Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The urgent recognition of phosphate resource scarcity and pollution
Thibaut L. M.
Martinon

Department of Chemistry, University of Minnesota Twin-Cities, Minneapolis, MN 55455, USA. E-mail: mart5691@umn.edu
First published on 5th October 2023
To quote Isaac Asimov (1974),1 “We may be able to substitute nuclear power for coal, and plastics for wood, and yeast for meat, and friendliness for isolation—but for phosphorus, there is neither substitute nor replacement”. In fact, phosphorus only accounts for 0.14% of the Earth’s crust, one of the rarest elements essential to organic life and what is considered “life’s bottleneck”. The chronic and inefficient use of nonrenewable synthetic fertilizers in the last century has led to vast amounts of phosphate eroding into surface-level water bodies all over the planet.2 The consequences manifest in a multifaceted threat to human society,3 where the most basic necessities of food and water hang in the balance.
An often-overlooked pollutant, the signs of phosphate contamination appear after a build-up of nutrient pollution fixed by cyanobacteria; these species will reproduce uncontrollably in a “bloom”, their decay resulting in hypoxic dead zones composed of excessive cyanotoxins. This eutrophication renders freshwater sources inaccessible and devastates the local biota’s biodiversity. The uncontrolled cycle of growth and death repeats until the excess nutrient is removed from the environment. A prominent example of this effect can be witnessed in Lake Erie, of which the western basin experienced blooms of Microcystis in 2014, which actively compromised the drinking water of half a million people in Toledo, Ohio.4 While this nutrient pollution was originally discovered in 1995, mitigation strategies and cooperation agreements between the United States and Canada have proved ineffective in preventing the re-eutrophication of this vital source of fresh water.5
The irony of the growing abundance of polluting phosphates is that sustained population growth has inadvertently led to the compounding dependence on scarce, nonrenewable phosphate rock following the Green Revolution of the 1960s.6 This resource is primarily monopolized between a dozen countries, with Morocco and occupied Western Sahara holding 70% of the world’s overland reserves (Fig. 1).7 Although scientists generally agree humans have over 50–100 years before synthetic phosphate reserves are depleted,8 the geopolitical ramifications of monopolized export of such a critical resource are likely to shape worldwide political/economic instability in the coming decades in a manner akin to the 1973 Organization of Petroleum Exporting Countries (OPEC) oil embargo.9 Some premonitions regarding this crisis have already manifested in surging fertilizer prices and countries such as China prioritizing domestic supply over exports critically desired by non-phosphate-producing countries.10
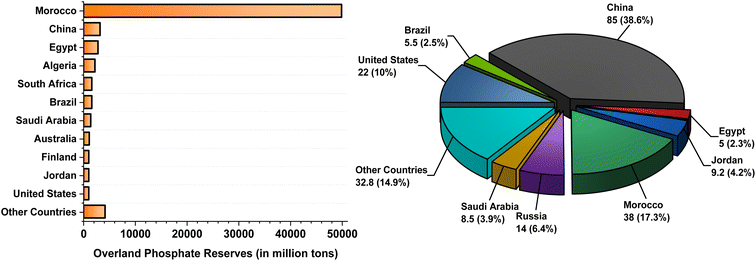 | ||
| Fig. 1 2021 total world phosphate rock reserves (left) and yearly production (right) by country in million tons. Data obtained from Jasinski7 and figures constructed with Origin 2021. | ||
As with all climate disasters, developing countries will bear the effect most heavily. As demonstrated by a 2007 study sponsored by the International Fertilizer and Development Center (IFDC), prices for fertilizers mount quickly due to compounding transport costs, leaving many impoverished farmers without access to crucial synthetic fertilizers.11 As phosphate prices continue to fluctuate, food security in Sub-Saharan Africa is expected to decrease should inter-state cooperation collapse in the scramble to address mounting scarcity.12 Recent events in Sri Lanka also reveal a foreboding omen for countries already dependent on synthetic fertilizers. The sudden transition from synthetic fertilizers resulted in varying catastrophes, including an estimated 33% reduction in rice and a 35% reduction in tea yields. These approximate damages alone totaled $425 million USD, contributing to the toppling of the ruling government within a year.13
Developed countries that manage these costs will instead find themselves footing an ever-increasing bill for pollution treatment and fertilizer costs. The United States public and private sectors alone have spent $1.9 trillion USD as of 2014 on abating surface-water pollution since the 1960s.14 These costs would accompany increased mass emigration as food insecurity displaces growing population centers, requiring major shifts in asylum laws along already congested borders.15 While one could not be blamed for assuming mitigating this disaster is inevitable, some progress has been made in the past decades. The Indian state of Sikkim, for example, was able to adopt an organic fertilizer economy through a gradual transition from 2003–2014, strongly supported via major public funding and education programs.16 Each region of the planet will need to adopt new strategies to tackle the unique aspects of its phosphate footprint, and the chemical sciences are excellently placed in leading these innovations.
