEs are within the specifications of EN 14214 and ASTM D6751.
4 Conclusion
We have used pyrazolyl nickel(II) and palladium(II) complexes to establish their ability to selectively hydrogenate methyl linoleate and biodiesel produced from jatropha, chinaberry and melon seed oils. Catalytic activities (in terms of the conversion of methyl linoleate) are in the orders of 1 > 3 > 2 and 5 > 4 > 6 for the nickel(II) and palladium(II) complexes, respectively. The hydrogenation is generally homogeneous as demonstrated with complex 6 and selective towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds over the carbonyl (C
C bonds over the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) moiety.
O) moiety.
It is significant to note that the bulk properties of the mixture of un-hydrogenated, partially hydrogenated, and fully hydrogenated of the three plant biodiesels in this study meet major international standards such as EN 14214 and ASTM D 6751. These include lower fire hazard compared to conventional diesel fuel and highly improved acid values compared to their un-hydrogenated biodiesels. Lastly, our study shows that if the desired product from the hydrogenation of the FAMEs studied in this report is to produce wax, then complex 5 can be used to make such waxes.
Author contributions
Oluwasegun E. Olaoye: investigation; formal analysis; methodology. Olayinka Oyetunji: conceptualization; funding acquisition; supervision; formal analysis; resources. Banothile C. E. Makhubela: conceptualization; supervision; formal analysis; resources. Gopendra Kumar: supervision; funding acquisition. James Darkwa: conceptualization; supervision; formal analysis; resources.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful for the funding provided by the Royal Society-Foreign Commonwealth and Development Office (RS-FCDO), United Kingdom. (Registered Charity Number 207043).References
- S. Alexander, V. Udayakumar and V. Gayathri, J. Mol. Catal., 2009, 314, 21–27 CrossRef CAS.
- Y. Liu, I. D. Gridnev and W. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1901–1905 CrossRef CAS PubMed.
- G. Amenuvor, B. C. E. Makhubela and J. Darkwa, ACS Sustain. Chem. Eng., 2016, 4, 6010–6018 CrossRef CAS.
- R. A. Farrar-tobar, Z. Wei, H. Jiao, S. Hinze and J. G. De Vries, Chem.–Eur. J., 2018, 24, 2725–2734 CrossRef CAS PubMed.
- T. A. Tshabalala and S. O. Ojwach, J. Organomet. Chem., 2018, 873, 35–42 CrossRef CAS.
- S. Naskar and M. Bhattacharjee, Tetrahedron Lett., 2007, 48, 465–467 CrossRef CAS.
- S. Bauri, S. N. R. Donthireddy, P. M. Illam and A. Rit, Inorg. Chem., 2018, 57, 14582–14593 CrossRef CAS PubMed.
- D. Spasyuk, S. Smith and D. G. Gusev, Angew. Chem., Int. Ed., 2012, 51, 2772–2775 CrossRef CAS PubMed.
- G. Zhang, Z. Yin and J. Tan, RSC Adv., 2016, 6, 22419–22423 RSC.
- P. Wang, H. Liu, M. Liu, R. Li and J. Ma, New J. Chem., 2014, 38, 1138–1143 RSC.
- G. Shang, W. Li and Z. Zhang, in Catalytic Asymmetric Synthesis, John Wiley and Sons, New York, 3rd edn, 2010 Search PubMed.
- F. Nerozzi, Plat. Met. Rev., 2012, 56, 236–261 CrossRef CAS.
- E. Bouwman, Handbook of Homogeneous Hydrogenation, Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, 2007 Search PubMed.
- Z. R. Dong, Y. Y. Li, S. L. Yu, G. S. Sun and J. X. Gao, Chin. Chem. Lett., 2012, 23, 533–536 CrossRef CAS.
- Y. Y. Li, S. L. Yu, W. Y. Shen and J. X. Gao, Acc. Chem. Res., 2015, 48, 2587–2598 CrossRef CAS PubMed.
