Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Determination of layered nickel hydroxide phases in materials disordered by stacking faults and interstratification†
Kurt
Lawson
 a,
Samuel P.
Wallbridge
a,
Samuel P.
Wallbridge
 a,
Amy E.
Catling
a,
Amy E.
Catling
 a,
Caroline A.
Kirk
b and
Sandra E.
Dann
*a
a,
Caroline A.
Kirk
b and
Sandra E.
Dann
*a
aDepartment of Chemistry, Loughborough University, Loughborough, UK. E-mail: S.E.Dann@lboro.ac.uk
bSchool of Chemistry, University of Edinburgh, Edinburgh, UK
First published on 6th December 2022
Abstract
The formation of stacking faults and phase interstratification disorder in layered nickel(II) hydroxides during the chemical precipitation synthesis of materials using nickel(II) nitrate and potassium hydroxide solutions has been investigated in the temperature range of 5 °C to 95 °C and time intervals from 1 hour to 1 week. Stacking faulted materials were identified by broadening of the 00l reflections, while interstratified materials were identified through the splitting of the 001 into two lines. In contrast to the disorder concepts presented in previous studies of these materials, this work has shown through vibrational spectroscopy that both the alpha-phase and beta-phase hydroxides are present in materials described with stacking fault disorder, while layered hydroxysalts were additionally present in the materials considered to be interstratified. Standard mixtures of Ni3(OH)4(NO3)2 and β-Ni(OH)2 were prepared to investigate if the intensity of particular vibrational bands could be correlated with the proportion of the particular phases in mixtures. The intensities of the C2v nitrate infrared and Raman bands at 990 cm−1 and 1315 cm−1 were shown to correlate with the amount of layered hydroxynitrate incorporated in the phase, theoretically providing a method to determine the components in mixed compositions. Since disorder and phase impurities in layered nickel hydroxide materials affect both their electroactive stability and performance as cathode materials, this work has important implications in several research fields.
Introduction
Electroactive layered nickel hydroxides have applications as the cathode material of rechargeable batteries,1 supercapacitors,2–5 electrochromic devices,6 and electrocatalysts.7 Two structures are commonly described for nickel hydroxide, a well-defined crystalline beta-phase and a poorly crystalline alpha-phase; the structure of the latter is not fully understood.8,9 The beta-phase of nickel hydroxide, β-Ni(OH)2, exists naturally as the emerald green coloured mineral theophrastite10 and is isostructural with Mg(OH)2 (brucite) and Ca(OH)2 (portlandite), and crystallises with the cadmium iodide (CdI2) type structure.11–13 The layered structure consists of a hexagonal close-packed (hcp) arrangement of the oxide anions with the nickel(II) cations on the octahedrally coordinated sites and the hydrogen of the hydroxide on the tetrahedral sites directed towards the adjacent layer.11,14 Alpha-phase nickel hydroxide, α-Ni(OH)2, is also a layered structure with the same edge-sharing octahedral layers as β-Ni(OH)2.15 Due to the relatively weak interactions between the layers, species can be intercalated between the layers resulting in an elongation along the c-direction of the unit cell. This also causes the layers to slip out of alignment and randomly orient relative to one another which can result in a structure often described as turbostratic.16,17 The crystal structures of β-Ni(OH)2 and α-Ni(OH)2 are shown in Fig. 1 (ref. 18 and 19) with ideal layer stacking given to α-Ni(OH)2 to highlight the different distances between adjacent octahedral layers that allow for water and anionic species to intercalate.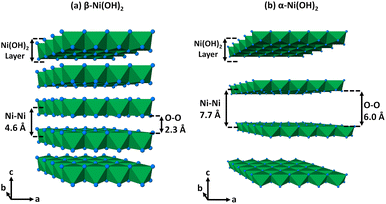 | ||
Fig. 1 Crystal structures drawn of (a) β-Ni(OH)2 and (b) α-Ni(OH)2 with ideal layer stacking to highlight the difference between the interlayer spacing adapted after data of the minerals theophrastite and jamborite respectively.18,19 Nickel(II) cations form coordination octahedra (green) with the oxygen of the hydroxide groups (blue spheres). β-Ni(OH)2 crystallises in the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 space group with dimensions of a, b = 3.11 Å and c = 4.62 Å with α, β = 90° and γ = 120° while ideal α-Ni(OH)2 crystallises in the R m1 space group with dimensions of a, b = 3.11 Å and c = 4.62 Å with α, β = 90° and γ = 120° while ideal α-Ni(OH)2 crystallises in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group with dimensions of a, b = 3.07 Å and c = 23.2 Å with α, β = 90° and γ = 120°. m space group with dimensions of a, b = 3.07 Å and c = 23.2 Å with α, β = 90° and γ = 120°. | ||
In addition, there are also disordered structures reported for layered nickel hydroxides including those with stacking faults and phase interstratification disorder as shown in the diagrams in Fig. 2. Stacking faults typically result in disorder of the crystallographic planes in the material. This phenomenon is encountered in layered metal hydroxides due to strong intralayer bonding and relatively weak interactions between them, typically causing misalignment of the layers in the c-direction. Beta-phase nickel hydroxides with stacking fault disorder are sometimes simply described as being ‘badly crystalline’ in the literature.9,17 Interstratified structures have been said to occur when the beta-phase and alpha-phase layer hydroxide materials are stacked in the c-direction of the unit cell within a single crystallite.
