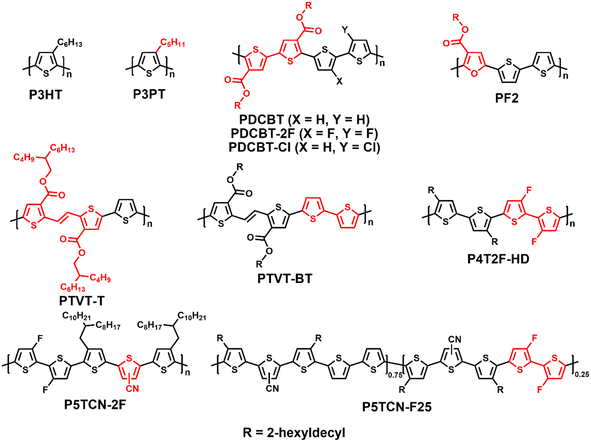
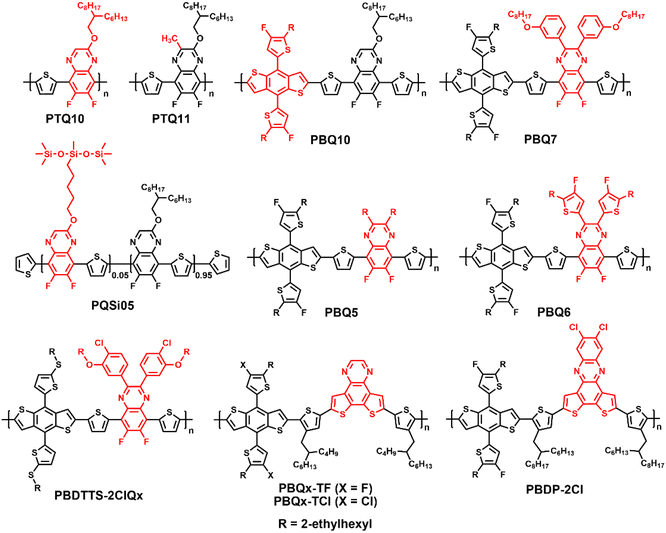
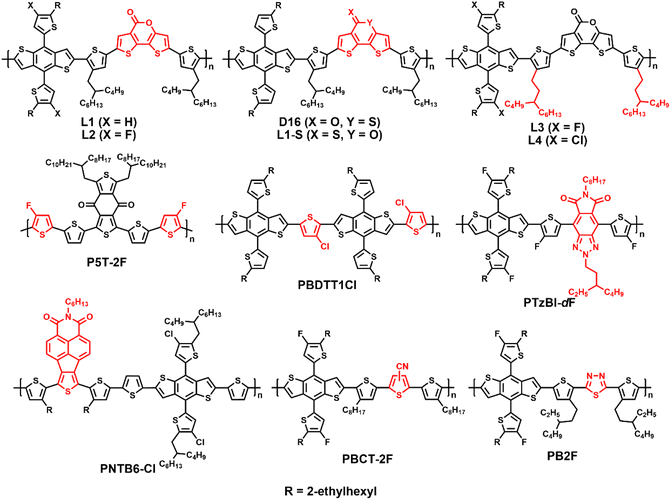
Wide-bandgap polymer donors for non-fullerene organic solar cells
Jiamin
Cao
 *a,
Lifei
Yi
a,
Lixiu
Zhang
c,
Yingping
Zou
*a,
Lifei
Yi
a,
Lixiu
Zhang
c,
Yingping
Zou
 *b and
Liming
Ding
*b and
Liming
Ding
 *c
*c
aKey Laboratory of Theoretical Organic Chemistry and Functional Molecule (MoE), Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China. E-mail: jiamincao@hnust.edu.cn
bCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: yingpingzou@csu.edu.cn
cCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: ding@nanoctr.cn
First published on 25th November 2022
Abstract
High-performance wide-bandgap (WBG) polymer donors are one of the key factors in determining the power conversion efficiencies (PCEs) of non-fullerene organic solar cells (OSCs). To date, thousands of polymer donors with different building blocks have been synthesized, but the reported efficient polymers need to be classified and analyzed to achieve better photovoltaic performances. Accordingly, in this work, we present a brief survey of the developments of WBG polymers and classify these efficient polymers into five categories. Moreover, the correlation among their chemical structures and optical properties, electronic properties, aggregation behavior, and photovoltaic performance are summarized and discussed. (i) PBDB-T and the fluorinated derivative PM6 are the most widely used polymer donors for evaluating photovoltaic properties and conducting theoretical research in OSCs. (ii) The D18 series polymers, which were first reported by Ding's group, are among the best polymer materials with over 19% PCEs in ternary OSCs. (iii) Polythiophenes (PTs) are the cheapest donors for commercial OSCs but exhibit intrinsically inferior photovoltaic performance compared with other D–A copolymers. However, the introduction of electron-withdrawing groups has enhanced the performance of PTs, achieving PCEs of over 17%. (iv) Quinoxaline (Qx)-based polymers exhibit excellent photovoltaic performance. Especially, PTQ10 shows great potential for commercial production due to its low cost and high performance. (v) There are also many other polymers with decent photovoltaic performances, such as lactone and 1,3,4-thiadiazole-based polymers. These results indicate the diversity of conjugated polymers and can inspire chemists to develop more effective polymer materials for commercialization. Finally, some perspectives for the further development of WBG polymers are provided and the bright future of OSCs is emphasized.
 Lifei Yi | Lifei Yi obtained her BS in 2020. Currently, she is a Master student at Hunan University of Science and Technology. Her work focuses on organic solar cells. |
1. Introduction
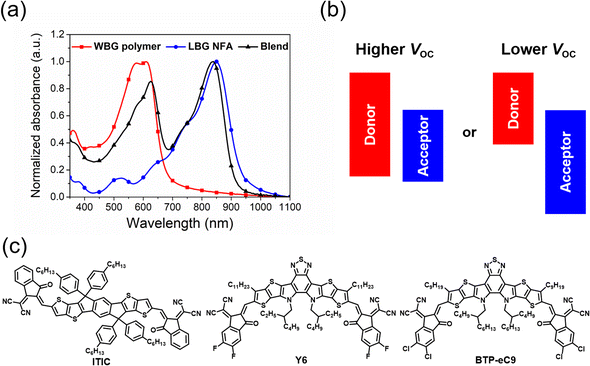
Solar cells, which can convert solar energy directly into electricity, are promising technology to achieve carbon neutrality. Nowadays, silicon solar cells still dominate the market, but there are also some new types of solar cells. Among them, organic solar cells (OSCs) have attracted much attention due to their merits of low cost, light weight and excellent mechanical flexibility.1–3 Conventional OSCs usually use fullerene derivatives as electron acceptors, but their power conversion efficiencies (PCEs) are limited to about 12%.4 Recent developments in donor/acceptor materials, device fabrication and morphological optimization have led to tremendous advances in the photovoltaic performance with efficiencies of over 19% achieved for single-junction devices.5–8 In particular, the emerge of non-fullerene acceptors (NFAs), such as the benchmark ITIC and Y6, has pushed the development of OSCs into a new era.2,9 Subsequently, hundreds of efficient NFAs have been developed, thus resulting in inspiring breakthroughs in PCEs. Moreover, these NFAs have revived the performance of polymer donors, with some of them demonstrating much lower PCEs in fullerene-based cells.10,11Generally, high-performance non-fullerene OSCs always employ wide-bandgap (WBG, Eoptg > 1.8 eV) polymer donors with strong absorption in the wavelength range of 400–700 nm and low-bandgap (LBG, Eoptg < 1.6 eV) NFAs with excellent light-harvesting ability in 600–900 nm, which are beneficial for complementary absorption and higher short-circuit current density (Jsc) (Fig. 1a).11,12 Alternatively, the open-circuit voltage (Voc) of devices is roughly proportional to the difference between the highest occupied molecular orbitals (HOMOs) of the polymer donors and the lowest unoccupied molecular orbitals (LUMOs) of NFAs, where this active material pair exhibits a much higher Voc than the inversive combination of LBG polymer donor/WBG NFA (Fig. 1b). This is because the appropriate LUMO offset (ΔELUMO(D–A)) between the polymer donors and NFAs is necessary for efficient exciton dissociation, while OSCs can work well with a small, even close to zero HOMO offset ΔEHOMO(D–A) value.13 Therefore, the perfect combination of WBG polymers and LBG NFAs utilizes the photons from the whole visible and even near-infrared (NIR) region and exhibits the maximum Voc, causing OSCs to reach a new level.11,12 It is clear that the development of high-performance WBG polymer donors plays a decisive role in the development of OSCs. Herein, we summarize and classify the recently reported efficient WBG polymer donors according to the polymer backbone and summarize the relationship between their structure and properties. Finally, the development trends of WBG polymers and the upcoming commercialization of OSCs are also highlighted.