The chemical sciences have been at the forefront of revolutionary innovations, as noted by Asimov. These same innovations have also equally been the architects of the crises that haunt our fragile planet. We have the duty to develop new strategies for mitigating the impending crisis by directing public policy. Our first step should be at a local level. Farmers are keenly aware of their contribution to this disaster and have access to many modern tools for collecting studies in cooperation with the scientific community, from sensors to monitor soil conditions to drones that analyze field conditions from above.17 The United States Department of Agriculture (USDA), for example, organizes critical Natural Resources Conservation Service (NRCS) conservation practices in tandem with farmers, saving $927 million USD’s worth of anhydrous ammonia in fields annually and, in some cases, reducing agricultural phosphate run-off by 75 percent.18 Taking advantage of these tools to mitigate phosphate run-off and overuse of fertilizers could be a key step in stopping phosphate contamination at its primary source.
We should also prioritize messaging around these economic impacts, because this provides a huge incentive for change on a political and individual level. Education is also crucial, spreading awareness of safeguarding water resources and food waste prevention to local community leaders with the trust and best interests of their communities. My own lab group at the University of Minnesota has led outreach efforts in our community to educate young students about phosphate pollution, and we strongly encourage our colleagues to do the same to combat the declining interest and trust in science.19
Immediate actions can also be taken to address nutrient pollution, particularly achieving international recognition in removing phosphates from detergents and personal care products. The role of detergents in nutrient pollution is well documented,20 and many countries have pursued legislation to limit or ban their use. Companies such as Proctor and Gamble, which composed 25% of the North American market, transitioned to phosphate-free alternatives in 2014.21 While this change will continue organically as phosphate prices increase, banning phosphate detergents prematurely should be pursued globally. In 2007, detergents alone made up 61% of all surface water pollution from industrial uses in Western Europe.22
Of course, activism will not be able to solve this issue alone. The re-eutrophication of Lake Erie serves as a dire warning that simple prevention methods alone will not suffice.5 Farmers need to be given viable alternatives to common fertilizers; this will come in the synthesis of cheaper, long-release fertilizers that will cushion the transition to a sustainable farming economy. Developing novel agents such as struvite has shown favorable advantages regarding slow-release properties, reduced heavy-metal contamination, and a salt index ideal for plant growth.23 The low water solubility, however, has raised questions about struvite’s agronomic utility,24 and hence further exploration into other slow-release fertilizers with either mineral or organic polymer coatings may be necessary.25
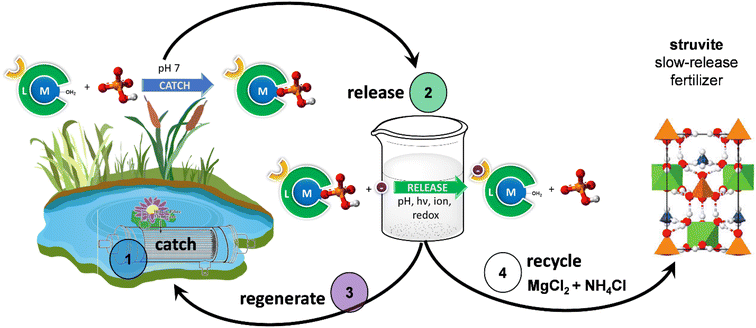