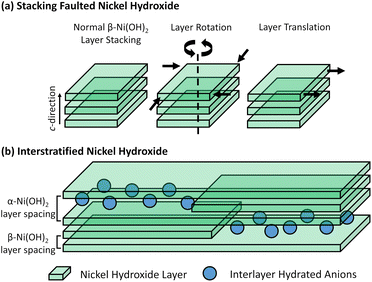 | ||
| Fig. 2 (a) Diagram of stacking fault disorder in adjacent beta-phase nickel hydroxide layers (left) including rotation about the c-axis (centre) and translation in the ab-plane (right).34 (b) Diagram of the phase interstratification that occurs within a single crystal of nickel hydroxide highlighting regions of beta-phase as well as alpha-phase nickel hydroxide with intercalating species.40 | ||
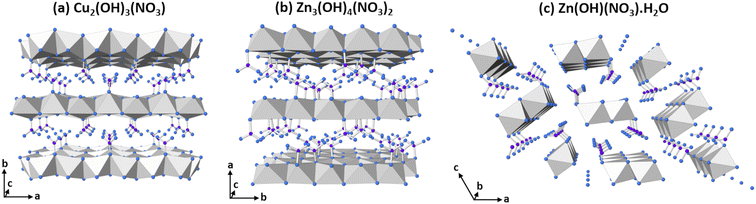
The study of disordered nickel(II) hydroxides is often challenging due to the poorly crystalline nature of these materials, thus limiting the utility of powder diffraction methods, and the determination of the phases present in a mixed-phase material can be difficult. The hypothetical structure and concomitant conducting properties of the alpha-phase hydroxide, with formula Ni(OH)2−x(H2O)x(An−)x/n, results from the movement of mobile protons in the partially protonated hydroxide layer leading to very low levels of weakly coordinated anions (ca. x = 0.1) between the layers20 to counteract the charge from the itinerant protons. Other phases of the form M(OH)2−x(An−)x/n that are derived from the parent brucite structure also exist, but with some of the hydroxides partially substituted for other anionic species that coordinate directly to the metal cations. Layered nickel hydroxynitrates are formed when the hydroxyl anions are substituted for nitrate and the three different compositions previously described are Ni2(OH)3(NO3) where x = 0.5,21 Ni3(OH)4(NO3)2 where x = 0.67,22 and Ni(OH)(NO3) where x = 1.0.23 While the structures of the nickel-containing analogues have not yet been determined, diffraction and compositional evidence in the literature suggest that these phases are isostructural with analogous layered metal hydroxynitrates with the same x value. The structures of Cu2(OH)3(NO3) where x = 0.5,24 Zn3(OH)4(NO3)2 where x = 0.67,25 and Zn(OH)(NO3)·H2O where x = 1.0 (ref. 26) are shown in Fig. 3. Alpha-phase hydroxides differ from layered hydroxysalt structures because the anion incorporation and subsequent hydration are variable and often lower, with these anions not bound to the cations. While alpha-phases also share similarities with hydrotalcite-type layered double hydroxide structures, they also differ from them by containing no trivalent cations.20
Due to the growing interest in the use of nickel oxides and hydroxides for electrochemical applications, the purity of the hydroxide phases is important and can affect the performance of compounds prepared from them, e.g. LiNiO2 for use in lithium-based batteries.27 Therefore, methods to distinguish between different hydroxide phases and estimate the amount of an impurity phase are of interest. Since the nickel hydroxide that is formed by the chemical precipitation method is dependent on the conditions, herein we investigate the nickel hydroxide phases and disorder formed from hydrated nickel(II) nitrate and base as a function of synthesis temperature and time. Material analysis using powder X-ray diffraction methods shows features in the data that are typically expected of stacking fault and phase interstratification behaviour with local structure synchrotron techniques additionally offering no further information about the structures formed. Analysis performed using infrared and Raman spectroscopy provided new insight into the phases present in these materials and show that mixed-phase samples may be easily mistaken for disorder in the materials. This emphasises the need to employ extra materials characterisation methods in addition to diffraction techniques for layered hydroxides that can successfully identify the presence of these poorly crystalline phases in a material.
Experimental section
Nickel hydroxide preparations
Nickel hydroxide phases were prepared as powders (ca. 1 g) by the homogenous precipitation synthesis method1 where aqueous potassium hydroxide (86%, Fischer Chemical) solutions (25 mL, 0.25 M or 0.50 M) were added slowly to aqueous nickel(II) nitrate (99%, Acros Organics) solutions (25 mL, 0.25 M). The desired synthesis temperatures were reached using a temperature-controlled refrigerator at 5 °C or an oven for temperatures at 50 °C and 95 °C. The precipitate that formed was collected by gravity filtration and was washed with water (ca. 250 mL) until the filtrate was neutral. The solid green product was dried at ambient temperature for 48 h.The nickel hydroxynitrate phase Ni3(OH)4(NO3)2 was prepared as a powder (ca. 5 g) by the thermolysis method described by Biswick et al.28 involving heating nickel(II) nitrate hexahydrate (ca. 5 g) in an oven at 220 °C for 2 h with stirring every 15 min. The solid product was cooled, washed with anhydrous ethanol (ca. 500 mL) then dried at 120 °C for 48 h.
Instrumentation
Powder X-ray diffraction (XRD) patterns were collected using a Bruker D8 Advance powder diffractometer with monochromatic Cu Kα1 radiation (λ = 1.5406 Å). The data were collected using a quartz standard calibrated Lynxeye detector over the 5–55° 2θ range with a step size of 0.014° 2θ. The International Centre for Diffraction Data (ICDD) powder diffraction file (PDF) database was used for phase identification.Synchrotron X-ray pair distribution function (XPDF) data were collected on the I15-1 beamline at Diamond Light Source, Didcot, UK. The powder samples (ca. 1–10 mg) were loaded into quartz capillaries with a diameter of 1.5 mm (internal radii 0.65 mm). Diffraction data were collected using a wavelength of 0.161669 Å that was filtered to 10% flux (12.5% actual) using aluminium plates at ambient temperature over 300 s. The scattering data (1.0 ≤ Q ≤ 30.0 Å−1) were processed into pair distribution function data using the GudrunX (2017) software.
Infrared (IR) absorption spectra were collected between 4000–250 cm−1 using a PerkinElmer Spectrum 100 FT-IR Spectrometer fitted with caesium iodide (CsI) optics. Potassium bromide (KBr) discs were initially used to record the spectra following previous literature methods but the KBr medium was abandoned and replaced with CsI. This was because KBr reacts with layered hydroxide phases,21,29 resulting in nitrate bands at ∼1380 cm−1 in all of the materials analysed regardless of the nitrate anion symmetry present.
Raman (R) data were collected between 4000–100 cm−1 using a Horiba Jobin Yvon HR LabRAM system in the backscatter configuration with a laser line at 633 nm originating from an argon ion (Ar+) laser filtered to 10% power so as not to heat and decompose the sample. The laser was focused onto the sample to a spot size of 1 μm.
Thermogravimetric analysis (TGA) measurements were carried out using a TA instruments SDT Q600 with sample masses of between 10–20 mg and a reference of alumina (Al2O3). A temperature ramp method was used with a heating rate of 5 °C min−1 and a gas flow of 100 mL min−1. Nickel hydroxide samples were heated under nitrogen to form nickel(II) oxide decomposition products.