2. PBDB-T series polymers
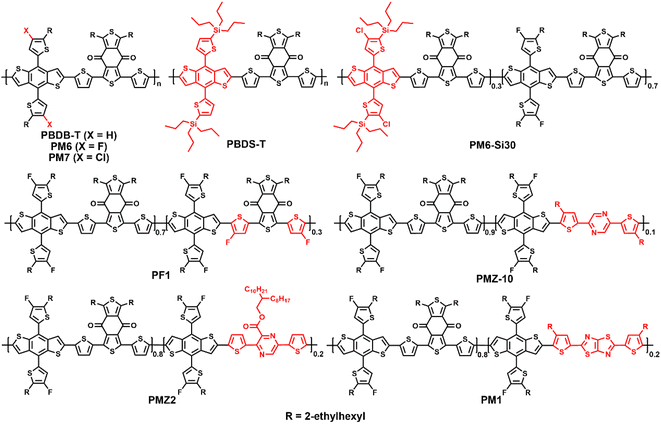
2-Alkylthiophene-substituted benzo[1,2-b:4,5-b′]dithiophene (BDTT) possesses a two-dimensional conjugated structure and weak electron-donating ability, and endows its copolymers with good hole mobilities and lower HOMO energy levels. Therefore, many efficient D–A copolymers have been developed by using BDTT as the donor unit.14,15 In 2015, Hou et al. reported the preparation of PBDB-T (PBDTBDD) via the copolymerization of BDTT with 1,3-bis(thiophen-2-yl)-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (BDD), achieving a decent PCE of 6.67% when combined with the fullerene acceptor PC61BM (Fig. 2).14 More importantly, PBDB-T also exhibited an impressive photovoltaic performance in non-fullerene OSCs.15 Recently, Bo et al. employed PBDB-T as the polymer donor and Y-series NFAs Y11 and SM16 as acceptors to fabricate ternary OSCs. As shown in Table 1, a PCE of 17.09% was achieved, which is the highest value for PBDB-T-based OSCs.16 Furthermore, fluorinated PM6 (PBDB-TF) and chlorinated PM7 (PBDB-TCl) were developed by adding two fluorine or chlorine atoms to the thiophene side chains of BDTT, achieving high PCEs of 19.6% and 17.04%, respectively.6,17 Especially, PM6 has become one of the best commercial polymer materials, which is widely used to estimate the photovoltaic performances of new NFAs. In addition, PM6 can be used in all-polymer solar cells (all-PSCs), where a ternary all-PSC based on PM6:PY2F-T:PYT exhibited impressively high PCE of 17.2% with superior light-soaking stability and photo-thermal stability.18 To reduce the synthetic complexity and energy loss (Eloss), Bi et al. replaced the alkyl side chains of BDTT with trialkylsilyl to obtain PBDS-T. After simple solvent vapor annealing (SVA), the OSCs based on PBDS-T:BTP-eC9 provided a decent PCE of 16.4%, with a low photon-energy loss of 0.53 eV and high internal quantum efficiency of 93%.19| Donor | E optg [eV] | HOMO/LUMO [eV] | Acceptor | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| PBDB-T | 1.80 | −5.34/−3.51 | Y14:SM16 | 0.844 | 27.49 | 73.60 | 17.09 | 16 |
| PM6:D18 | 1.83 | −5.50/−3.61 | L8-BO | 0.896 | 26.7 | 81.9 | 19.6 | 6 |
| PM7 | 1.80 | −5.52/−3.57 | Y6 | 0.882 | 25.61 | 73.3 | 17.04 | 17 |
| PM6 | — | — | PY2F-T:PYT | 0.90 | 25.2 | 76.0 | 17.2 | 18 |
| PBDS-T | 1.86 | −5.38/−3.17 | BTP-eC9 | 0.86 | 25.5 | 74 | 16.4 | 19 |
| PM6:PM6-Si30 | 1.85 | −5.56/−3.19 | C9 | 0.870 | 26.90 | 78.04 | 18.27 | 21 |
| PF1 | 1.81 | −5.52/−3.71 | Y6 | 0.87 | 26.3 | 76 | 17.3 | 20 |
| PMZ-10 | 1.84 | −5.63/−3.62 | Y6 | 0.834 | 27.96 | 78.2 | 18.23 | 22 |
| PMZ2 | 1.84 | −5.55/−3.70 | Y6 | 0.854 | 26.9 | 77.2 | 17.8 | 23 |
| PM1 | 1.86 | −5.52/−3.59 | Y6 | 0.87 | 25.9 | 78 | 17.6 | 24 |
| PM1 | — | — | L8-BO:BTP-2F2Cl | 0.881 | 27.15 | 80.14 | 19.17 | 7 |
By introducing a third monomer into the backbone of the host donor polymer, terpolymerization is an efficient strategy to adjust the energy levels, absorption spectrum, intermolecular aggregation and active layer morphology for enhanced exciton dissociation, charge transport capability, and photovoltaic performances.7,20 Recently, Peng et al. reported the preparation of a random terpolymer PM6-Si30, and added 15 wt% to the PM6:C9 blend. The ternary active layer exhibited enhanced π–π stacking, nanofiber-like microstructure, balanced charge carrier mobilities, and reduced Eloss, and thus a much higher PCE of 18.27% was obtained for ternary OSCs compared to the 17.38% of the control PM6:C9 cells.21 To regulate the molecular aggregation, Zhang et al. copolymerized 70 mol% BDD and 30 mol% fluorinated BFT units with fluorinated BDTT, to obtain the terpolymer donor PF1. PF1 exhibited deeper energy levels, slightly red-shifted absorption, improved crystallinity and aggregation, and optimized morphology with Y6. Consequently, the PF1:Y6 cells demonstrated a decent PCE of 17.3%.20 In 2021, Li et al. synthesized the terpolymer PMZ-10via a random ternary polymerization strategy by incorporating 10% of the low-cost 2,5-bis(4-(2-ethylhexyl)thiophen-2-yl)pyrazine (PZ-T) in the state-of-the-art PM6 polymer backbone and the HOMO energy level shifted down efficiently compared with the control PM6. The PMZ-10:Y6 cell exhibited efficient exciton dissociation, higher charge carrier mobility, desirable aggregation behavior, and much higher PCE of 18.23% than that of the PM6:Y6 cell (16.63%).22 Recently, Zhang et al. incorporated the electron-deficient building block 3,6-dithiophenyl-2-carboxylate pyrazine (DTCPz) in the PM6 polymer backbone as the third building component to obtain the D–A1–D–A2-type terpolymer PMZ2. Due to the down-shifted HOMO energy level, enhanced molecular planarity, better crystallinity, and favorable phase aggregation, the PMZ2:Y6 cell delivered a decent PCE of 17.8% with Voc of 0.854 V, which were much better than that of the PM6:Y6 cell.23 In 2020, Min et al. reported the preparation of the random terpolymer PM1 by incorporating 20% of thiophene-thiazolothiazole (TTz) in the PM6 polymer backbone. The PM1:Y6 blend exhibited a favorable morphology with superb phase separation and good crystallinity due to the enhanced exciton dissociation, charge transport, and reduced bimolecular recombination. Consequently, the PM1:Y6 cell afforded a high PCE of 17.6% with excellent batch-to-batch reproducibility.24 Recently, the same group used PM1 as the donor and L8-BO:BTP-2F2Cl as the acceptor to fabricate a ternary OSCs. This cell showed a higher luminescence quantum efficiency, suppressed nonradiative recombination, and lower Eloss than that of the binary PM1:L8-BO device. Consequently, the ternary cell demonstrated a notable PCE of 19.17% with a Jsc of 27.15 mA cm−2 and fill factor (FF) of 80.14%, which is among the highest efficiency reported for single-junction OSCs.7 These results indicate that the classic PBDB-T series polymers keep advancing via the ternary copolymerization strategy, and consequently some promising terpolymers have been developed.