- R. J. Liua, P. A. Croziera, C. M. Smith, D. A. Huculc, J. Blacksond and G. Salaita, Appl. Catal. Gen., 2005, 282, 111–121 CrossRef.
- I. M. Angulo, A. M. Kluwer and E. Bouwman, Chem. Commun., 1998, 2689–2690 RSC.
- I. M. Angulo and E. Bouwman, J. Mol. Catal. A: Chem., 2001, 175, 65–72 CrossRef CAS.
- I. M Angulo, E. Bouwman, R. van Gorkum, S. M. Lok, M. Lutz and A. L. Spek, J. Mol. Catal. A: Chem., 2003, 202, 97–106 CrossRef.
- M. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyl, L. C. Campeau and P. J. Chirik, J. Am. Chem. Soc., 2016, 138, 3562–3569 CrossRef CAS PubMed.
- E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley and Sons, New York, 2002 Search PubMed.
- F. A. Harraz, S. E. El-Hout, H. M. Killa and I. A. Ibrahim, J. Catal., 2012, 286, 184–192 CrossRef CAS.
- P. Pelagatti, A. Bacchi, M. Carcelli, M. Costa, A. Fochi, P. Ghidini, E. Leporati, M. Masi, C. Pelizzi and G. Pelizzi, J. Organomet. Chem., 1999, 583, 94–105 CrossRef CAS.
- A. Bacchi, M. Carcelli, M. Costa, A. Leporati, E. Leporati, P. Pelagatti, C. Pelizzi and G. J. Pelizzi, Organomet. Chem., 1997, 535, 107–120 CrossRef CAS.
- B. Ding, Z. Zhang, Y. Liu, M. Sugiya, T. Imamoto and W. Zhang, Org. Lett., 2013, 15, 3690–3693 CrossRef CAS PubMed.
- M. Boronat, A. Corma, C. González-Arellano, M. Iglesias and F. Sánchez, Organometallics, 2010, 29, 134–141 CrossRef CAS.
- M. W. Van Laren and C. J. Elsevier, Angew. Chem., Int. Ed., 1999, 38, 3715–3717 CrossRef CAS.
- S. H. Teo, A. Islam and Y. H. Taufiq-Yap, Chem. Eng. Res. Des., 2016, 111, 362–370 CrossRef CAS.
- B. Aghabarari, N. Dorostkar and M. V. Martinez-Huerta, Fuel Process. Technol., 2014, 118, 296–301 CrossRef CAS.
- Y. A. Elsheikh, Process Saf. Environ. Prot., 2014, 92, 828–834 CrossRef CAS.
- M. S. M. Zaharin, N. R. Abdullah, G. Najafi, H. Sharudin and T. Yusaf, Renew. Sustain. Energy Rev., 2017, 79, 475–493 CrossRef CAS.
- N. Rasimoglu and H. Temur, Energy, 2014, 68, 57–60 CrossRef CAS.
- J. Elguero and E. J. R. Jacquier, Bull. Soc. Chim. Fr., 1968, 2, 707–711 Search PubMed.
- K. Niedenzu, J. Sorwatowski and S. Trofimenko, Inorg. Chem., 1991, 30, 524–527 CrossRef CAS.
- G. K. Anderson, M. Lin, A. Sen and E. Gretz, Inorg. Synth., 1990, 28, 60–63 CrossRef CAS.
- E. Ocansey, J. Darkwa and B. C. E. Makhubela, Polyhedron, 2019, 166, 52–59 CrossRef CAS.
- S. M. Nelana, J. Darkwa, I. A. Guzei and S. F. Mapolie, J. Organomet. Chem., 2004, 689, 1835–1842 CrossRef CAS.
- G. Knothe, J. Am. Oil Chem. Soc., 2006, 83, 823–833 CrossRef CAS.
- K. Li, J. Darkwa, I. A. Guzei and S. F. Mapolie, J. Organomet. Chem., 2002, 660, 108–115 CrossRef CAS.