Results and discussion
Identification of disordered nickel hydroxide materials
The nickel hydroxide synthesis temperature, chemical ageing duration and reagent concentrations were based on preliminary studies (Tables S1 and S2†). Initially, five temperature intervals (5 °C, 25 °C, 50 °C, 75 °C and 95 °C) and six chemical ageing durations (1 h, 6 h, 1 d, 2 d, 4 d, 1 w) were investigated. Powder X-ray diffraction analysis of these thirty materials indicated crystalline beta-phases were typically formed at both higher temperatures and longer ageing times, while poorly crystalline materials were formed at lower temperatures and shorter ageing durations. Several mixed phase materials were also formed at intermediate temperatures and ageing durations. Therefore, the conditions selected were based on the preliminary experiments summarised in Table S1† in order to ensure that the required material was reproducibly isolated. It was also found that changing the ratio of nickel(II) cations to hydroxide anions in solution so that the base would be in excess no longer had a significant effect on the disorder formation. At both a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, only one type of disordered nickel hydroxide material was formed regardless of the temperature and ageing duration conditions used.
4 ratio, only one type of disordered nickel hydroxide material was formed regardless of the temperature and ageing duration conditions used.
Six nickel hydroxide materials were targeted (Table 1) using three sets of temperature and ageing duration, each prepared using two potassium hydroxide solution concentrations to give a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
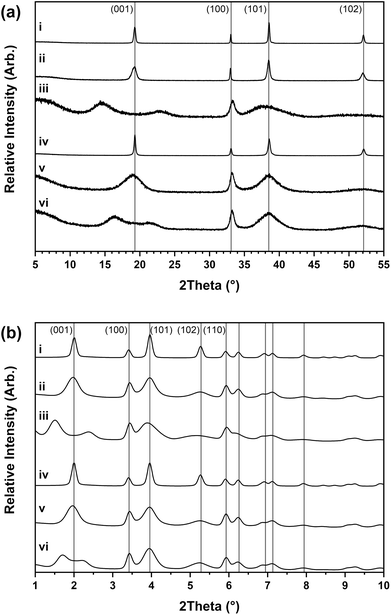
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. Three distinct material types were identified through the powder X-ray diffraction analysis shown in Fig. 4a. The first material type formed at 95 °C over 1 week had narrow reflections that matched with the crystalline beta-phase of nickel hydroxide (β-Ni(OH)2) as theophrastite (ICDD PDF 14-117).18
2 ratio. Three distinct material types were identified through the powder X-ray diffraction analysis shown in Fig. 4a. The first material type formed at 95 °C over 1 week had narrow reflections that matched with the crystalline beta-phase of nickel hydroxide (β-Ni(OH)2) as theophrastite (ICDD PDF 14-117).18
| Material assigned | Description | Synthesis conditions | Ni2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OH− ratio OH− ratio |
|
|---|---|---|---|---|
| i | β-Ni(OH)2 | Beta-phase nickel hydroxide | 95 °C, 1 w | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| ii | βSF-Ni(OH)2 | Stacking faulted nickel hydroxide | 50 °C, 2 d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| iii | βIS-Ni(OH)2 | Interstratified nickel hydroxide | 5 °C, 1 w | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| iv | β-Ni(OH)2 | Beta-phase nickel hydroxide | 95 °C, 1 w | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| v | βSF-Ni(OH)2 | Stacking faulted nickel hydroxide | 50 °C, 2 d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| Vi | βIS-Ni(OH)2 | Interstratified nickel hydroxide | 5 °C, 1 w | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
The second type that formed at 50 °C over 2 days had the same reflections as β-Ni(OH)2 but with anisotropic peak widths where the 001, 101 and 201 are broader than the 100 which indicates beta-phase nickel hydroxide with stacking fault disorder (βSF-Ni(OH)2). The origin of this disorder is much more complex than might initially be expected from the description.30,31 First Barnard32 and then Tessier33 described two structural dislocations which could lead to this unusual broadening of powder X-ray diffraction patterns. These dislocations are referred to as ‘growth faults’ and ‘deformation faults’ which typically occur because of rapid or low-temperature crystal growth. Broadening of the 00l reflections is commonly observed in the powder X-ray diffraction pattern of layered hydroxide materials since this group of reflections relates to disorder in the c-direction.33–35 Additionally, hk0 broadening is also observed if the crystallites are small and there is good agreement between the size of the crystals with the broadening of both 00l and hk0 reflections, determined through the Debye–Scherrer formula and direct domain size determination by electron microscopy.34 However, abnormal broadening can also be observed for the 10l and 20l (where l is not zero) which cannot be explained by particle size arguments alone.
The third material type formed at 5 °C over 1 week also had the broadened reflections of β-Ni(OH)2 but with an apparent splitting of the 001 reflection, identified previously as characteristic of interstratification disorder (βIS-Ni(OH)2).26,27 Structures with interstratified phases are described for clay minerals where there is a mix of two or more types of layers, e.g. dioctahedral and trioctahedral clays that are stacked in the c-direction of the unit cell, producing a wide range of mixed-layer clay phases, e.g. kaolinite-smectite and talc-smectite.36,37 These interstratified phases produce powder X-ray diffraction patterns which are composites of the two types of layer, but often with much broadening in the observed reflections since long-range order in the c-direction is lost. Interstratification of the nickel hydroxides has been suggested to occur in the same way as clays, consisting of layers of both the beta-phase and the alpha-phase with interlayer species intermixed.38,39 In contrast to other interstratified phases that are typically formed of two crystalline phases, the turbostratic alpha-phase of nickel hydroxide often has no long-range ordering to the structure. Therefore the diffraction patterns generated by interstratified nickel hydroxides are different to a simple mixture of the two independent crystalline phases and the reflections relating to the c-direction can be shifted, broadened or absent.40 For example, in the powder X-ray diffraction patterns collected by Kamath38,39 both the expected first reflection of the ordered alpha-phase (∼7–10 Å) and beta-phase (∼4.6 Å) are missing.
Fig. 4b shows high-resolution powder X-ray diffraction data collected for these materials using a synchrotron radiation source and the reflections observed for the β-Ni(OH)2 and βSF-Ni(OH)2 materials match beta-phase nickel hydroxide but with differences in the crystallinity. The diffraction patterns of the βIS-Ni(OH)2 materials have broad and split 001 reflections with the maxima observed being different, with Fig. 4b(iii) having d-spacing values at ∼6.12 Å and ∼3.90 Å while in Fig. 4b(vi) at ∼5.45 Å and ∼4.16 Å. These values are also different from the interlayer d-spacing values expected in β-Ni(OH)2 which is 4.60 Å and α-Ni(OH)2 which is approximately between 7–10 Å.11 This suggests these values are not derived from the interlayer distances of the parent phases similar to those usually observed in interstratified clay materials, implying the disorder in layered hydroxides is different. Since clays form in the earth over long periods through weathering, the layer arrangement can be more ordered with larger domains in the c-direction of a phase, making it possible to see reflections characteristic of the stacking sequences of the individual phases. In contrast, these nickel hydroxide formation experiments were carried out over short periods and crystals that formed rapidly are likely to be significantly smaller and have a less ordered structure.