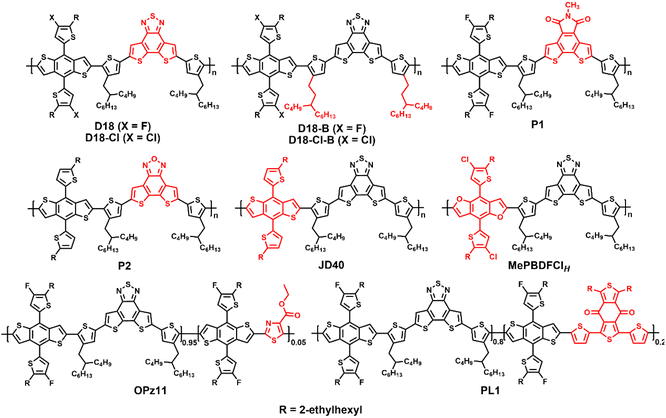
3. D18 series polymers
Dithieno[3′,2′:3,4;2′′,3′′:5,6]benzo[1,2-c][1,2,5]thiadiazole (DTBT), which is benzo[1,2,5]thiadiazole with two fused thiophene units, is an excellent unit for organic electronics and its larger molecular plane endows copolymers with good hole mobility and photovoltaic performance.3,9 In 2020, Ding et al. reported the preparation of the novel donor polymer D18 based on DTBT and fluorinated BDTT, which an offered impressively high PCE of 18.22% when blended with Y6 (Fig. 3 and Table 2). This is the first single-junction OSC with an efficiency of over 18%.3 Next, the same group added a small amount of PC61BM to the D18:Y6 blend, and PCEs of over 16% were achieved for the ternary cells with an active layer thickness of over 300 nm due to the faster and more balanced charge transport and suppressed trap-assisted charge recombination.25 Subsequently, D18 has attracted great attention from many researchers, becoming the star polymer donor. For example, Zhang et al. fabricated semitransparent cells based on D18:N3 with a PCE of 12.91% and an average visible transmittance (AVT) of 22.49%.26 Recently, Jen et al. and Liu et al. prepared ternary OSCs based on D18:BS3TSe-4F:Y6-O and D18:PM6:L8-BO with high PCEs of 19.03% and 19.6%, respectively.5,6 Bo et al. revealed that D18 exhibited universal compatibility and could work well with some cost-effective non-fused NFAs.27,28 Furthermore, Ding et al. developed the highly efficient chlorinated copolymer donor D18-Cl by replacing the fluorine atoms in D18 with chlorine atoms. The OSCs based on D18-Cl:N3 and D18-Cl:N3:PC61BM solar cells exhibited promising PCEs of 18.13% and 18.69%, respectively, which are among the highest efficiency for chlorinated polymer donors.29,30 By employing 1,4-diiodobenzene (DIB) as the volatile solid additive, Sun and Lu et al. fabricated binary OSCs based on D18-Cl:N3 and D18-Cl:L8-BO with high PCEs of 18.42% and 18.7%, respectively.31,32 Ding et al. further developed D18-B and D18-Cl-Bvia side chain engineering. After optimization of the number-average molecular weight (Mn) of the polymers, these two copolymers with moderate Mn exhibited outstanding PCEs of 18.53% and 18.74% for D18-B:N3:PC61BM and D18-Cl-B:N3:PC61BM cells, respectively.33 The same group also tried to develop similar fused-ring acceptors, i.e., 5-methyl-4H-dithieno[3,2-e:2′,3′-g]isoindole-4,6(5H)-dione (MDTID) and dithieno[3′,2′:3,4;2′′,3′′:5,6]benzo[1,2-c][1,2,5]oxadiazole (DTBO), to construct efficient polymers P1 and P2, achieving decent PCEs of 14.52% and 15.64% for the P1:N3 and P2:Y6 cells, respectively.34,35| Donor | E optg [eV] | HOMO/LUMO [eV] | Acceptor | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| D18 | 1.98 | −5.51/−2.77 | Y6 | 0.859 | 27.70 | 76.6 | 18.22 | 3 |
| D18 | — | — | BS3TSe-4F:Y6-O | 0.845 | 29.41 | 76.56 | 19.03 | 5 |
| D18:PM6 | — | — | L8-BO | 0.896 | 26.7 | 81.9 | 19.6 | 6 |
| D18-Cl | 1.99 | −5.56/−2.78 | N3 | 0.859 | 27.85 | 75.7 | 18.13 | 29 |
| D18-Cl | — | — | N3:PC61BM | 0.849 | 28.22 | 78.0 | 18.69 | 30 |
| D18-Cl | — | — | N3 | 0.860 | 27.18 | 78.8 | 18.42 | 31 |
| D18-Cl | — | — | L8-BO | 0.922 | 26.6 | 75.6 | 18.7 | 32 |
| D18-B | 1.97 | −5.51/−2.71 | N3:PC61BM | 0.823 | 28.50 | 79.0 | 18.53 | 33 |
| D18-Cl-B | 1.98 | −5.56/−2.68 | N3:PC61BM | 0.836 | 28.50 | 78.7 | 18.74 | 33 |
| P1 | 2.06 | −5.54/−2.78 | N3 | 0.90 | 24.52 | 65.8 | 14.52 | 34 |
| P2 | 1.96 | −5.45/−3.49 | Y6 | 0.83 | 26.72 | 70.6 | 15.64 | 35 |
| JD40 | 1.97 | −5.38/−3.32 | PJTVT | 0.89 | 23.75 | 76.40 | 16.13 | 36 |
| MePBDFClH | 1.94 | −5.50/−3.53 | Y6 | 0.88 | 24.96 | 68.32 | 15.06 | 37 |
| OPz11 | 1.98 | −5.58/−3.68 | Y6 | 0.865 | 27.02 | 78.71 | 18.42 | 38 |
| PL1 | 1.91 | −5.55/−3.42 | BTP-eC9-4F | 0.876 | 27.11 | 76.41 | 18.14 | 39 |
Some D18 derivatives have also been reported by other groups. In 2021, Huang et al. synthesized the D–A copolymer JD40, which can be considered as non-fluorinated D18. When it was blended with the polymerized small molecular acceptor (PSMA) PJTVT, the all-PSC showed a high PCE of 16.13% with superior thickness-insensitivity and long-term stability.36 In 2022, Huo et al. synthesized a benzo[1,2-b:4,5-b′]difuran (BDF)-based mesopolymer MePBDFClH with a Mn of 9.9 kDa. The corresponding MePBDFClH:Y6 cells delivered a record PCE of 15.06% for the BDF-based single-junction OSCs, indicating that mesopolymers are good for reducing the batch-to-batch variation.37 Recently, Lu et al. reported the preparation of the terpolymer donor OPz11 by adding 5% ester-substituted thiazole (E-Tz) acceptor unit to the D18 polymer to regulate the energy levels, molecular aggregation and phase separation. Consequently, the OPz11:Y6 cells exhibited an exciting PCE of 18.42% with a higher Voc of 0.865 V and FF of 78.71% than that of the control D18:Y6 cell.38 Bo et al. developed the terpolymer donor PL1, which was a combination of D18 and PM6. PL1 exhibited reduced regioregularity (RR) and good solubility in non-halogenated solvents, such as o-xylene. Thus, the PL1:BTP-eC9-4F cell was fabricated via eco-friendly solvent processing and an excellent PCE of 18.14% was achieved, which is much higher than that of PM6 (15.16%) and D18 (16.18%).39 These results indicate that D18 and its derivatives are promising polymer donors and random ternary copolymerization is a flexible and promising strategy for the preparation of high-performance OSCs for special applications.
4. Polythiophenes
Due to its simple chemical structure and facile synthesis, poly(3-hexylthiophene) (P3HT) has attracted extensive attention and shown great potential in commercial applications (Fig. 4).40–43 However, the PCEs of most P3HT-based OSCs remain low (∼7%) when using fullerene derivatives as acceptors because of their inferior absorption in the visible region.44 In 2020, Hou et al. synthesized a NFA ZY-4Cl with 5,6-dichloro-1,3-indanedione end groups to tune its energy levels and reduce its miscibility with P3HT. Consequently, the corresponding P3HT:ZY-4Cl cell demonstrated a record PCE of 9.46% for P3HT (Table 3). Meanwhile, the typical NFA BTP-4Cl with 5,6-difluoro-1,1-dicyanomethylene-3-indanone end groups only offered a poor PCE of 1.01%.42 Recently, Ye et al. improved the regioregularity of P3HT effectively by tuning the molar ratio of two ligands via direct arylation polycondensation and investigated the relationship among regioregularity and crystallinity, phase separation, and device performance. When blended with ZY-4Cl, the P3HT batch with an RR of 95% eventually exhibited a decent PCE of 10.82%, which was much higher than that of 9.24% from its counterpart with 90% RR.45 Simultaneously, Ye et al. synthesized a series of poly(3-alkylthiophene) ranging from butyl to octyl via direct arylation polymerization, and revealed that P3PT with pentyl side-chain is a promising donor with a higher PCE of 9.79% than that of P3HT in poly(3-alkylthiophene):ZY-4Cl cells.46| Donor | E optg [eV] | HOMO/LUMO [eV] | Acceptor | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| P3HT | 1.90 | −5.06/−2.76 | ZY-4Cl | 0.88 | 16.49 | 65 | 9.46 | 42 |
| P3HT | — | — | ZY-4Cl | 0.90 | 17.5 | 68.7 | 10.82 | 45 |
| P3PT | 1.90 | −5.06/−2.76 | ZY-4Cl | 0.91 | 15.2 | 70.8 | 9.79 | 46 |
| PDCBT | 1.90 | −5.26/−3.36 | ITIC | 0.94 | 16.50 | 65.67 | 10.16 | 48 |
| PDCBT-2F | 1.93 | −5.60/−3.67 | IT-4F | 0.92 | 17.8 | 69 | 11.6 | 49 |
| PDCBT-Cl | 1.91 | −5.34/−3.01 | ITIC-Th1 | 0.94 | 18.50 | 71.2 | 12.38 | 50 |
| PF2 | 1.82 | −5.37/−3.16 | m-ITIC:PC71BM | 0.86 | 18.52 | 77.6 | 12.40 | 51 |
| PTVT-T | 1.76 | −5.28/−3.02 | eC9 | 0.79 | 26.22 | 78 | 16.20 | 52 |
