Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced sensitivity towards hydrogen by a TiN interlayer in Pd-decorated SnO2 nanowires†
Clémence
Badie‡
 a,
Jae-Hyoung
Lee‡
a,
Jae-Hyoung
Lee‡
 b,
Ali
Mirzaei
c,
Hyoun Woo
Kim
*d,
Syreina
Sayegh
b,
Ali
Mirzaei
c,
Hyoun Woo
Kim
*d,
Syreina
Sayegh
 e,
Mikhael
Bechelany
e,
Mikhael
Bechelany
 *ef,
Lionel
Santinacci
*ef,
Lionel
Santinacci
 *a and
Sang Sub
Kim
*a and
Sang Sub
Kim
 *g
*g
aAix Marseille Univ, CNRS, CINaM, Marseille, France. E-mail: lionel.santinacci@univ-amu.fr
bElectronic Materials Research Center, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea
cDepartment of Materials Science and Engineering, Shiraz University of Technology, Shiraz 715557-13876, the Islamic Republic of Iran
dDivision of Materials Science and Engineering, Hanyang University, Seoul 04763, Republic of Korea. E-mail: hyounwoo@hanyang.ac.kr
eInstitut Européen des Membranes, IEM – UMR 5635, University of Montpellier, CNRS, ENSCM, Place Eugène Bataillon, Montpellier 34095, France. E-mail: mikhael.bechelany@umontpellier.fr
fGulf University for Science and Technology, GUST, Kuwait
gDepartment of Materials Science and Engineering, Inha University, Incheon 22212, Republic of Korea. E-mail: sangsub@inha.ac.kr
First published on 15th May 2023
Abstract
In this study, we designed a new structure based on Pd-decorated TiN-coated SnO2 nanowires (NWs) for the selective detection of H2 gas. Initially, SnO2 NWs were prepared by a simple vapor–liquid–solid growth method. Then, atomic layer deposition (ALD) was used to grow a continuous TiN layer and, subsequently, Pd nanoparticles on the NW networks. The TiN thickness was precisely set to 0.5, 1, 2, and 5 nm, while the Pd loading was adjusted by varying the number of ALD cycles (25 to 200 cycles). Various characterization techniques revealed the amorphous nature of TiN, a homogeneous dispersion of Pd NPs and the uniform morphology and single crystallinity of the SnO2 NWs. H2 gas sensing studies revealed that the sensor with a TiN thickness of 1 nm exhibited the highest response. Pd decoration further improved the response to H2 gas. Hence, the Pd-decorated gas sensor with a 1 nm–thick TiN layer showed the highest H2 sensing performance at 250 °C among all gas sensors. Due to the unique chemical reaction between Pd and hydrogen, the fabricated sensor shows excellent performance in detecting hydrogen gas. The underlying sensing mechanism is discussed in detail. The optimized sensor has a sensitivity of 8.18 for hydrogen gas, which is four times higher than that of other gas species, showing that it is suitable for detecting hydrogen gas. We believe that this new design is a highly valuable gas sensor for the real application of H2 monitoring with high selectivity.
1 Introduction
Nowadays, due to high pollution and harms caused by the use of fossil resources, the development of sustainable and green energy is the general trend.1 Hydrogen (H2) is the most abundant element in the universe,2 and its consumption does not generate toxic emissions.3 It is, indeed, regarded as the most promising new fuel for vehicles.4 Currently, it has many applications in different fields, such as biorefining, pharmaceuticals, metallurgy,5 aerospace,6 and automobile industry.7However, its small kinetic diameter (0.289 nm) and high diffusion coefficient (0.61 cm2 s−1) cause leakage from gas processing equipment.8 Due to its flammable nature with a low explosive limit of 4.0 vol%, high heat of combustion, high flame propagation velocity, and low ignition energy, its leakage can lead to catastrophic accidents. In addition, its colorless and odorless nature makes it impossible to be perceived by human kinds,9 and thus, it is important to develop highly efficient H2 gas sensors capable of detecting very low concentrations to avoid dangerous accidents.8,10,11 Furthermore, H2 gas is a biomarker, and therefore, its presence in exhaled breath can be used for diagnosing some diseases,12 such as small intestinal bacterial overgrowth, digestive disorders of dietary sugars (sucrose, fructose, lactose, and sorbitol), and normal food transport in the small intestine. Hence, the development of highly sensitive and selective H2 gas sensors is important13 not only from a safety point of view, but also from a medical standpoint.
There are many different types of gas sensors, such as work function,12 electrochemical,13 optical,14 surface acoustic wave,15 gasochromic,16 colorimetric,17 and electrical resistivity.18 Among them, resistivity-based gas sensors are highly appreciated for gas sensing owing to their high response, high stability, fast dynamics, and low price.19–21 In this type of gas sensors, the variations in resistance in the presence of the target gas is the basis of detection.22 Many semiconducting metal oxides have been used for gas detection. Among them, SnO2 is the most widely used for this purpose23,24 because it exhibits many advantageous features for sensing applications, such as the high mobility of charge carriers, high stability, abundance and low price.25–27 However, like other materials, such as ZnO and WO3, SnO2 gas sensors have no good selectivity especially in pristine form. Furthermore, they often need high operating temperatures to activate the sensor and to increase the number of charge carriers in the conduction band, which might be a drawback for some (not all) applications.