Synchrotron X-ray pair distribution function (XPDF) analysis
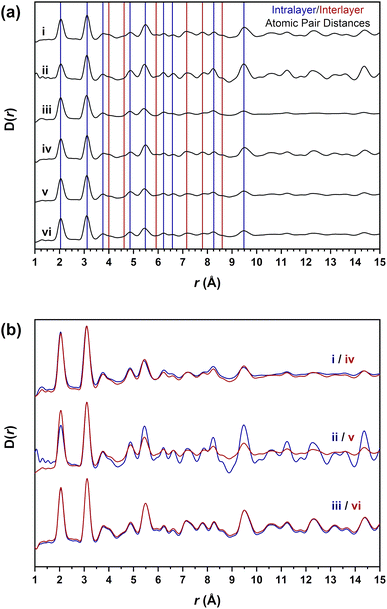
The X-ray pair distribution function D(r) data in Fig. 5a were obtained from the collected synchrotron diffraction data to observe the local structure bonding environments present in the materials. The atomic pair distances observed are assigned in Table 2 to the expected atom pair distances extracted from the crystal structure of beta-phase nickel hydroxide (Fig. S1†). The intralayer values represent the distances between the atom pairs within a single nickel hydroxide layer formed by the metal-hydroxide octahedra. These distances are present in both the beta-phase and alpha-phases of nickel hydroxide and would be expected to be observed in all the materials. Interlayer distances will also be apparent due to the atom pairs across adjacent nickel hydroxide octahedral layers. These distances will only be present in the beta-phase but not the alpha-phase and changes or absences of these distances will indicate differences in the short-range layer stacking.| Atomic pair assignment | XPDF atom pair distance (Å) | ||||||
|---|---|---|---|---|---|---|---|
| Intralayer atom pair | Distance (Å) | i | ii | iii | iv | v | vi |
| Ni–O | 2.13 | 2.04 | 2.06 | 2.06 | 2.06 | 2.06 | 2.06 |
| Ni–Ni/O–O | 3.11 | 3.12 | 3.10 | 3.10 | 3.12 | 3.10 | 3.10 |
| Ni–O | 3.77 | 3.76 | 3.76 | 3.76 | 3.76 | 3.76 | 3.76 |
| Ni–O | 4.88 | 4.86 | 4.88 | 4.86 | 4.88 | 4.88 | 4.86 |
| Ni–Ni/O–O | 5.39 | 5.48 | 5.44 | 5.42 | 5.50 | 5.44 | 5.44 |
| Ni–Ni/O–O | 6.22 | 6.22 | 6.24 | 6.24 | 6.24 | 6.22 | 6.22 |
| O–O | 6.88 | 6.58 | 6.62 | 6.58 | 6.62 | 6.62 | 6.62 |
| Ni–Ni/O–O | 8.24 | 8.26 | 8.26 | 8.24 | 8.26 | 8.26 | 8.26 |
| Ni–Ni/O–O | 9.34 | 9.48 | 9.46 | 9.48 | 9.50 | 9.48 | 9.50 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Interlayer atom pair | Distance (Å) | i | ii | iii | iv | v | vi |
| Ni–O | 3.90 | 4.00 | 4.06 | — | 4.04 | 4.06 | — |
| Ni–Ni/O–O | 4.62 | 4.62 | — | — | 4.60 | — | — |
| Ni–O | 5.88 | 5.92 | 5.96 | — | 5.92 | — | — |
| O–O | 7.15 | 7.16 | 7.18 | 7.22 | 7.14 | 7.18 | 7.20 |
| Ni–Ni/O–O | 7.75 | 7.80 | 7.80 | 7.84 | 7.82 | 7.84 | 7.82 |
| O–O | 8.40 | 8.60 | 8.58 | — | 8.62 | 8.62 | — |
The datasets in Fig. 5b are a direct overlay of the same β-Ni(OH)2, βSF-Ni(OH)2 and βIS-Ni(OH)2 material types with both intralayer and interlayer distances in all of the materials assigned. These data show there is not a significant difference in the atomic pair distances present, with only the βSF-Ni(OH)2 materials having different relative peak intensities which are more intense in Fig. 5b(ii) and (v). While all of the intralayer distances up to 10 Å are accounted for across all materials, several interlayer distances present in the β-Ni(OH)2 materials are absent in the disordered βSF-Ni(OH)2 and βIS-Ni(OH)2 examples. This suggests that the broadening observed in the diffraction data is due only to the long-range layer stacking disorder occurring along the c-direction of the unit cell. There is no change to the local short-range structure between these materials, indicating that regardless of the disorder, individual layers of octahedra are formed in all the materials studied. Therefore, it is not possible to establish any differences in the structures of these materials through these datasets and alternative methods are required.