| PTVT-BT | 1.78 | −5.38/−3.02 | eC9 | 0.791 | 26.75 | 77.14 | 16.31 | 53 |
| P4T2F-HD | 1.95 | −5.35/−3.43 | Y6-BO | 0.72 | 24.39 | 75.30 | 13.65 | 54 |
| P5TCN-2F | 1.89 | −5.54/−3.65 | Y6 | 0.85 | 25.07 | 75.0 | 16.1 | 55 |
| P5TCN-F25 | 1.88 | −5.44/−3.56 | Y6 | 0.79 | 27.13 | 77.1 | 16.6 | 56 |
| P5TCN-F25 | — | — | Y6:PC71BM | 0.80 | 27.55 | 77.7 | 17.2 | 56 |
However, the photovoltaic performance of P3HT is restricted by its photovoltage and photocurrent, resulting from the high HOMO energy level (−5.06 eV), poor absorbance, and hyper-miscibility between P3HT and NFA. Accordingly, the introduction of electron-withdrawing groups, such as carboxylate, fluorine, and cyano groups, in polythiophenes (PTs) can be an effective strategy to reduce the HOMO energy level and tune the miscibility.47–50 In 2016, Hou et al. combined the ester-substituted polymer PDCBT with ITIC, and the corresponding fullerene-free OSCs exhibited an impressive PCE of 10.16% with enhanced Voc of 0.94 V. Meanwhile, the control P3HT:ITIC-based devices only showed a poor PCE of 1.25% with low Voc of 0.52 V due to the amorphous morphology for restricted exciton dissociation and charge transport.48 Then, the PCE was improved to 11.6% by adding fluorine to both PDCBT and ITIC simultaneously to obtain PDCBT-2F and IT-4F by the same group.49 In 2019, Geng et al. synthesized a series of halogen atom-substituted PDCBT and studied the effects of chlorination and fluorination on its photophysical and charge transport properties, molecular packing, and morphology. Consequently, the OSCs based on the monochloride-PTs PDCBT-Cl and ITIC-Th1 exhibited a decent PCE of 12.38% with a high Voc of 0.94 V, which was the highest value for polythiophene derivatives at that time.50 Lei et al. reported a facile polymer PF2 consisting of furan-3-carboxylate and 2,2′-bithiophene and the optimized PF2:m-ITIC:PC71BM cells yielded a much higher PCE of 12.40%, resulting from the larger conjugated plane, higher charge mobility, larger dielectric constant, and deeper electronic energy level than its all-thiophene counterpart PT-E (6.41%).51 In 2021, Hou et al. synthesized an ester group-substituted (E)-1,2-di(thiophen-2-yl)ethene (TVT) unit and combined it with thiophene to obtain a polythiophene derivative PTVT-T. This polymer exhibited a planar backbone structure, strong aggregation, excellent molecular packing and morphology in blend films, and therefore the cells based on PC71BM, IT-4F and eC9 acceptors afforded a promising photovoltaic performance of 7.25%, 11.28% and 16.20%, respectively.52 Recently, Hou et al. developed the low-cost polymer donor PTVT-BT by replacing the thiophene unit on the aforementioned PTVT-T with 2,2′-bithiophene and optimization of the side chains. PTVT-BT demonstrated strong intermolecular interaction in solution and an impressive high hole mobility (1 × 10−2 cm−2 V−1 s−1). Benefiting from its dominant face-on orientation, fiber-like nanostructures, lower non-radiative energy loss (Enon-radloss), and enhanced dissociation efficiency, the PTVT-BT:eC9 cells exhibited a remarkable PCE of 16.31%. Importantly, PTVT-BT also exhibited decent photovoltaic properties in indoor and tandem cells.53 These results indicate that these PTs based on TVT can be a promising donor for the future industrialization of OPV due to their low cost and promising photovoltaic performances.
Simultaneously, Yip et al. reported the preparation of the fluorinated polythiophene derivative P4T2F-HD, which exhibited moderate miscibility with the popular NFA Y6-BO and nanoscale phase separation was observed in the BHJ blend film. Consequently, the OSCs based on P4T2F-HD:Y6-BO achieved a decent PCE of 13.65%, whereas the P3HT-based cell offered a poor PCE of 0.38% due to the hyper-miscibility and completely mixed P3HT:Y6-BO blend film.54 Duan et al. introduced 3-cyanothiophene (CT) in the backbone of P4T2F-HD and the WBG polymer donor P5TCN-2F was synthesized. P5TCN-2F exhibited a deeper-lying HOMO energy level, achieving a higher Voc, due to its electron-deficient cyano group. Compared with P4T2F-HD, P5TCN-2F showed more ordered packing and enhanced crystallinity for a higher Jsc (Fig. 5a). Moreover, P5TCN-2F could work well in combination with many NFAs, and the OSCs based on P5TCN-2F:Y6 and P5TCN-2F:Y6-BO cells demonstrated promising PCEs of 16.1% and 15.8%, respectively (Fig. 5b). Moreover, these OSCs based on P5TCN-2F exhibited an average figure of merit (AFOM) of 3.54 and 3.60, respectively, indicating that this polymer is much more cost-effective than BDTT-based polymers (Fig. 5c).55 Recently, Duan et al. developed a series of terpolymer P5TCN-Fx based on 3-cyanothiophene, 3,3′-difluoro-2,2′-bithiophene and 2,2′-bithiophene. The effects of the degree of fluorination on interchain interaction, crystallinity and miscibility were studied comprehensively. Consequently, the binary P5TCN-F25:Y6 and ternary P5TCN-F25:Y6:PC71BM cells offered remarkable PCEs of 16.6% and 17.2%, respectively.56 These are the best photovoltaic performances for PTs, indicating the renaissance of PT-based OSCs with low production cost and excellent performance as high as D–A polymer donors.56,57
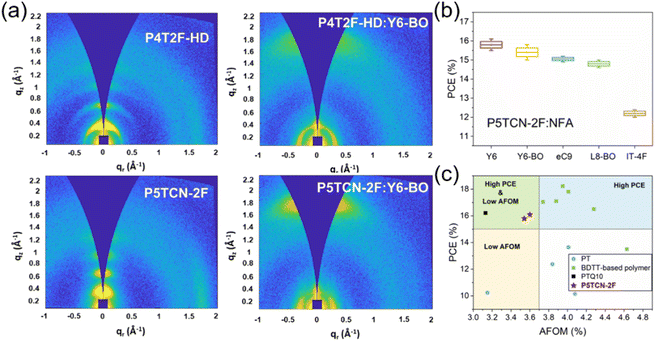 | ||
| Fig. 5 (a) 2D-GIWAXS pattern of the neat and blend films of P4T2F-HD and P5TCN-2F. (b) PCEs of the OSCs based on P5TCN-2F and different acceptors. (c) PCE versus AFOM for OSCs based on representative BDTT-based polymers, PTQ10, and PTs. Reproduced with permission.55 Copyright 2022, Wiley-VCH. | ||
5. Quinoxaline (Qx)-based polymers
Quinoxaline (Qx) and its derivatives are excellent building blocks for WBG D–A copolymers due to their weak electron-deficient ability, strong quinoid form, and facile modification on many positions (2, 3, 6, and 7 positions). Therefore, many Qx-based polymers have been developed to match with the traditional fullerene derivatives. Moreover, these polymers also exhibited promising photovoltaic properties in non-fullerene OSCs.58 In 2018, Li et al. reported the preparation of the low-cost polymer donor PTQ10 based on 6,7-difluoro-2-((2-hexyldecyl)oxy)quinoxaline and thiophene (Fig. 6). PTQ10 was synthesized via a two-step reaction from cheap raw materials with a high overall yield of 87.4% and exhibited great potential in commercial production. PTQ10:IDIC cells were fabricated and afforded a promising PCE of 12.70% and exhibited insensitivity of the active layer thickness for large-area devices (Table 4).59 Subsequently, some more efficient devices based on PTQ10 were obtained. Cui et al. and Peng et al. reported the preparation of ternary OSCs based on PTQ10:m-BTP-PhC6:PC71BM and PTQ10:BTP-FTh:IDIC with outstanding PCEs of 18.89% and 19.05%, respectively, after volatilizable solid additive-assisted treatment, indicating that PTQ10 is a promising polymer donor for the fabrication of low-cost and high-performance OSCs.60,61| Donor | E optg [eV] | HOMO/LUMO [eV] | Acceptor | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| PTQ10 | 1.92 | −5.54/−2.98 | IDIC | 0.969 | 17.81 | 73.60 | 12.70 | 59 |
| PTQ10 | — | — | m-BTP-PhC6:PC71BM | 0.869 | 26.99 | 80.6 | 18.89 | 60 |
| PTQ10 | — | — | BTP-FTh:IDIC | 0.870 | 27.17 | 80.6 | 19.05 | 61 |
| PTQ11 | 1.95 | −5.52/−2.76 | TPT10 | 0.88 | 24.79 | 74.8 | 16.32 | 13 |
| PBQ7 | 1.79 | −5.38/−3.14 | Y6 | 0.81 | 25.83 | 64.3 | 13.45 | 62 |
| PBQ10 | 1.92 | −5.47/−2.89 | Y6 | 0.85 | 25.77 | 74.6 | 16.34 | 62 |
| PQSi05 | 1.93 | −5.67/−3.74 | IT-4F | 0.911 | 21.42 | 69.48 | 13.56 | 63 |
| PBQ5 | 1.88 | −5.55/−2.91 | Y6 | 0.844 | 26.02 | 70.81 | 15.55 | 64 |
| PBQ6 | 1.71 | −5.64/−3.18 | Y6 | 0.851 | 26.58 | 77.91 | 17.62 | 64 |