For phosphate already contaminating surface-level water bodies, several recent approaches have been studied in close context in the past decades, regarding phosphate removal and recovery.6,26 Phosphate removal is principally performed via chemical precipitation of bioavailable phosphate with cations of either calcium, iron, or aluminum. While great in the short term in preventing eutrophication witnessed in China,27 there are concerns the phosphate salts could contaminate waterbeds and lead to long-term elevated phosphate/cation levels. Phosphate recovery instead solves both the scarcity and pollution issues at once, and is principally utilized in the treatment of phosphate-contaminated wastewater. Enhanced biological phosphorous removal of activated sludge with polyphosphate-accumulating organisms is one of the primary methods to refine phosphate into an aqueous state.6 Phosphates harvested in this manner are unfortunately well-known to bioaccumulate heavy metals through stabilization,28 unideal for phosphate capture and leaving over 60% of recovered phosphate to languish in landfills as heavy-metal-contaminated waste.2 An answer is likely found within supramolecular phosphate receptors and membrane technologies.29 Lanthanide-based receptors have proved to be of particular interest due to their unique properties in forming labile, selective bonds with phosphates, surpassing the hydration energy of phosphate in water (Fig. 2).30–32
These innovations will require vertical integration to facilitate novel discoveries; lanthanides used in phosphate binders could be more readily accessible. However, limitations in the purification process have led to higher prices, despite them being more abundant than traditional metals such as gold.33 While this sprawling field is already gaining traction, due to the increased requirement of rare earth elements (REEs) in electrics, novel lanthanide ligands34 and separation methods35 should be explored to incur fewer environmental costs and increase accessibility. It should also be noted that some alternatives to solve climate change should also be re-evaluated, particularly biofuels. First-generation biofuels require heavy uses of phosphate-based fertilizers, and their impact in depleting inorganic phosphorous (Pi) sources outweighs the benefit of combating climate change.36 Our fight against climate change should not jeopardize the security of the most basic resources required for human life. Neither should we idle as the 50th anniversary of Asimov’s warning comes into view, and should continue to build a path forward for the coming generations.
References
- I. Asimov, Asimov on Chemistry, Doubleday, New York NY, 1974, ISBN-100385041004 Search PubMed.
- Z. Yuan, S. Jiang, H. Sheng, X. Liu, H. Hua, X. Liu and Y. Zhang, Human perturbation of the global phosphorus cycle: Changes and consequences, Environ. Sci. Technol., 2018, 52, 2438–2450, DOI:10.1021/acs.est.7b03910.
- K. Ashley, D. Cordell and D. Mavinic, A brief history of phosphorus: from the philosopher’s stone to nutrient recovery and reuse, Chemosphere, 2011, 84(6), 737–746, DOI:10.1016/j.chemosphere.2011.03.001.
- M. M. Steffen, B. S. Belisle, S. B. Watson, G. L. Boyer and S. W. Wilhelm, Status, causes and controls of cyanobacterial blooms in Lake Erie, J. Great Lakes Res., 2014, 40(2), 215–225, DOI:10.1016/j.jglr.2013.12.012.
- S. B. Watson, C. Miller, G. Arhonditsis and G. L. Boyer, et al, The re-eutrophication of Lake Erie: harmful algal blooms and hypoxia, Harmful Algae, 2016, 56, 44–66, DOI:10.1016/j.hal.2016.04.010.
- S. D. A. Callegari, A. Capodaglio and D. Vaccari, The potential phosphorus crisis: resource conservation and possible escape technologies: a review, Resources, 2018, 7(2), 37, DOI:10.3390/resources7020037.