Spectroscopic analysis of disordered nickel hydroxide materials
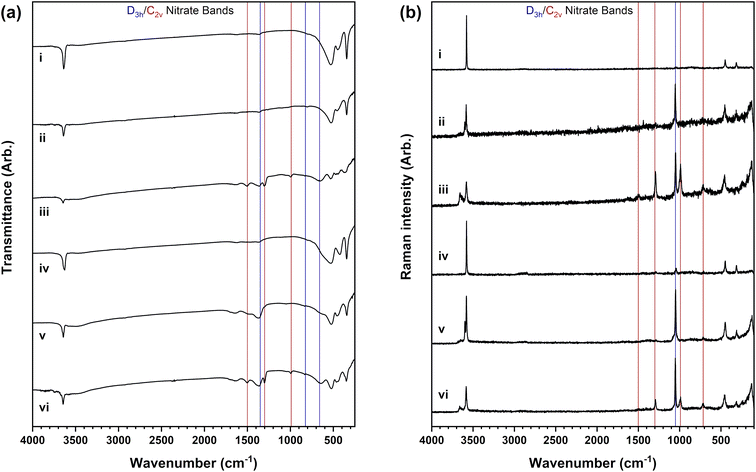
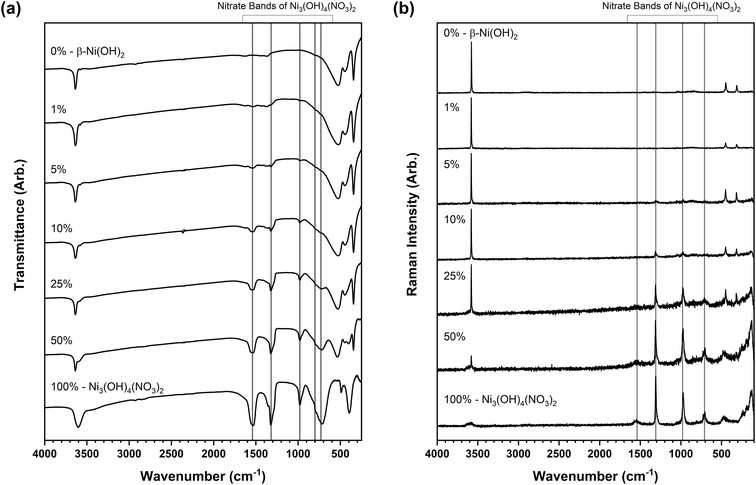
Spectroscopic analysis affords information on the bonding present in the different nickel hydroxide materials and importantly reveals the incorporation of nitrate anions from the nickel(II) salt reagent used in most of the samples analysed. Differences are observed in the infrared spectra in Fig. 6a and Raman spectra in Fig. 6b of the β-Ni(OH)2, βSF-Ni(OH)2 and βIS-Ni(OH)2 material types. Crystalline β-Ni(OH)2 materials have a highly ordered structure with no nitrate anions detected as observed in Fig. 6a(i) and b(i). The infrared spectrum in Fig. 6a(iv) shows no nitrate anions are detected but the Raman spectrum in Fig. 6b(iv) suggests a small amount to be present from a phase impurity. Differently, intense bands of nitrate are present in the disordered βSF-Ni(OH)2 and βIS-Ni(OH)2 types. The assignment of these bands listed in Table 3 allows for two nitrate anion symmetries to be identified which indicates there are different layered hydroxide structures present.| Infrared band assignment | Ref. value | i | ii | iii | iv | v | vi | |
|---|---|---|---|---|---|---|---|---|
| Hydroxide stretch | ν(O–H) | 3570–3650 | 3638 | 3641 | 3647 | 3627 | 3643 | 3645 |
| Hydroxide bend | δ(O–H) | 510–553 | 542 | 531 | 532 | 532 | 529 | 533 |
| Lattice modes | δ(Ni–O) | 350–475 | 450 | 455 | 437 | 418 | 456 | 465 |
| Hydroxide bend | γ(O–H) | 340–354 | 345 | 345 | 348 | 345 | 344 | 349 |
| Water stretch | ν(O–H) | ∼3570–3650 | — | — | ∼3500 | — | ∼3500 | ∼3500 |
| Water bend | δ(O–H) | 1600–1650 | — | — | 1650 | — | 1647 | 1651 |
| D 3h nitrate stretch (E′) | ν as(NO3) | 1350 | — | 1364 | 1378 | — | 1363 | 1368 |
| D 3h nitrate bend (A′′2) | γ(NO3) | 830 | — | 831 | 838 | — | 834 | 833 |
| C 2v nitrate stretch (B2) | ν s(NO2) | 1400–1500 | — | — | 1514 | — | — | 1500 |
| C 2v nitrate stretch (A1) | ν as(NO) | 1290 | — | — | 1301 | — | — | 1303 |
| C 2v nitrate stretch (A1) | ν s(NO) | 1000 | — | — | 995 | — | — | 994 |
| C 2v nitrate bend (B2) | δ(NO3) | 715 | — | — | 715 | — | — | 712 |
| Raman band assignment | Ref. value | i | ii | iii | iv | v | vi | |
|---|---|---|---|---|---|---|---|---|
| Hydroxide stretch | ν(O–H) | 3580–3600 | 3580 | 3601, 3584 | 3659, 3580 | 3580 | 3600, 3580 | 3662, 3583 |
| Hydroxide bend | γ(O–H) | 306–319 | 315 | 315 | 315 | 314 | 311 | 313 |
| Lattice modes | δ(Ni–O) | 445–450 | 450 | 453 | 455 | 449 | 448 | 455 |
| D 3h nitrate stretch (A′1) | ν s(NO3) | 1050 | — | 1052 | 1050 | 1047 | 1049 | 1052 |
| C 2v nitrate stretch (B2) | ν s(NO2) | 1400–1500 | — | — | 1498 | — | — | 1503 |
| C 2v nitrate stretch (A1) | ν as(NO) | 1290 | — | — | 1294 | — | — | 1293 |
| C 2v nitrate stretch (A1) | ν s(NO) | 1000 | — | — | 988 | — | — | 989 |
| C 2v nitrate bend (B2) | δ(NO3) | 715 | — | — | 714 | — | — | 718 |
The nitrate in the βSF-Ni(OH)2 materials is present in a D3h symmetry which has four vibrational modes that are A′1, A′′2, and 2E′ (ν1 to ν4) with ν1 being infrared inactive and ν2 being Raman inactive.41–44 This anion symmetry is established in these materials by the infrared inactive nitrate symmetric stretch at ∼1000 cm−1 which is absent in the infrared spectra but present in the Raman spectra. Moreover, the infrared spectra also have bands of water present including the broad O–H stretch at ∼3500 cm−1 and the O–H bend at ∼1650 cm−1. Therefore, it can be predicted that the nitrate anions are hydrated with trigonal geometry and are incorporated between the layers similar to the anions present in layered double hydroxide structures. This suggests that the alpha-phase hydroxide is present and implies that materials described as βSF-Ni(OH)2 by powder diffraction are instead a mixture, or possibly an interstratification, between the beta-phase and alpha-phase nickel hydroxides.
The βSF-Ni(OH)2 types also have an intense Raman O–H stretch at ∼3600 cm−1 in addition to the band at ∼3580 cm−1 present in the β-Ni(OH)2 materials. This has previously been identified as a unique feature of layer stacking faulted beta-phase nickel hydroxide.45 The intensity of the band was shown to increase as the layer stacking in the c-direction became more disordered and was only observed to coexist with the parent beta-phase nickel hydroxide. In the case where both layered hydroxide phases are present, it can be expected that the differences in the crystallinity between the beta-phase and alpha-phase of nickel hydroxide would result in non-ideal layer stacking similar to what is described as simple layer disordering.