- K. Li, M. S. Mohlala, T. V. Segapelo, P. M. Shumbula, I. A. Guzei and J. Darkwa, Polyhedron, 2008, 27, 1017–1023 CrossRef CAS.
- F. K. Keter, S. Kanyanda, S. Lyantagaye, J. Darkwa, J. Rees and M. Meyer, Cancer Chem. Pharm., 2008, 63, 127–138 CrossRef CAS PubMed.
- (a) O. E Olaoye, O. Oyetunji, B. C. E. Makhubela, A. Muyaneza, G. Kumar and J. Darkwa, S. Afr. J. Chem., 2021, 74, 50–56 CrossRef; (b) C. C. Chou, C. C. Yang, H. B. Syu and T. S. Kuo, J. Organomet. Chem., 2013, 745–746, 387–392 CrossRef CAS; (c) E. Ocansey, J. Darkwa and B. C. E. Makhubela, RSC Adv., 2018, 8, 13826–13834 RSC; (d) E. Ocansey, J. Darkwa and B. C. E. Makhubela, Polyhedron, 2019, 166, 52–59 CrossRef CAS; (e) A. Sanchez-Mendez, E. de Jesus, J. C. Flores and P. Gomez-Sal, Eur. J. Inorg. Chem., 2010, 1881–1887 CrossRef CAS.
- P. Tolvanen, T. Kilpiö, P. Mäki-Arvela, D. Y. Murzin and T. Salmi, ACS Sustain. Chem. Eng., 2014, 2, 537–545 CrossRef CAS.
- W. Liu, L. Xu, G. Lu and H. Zhang, ACS Sustain. Chem. Eng., 2017, 5, 1368–1375 CrossRef CAS.
- G. M. Whitesides, M. Hackett, R. L. Brainard, J. P. M. Lavalleye, A. F. Sowinski, A. N. Izumi, S. S. Moore, D. W. Brown and E. M. Staudt, Organometallics, 1985, 4, 1819–1830 CrossRef CAS.
- F. Zaccheria, R. Psaro, N. Ravasio and P. Bondioli, Eur. J. Lipid Sci. Technol., 2012, 114, 24–30 CrossRef CAS.
- A. Bouriazos, C. Vasiliou, A. Tsichla and G. Papadogianakis, Catal. Today, 2015, 247, 20–32 CrossRef CAS.
- C. Thunyaratchatanon, J. Jitjamnong, A. Luengnaruemitchai, N. Numwong, N. Chollacoop and Y. Yoshimura, Appl. Catal., A, 2016, 520, 170–177 CrossRef CAS.
- C. Thunyaratchatanon, A. Luengnaruemitchai, J. Jitjamnong, N. Numwong, N. Chollacoop, S.-Y. Chen and Y. Yoshimura, Energy Fuels, 2018, 32, 9744–9755 CrossRef CAS.
- I. Barabás and I. A. Todoruţ, Biodiesel Quality, Standards and Properties, Biodiesel- Quality, Emissions and By-Products, InTech, Shanghai, 2011 Search PubMed.
- V. Makareviciene, K. Kazancev and I. Kazanceva, Renew. Energy, 2015, 75, 805–807 CrossRef CAS.
- S. Nainwal, N. Sharma, A. S. Sharma, S. Jain and S. Jain, Energy, 2015, 89, 702–707 CrossRef CAS.
- A. M. Ashraful, H. H. Masjuki, M. A. Kalam, I. M. Rizwanul Fattah, S. Imtenan, S. A. Shahir and H. M. Mobarak, Energy Convers. Manag., 2014, 80, 202–228 CrossRef CAS.
- M. Ahmad, S. Rashid, M. A. Khan, M. Sultan and S. Gulzar, Afr. J. Biotechnol., 2009, 8, 441–443 CAS.
- P. K. Barua, Int. J. Energy Inform. Commun., 2011, 2, 63–65 Search PubMed.
- K. Yamane, A. Ueta and Y. Shimamoto, Int. J. Engine Res., 2001, 4, 249–261 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00254c |
| This journal is © The Royal Society of Chemistry 2023 |