Since 1-dimension and morphology affect the sensing properties, SnO2 nanostructures (e.g. wires, rods, belts, needles, whiskers, and sheets) have thus been implemented to improve the gas sensing properties such as, high surface area, fast response/recovery times and low power consumption.28 In addition, for selectivity enhancement of SnO2-based H2 sensor devices, further strategies such as electron beam29 or ion-beam irradiations,30 heterojunction formation,31 and noble metal functionalization32,33 have been proposed so far.
Noble metals such as Pd, Pt, and Au have very high catalytic activities and they can reduce the gas adsorption energy and, hence, enhance the response and selectivity toward a specific gas at a lower sensing temperature. Furthermore, when the Fermi levels of noble metals are lower than the sensing layer, the energy bands of the sensing layer are bent at the interfaces, and the Schottky barriers will be formed due to the electron flow from the semiconductor to the noble metal. Changes in the height of the Schottky barrier in a H2 atmosphere can significantly modify the resistance of the gas sensor, leading to the generation of an enhanced sensing signal.34 Among different noble metals, Pd is particularly appropriated for H2 detection.35 Pd can easily dissociate adsorbed H2 gas, converting it into atomic H, and then lead to a spillover effect, in which atomic species can be moved to the neighboring metal oxides and react with already adsorbed oxygen species. Furthermore, H atoms can diffuse into interstitial sites in the Pd lattice and convert it into PdHx, exhibiting a higher resistance relative to metallic Pd. In addition, nanoparticles are more appropriate than continuous films due to the larger surface area developed. These effects can positively contribute to enhance H2 selective sensing.36 Finally, as mentioned by Yamazoe et al.37 in contact with air, an electron depletion layer (EDL) is formed on the surface of sensing layers, due to partial oxidation of Pd into PdO. However, the EDL disappears when PdO is reduced into Pd in reducing atmospheres such as H2 gas atmosphere. Accordingly, many researchers have used Pd for H2 sensing enhancement.38,39
The combination of catalytic particles such as Pd or Pt with SnO2 therefore appears to be one of the most efficient strategies. However, it has been shown that the catalytic activity of metallic particles can further be enhanced depending on the nature of the support. This improvement is known for a long time on oxidized supports for various processes.40,41 For instance, oxides are still investigated as possible carbon support replacement in electrocatalysis (e.g. nanostructured TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 or SnO2 interlayer43), and a thin porous Al2O3 layer grown by molecular layer deposition (MLD) on SnO2 nanowires (NWs) has been used as an efficient humidity sensor.44 However, nitrides are now scrutinized with a strong interest. Recently, it has been shown that a BN interlayer enhances the sensing properties of supported Pd nanoparticles (NPs).45 Transition metal nitrides have also been shown as possible replacement catalysts to Pt.46 Among nitrides, TiN is very interesting since it is often used in microelectronics as a conductive layer47–49 due to its stability at high temperatures and good ohmic contact (metal-like conductivity). It also prevents corrosion with good wear resistance.50 Finally, TiN has been reported to be an efficient support for Pt minimization or replacement in the field of electrocatalysis51 or photocatalysis.52 Research on H2 separation devices currently in progress in our groups reveals that TiN layer blocks the H2 molecules while atomic H generated by Pd particles deposited on the TiN can cross it. Previous works have shown the sensing potential of SnO2 nanowires (NWs).44,53,54 Thus, the combination of a TiN thin layer as an interlayer between SnO2 NWs and Pd NPs can be an efficient and novel strategy to enhance the overall H2 gas sensing performance.
42 or SnO2 interlayer43), and a thin porous Al2O3 layer grown by molecular layer deposition (MLD) on SnO2 nanowires (NWs) has been used as an efficient humidity sensor.44 However, nitrides are now scrutinized with a strong interest. Recently, it has been shown that a BN interlayer enhances the sensing properties of supported Pd nanoparticles (NPs).45 Transition metal nitrides have also been shown as possible replacement catalysts to Pt.46 Among nitrides, TiN is very interesting since it is often used in microelectronics as a conductive layer47–49 due to its stability at high temperatures and good ohmic contact (metal-like conductivity). It also prevents corrosion with good wear resistance.50 Finally, TiN has been reported to be an efficient support for Pt minimization or replacement in the field of electrocatalysis51 or photocatalysis.52 Research on H2 separation devices currently in progress in our groups reveals that TiN layer blocks the H2 molecules while atomic H generated by Pd particles deposited on the TiN can cross it. Previous works have shown the sensing potential of SnO2 nanowires (NWs).44,53,54 Thus, the combination of a TiN thin layer as an interlayer between SnO2 NWs and Pd NPs can be an efficient and novel strategy to enhance the overall H2 gas sensing performance.