| PBDTTS-2ClQx | 1.67 | −5.44/−3.77 | BTP-eC9 | 0.85 | 25.6 | 74.0 | 16.1 | 65 |
| PBQx-TCl | 2.05 | −5.39/−2.86 | BTP-eC9:BTA3 | 0.84 | 26.9 | 79.6 | 18.0 | 66 |
| PBQx-TF | 2.07 | −5.41/−2.82 | eC9-2Cl:F-BTA3 | 0.879 | 26.7 | 80.9 | 19.0 | 8 |
| PBDP-2Cl | 1.73 | −5.59/−3.86 | BTIC-BO-4Cl | 0.88 | 24.41 | 62.49 | 13.34 | 67 |
To enhance the molecular crystallization and hole mobility, Li et al. developed the WBG polymer PTQ11 derived from PTQ10 with a methyl substituent on its quinoxaline unit. When it was combined with the Y-series NFA TPT10 with the same HOMO energy level, the corresponding non-fullerene OSCs achieved a much higher PCE of 16.32% with Jsc of 24.79 mA cm−2 than that of the PTQ10:TPT10 cell (9.24% and 17.25 mA cm−2).13 In 2020, Li et al. reported the preparation of the PBQ10 polymer based on fluorinated BDTT and monoalkoxy-substituted difluoroquinoxaline (DFQ) unit, which exhibited a lower HOMO energy level, preferential face-on molecular orientation, better π–π stacking, and higher hole mobility and decent PCEs of 16.34%, whereas its counterpart PBQ7 based on dialkoxyphenyl-substituted difluoroquinoxaline just afforded an inferior PCE of 13.45%.62 In 2020, Chen et al. introduced 5 mol% combinatory side chain integrating siloxane terminal and alkoxy groups in PTQ10 to synthesize the terpolymer PQSi05, which showed enhanced aggregation, low film surface energy, weakened miscibility with IT-4F and good morphology. The resulting PQSi05:IT-4F cell afforded a notable PCE of 13.56% and was one of the most cost-effective.63 In 2021, Li et al. developed two DFQ-based polymer donors, PBQ5 and PBQ6, with fluorine-substituted BDTT. Compared with PBQ5 with two alkyl side chains on DFQ, PBQ6 substituted by 5-alkyl-4-fluorothiophene showed a red-shifted absorption, stronger intermolecular interaction, and higher hole mobility, resulting in a much better photovoltaic performance. When blended with Y6, PBQ6 exhibited a much higher PCE of 17.62% than that of the PBQ5:Y6 cells (15.55%) due to its better charge carrier mobilities, less charge carrier recombination, and better morphology, indicating that the side-chain engineering played an important role in the improved photovoltaic performance of the quinoxaline-based polymer donors.64 Recently, Liu et al. also employed side-chain engineering to develop the chlorinated copolymer PBDTTS-2ClQx, which achieved a decent PCE of 16.1% when blended with NFA BTP-eC9.65
Recently, Hou et al. fused Qx with two thiophenes to develop the fused-ring acceptor unit dithieno[3,2-f:2′,3′-h]quinoxaline (DTQx), which showed a similar structure to the excellent building block dithieno[3,2-f:2′,3′-h]benzodithiazole from the D18 series polymers.3,66PBQx-TCl was firstly synthesized by copolymerizing DTQx with chlorinated BDTT in good yield. Combined with the medium-bandgap acceptor BTA3 and LBG acceptor BTP-eC9, the ternary OSCs exhibited an outstanding PCE of 18.0% under AM 1.5 G illumination conditions. Under 1000 lux light-emitting diode (LED) illumination, these ternary OSCs offered a promising PCE of 28.5% and 26.0% for the 1 cm2 and 10 cm2 devices, respectively. Moreover, the device based on PBQx-TCl:BTA3 also delivered a decent OLED performance with an electroluminescence quantum efficiency (EQEEL) approaching 0.2%.66 Furthermore, PBQx-TF based on DTQx and fluorinated BDTT was developed by the same group and a remarkable PCE of 19.0% was achieved for the ternary PBQx-TF:eC9-2Cl:F-BTA3 cell, which was the record efficiency for single-junction OSCs at that time.8 Recently, He et al. reported the preparation of the chlorinated fused-ring acceptor unit dithieno[3,2-a:2′,3′-c]phenazine unit, where its copolymer PBDP-2Cl can be considered as a PBQx-TF derivative fused by dichlorobenzene. The PBDP-2Cl:BTIC-BO-4Cl cell exhibited a decent PCE of 13.34% due to its enhanced Voc and optimized morphology with uniform fiber network. In contrast, the analogues with one or without Cl atom yielded gradually reduced PCEs of 9.84% and 6.66%, respectively.67 These results indicate that Qx and its derivatives are among the best building blocks for the construction of various D–A conjugated polymers with promising photovoltaic performances.
6. Other polymers
Besides the above-mentioned efficient WBG polymers, many other high-performance D–A copolymers have been developed (Fig. 7). In the last few years, Ding et al. developed a series of lactam, lactone and thiolactone polymer donors, and their photovoltaic performance improved from 10% to 18%.68–72 Among them, L1 based on a 5H-dithieno[3,2-b:2′,3′-d]pyran-5-one unit and BDTT presented the wide bandgap of 1.96 eV and deep HOMO level of −5.45 eV for high Voc. As shown in Table 5, a decent PCE of 16.12% was achieved for the binary L1:BTP-eC9 cell, and this promising result inspired continuous work for the development of high-performance fused-ring aromatic lactone (FRAL)-based polymer donors.68,69 The first modified derivative was L2 based on a fluorinated BDTT donor unit and a high PCE of 16.69% was achieved by the L2:N3 cell.68,70 Subsequently, Ding et al. replaced the lactone of L1 with a thiolactone unit, i.e., 5H-dithieno[3,2-b:2′,3′-d]thiopyran-5-one (DTTP), to obtain the rare polymer D16 with enhanced π–π stacking and higher hole mobility. The best D16:Y6 cells afforded a PCE of 16.72% with a Voc of 0.85 V, Jsc of 26.61 mA cm−2 and FF of 73.8%, which were much higher than that of the L1:Y6 cell.71 Next, they synthesized a derivative L1-Svia simple post-sulphuration of L1, endowing the polymer with a deeper HOMO level, stronger light absorption, and higher hole mobility, resulting in the remarkable photovoltaic performance of 17.73% for the L1-S:BTP-eC9 cell (Fig. 8a).69 Moreover, Ding et al. employed excellent side chain engineering with D18 derivatives to optimize L1 and developed two cost-effective lactone polymers L3 and L4 by using fluorinated or chlorinated BDTT, respectively. These two polymers exhibited enhanced backbone planarity, improved hole mobility, optimized morphology with typical nanofibers, and superior photovoltaic performance of 17.81% or 18.10% for the corresponding ternary polymer:N3:PC61BM cells, respectively.70,72| Donor | E optg [eV] | HOMO/LUMO [eV] | Acceptor | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| L1 | 1.96 | −5.45/−2.79 | BTP-eC9 | 0.831 | 25.94 | 74.8 | 16.12 | 69 |
| L2 | 1.95 | −5.52/−2.91 | N3 | 0.870 | 24.99 | 76.8 | 16.69 | 70 |
| D16 | 1.95 | −5.48/−2.83 | Y6 | 0.85 | 26.61 | 73.8 | 16.72 | 71 |
| L1-S | 1.88 | −5.50/−2.94 | BTP-eC9 | 0.845 | 27.22 | 77.1 | 17.73 | 69 |
| L3 | 1.93 | −5.50/−2.92 | N3:PC61BM | 0.864 | 26.97 | 76.4 | 17.81 | 70 |
| L4 | 1.92 | −5.52/−2.95 | N3:PC61BM | 0.850 | 27.07 | 78.7 | 18.10 | 72 |
| P5T-2F | 1.79 | −5.51/−3.56 | eC9 | 0.837 | 26.5 | 79.8 | 17.7 | 73 |
| PBDTT1Cl | 2.03 | −5.47/−3.48 | Y18-1F | 0.87 | 27.70 | 71.35 | 17.10 | 74 |
| PTzBI-dF | 1.72 | −5.55/−3.83 | Y6:PC71BM | 0.85 | 26.42 | 76.64 | 17.26 | 76 |
| PNTB6-Cl | 1.98 | −5.47/−3.49 | N3 | 0.857 | 26.58 | 77.3 | 17.59 | 78 |
| PBCT-2F | 1.92 | −5.58/−3.66 | Y6 | 0.85 | 27.2 | 74.3 | 17.1 | 79 |
| PB2F:PM6 | 2.12 | −5.64/−3.52 | BTP-eC9 | 0.863 | 26.8 | 80.4 | 18.6 | 80 |
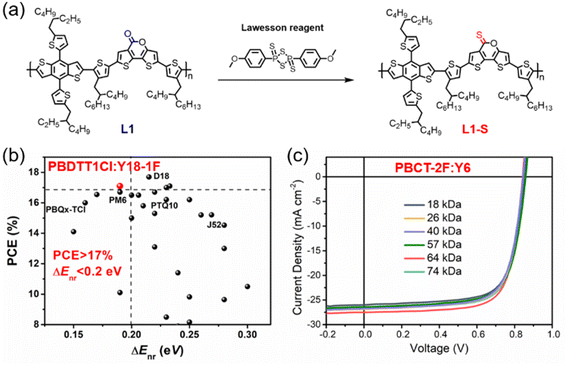 | ||
| Fig. 8 (a) Post-sulphuration of L1 to L1-S. Reproduced with permission.69 Copyright 2022, IOP Publishing. (b) PCE-ΔEnr data of PBDTT1Cl:Y18-1F cell and other efficient OPVs reported in recent years. Reproduced with permission.74 Copyright 2022, Wiley-VCH. (c) J–V characteristics of the PBCT-2F:Y6 solar cells with different PBCT-2F batches. Reproduced with permission.79 Copyright 2020, The Royal Society of Chemistry. | ||