Nitrate anions in a D3h symmetry are present in the βIS-Ni(OH)2 materials but there are also additional bands of the C2v symmetry of nitrate observed. This nitrate symmetry is observed in layered metal hydroxynitrate structures where one N–O bond is in a different environment from the other two because the nitrate anions are covalently coordinated to the nickel(II) cations through the oxygen atoms (Fig. S2†).42 This behaviour can be identified through the six vibrational modes of the C2v symmetry that arise due to the splitting of the two doubly degenerate (E′) modes of D3h symmetry and are all infrared and Raman active.41–44 Not all six bands expected for C2v nitrate are observed, however, the symmetric N–O stretch that is infrared inactive for the D3h symmetry is observed in both the infrared and Raman spectra at ∼1000 cm−1. The change in the infrared activity of this band between the alpha-phase hydroxide and hydroxynitrate phases allows for a diagnostic method of determining if hydroxynitrate phases are present in mixed-phase materials.
The position of the symmetric N–O stretching band can also be used to identify the nickel hydroxynitrate phase present by comparison to spectroscopic data collected on the individual nickel hydroxynitrate phases (Fig. S3†). Three nickel hydroxynitrate phases were targeted by different synthesis methods, namely Ni2(OH)3(NO3) (where x = 0.5) by Petrov precipitation,46 Ni3(OH)4(NO3)2 (where x = 0.67) by nickel(II) nitrate thermolysis28 and Ni(OH)(NO3)·H2O (where x = 1.0) by mechanochemical methods.47 The band positions in Fig. 6b(iii) and (vi) correlate to those of Ni3(OH)4(NO3)2 (where x = 0.67) that are observed at 984 cm−1 in the infrared and 974 cm−1 in the Raman data. This was the phase determined because the values of Ni(OH)(NO3)·H2O (where x = 1.0) are higher at ∼1050 cm−1 while Ni2(OH)3(NO3) (where x = 0.5) could not be prepared and instead a mixed-phase product was formed. The presence of nitrate in a C2v symmetry determined in the βIS-Ni(OH)2 materials suggests phase interstratification disorder is not a simple mixture or an interstratification between only the beta-phase and alpha-phase hydroxides as previously reported by Kamath39 because there are multiple disordered phases present. While the nitrate in C2v symmetry could be observed due to any remaining nickel(II) nitrate starting reagent, it is not detected as an impurity phase by powder diffraction analysis. The Raman band position of the nitrate symmetric N–O stretch is also observed to be higher in the nickel(II) nitrate reagent at 1057 cm−1 (Fig. S4†) than any of the layered hydroxide phases. In addition, if the interstratification was solely between alpha-phase and beta-phase nickel hydroxides, the nitrate would only be present as loosely coordinated anions between the layers with D3h symmetry and the covalently bonded nitrate anions in C2v symmetry would not be observed. This suggests that the materials described with interstratification disorder can be considered an extension of the βSF-Ni(OH)2 materials that have both the alpha-phase and beta-phase hydroxides, but with the nickel hydroxynitrate phase Ni3(OH)4(NO3) additionally present.
These findings suggest that the previous concepts of stacking faulted and interstratified disorder in nickel hydroxide materials are more complex than the simple structural disorder concepts they are named after. The broadening of the beta-phase nickel hydroxide reflections typically attributed to stacking faults is instead likely a result of an interstratification of the beta-phase and alpha-phases hydroxides. Similarly, materials with split beta-phase nickel hydroxide 001 reflections attributed to the phase interstratified disorder are also instead multi-phase with both beta-phase and alpha-phase nickel hydroxides as well as nickel hydroxynitrate phases present. It is proposed that these are more plausible as interstratification of multiple phases with the beta-phase and alpha-phase hydroxides intergrown. This is in contrast to a simple mixture of the phases which would have reflections corresponding to the individual phases that would indicate regions of ordered layering in the c-direction of the unit cell.
Nickel hydroxynitrate identification in mixed-phase materials
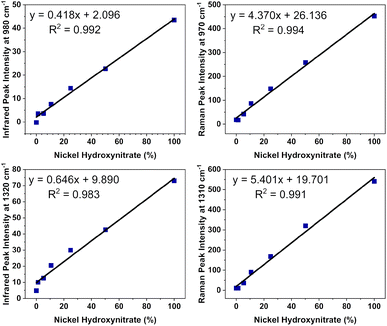
The ability to identify impurity phases is crucial from a practical application standpoint for developing phase-pure materials with predictable electroactive properties. The detection of layered hydroxynitrate phases in these materials is exceedingly difficult by powder X-ray diffraction analysis which is unsuitable as the sole characterisation method for these materials. Features in the infrared and Raman spectroscopy datasets, particularly the different bonding and vibrational band positions of the nitrate anions, offer conclusive identities of the layered hydroxide and hydroxynitrate phases present. However, this ability is limited only to a few polyatomic anions that display the differences in symmetry required such as nitrates and carbonates. This makes it difficult to identify phases where for example nickel(II) halide starting reagents are used. To examine whether small amounts of a nickel hydroxynitrate phase could be detected and quantified by spectroscopic analysis in disordered materials, standard mixtures were prepared of β-Ni(OH)2 and Ni3(OH)4(NO3)2 at different proportions. These phases were selected as they were identified in the disordered materials and were used to form mixtures at five intervals between 1 and 50 wt% with the two pure phases forming 0 and 100 wt%. Powder X-ray diffraction data were also collected on these standard mixture samples (Fig. S5†) and the intensities of the individual phase reflections were found to also increase as the wt% increased. However, as the diffraction patterns of the disordered phases do not have the specific reflections of the phases identified (Fig. 4), a similar analysis approach could not be performed.Fig. 7a and b show the infrared and Raman spectra respectively of the standard mixture samples prepared where the band intensity of the C2v symmetry nitrate increased with increased wt%. Calibration plots were prepared from the datasets using the intensities of the infrared and Raman band which were background corrected using an average intensity between 1150–1200 cm−1, a region where there are no vibrational modes expected for the hydroxide or hydroxynitrate phases. Fig. 8 shows regression analysis gave a good fit of the calibration plots, indicating linear correlations between the amount of C2v nitrate present and the peak intensities of the infrared bands at 980 and 1320 cm−1 as well as the Raman bands at 970 cm−1 and 1310 cm−1. These plots were used to estimate the amount of Ni3(OH)4(NO3)2 present in the i–vi nickel hydroxide materials and the results are listed in Table 4.