The target sensing system requires a compact and uniform TiN thin film as an interlayer to protect pristine SnO2 underneath, allowing H atoms to diffuse through it. In addition, catalytic Pd NPs should be monodispersed on the overall 3D-SnO2 NW network. No material accumulation or damage should occur during both TiN and Pd NP coatings to ensure that the high surface area of the SnO2 NW network is maintained. Atomic layer deposition (ALD) is the method of choice to achieve such objectives while physical or chemical vapor deposition techniques exhibit coverage limitations.55–58 Several examples cited above have already shown the interest of this deposition technique for such purpose (see, e.g. ref. 58 for review).
Initially, TiN films were mainly deposited using TiCl4 associated with NH3.59 However, TiCl4 requires high process temperatures and its by-products are corrosive. Then, organometallic precursors such as amides have become mostly used. Among them, tetrakis(dimethylamino)titanium (TDMAT)60,61 is now predominant. TiN films exhibiting low carbon contamination, low roughness and low resistivity have already been conformally deposited using TDMAT and NH3 onto different structured substrates such as nanoporous alumina and macroporous silicon.62,63
The ALD of Pd has been successfully performed on various substrates such as Ni, BN, and TiO2.42,43,64,65 Compared to TiN deposition, where both precursors, TDMAT and NH3, are involved in the growth of the film, the Pd process deals with the reduction to metallic Pd and the elimination of the ligands of the Pd precursor by the co-reactant. In this case, after each cycle, metallic Pd atoms are deposited onto the substrate, forming nuclei. Then, in the following cycle, the deposition is more promoted to grow on the nuclei than on the substrate. This explains why metal particles, usually monocrystalline, are achieved instead of a compact film in the case of metals such as Pd, Pt or Ru, as reported by Mackus et al.66 and Ru by Federov et al.67 In this work, Pd clusters are deposited by a direct ALD process, which uses palladium hexafluoroacetylacetone (Pd(hfac)2) and formaldehyde, as previously described.68–71
According to the above-mentioned motivations, we report, for the first time, the fabrication process of a H2 sensor device that involves coating SnO2 NWs with a continuous ultra-thin TiN layer that is subsequently decorated with Pd NPs. SnO2 NWs were synthesized by a simple vapor–liquid–solid (VLS) growth method. Both TiN and Pd depositions were performed by ALD with the possibility of exact control over the synthesis parameters. The gas sensing performance of bare SnO2 NW, TiN/SnO2 NW, Pd/SnO2 NW and Pd/TiN/SnO2 NW sensors was deeply studied. The results are discussed and they revealed that Pd/TiN/SnO2 NW gas sensors have enhanced H2 gas sensing performance in terms of sensitivity and selectivity in comparison with other H2 gas sensors.
2 Experimental
2.1 Growth of SnO2 NWs
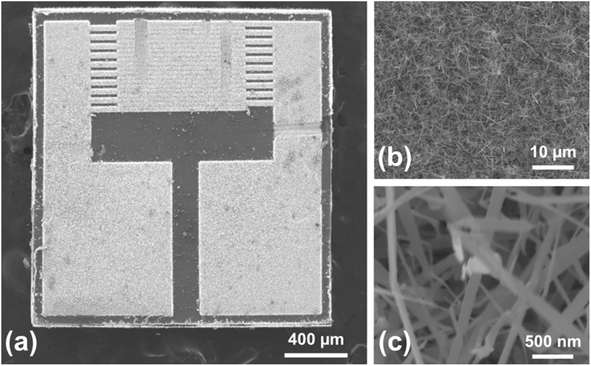
SnO2 NWs were fabricated by a VLS growth method. First, tri-layered Ti (50 nm), Pt (200 nm), and Au (5 nm) electrodes were fabricated in an interdigitated pattern by sputtering these metals onto an oxidized Si substrate (SiO2 grown on Si). Then, the VLS method was used to grow networked SnO2 NWs. Substrates with interdigitated electrodes were placed in a quartz tube furnace loaded with an Al2O3 crucible containing a metallic Sn powder (Aldrich, 99.9%). The furnace was heated to 950 °C for 1 h under the flow of Ar and O2 gases simultaneously at rates of 300 and 10 sccm, respectively. Under these conditions, networked SnO2 NWs grew selectively on the substrate. Fig. S1a† schematically illustrates the SnO2 NW growth method.2.2 ALD of TiN films
TiN deposition was performed on both SnO2 NWs and HF (5%) cleaned Si(100) wafer pieces to monitor the thickness. TiN films were grown by thermal ALD in a shower-head geometry reactor Fiji 200 (Veeco/Cambridge Nanotech) according to the previous studies.62,63 TDMAT (99.99%, Strem Chemicals) and NH3 (≥99.999%, from Linde Electronics) were used as the Ti precursor and N-source, respectively, and Ar (≥99.999%, from Linde Electronics) served as the vector gas. TDMAT was maintained at 70 °C and the chamber temperature was fixed at TTiN = 200 °C. ALD sequences consist of pulsing, exposure and long purging successively TDMAT and NH3 for defined durations (0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 s). The thickness of the films (tTiN) was adjusted by varying the number of ALD cycles (NTiN) according to the growth per cycle of 1.1 Å/cycle (the surface reactions are schematically described in Fig. S2†).
20 s). The thickness of the films (tTiN) was adjusted by varying the number of ALD cycles (NTiN) according to the growth per cycle of 1.1 Å/cycle (the surface reactions are schematically described in Fig. S2†).