In 2021, Hou et al. designed and synthesized P5T-2F based on fluorinated quaterthiophene and BDD unit to control its miscibility by tuning its electrostatic interactions. As expected, P5T-2F showed enhanced conformational stability, reduced electrostatic potentials (ESP), and appropriate miscibility, and thus the P5T-2F:eC9 cell afforded a much higher PCE of 17.7% than that of its non-fluorinated analogue.73 In 2022, Bo et al. reported the preparation of the regioregular polymer PBDTT1Cl based on BDTT and 3-chlorothiophene with suitable π–π stacking, high and balanced charge carrier mobilities, and efficient charge separation when blended with NFA Y18-1F. Consequently, the PBDTT1Cl:Y18-1F cell yielded a promising photovoltaic performance of 17.10%, with impressively low Enon-radloss of 0.19 eV (Fig. 8b). Moreover, PBDTT1Cl also exhibits good universality to match with many fullerene acceptors and NFAs and showed a low FOM value of 2.31, indicating its great potential in future industrial applications.74 Recently, Huang et al. reported a series of WBG polymers based on electron-deficient units [1,2,3]triazolo[4,5-f]isoindole-5,7(2H,6H)-dione (TzBI) and these polymers showed remarkable performances in non-fullerene OSCs, all-polymer solar cells, and photodetectors.75,76 Especially, PTzBI-dF with optimized fluorinated π-bridge exhibited planar molecular conformation, notably enhanced charge transport, preferential face-on orientation, and outstanding PCEs of 16.8% and 17.26% for the binary PTzBI-dF:Y6 cell and ternary PTzBI-dF:Y6:PC71BM cell, respectively.76 In 2021, Wu et al. reported a pentacyclic acceptor unit naphthalene-thiophene imide (NTI) and developed a series of D–A copolymers with low HOMO energy levels due to the electron-deficient imide group and aromatic five-membered ring of NTI.77 For example, PNTB6-Cl based on chlorinated BDTT and NTI with a linear hexyl chain showed poor solubility in chloroform due to its stronger intermolecular interactions and shorter π–π stacking distance. Consequently, the layer-by-layer (LBL) processed OSCs based on PNTB6-Cl:N3 exhibited an impressive PCE of 17.59% with excellent batch-to-batch reproducibility.78
In 2021, Duan et al. reported a facile polymer donor based on cyano-substituted terthiophene and fluorinated BDT, namely, PBCT-2F. PBCT-2F exhibited a WBG of 1.92 eV, ideal energy levels, proper aggregation, and high charge carrier mobilities, resulting in an excellent PCE of 17.1% for the PBCT-2F:Y6 cells. More importantly, these cells afforded notable PCEs of 15.9–17.1% even with the wide molecular weight range of PBCT-2F (18–74 kDa), indicating its good batch-to-batch reproducibility (Fig. 8c). These results endowed PBCT-2F with great potential in large-scale synthesis and commercial application.79 In 2021, Hou et al. employed the good building block 1,3,4-thiadiazole to construct the conjugated polymer PB2F with a very deep HOMO level of −5.64 eV, wide bandgap of 2.12 eV, and high EQEEL of 3.9 × 10−3 in neat film. The ternary OSCs based on PB2F:PBDB-TF:BTP-eC9 achieved an outstanding PCE of 18.6% due to the improved Voc, reduced Enon-radloss and ΔECT, and optimized morphology.80 These recent advances in other efficient polymers have also attracted much attention and promoted the development of organic photovoltaics.
7. Conclusion
Recently, the photovoltaic performance of OSCs has been greatly improved to over 19%, which is mainly due to the development of efficient donor/acceptor materials, improved device fabrication, optimized morphology control, and in-depth understanding of their working mechanism. In this review, we summarized and classified the recent high-performance WBG polymer donors as PBDB-T series polymers, D18 series polymers, polythiophenes, quinoxaline-based polymers, and other polymers. To date, it is clear that the best OSCs always employ terpolymers or ternary systems, which can tap the potential of the currently available polymers directly. These polymers play a vital role in improving the PCEs of OSCs, even in all-polymer cells, LBL cells, tandem cells, large-area cells and indoor cells.10,18,81–86However, some key issues need to be further studied for their commercialization. (1) Efficient polymers with both low cost and good batch-to-batch reproducibility are rare. As shown above, most efficient polymer use BDTT as the donor units and these building blocks require tedious synthetic routes and high cost for their mass production. Moreover, the batch-to-batch reproducibility of polymer donors is crucial for commercial devices.79,87 (2) Good stability, especially for photoactive materials and nanoscale morphology, is critical for commercial OSCs, which have to operate for long periods.88–91 Some studies on chemical structure modification and interfacial layers have been performed, but further research regarding long-term stability is still needed.92,93 (3) The correlation between Eloss and the molecular structures of the donors/acceptors needs to be well studied, which can further improve the photovoltaic performance without sacrificing the photocurrent and FF.94–97 Accordingly, we firmly believe that more excellent polymer donors that are well-matched with LBG NFAs will be developed through better understanding of their structure–property relationship and fine molecular structure optimization in the future. Combined with improved device fabrication and morphology optimization, over 20% efficiency will be achieved soon and the commercialization of organic photovoltaics is ongoing.
Author contributions
L. Ding and J. Cao designed the draft. J. Cao, L. Yi and L. Zhang contributed to the writing of the manuscript. J. Cao and L. Yi drew the figures. L. Ding and Y. Zou edited the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
J. Cao thanks the National Natural Science Foundation of China (21604021), Hunan Provincial Natural Science Foundation (2018JJ3141), Scientific Research Fund of Hunan Provincial Education Department (21B0447), and the Innovation Team of Huxiang High-level Talent Gathering Engineering (2021RC5028). Y. Zou thanks the National Natural Science Foundation of China (21875286). L. Ding thanks the open research fund of Songshan Lake Materials Laboratory (2021SLABFK02) and the National Natural Science Foundation of China (21961160720).References
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272–275 CrossRef CAS.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- W. Gao, F. Qi, Z. Peng, F. R. Lin, K. Jiang, C. Zhong, W. Kaminsky, Z. Guan, C.-S. Lee, T. J. Marks, H. Ade and A. K.-Y. Jen, Adv. Mater., 2022, 34, 2202089 CrossRef CAS PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, 2110147 CrossRef CAS PubMed.
- Y. Cui, Y. Xu, H. Yao, P. Bi, L. Hong, J. Zhang, Y. Zu, T. Zhang, J. Qin, J. Ren, Z. Chen, C. He, X. Hao, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2102420 CrossRef CAS PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- C. An, Z. Zheng and J. Hou, Chem. Commun., 2020, 56, 4750–4760 RSC.
- H. Yang, C. Cui and Y. Li, Acc. Mater. Res., 2021, 2, 986–997 CrossRef CAS.
- J. Cao, G. Nie, L. Zhang and L. Ding, J. Semicond., 2022, 43, 070201 CrossRef.
- C. Sun, S. Qin, R. Wang, S. Chen, F. Pan, B. Qiu, Z. Shang, L. Meng, C. Zhang, M. Xiao, C. Yang and Y. Li, J. Am. Chem. Soc., 2020, 142, 1465–1474 CrossRef CAS PubMed.
- D. Qian, L. Ye, M. Zhang, Y. Liang, L. Li, Y. Huang, X. Guo, S. Zhang, Z. Tan and J. Hou, Macromolecules, 2012, 45, 9611–9617 CrossRef CAS.
- C. An and J. Hou, Acc. Mater. Res., 2022, 3, 540–551 CrossRef CAS.
- H. Lu, W. Liu, H. Jin, H. Huang, Z. Tang and Z. Bo, Adv. Funct. Mater., 2022, 32, 2107756 CrossRef CAS.