- S. M. Jasinski, Mineral Commodity Summaries: Phosphate Rock, January 2022; U.S. Geological Survey, Reston, VA, 2022, available at: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-phosphate.pdf Search PubMed.
- D. Cordell, J.-O. Drangert and S. White, The story of phosphorus: global food security and food for thought, Global Environ. Change, 2009, 19(2), 292–305, DOI:10.1016/j.gloenvcha.2008.10.009.
- OPEC Oil Embargo 1973-1974, United States Office of the Historian, US Department of State, Washington, DC, 2014, available at: https://history.state.gov/milestones/1969-1976/oil-embargo Search PubMed.
- F. K. Nti, International Agricultural Trade Report: Impacts and Repercussions of Price Increases on the Global Fertilizer Market; United States Department of Agriculture, Washington, DC, 2022, available at: https://www.fas.usda.gov/data/impacts-and-repercussions-price-increases-global-fertilizer-market Search PubMed.
- Fertilizer Supply and Costs in Africa; Chemonics International; International Center for Soil Fertility and Agricultural Development, Muscle Shoals, AL, 2007, available at: https://www.inter-reseaux.org/wp-content/uploads/IFDC__ChemonicsFertilizerSupplyandCostsinAfrica_Study_for_BMGF-2.pdf Search PubMed.
- D. Magnone, V. J. Niasar, A. F. Bouwman, A. H. W. Beusen, S. E. A. T. M. Van Der Zee and S. Z. Sattari, The impact of phosphorus on projected Sub-Saharan Africa food security futures, Nat. Commun., 2022, 13(1), 6471, DOI:10.1038/s41467-022-33900-x.
- M. J. B. A. Galappattige, Sri Lanka: Restricting Import of Fertilizers and Agrochemicals; United States Department of Agriculture, Washington, DC, 2021, available at: https://www.fas.usda.gov/data/sri-lanka-sri-lanka-restricts-and-bans-import-fertilizers-and-agrochemicals Search PubMed.
- D. A. Keiser, C. L. Kling and J. S. Shapiro, The low but uncertain measured benefits of US water quality policy, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(12), 5262–5269, DOI:10.1073/pnas.1802870115.
- M. A. Carney and K. C. Krause, Immigration/migration and healthy publics: the threat of food insecurity, Palgrave Commun., 2020, 6(1), 93, DOI:10.1057/s41599-020-0461-0.
- The “100% Organic State” Sikkim in India Wins Gold. Policies from Brazil, Denmark and Ecuador Honoured with Silver Awards, Food and Agriculture Organization, Rome, 2018, available at: https://www.fao.org/agroecology/slideshow/news-article/en/c/1157015/ Search PubMed.
- L. Goedde, J. Katz, A. Ménard and J. Revellat. Agriculture’s Connected Future: How Technology Can Yield New Growth; McKinsey & Co., London, 2020, available at: https://www.mckinsey.com/industries/agriculture/our-insights/agricultures-connected-future-how-technology-can-yield-new-growth Search PubMed.
- E. Creech, Farmers Keeping Nutrients on the Field, Out of Streams; United States Department of Agriculture, Washington DC, 2021, available at: https://www.usda.gov/media/blog/2017/12/13/farmers-keeping-nutrients-field-out-streams Search PubMed.
- S. M. Harris, K. L. Peterson, K. M. Bailie, C. D. Tower, B. K. Rundle, T. R. Ricks and V. C. Pierre, Design and evaluation of the environmental outreach activity for middle school students, ACS Omega, 2020, 5(39), 25175–25187, DOI:10.1021/acsomega.0c03194.
- J. Köhler, Detergent phosphates: an EU policy assessment, J. Bus. Chem., 2006, 3(2), 15–30 Search PubMed , ISSN 1613-9615.
- E. Gies, Proctor and Gamble Touts ’win-Win’ of Cutting Phosphates in All Laundry Soaps. The Guardian, 2014, available at: https://www.theguardian.com/sustainable-business/proctor-gamble-remove-phosphates-laundry-soap Search PubMed.