2.3 ALD of Pd particles
Palladium NPs were deposited onto a SnO2–TiN sensor in a home-built ALD reactor;41,59,72 the deposition was performed at 220 °C using Pd(hfac)2 (95% from Strem chemicals) heated at 70 °C and formalin (37% formaldehyde in water with 10–15% of methanol from Sigma-Aldrich) as the precursor and co-reactant, respectively. One cycle of ALD consisted of 5 s Pd pulse, 15 s exposure and 10 s Ar purge followed by 1 s pulse of CH2O, 15 s exposure and 60 s Ar purge. A total of NPd from 25 to 200 cycles was repeated to vary the desired Pd NP amount (the deposition mechanism is summarized in Fig. S3†).2.4 Characterization methods
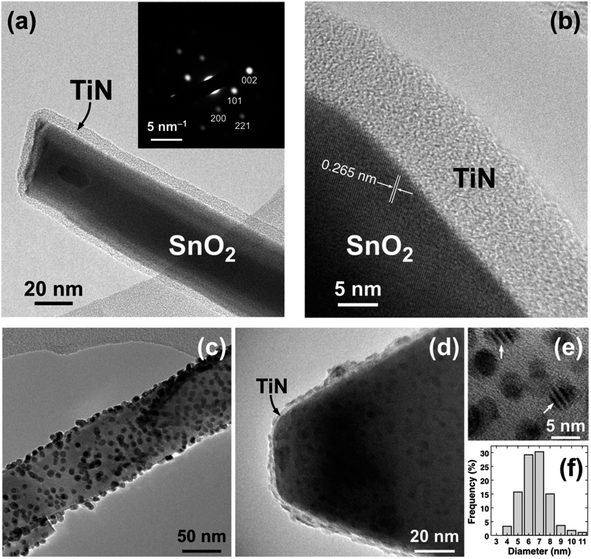
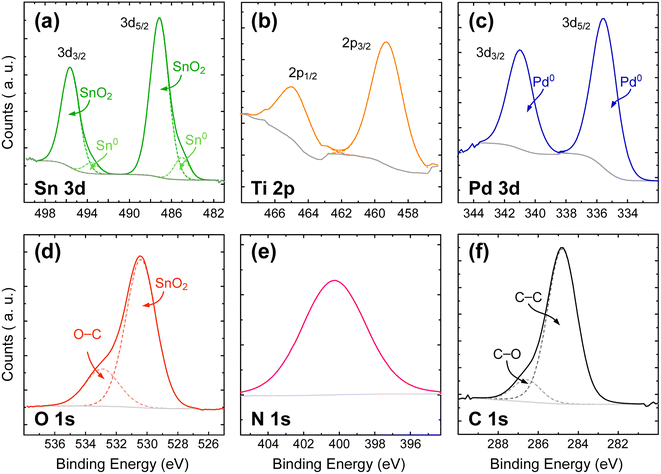
The TiN thickness was measured by in situ spectroscopic ellipsometry using a M2000V (J. A. Woollam Inc). Scanning and transmission electron microscopies (SEM and TEM) using a JSM 7900F (JEOL Ltd) and a JEM 2010 (JEOL Ltd), respectively, were performed to investigate the morphology of TiN films as well as the Pd NPs grown successively on SnO2 NWs. Selected area electron diffraction (SAED) was performed in the TEM to locally study the crystal structure. The chemical composition was determined by energy-dispersive spectroscopy (EDS) using a Quantax FlatQuad (Bruker) and by X-ray photoelectron spectroscopy (XPS) using a Kratos Axis Ultra spectroscope (Kratos Analytical, UK) equipped with a monochromatic Al Kα source (1486.6 eV). The binding energy (BE) was corrected using the C 1s peak at 284.8 eV as an internal standard.2.5 Sensor device fabrication
First, a Ti/Au bilayer electrode was deposited onto an alumina substrate. Then a paste was prepared by mixing the sensing powders with α-terpineol (20 μL) and then screen-printed on the sensor substrate (Fig. S1b†); subsequently, it was dried at 60 °C for 8 h.2.6 Sensing measurements
The fabricated gas sensors were exposed to different gases at different temperatures using a specifically home-made designed sensing system (Fig. S1c†). The concentration of the target gas was precisely controlled by adjusting the mixing ratio between the target gas and dry air using accurate mass flow controllers (total flow rate = 500 sccm). During the sensing measurement process, the change in the resistance of the sensor in air (Ra) and in the presence of target gas (Rg) was continuously measured and recorded using a computer. The response was defined as R = Ra/Rg. The response and recovery times were defined as the time to reach 90% of the final resistance after exposure to the target gas and the time to recover 90% of the initial resistance of the sensor after removing the target gas, respectively. Details of gas sensing measurements can be found in.73,743 Results and discussions
3.1 Morphological and chemical studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 but tTiN is too thin to produce a significant signal. Pd 3d3/2 and 3d5/2 peaks located at BE = 341.0 and 335.5 eV, respectively (Fig. 3c), are sharp and show no shoulder. The doublet exhibits a SOS of 5.5 eV near the expected value of 5.25 eV. This demonstrates that Pd0 NPs are deposited onto a thin composite film composed of TiN/TiOxNy/TiO2. Finally, Fig. 3f shows the reference C 1s peak. As mentioned in the experimental section, C–C at BE = 284.8 eV is used as reference to calibrate all the spectra. A second contribution at a higher energy (BE = 286.6 eV) corresponds to the superficial contamination layer.