- R. Ma, T. Liu, Z. Luo, Q. Guo, Y. Xiao, Y. Chen, X. Li, S. Luo, X. Lu, M. Zhang, Y. Li and H. Yan, Sci. China: Chem., 2020, 63, 325–330 CrossRef CAS.
- R. Sun, W. Wang, H. Yu, Z. Chen, X. Xia, H. Shen, J. Guo, M. Shi, Y. Zheng, Y. Wu, W. Yang, T. Wang, Q. Wu, Y. Yang, X. Lu, J. Xia, C. J. Brabec, H. Yan, Y. Li and J. Min, Joule, 2021, 5, 1548–1565 CrossRef CAS.
- H. Bin, T. P. A. van der Pol, J. Li, B. T. van Gorkom, M. M. Wienk and R. A. J. Janssen, Chem. Eng. J., 2022, 435, 134878 CrossRef CAS.
- Q. Guo, J. Lin, X. Dong, L. Zhu, X. Guo, F. Liu and M. Zhang, Chem. Eng. J., 2022, 431, 134117 CrossRef CAS.
- W. Peng, Y. Lin, S. Y. Jeong, Z. Genene, A. Magomedov, H. Y. Woo, C. Chen, W. Wahyudi, Q. Tao, J. Deng, Y. Han, V. Getautis, W. Zhu, T. D. Anthopoulos and E. Wang, Nano Energy, 2022, 92, 106681 CrossRef CAS.
- L. Zhou, L. Meng, J. Zhang, C. Zhu, S. Qin, I. Angunawela, Y. Wan, H. Ade and Y. Li, Adv. Funct. Mater., 2022, 32, 2109271 CrossRef CAS.
- J. Wu, X. Guo, M. Xiong, X. Xia, Q. Li, J. Fang, X. Yan, Q. Liu, X. Lu, E. Wang, D. Yu and M. Zhang, Chem. Eng. J., 2022, 446, 137424 CrossRef CAS.
- J. Wu, G. Li, J. Fang, X. Guo, L. Zhu, B. Guo, Y. Wang, G. Zhang, L. Arunagiri, F. Liu, H. Yan, M. Zhang and Y. Li, Nat. Commun., 2020, 11, 4612 CrossRef CAS PubMed.
- J. Qin, L. Zhang, Z. Xiao, S. Chen, K. Sun, Z. Zang, C. Yi, Y. Yuan, Z. Jin, F. Hao, Y. Cheng, Q. Bao and L. Ding, Sci. Bull., 2020, 65, 1979–1982 CrossRef CAS.
- C. Xu, K. Jin, Z. Xiao, Z. Zhao, X. Ma, X. Wang, J. Li, W. Xu, S. Zhang, L. Ding and F. Zhang, Adv. Funct. Mater., 2021, 31, 2107934 CrossRef CAS.
- X. Wang, H. Lu, Y. Liu, A. Zhang, N. Yu, H. Wang, S. Li, Y. Zhou, X. Xu, Z. Tang and Z. Bo, Adv. Energy Mater., 2021, 11, 2102591 CrossRef CAS.
- Y. Zhou, M. Li, H. Lu, H. Jin, X. Wang, Y. Zhang, S. Shen, Z. Ma, J. Song and Z. Bo, Adv. Funct. Mater., 2021, 31, 2101742 CrossRef CAS.
- J. Qin, L. Zhang, C. Zuo, Z. Xiao, Y. Yuan, S. Yang, F. Hao, M. Cheng, K. Sun, Q. Bao, Z. Bin, Z. Jin and L. Ding, J. Semicond., 2021, 42, 010501 CrossRef CAS.
- K. Jin, Z. Xiao and L. Ding, J. Semicond., 2021, 42, 060502 CrossRef.
- J. Qin, Q. Yang, J. Oh, S. Chen, G. O. Odunmbaku, N. A. N. Ouedraogo, C. Yang, K. Sun and S. Lu, Adv. Sci., 2022, 9, 2105347 CrossRef CAS PubMed.
- G. Cai, Z. Chen, X. Xia, Y. Li, J. Wang, H. Liu, P. Sun, C. Li, R. Ma, Y. Zhou, W. Chi, J. Zhang, H. Zhu, J. Xu, H. Yan, X. Zhan and X. Lu, Adv. Sci., 2022, 9, 2200578 CrossRef CAS PubMed.
- X. Meng, K. Jin, Z. Xiao and L. Ding, J. Semicond., 2021, 42, 100501 CrossRef.
- A. Sun, J. Xu, G. Zong, Z. Xiao, Y. Hua, B. Zhang and L. Ding, J. Semicond., 2021, 42, 100502 CrossRef CAS.
- X. Li, J. Xu, Z. Xiao, X. Wang, B. Zhang and L. Ding, J. Semicond., 2021, 42, 060501 CrossRef CAS.
- J. Zhang, C.-H. Tan, K. Zhang, T. Jia, Y. Cui, W. Deng, X. Liao, H. Wu, Q. Xu, F. Huang and Y. Cao, Adv. Energy Mater., 2021, 11, 2102559 CrossRef CAS.
- B. Zheng, J. Ni, S. Li, Y. Yue, J. Wang, J. Zhang, Y. Li and L. Huo, Adv. Sci., 2022, 9, 2105430 CrossRef CAS PubMed.
- Z. Liao, D. Hu, H. Tang, P. Huang, R. Singh, S. Chung, K. Cho, M. Kumar, L. Hou, Q. Chen, W. Yu, H. Chen, K. Yang, Z. Kan, F. Liu, Z. Xiao, G. Li and S. Lu, J. Mater. Chem. A, 2022, 10, 7878–7887 RSC.
- H. Lu, H. Wang, G. Ran, S. Li, J. Zhang, Y. Liu, W. Zhang, X. Xu and Z. Bo, Adv. Funct. Mater., 2022, 32, 2203193 CrossRef CAS.
- Q. Wang, Y. Qin, M. Li, L. Ye and Y. Geng, Adv. Energy Mater., 2020, 10, 2002572 CrossRef CAS.
- S. Chatterjee, S. Jinnai and Y. Ie, J. Mater. Chem. A, 2021, 9, 18857–18886 RSC.
- C. Yang, S. Zhang, J. Ren, M. Gao, P. Bi, L. Ye and J. Hou, Energy Environ. Sci., 2020, 13, 2864–2869 RSC.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114–2121 RSC.
- X. Guo, C. Cui, M. Zhang, L. Huo, Y. Huang, J. Hou and Y. Li, Energy Environ. Sci., 2012, 5, 7943–7949 RSC.
- Y. Liu, K. Xian, X. Zhang, M. Gao, Y. Shi, K. Zhou, Y. Deng, J. Hou, Y. Geng and L. Ye, Macromolecules, 2022, 55, 3078–3086 CrossRef CAS.
- Y. Liu, K. Xian, R. Gui, K. Zhou, J. Liu, M. Gao, W. Zhao, X. Jiao, Y. Deng, H. Yin, Y. Geng and L. Ye, Macromolecules, 2022, 55, 133–145 CrossRef CAS.
- Q. Wang, M. Li, Z. Peng, N. Kirby, Y. Deng, L. Ye and Y. Geng, Sci. China: Chem., 2021, 64, 478–487 CrossRef CAS.
- Y. Qin, M. A. Uddin, Y. Chen, B. Jang, K. Zhao, Z. Zheng, R. Yu, T. J. Shin, H. Y. Woo and J. Hou, Adv. Mater., 2016, 28, 9416–9422 CrossRef CAS PubMed.
- H. Yao, D. Qian, H. Zhang, Y. Qin, B. Xu, Y. Cui, R. Yu, F. Gao and J. Hou, Chin. J. Chem., 2018, 36, 491–494 CrossRef CAS.
- Q. Wang, M. Li, X. Zhang, Y. Qin, J. Wang, J. Zhang, J. Hou, R. A. J. Janssen and Y. Geng, Macromolecules, 2019, 52, 4464–4474 CrossRef CAS.
- Y. Gao, M. Cui, S. Qu, H. Zhao, Z. Shen, F. Tan, Y. Dong, C. Qin, Z. Wang, W. Zhang, Z. Wang and Y. Lei, Small, 2022, 18, 2104623 CrossRef CAS PubMed.
- J. Ren, P. Bi, J. Zhang, J. Liu, J. Wang, Y. Xu, Z. Wei, S. Zhang and J. Hou, Natl. Sci. Rev., 2021, 8, nwab031 CrossRef CAS PubMed.
- P. Bi, J. Ren, S. Zhang, J. Wang, Z. Chen, M. Gao, Y. Cui, T. Zhang, J. Qin, Z. Zheng, L. Ye, X. Hao and J. Hou, Nano Energy, 2022, 100, 107463 CrossRef CAS.
- J. Xiao, X. Jia, C. Duan, F. Huang, H.-L. Yip and Y. Cao, Adv. Mater., 2021, 33, 2008158 CrossRef CAS PubMed.
- X. Yuan, Y. Zhao, Y. Zhang, D. Xie, W. Deng, J. Li, H. Wu, C. Duan, F. Huang and Y. Cao, Adv. Funct. Mater., 2022, 32, 2201142 CrossRef CAS.