| Nickel hydroxide material | Assignment by powder X-ray diffraction | Phase(s) identified by infrared and Raman spectroscopy | Wt% of nickel hydroxynitrate (infrared data) | Wt% of nickel hydroxynitrate (Raman data) | TGA weight loss (%) | Additional TGA weight loss from β-Ni(OH)2 |
|---|---|---|---|---|---|---|
| i | β-Ni(OH)2 | β-Ni(OH)2 | 0.6 (±6.1) | <0.1 (±1.5) | 20.2 | +0.8 |
| ii | βSF-Ni(OH)2 | β-Ni(OH)2 and α-Ni(OH)2 | 5.0 (±6.4) | 0.9 (±0.5) | 21.6 | +2.2 |
| iii | βIS-Ni(OH)2 | β-Ni(OH)2, α-Ni(OH)2, and Ni3(OH)4(NO3)2 | 33.1 (±32.4) | 58.4 (±26.1) | 31.7 | +12.3 |
| iv | β-Ni(OH)2 | β-Ni(OH)2 | 6.3 (±2.0) | <0.1 (±3.7) | 20.3 | +0.9 |
| v | βSF-Ni(OH)2 | β-Ni(OH)2 and α-Ni(OH)2 | 11.4 (±19.4) | 2.4 (±0.5) | 26.5 | +7.1 |
| vi | βIS-Ni(OH)2 | β-Ni(OH)2, α-Ni(OH)2, and Ni3(OH)4(NO3)2 | 35.0 (±36.6) | 37.1 (±15.3) | 29.2 | +9.8 |
Through this analysis, it was determined that the β-Ni(OH)2 and βSF-Ni(OH)2 type materials contained small quantities of the layered hydroxynitrate phase, varying somewhat by technique at ∼10 wt% by infrared but only ∼5 wt% by Raman. In contrast, considerable amounts of layered hydroxynitrate were present in the βIS-Ni(OH)2 type materials, determined to be in the approximate ranges of 30 to 35 wt% by infrared and 35 to 60 wt% by Raman. The variation between the values obtained by infrared and Raman as well as the observed errors on each value result from several sources, including inhomogeneity of the samples, variations in instrument resolution and band width, and significant differences in background intensity leading to more difficult background subtraction. Comparing the two, the Raman datasets have lower backgrounds and more narrow bands and these data are likely to be more reliable. There is also a difference in the samples analysed, where the standard mixtures were physical mixtures of crystallites of the individual phases whereas the disordered materials are proposed to be an interstratification of the nickel hydroxide phases. Despite this, however, the analysis shows that the interstratified phases have significant amounts of the impurity hydroxynitrate present.
The phases identified in the six nickel hydroxide materials are supported by the total weight losses listed in Table 4 determined by TG analysis and additional weight losses in the profile (Fig. S6†). The difference in the values obtained to the 19.4% theoretical weight loss of beta-phase nickel hydroxide are also listed and are within 1% for the β-Ni(OH)2 type materials. The weight loss values are slightly higher for the βSF-Ni(OH)2 materials which would be expected from the hydrated nitrate anions present in alpha-phase nickel hydroxide (Fig. S7†). If these materials were solely only stacking faulted beta-phase nickel hydroxide, the weight loss value should match the expected value for β-Ni(OH)2. The βIS-Ni(OH)2 type materials have significantly higher weight losses than the β-Ni(OH)2 and βSF-Ni(OH)2 types, being approximately at the midpoint between the values of β-Ni(OH)2 at 19.4% and Ni3(OH)4(NO3)2 at 39.1%. Furthermore, there is a weight loss visible between 250 and 400 °C that is characteristic of the Ni3(OH)4(NO3)2 phase (Fig. S7†) as well as the low temperature loss indicative of coordinated water in the alpha phase. This is consistent with the wt% of the hydroxynitrate phases determined by Raman spectroscopy, but because there are three phases identified and significant overlap between alpha phase and hydroxynitrate phase weight losses, quantification of the individual phases is not possible.
This analysis further highlights the complexity of studying poorly crystalline nickel hydroxides and emphasises that assigning layered hydroxide phases based on the diffraction patterns alone is not sufficient. Spectroscopic techniques together with thermogravimetric analysis can be used, not only to identify the different phases in the poorly crystalline and disordered nickel hydroxide materials but to approximate their relative proportions. This was possible due to nitrate anions being present from the nickel(II) nitrate starting reagents used during synthesis that were incorporated with different symmetries into the layered structures. The approach described here would be universally applicable to all layered metal hydroxide materials, including layered hydroxysalts and layered double hydroxide where polyatomic anions are present.48–50
Conclusions
Beta-phase nickel hydroxide materials with stacking fault and phase interstratification disorder can be targeted through the careful control of time and temperature during chemical precipitation synthesis. X-ray diffraction techniques including synchrotron X-ray pair distribution function analyses were unable to distinguish between different materials, and instead, only indicated long-range disorder with no change to the short-range intralayer and interlayer atomic pair distances. Characterisation using infrared and Raman spectroscopy identified different nitrate anion geometries present in the disordered materials, with the D3h symmetry species present in the stacking faulted materials and both D3h and C2v symmetries present in the phase interstratified materials. This indicates the materials considered to be disordered are multi-phase materials with the indication that alpha-phase nickel hydroxide and nickel hydroxynitrate phases are formed. The amount of nickel hydroxynitrate in the interstratified βIS-Ni(OH)2 materials was also estimated using standard mixtures prepared between β-Ni(OH)2 and Ni3(OH)4(NO3)2, being present at approximately 30 to 60 wt%. This was not detectable by powder X-ray diffraction methods meaning it is insufficient to characterise layered hydroxide materials by these methods alone.Author contributions
Kurt Lawson: methodology, investigation and writing – original draft. Sam Wallbridge: methodology, investigation and writing – original draft. Amy Catling: methodology and investigation. Caroline Kirk: review and editing, supervision, project administration and funding acquisition Sandra Dann: conceptualization, resources, writing – original draft, review and editing, supervision, project administration and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the ESPRC/STFC for funding for KL and the University for the DTP/EPSRC for the Studentship for SW. SCI are also thanked for their award of the Sydney Andrew Scholarship for SW.References
- J. McBreen, Handbook of Battery Materials, VCH, 1997 Search PubMed.
- H. Cheng, A. D. Su, S. Li, S. T. Nguyen, L. Lu, C. Y. H. Lim and H. M. Duong, Chem. Phys. Lett., 2014, 601, 168–173 CrossRef CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, J. Solid State Electrochem., 2014, 18, 1569–1584 CrossRef CAS.
- T. Brezesinski, J. Wang, S. H. Tolbert and B. Dunn, Nat. Mater., 2010, 9, 146–151 CrossRef CAS PubMed.
- X. Yi, H. Sun, N. Robertson and C. Kirk, Sustainable Energy Fuels, 2021, 5, 5236–5246 RSC.