77 but tTiN is too thin to produce a significant signal. Pd 3d3/2 and 3d5/2 peaks located at BE = 341.0 and 335.5 eV, respectively (Fig. 3c), are sharp and show no shoulder. The doublet exhibits a SOS of 5.5 eV near the expected value of 5.25 eV. This demonstrates that Pd0 NPs are deposited onto a thin composite film composed of TiN/TiOxNy/TiO2. Finally, Fig. 3f shows the reference C 1s peak. As mentioned in the experimental section, C–C at BE = 284.8 eV is used as reference to calibrate all the spectra. A second contribution at a higher energy (BE = 286.6 eV) corresponds to the superficial contamination layer.
3.2 Electrical and gas sensing studies
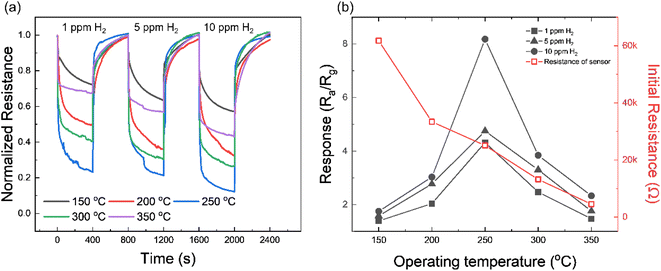
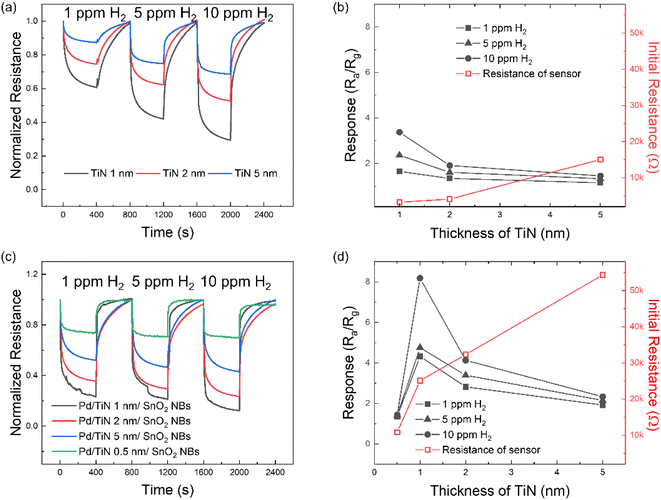
Fig. S5† presents the I–V characteristics of Pd/TiN/SnO2 NW gas sensors with different thicknesses of TiN layers (0.5, 1, 2, and 5 nm). In general, all gas sensors showed the Schottky contact between the sensing layer and electrodes. TiN tends to show an ohmic junction due to its unique conductivity, but in this study, it appears to be a Schottky junction due to the presence of SnO2 and Pd.In the first step and to find the optimal working temperature of gas sensors, we exposed the Pd/TiN (1 nm)/SnO2 NW gas sensor to 1, 5 and 10 ppm H2 gas at various temperatures, as shown in Fig. 4a. All gas sensors showed a n-type gas response originating from the n-type nature of SnO2, where the resistance decreased in the presence of H2 gas. The resistance is also modified depending on the working temperature from 150 to 350 °C. To have a better insight, the gas response was plotted against the sensing temperature in Fig. 4b. It depicts the variations in the initial resistance of the gas sensor versus temperature where, as it was expected, the resistance decreased upon increasing the sensing temperature, reflecting the semiconducting nature of the sensing material. The sensor shows a bell-shaped behavior, where the response is low at temperatures <250 °C until showing a maximum at 250 °C and decreases again with further heating. Chemisorption occurs when bonds form between the adsorbate and the sensing layer by transferring electrons similar to the case of oxygen adsorption on the surface of gas sensors in which a monolayer of oxygen ions form on sensor surface.78 At low operating temperatures, and due to the unavailability of electrons in the conduction band, physisorption is dominant and no significant electron transfer occurs between adsorbed oxygen species and sensing layer. However, at higher temperatures, more electrons jump to the conduction band of sensing materials, and hence, there are more available electrons to be abstracted by oxygen species. Therefore, at higher temperatures, it is expected that more chemisorbed oxygen species react with H2 gas molecules and a higher response is expected. However, at very high temperatures, the desorption rate of both oxygen and target gas molecules is higher than the adsorption rate, leading to a further decrease in the gas response.79 To assess the activation energy of oxygen chemisorption on the surface of materials, analysis of the kinetics of interaction between oxygen and the material is required. Hence the study of such mechanisms is really complex and should take into consideration several parameters which are beyond the scope of this work.80,81 Moreover, sensors with different tTiN (Pd/TiN (0.5, 2, 5 nm)/SnO2 NWs) were studied to find their optimal sensing temperature. Transient resistance curves and response/initial resistance versus temperature for Pd/TiN (0.5 nm)/SnO2 NWs (Fig. S6a and b†), Pd/TiN (2 nm)/SnO2 NWs (Fig. S6c and d†), and Pd/TiN (5 nm)/SnO2 NWs (Fig. S6e and f†) were obtained. The optimized sensing temperatures were 200, 250 and 300 °C for the sensor with 0.5, 2 and 5 nm–thick TiN layers, respectively. The response of these gas sensors at their optimal working temperatures was lower than that of the Pd/TiN (1 nm)/SnO2 gas sensor at its optimal sensing temperature (250 °C). Therefore, other sensing studies were performed at 250 °C.