- X. Yuan, Y. Zhao, D. Xie, L. Pan, X. Liu, C. Duan, F. Huang and Y. Cao, Joule, 2022, 6, 647–661 CrossRef CAS.
- K. Xian, Y. Geng and L. Ye, Joule, 2022, 6, 941–944 CrossRef CAS.
- C. Sun, C. Zhu, L. Meng and Y. Li, Adv. Mater., 2022, 34, 2104161 CrossRef CAS PubMed.
- C. Sun, F. Pan, H. Bin, J. Zhang, L. Xue, B. Qiu, Z. Wei, Z.-G. Zhang and Y. Li, Nat. Commun., 2018, 9, 743 CrossRef PubMed.
- S. Bao, H. Yang, H. Fan, J. Zhang, Z. Wei, C. Cui and Y. Li, Adv. Mater., 2021, 33, 2105301 CrossRef CAS PubMed.
- K. Chong, X. Xu, H. Meng, J. Xue, L. Yu, W. Ma and Q. Peng, Adv. Mater., 2022, 34, 2109516 CrossRef CAS PubMed.
- C. Sun, F. Pan, B. Qiu, S. Qin, S. Chen, Z. Shang, L. Meng, C. Yang and Y. Li, Chem. Mater., 2020, 32, 3254–3261 CrossRef CAS.
- D. Yuan, F. Pan, L. Zhang, H. Jiang, M. Chen, W. Tang, G. Qin, Y. Cao and J. Chen, Sol. RRL, 2020, 4, 2000062 CrossRef CAS.
- C. Zhu, L. Meng, J. Zhang, S. Qin, W. Lai, B. Qiu, J. Yuan, Y. Wan, W. Huang and Y. Li, Adv. Mater., 2021, 33, 2100474 CrossRef CAS PubMed.
- Q. Zhang, X. Song, R. Singh, S. Chung, Z. Zhou, Y. Lu, B. Zhang, K. Cho, W. Zhu and Y. Liu, Chem. Eng. J., 2022, 437, 135182 CrossRef CAS.
- Y. Xu, Y. Cui, H. Yao, T. Zhang, J. Zhang, L. Ma, J. Wang, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2101090 CrossRef CAS PubMed.
- M. Pu, C. Cao, H. Chen, Y. Zhu, P. Tan, X. Lai and F. He, Chem. Eng. J., 2022, 437, 135198 CrossRef CAS.
- J. Liu, L. Liu, C. Zuo, Z. Xiao, Y. Zou, Z. Jin and L. Ding, Sci. Bull., 2019, 64, 1655–1657 CrossRef CAS.
- Y. Jiang, K. Jin, X. Chen, Z. Xiao, X. Zhang and L. Ding, J. Semicond., 2021, 42, 070501 CrossRef CAS.
- Z. Ou, J. Qin, K. Jin, J. Zhang, L. Zhang, C. Yi, Z. Jin, Q. Song, K. Sun, J. Yang, Z. Xiao and L. Ding, J. Mater. Chem. A, 2022, 10, 3314–3320 RSC.
- J. Xiong, K. Jin, Y. Jiang, J. Qin, T. Wang, J. Liu, Q. Liu, H. Peng, X. Li, A. Sun, X. Meng, L. Zhang, L. Liu, W. Li, Z. Fang, X. Jia, Z. Xiao, Y. Feng, X. Zhang, K. Sun, S. Yang, S. Shi and L. Ding, Sci. Bull., 2019, 64, 1573–1576 CrossRef CAS.
- K. Jin, Z. Ou, L. Zhang, Y. Yuan, Z. Xiao, Q. Song, C. Yi and L. Ding, J. Semicond., 2022, 43, 050501 CrossRef.
- L. Ma, S. Zhang, J. Wang, J. Ren, M. Gao, J. Zhang, T. Zhang, H. Yao, L. Ye and J. Hou, ACS Mater. Lett., 2021, 3, 1276–1283 CrossRef CAS.
- H. Wang, H. Lu, Y.-N. Chen, G. Ran, A. Zhang, D. Li, N. Yu, Z. Zhang, Y. Liu, X. Xu, W. Zhang, Q. Bao, Z. Tang and Z. Bo, Adv. Mater., 2022, 34, 2105483 CrossRef CAS PubMed.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746–752 CrossRef CAS.
- B. Fan, M. Li, D. Zhang, W. Zhong, L. Ying, Z. Zeng, K. An, Z. Huang, L. Shi, G. C. Bazan, F. Huang and Y. Cao, ACS Energy Lett., 2020, 5, 2087–2094 CrossRef CAS.
- H. Ning, G. Zhang, H. Chen, Z. Wang, S. Ni, F. Lu, F. Liu, L. Dang, J. Liu, F. He and Q. Wu, Chem. Mater., 2021, 33, 1976–1982 CrossRef CAS.
- H. Ning, Q. Jiang, P. Han, M. Lin, G. Zhang, J. Chen, H. Chen, S. Zeng, J. Gao, J. Liu, F. He and Q. Wu, Energy Environ. Sci., 2021, 14, 5919–5928 RSC.
- X. Yuan, Y. Zhao, T. Zhan, J. Oh, J. Zhou, J. Li, X. Wang, Z. Wang, S. Pang, P. Cai, C. Yang, Z. He, Z. Xie, C. Duan, F. Huang and Y. Cao, Energy Environ. Sci., 2021, 14, 5530–5540 RSC.
- T. Zhang, C. An, P. Bi, Q. Lv, J. Qin, L. Hong, Y. Cui, S. Zhang and J. Hou, Adv. Energy Mater., 2021, 11, 2101705 CrossRef CAS.
- B. Wu, B. Yin, C. Duan and L. Ding, J. Semicond., 2021, 42, 080301 CrossRef CAS.
- J. Chen, J. Cao, L. Liu, L. Xie, H. Zhou, J. Zhang, K. Zhang, M. Xiao and F. Huang, Adv. Funct. Mater., 2022, 32, 2200629 CrossRef CAS.
- X. Du, N. Li and L. Ding, J. Semicond., 2021, 42, 110201 CrossRef CAS.
- M. Li, J. Wang, L. Ding and X. Du, J. Semicond., 2022, 43, 060201 CrossRef.
- E. Feng, Y. Han, J. Chang, H. Li, K. Huang, L. Zhang, Q. Luo, J. Zhang, C. Ma, Y. Zou, L. Ding and J. Yang, J. Semicond., 2022, 43, 100501 CrossRef.
- W. Pan, Y. Han, Z. Wang, Q. Luo, C. Ma and L. Ding, J. Semicond., 2021, 42, 050301 CrossRef CAS.
- C. Duan and L. Ding, Sci. Bull., 2020, 65, 1422–1424 CrossRef CAS.
- W. Yang, W. Wang, Y. Wang, R. Sun, J. Guo, H. Li, M. Shi, J. Guo, Y. Wu, T. Wang, G. Lu, C. J. Brabec, Y. Li and J. Min, Joule, 2021, 5, 1209–1230 CrossRef.
- X. Du, T. Heumueller, W. Gruber, A. Classen, T. Unruh, N. Li and C. J. Brabec, Joule, 2019, 3, 215–226 CrossRef CAS.
- Y. Liu, J. Song and Z. Bo, Chem. Commun., 2021, 57, 302–314 RSC.
- J. Song and Z. Bo, Sci. China: Chem., 2019, 62, 9–13 CrossRef CAS.
- Q. Liao, Q. Kang, Y. Yang, Z. Zheng, J. Qin, B. Xu and J. Hou, CCS Chem., 2022, 4, 938–948 CrossRef CAS.
- W. Liu, J. Yuan, C. Zhu, Q. Wei, S. Liang, H. Zhang, G. Zheng, Y. Hu, L. Meng, F. Gao, Y. Li and Y. Zou, Sci. China: Chem., 2022, 65, 1374–1382 CrossRef CAS.
- L. Xie, Y. Zhang, W. Zhuang, S. Y. Jeong, Q. Bian, H. Li, J. Cao, W. Liu, H. Tan, H. Y. Woo, J. Zhang and E. Wang, Chem. Eng. J., 2022, 427, 131674 CrossRef CAS.
- J. Yuan, C. Zhang, H. Chen, C. Zhu, S. H. Cheung, B. Qiu, F. Cai, Q. Wei, W. Liu, H. Yin, R. Zhang, J. Zhang, Y. Liu, H. Zhang, W. Liu, H. Peng, J. Yang, L. Meng, F. Gao, S. So, Y. Li and Y. Zou, Sci. China: Chem., 2020, 63, 1159–1168 CrossRef CAS.
- Z. Tang and L. Ding, J. Semicond., 2023, 44, 010202 Search PubMed.
- J. Yuan, H. Zhang, R. Zhang, Y. Wang, J. Hou, M. Leclerc, X. Zhan, F. Huang, F. Gao, Y. Zou and Y. Li, Chem, 2020, 6, 2147–2161 CAS.
| This journal is © The Royal Society of Chemistry 2023 |