- T. Wind, The Role of Detergents in the Phosphate-Balance of European Surface Waters. E-Water: Official Publication of the European Water Association, 2007, available at: https://www.ewa-online.eu/tl_files/_media/content/documents_pdf/Publications/E-WAter/documents/25_2007_03.pdf Search PubMed.
- M. Latifian, J. Liu and B. Mattiasson, Struvite-based fertilizer and its physical and chemical properties, Environ. Technol., 2012, 33(24), 2691–2697, DOI:10.1080/09593330.2012.676073.
- A. J. Hertzberger, R. D. Cusick and A. J. Margenot, A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer, Soil Sci. Soc. Am. J., 2020, 84(3), 653–671, DOI:10.1002/saj2.20065.
- Y. P. Timilsena, R. Adhikari, P. Casey, T. Muster, H. Gill and B. Adhikari, Enhanced efficiency fertilizers: a review of formulation and nutrient release patterns, J. Sci. Food Agric., 2015, 95(6), 1131–1142, DOI:10.1002/jsfa.6812.
- L. Peng, H. Dai, Y. Wu, Y. Peng and X. Lu, A comprehensive review of the available media and approaches for phosphorus recovery from wastewater, Water, Air, Soil Pollut., 2018, 229(4), 115, DOI:10.1007/s11270-018-3706-4.
- H. Yang, K. He, D. Lu, J. Wang, D. Xu, Z. Jin, M. Yang and J. Chen, Removal of phosphate by aluminum-modified clay in a heavily polluted lake, Southwest China: effectiveness and ecological risks, Sci. Total Environ., 2020, 705, 135850, DOI:10.1016/j.scitotenv.2019.135850.
- A. Nzihou and P. Sharrock, Role of phosphate in the remediation and reuse of heavy metal polluted wastes and sites, Waste Biomass Valorization, 2010, 1(1), 163–174, DOI:10.1007/s12649-009-9006-x.
- S. M. Ribet, B. Shindel, R. Dos Reis, V. Nandwana and V. P. Dravid, Phosphate Elimination and Recovery Lightweight (PEARL) membrane: a sustainable environmental remediation approach, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(23), e2102583118, DOI:10.1073/pnas.2102583118.
- V. C. Pierre and R. K. Wilharm, Design principles and applications of selective lanthanide-based receptors for inorganic phosphate, Front. Chem., 2022, 10 DOI:10.3389/fchem.2022.821020.
- T. L. M. Martinon and V. C. Pierre, Luminescent lanthanide probes for inorganic and organic phosphates, Chem.–Asian J., 2022, 17(16), e202200495, DOI:10.1002/asia.202200495.
- S.-Y. Huang, M. Qian and V. C. Pierre, A combination of factors: tuning the affinity of europium receptors for phosphate in water, Inorg. Chem., 2019, 58(23), 16087–16099, DOI:10.1021/acs.inorgchem.9b02650.
- The Rare-Earth Elements-Vital to Modern Technologies and Lifestyles; United States Geological Survey, Reston, VA, 2014, available at: https://pubs.usgs.gov/fs/2014/3078/pdf/fs2014-3078.pdf Search PubMed.
- E. J. Werner and S. M. Biros, Supramolecular ligands for the extraction of lanthanide and actinide ions, Org. Chem. Front., 2019, 6(12), 2067–2094, 10.1039/c9qo00242a.
- S. K. Pradhan and B. Ambade, Extractive separation of rare earth elements and their determination by inductively coupled plasma optical emission spectrometry in geological samples, J. Anal. At. Spectrom., 2020, 35(7), 1395–1404, 10.1039/d0ja00190b.
- L. Hein and R. Leemans, The impact of first-generation biofuels on the depletion of the global phosphorus reserve, Ambio, 2012, 41(4), 341–349, DOI:10.1007/s13280-012-0253-x.
| This journal is © The Royal Society of Chemistry 2023 |