In the next step, TiN/SnO2 NW gas sensors with 1, 2 and 5 nm TiN thicknesses were exposed to 1, 5 and 10 ppm of H2 gas at 250 °C. Fig. 5a shows the dynamic resistance curves and Fig. 5b the corresponding response versus tTiN. For all tested concentrations, the response of the sensor with a 1 nm–thick TiN layer is higher than that of other gas sensors, demonstrating, therefore, that it is the optimal thickness. Variations in the initial sensor resistance are depicted. Sensor with the thickest TiN layer showed the highest initial resistance. TiN is known as a conductive material, but in this study, resistance tends to increase with the increase in thickness. This seems to be due to the influence of very small amounts of TiO2 and TiOxNy shown in the XPS data of Fig. 3. The above-mentioned experiments were repeated for Pd/TiN (0.5, 1, 2, and 5 nm)/SnO2 NW gas sensors, and the results are presented in Fig. 5c and d. The sensor with a TiN layer with a thickness of 1 nm showed the highest response to H2 gas for all concentrations.
To study the effect of Pd NPs, the H2 gas sensing tests were performed by controlling the number of deposition cycles from 25 to 200 to make Pd (25, 50, 100, and 200 cycles)/TiN (1 nm)/SnO2 NW gas sensors. Fig. S7a† gives the dynamic resistance curves of different gas sensors with various Pd NP cycles. Based on Fig. S7b,† since the response of the sensor with 100 cycles of Pd was higher than the response of other gas sensors, remaining tests were performed using the sensor with 100 cycles of Pd deposition.
We also tested the responses of pristine SnO2 NWs and Pd/SnO2 NWs to 1–10 ppm of H2 gas at 250 °C, as shown in Fig. S8a and b,† respectively. To better understand the performance of different gas sensors, their response to 1, 5 and 10 ppm H2 gas is summarized in Fig. S9.† Based on these data, the (100 cycles)Pd/TiN (1 nm)/SnO2 NW gas sensor showed the optimal gas sensing results in this study.
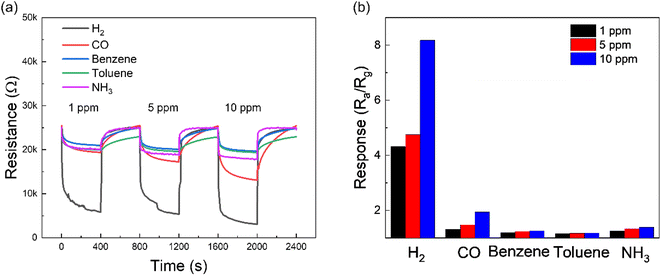
Since selectivity is of importance for practical applications, we also measured transient resistance curves of optimal gas sensors by exposing it to 1, 5 and 10 ppm of various gases (H2, CO, C6H6, C7H8 and NH3) as shown in Fig. 6a. The corresponding responses to different gases are plotted in Fig. 6b. Obviously, the response to H2 gas is higher than that for other gases, reflecting the high H2-sensing selectivity of the optimized gas sensor. Moreover, Fig. S10† shows the response and recovery times of Pd/TiN (1 nm)/SnO2 NW gas sensors to 10 ppm of various gases. Both response and recovery times were shorter than other tested gases, confirming that the as-fabricated gas sensor could detect H2 gas molecules faster than other gases.
Fig. S11a† offers the dynamic resistance curves of Pd/TiN (1 nm)/SnO2 NW gas sensors to 10 ppm H2 gas in the presence of different relative humidity (RH) levels (from 20 to 60%), and corresponding response versus RH (%) are presented in Fig. S11b.† Increasing RH from 20 to 60%, the response of the gas sensor decreased from 6.63 to 4.56. When water vapor is present in the environment, water molecules adsorb onto the surface of the sensing layer and occupy some adsorption sites. Hence, the number of available sites for incoming H2 gas molecules decreases. Therefore, reduced amounts of H2 gas can be adsorbed onto the surface, resulting in a lower response in the presence of humidity. Fig. S12a† shows the dynamic resistance curves of Pd/TiN (1 nm)/SnO2 NW gas sensors to 10 ppm H2 gas after two years, and Fig. S12b† shows the corresponding response versus the cycle number. Moreover, for comparison, the response of the fresh gas sensor is shown. The fresh sensor shows a response of 8.18 to 10 ppm H2 gas, and after two years, the response during different cycles was varied between 7 and 8.66, reflecting the good stability of the optimized gas sensor even after two years.