- R. J. Mortimer, M. Z. Sialvi, T. S. Varley and G. D. Wilcox, J. Solid State Electrochem., 2014, 18, 3359–3367 CrossRef CAS.
- Y. Chen, K. Rui, J. Zhu, S. X. Dou and W. Sun, Chem.–Eur. J., 2019, 25, 703–713 CrossRef CAS PubMed.
- H. Bode, K. Dehmelt and J. Witte, Electrochim. Acta, 1966, 11, 1079–1087 CrossRef CAS.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Proc. R. Soc. A, 2015, 471, 20140792 CrossRef PubMed.
- T. Marcopoulos and M. Economou, Am. Mineral., 1981, 66, 1020–1021 CAS.
- R. S. McEwen, J. Phys. Chem., 1971, 75, 1782–1789 CrossRef CAS.
- A. Szytula, A. Murasik and M. Balanda, Phys. Status Solidi, 1971, 43, 125–128 CrossRef CAS.
- C. Greaves and M. A. Thomas, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1986, 42, 51–55 CrossRef.
- M. Mookherjee and L. Stixrude, Am. Mineral., 2006, 91, 127–134 CrossRef CAS.
- P. Oliva, J. Leonardi, J. F. Laurent, C. Delmas, J. J. Braconnier, M. Figlarz, F. Fievet and A. d. Guibert, J. Power Sources, 1982, 8, 229–255 CrossRef CAS.
- A. Livingstone and D. L. Bish, Mineral. Mag., 1982, 46, 1–5 CrossRef CAS.
- C. Faure, C. Delmas and M. Fouassier, J. Power Sources, 1991, 35, 279–290 CrossRef CAS.
- R. W. Cairns and E. Ott, J. Am. Chem. Soc., 1933, 55, 527–533 CrossRef CAS.
- O. Glemser and J. Einerhand, Z. Anorg. Chem, 1950, 261, 43–51 CrossRef CAS.
- P. V. Kamath, G. H. Annal Therese and J. Gopalakrishnan, J. Solid State Chem., 1997, 128, 38–41 CrossRef CAS.
- K. Petrov, N. Zotov, E. Mirtcheva, O. García-Martínez and R. M. Rojas, J. Mater. Chem., 1994, 4, 611–614 RSC.
- P. Gallezot and M. Prettre, Bull. Soc. Chim., 1969, 2, 407–409 Search PubMed.
- M. Louër, D. Louër and D. Grandjean, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 1696–1703 CrossRef.
- B. Bovio and S. Locchi, J. Crystallogr. Spectrosc. Res., 1982, 12, 507–517 CrossRef CAS.
- M. Louër, D. Grandjean and D. Weigel, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 1703–1706 CrossRef.
- L. Eriksson, D. Louër and P.-E. Werner, J. Solid State Chem., 1989, 81, 9–20 CrossRef CAS.
- M. Bianchini, M. Roca-Ayats, P. Hartmann, T. Brezesinski and J. Janek, Angew. Chem., Int. Ed., 2019, 58, 10434–10458 CrossRef CAS PubMed.
- T. Biswick, W. Jones, A. Pacuła and E. Serwicka, J. Solid State Chem., 2006, 179, 49–55 CrossRef CAS.
- N. Iyi, F. Geng and T. Sasaki, Chem. Lett., 2009, 38, 808–809 CrossRef CAS.
- D. F. Wong, K. Young, L. Wang, J. Nei and K. Y. S. Ng, J. Alloys Compd., 2017, 695, 1763–1769 CrossRef CAS.
- T. N. Ramesh, Mater. Chem. Phys., 2009, 114, 618–623 CrossRef CAS.
- R. Barnard, C. F. Randell and L. F. Tye, Power Sources, 1980, 8, 401–425 Search PubMed.
- C. Delmas and C. Tessier, J. Mater. Chem., 1997, 7, 1439–1443 RSC.
- C. Tessier, J. Electrochem. Soc., 1999, 146, 2059–2067 CrossRef CAS.
- T. N. Ramesh and P. V. Kamath, Mater. Res. Bull., 2008, 43, 2827–2832 CrossRef CAS.
- R. C. Reynolds, Crystal Structures of Clay Minerals and Their X-Ray Identification, Mineralogical Society of Great Britain and Ireland, 1980 Search PubMed.
- J. Cuadros, Clay Miner., 2012, 47, 147–164 CrossRef CAS.
- M. Rajamathi, P. Vishnu Kamath and R. Seshadri, J. Mater. Chem., 2000, 10, 503–506 RSC.
- M. Rajamathi, G. N. Subbanna and P. Vishnu Kamath, J. Mater. Chem., 1997, 7, 2293–2296 RSC.
- L. Guerlou-Demourgues, C. Denage and C. Delmas, J. Power Sources, 1994, 52, 269–274 CrossRef CAS.
- K. Nakamoto, Handbook of Vibrational Spectroscopy, Wiley, 2006 Search PubMed.
- D. N. Sathyanarayana, Vibrational Spectroscopy: Theory and Applications, New Age International Limited, 2015 Search PubMed.
- G. Adachi, N. Imanaka and Z. C. Kang, Binary Rare Earth Oxides, Springer, Netherlands, 2006 Search PubMed.
- V. R. Sastri, J. R. Perumareddi, V. R. Rao, G. V. S. Rayudu and J. C. G. Bünzli, Modern Aspects of Rare Earths and Their Complexes, Elsevier Science, 2003 Search PubMed.
- D. S. Hall, D. J. Lockwood, S. Poirier, C. Bock and B. R. MacDougall, J. Phys. Chem. A, 2012, 116, 6771–6784 CrossRef CAS.
- L. Markov, K. Petrov and V. Petkov, Thermochim. Acta, 1986, 106, 283–292 CrossRef CAS.
- N. Thomas, Mater. Res. Bull., 2012, 47, 3568–3572 CrossRef CAS.
- Z. Pan, Y. Jiang, P. Yang, Z. Wu, W. Tian, L. Liu, Y. Song, Q. Gu, D. Sun and L. Hu, ACS Nano, 2018, 12, 2968–2979 CrossRef CAS PubMed.
- X. Liu, R. Ma, Y. Bando and T. Sasaki, Adv. Mater., 2012, 24, 2148–2153 CrossRef CAS PubMed.
- Y. Jiang, Y. Song, Y. Li, W. Tian, Z. Pan, P. Yang, Y. Li, Q. Gu and L. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 37645–37654 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07655a |
| This journal is © The Royal Society of Chemistry 2023 |