To explore the experimental detection limit, the optimal gas sensor was exposed to various H2 concentrations (0.1–50 ppm), and the corresponding results are shown in Fig. S13a and b.† As presented in results, the fabricated gas sensor detects very low concentrations of hydrogen gas. Therefore, the present sensor successfully detected the very low concentrations of H2 gas. Then, the detected concentrations of H2 gas are much lower than the explosive limit of H2 gas. Hence, the present optimal gas sensor can reliably detect very low concentrations of H2 leakages in real applications and prevent the explosions caused by H2 leakage as the explosion limit of H2 gas is 4%.
The response of the Pd/TiN (1 nm)/SnO2 NW sensor is compared with those reported in the literature, as summarized in Table 1. In a comparative view, it can be deduced that the gas sensor fabricated in this study exhibited the excellent hydrogen detection properties, in terms of operating temperature as well as response. In particular based on the sensitivity factor which is defined as “response/concentration”, the present sensor shows much enhanced performance relative to other gas sensors listed in Table 1.
| Sensor | Conc. (ppm) | Temp. (°C) | Response | Sensitivity factor | Ref. |
|---|---|---|---|---|---|
| a R a/Rg. b ΔR/R0 × 100%. | |||||
| Pd/TiN (1 nm)/SnO2 NWs | 10 | 250 | 8.18a | 0.818 | This study |
| SnO2 NWs | 10 | 250 | 2.72a | 0.272 | 82 |
| Pd-SnO2 NWs | 10 | 400 | ∼2.5a | 0.250 | 83 |
| SnO2 thin film sensitized with microsized Pd islands | 100 | 300 | ∼2a | 0.020 | 81 |
| 2D SnO2 disks | 100 | 350 | ∼6.5a | 0.065 | 84 |
| Pd/SnS2/SnO2 | 500 | 300 | ∼7.5a | 0.015 | 8 |
| SnO2-Cr2O3 | 400 | 250 | ∼30%b | 0.075 | 85 |
| SnO2 NSs | 50 | 300 | 3.2a | 0.064 | 86 |
| Pd doped rGO/ZnO–SnO2 | 100 | 300 | ∼4.5a | 0.045 | 87 |
3.3 Gas sensing mechanism
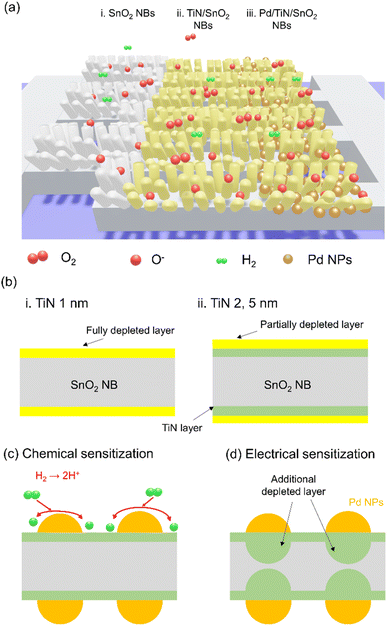
Fig. 7a illustrates the various sensing materials, namely, SnO2 NWs, TiN/SnO2 NWs and Pd/TiN/SnO2 NWs grown on the interdigitated pattern, and herein, we will discuss the sensing mechanisms of different sensing materials. Initially, in air, oxygen molecules adsorb onto the surface of the sensing layer, taking electrons due to their high electron affinity. The relevant reactions can be shown as follows:22| O2(g) → O2(ads) | (1) |
| O2(ads) + e− → O−2(ads) | (2) |
| O−2(ads) + e− → 2O− | (3) |
| O− + e− → O2− | (4) |
It should be noted that each oxygen ionic species is stable in the temperature range. For example, at high temperatures (>300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C), O2− species are dominant.88 Therefore, in this study, dominant species are O− ions because the working temperature is set to 250 °C.
°C), O2− species are dominant.88 Therefore, in this study, dominant species are O− ions because the working temperature is set to 250 °C.
As a result, the so-called EDL will be formed on the SnO2 NW surface, limiting the conductivity to inner parts on the SnO2 NW, named conduction channels in the following. In air, the diameter of the conduction channels is smaller than when the sensor is in a pure N2 gas atmosphere, where there is no adsorbed oxygen. By subsequent exposure to H2 gas, the following reaction can take place:89
| H2 + O− → H2O + e− | (5) |
According to the above-mentioned reaction, electrons are released upon reaction between H2 and adsorbed oxygen species. This leads to narrowing the EDL and increasing the diameter of the conduction channel inside of SnO2 NWs. This ultimately leads to a decrease in the resistance, as observed in dynamic resistance curves. Furthermore, due to the networked nature of SnO2 NWs, many contact areas and potential barriers can be created at SnO2–SnO2 homojunctions in air. Upon exposure to H2 gas, the height of the potential barrier decreases, and a remarkable modulation in the resistance of the sensor occurs.90
Fig. S14† depicts the band diagram of Pd/TiN/SnO2 upon intimate contact. The work function of SnO2 (4.55 eV)91 is lower than that of Pd (5.6 eV) and TiN (4.65 eV).92 Thus, electrons flow from SnO2 to Pd and TiN, creating a Schottky junction with a potential barrier to electron flow in air. As a result, the electron depletion layer will appear inside SnO2 in interfaces between SnO2/Pd and SnO2/TiN. Upon exposure to H2 gas, the height of the formed junctions decreases, leading to the narrowing of electron depletion layers inside SnO2, and eventually decreases the sensor resistance.
Furthermore, since some parts of the TiN film are exposed to air in TiN-coated and Pd-NPs-TiN-coated SnO2 NW sensors, these areas will also be depleted from electrons by adsorbed oxygen ions. Accordingly, in a H2 gas atmosphere and by releasing back of electrons, more modulation of the resistance is expected. It was found that the sensor with a TiN layer thickness of 1 nm showed the highest response to H2 gas. Indeed, we should consider two factors that contribute to the sensing signal. The first one is the thickness of the TiN layer and the second one is the total thickness of the SnO2 + TiN layer. For the sensor with the thinnest layer of TiN (0.5 nm), even though the whole TiN layer may be depleted from electrons in air (Fig. 7b), that thickness is much smaller than the overall SnO2 + TiN thickness and upon exposure to H2 gas, the resistance modulation is not significant and the response value is lower than that of the sensor with a thicker TiN layer. Moreover, for the sensors with a TiN layer thicker than 1 nm, the overall thickness of TiN is not depleted from the electrons and upon exposure to H2 gas, no significant modulation of resistance occurs again. However, for the sensor with a TiN layer of 1 nm, it seems that not only the whole thickness of TiN become depleted from electrons, but also the ratio of TiN thickness to overall SnO2 + TiN thickness is noticeable. Hence, upon exposure to H2 gas, a large modulation of the electrical resistance occurs, resulting in a higher response for the sensor with a TiN layer of 1 nm.
Pd decoration led to a higher response of H2 gas sensors. As shown in TEM images (Fig. 2), Pd NPs are dispersed on the surface of SnO2 NWs. Hence, the gas response significantly increases by the catalytic activity of Pd on H2. In the H2 gas ambient, Pd facilitates the dissociation of molecular H2 into H atoms (Fig. 7c), which migrate to the TiN surface by the so-called spillover mechanism. H atoms then react with the adsorbed oxygen ions on the TiN surface and the electrons are returned back to SnO2. The relevant reactions are as follows:93
| H2 → H + H | (6) |
| H → H+ + e− | (7) |
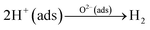 | (8) |
The above-mentioned reactions lead to a significant decrease in resistance, yielding to a high response. The modulation of the resistance in the TiN/Pd heterojunction also contributes to the evolution in the sensing activity of the gas sensor. Due to the work function difference of materials as mentioned above, the electron-depletion regions are generated in TiN, and a subsequent exposure to H2 gas, will result in a greater resistance modulation (Fig. 7d). Moreover, the H2 gas can be directly dissolved in octahedral sites of Pd, changing its metallic state (Pd0) into a hybrid form (PdHx), which ultimately will affect the overall resistance of the gas sensor. The formation of PdHx was confirmed in a recent study by our group using XPS studies.94
In addition to the promising catalytic role of Pd towards H2 gas, the selectivity of the optimized H2 gas sensor can be related to the small kinetic diameter of the H2 gas molecule (2.89 Å), relative to C6H6 (5.85 Å), CO and C7H8 (5.8 Å) molecules. Accordingly, H2 gas molecules can more easily penetrate the SnO2 NW gas sensor structure, resulting in a higher gas response.94
4 Conclusions
In brief, we introduced a novel gas sensor based on Pd-decorated TiN-coated SnO2 NWs. TiN with thicknesses of 0.5, 1, 2 and 5 nm was deposited over a SnO2 NW network, followed by Pd decoration with different amounts depending on the number of cycles applied (from 25 to 200 cycles). ALD was used to conformally deposit TiN and uniformly distribute Pd NPs. Based on the characterization results, amorphous TiN films were conformally coating the SnO2 NWs, as well as the crystalline Pd NPs were homogeneously dispersed over TiN. H2 gas sensing results indicated that the sensor with 100 cycles-Pd decoration and a 1 nm–thick TiN layer had the highest response to H2 gas. Enhanced gas response and high H2 sensing selectivity were related to different causes: the catalytic effect of Pd towards H2 gas, small kinetic diameter of H2 gas, high surface area of SnO2 NWs, formation of SnO2/TiN heterojunctions as well as Pd/TiN heterojunctions. Successful results obtained in this study can be extended to other similar systems to improve the overall gas sensing performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge D. Chaudanson and A. Altié (CINaM) for their valuable help with the electron microscopy. This collaborative work was supported by the French Agence Nationale de la Recherche (ANR) under grant ANR-17-CE09-0049-03 (project MENINA). Sang Sub Kim also acknowledges the support from the Korea Polar Research Institute (KOPRI) grant funded by the Ministry of Oceans and Fisheries (KOPRI project No. PE22900), and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [No. 2021R1A2C1009790].References
- Z. Shi, J. Feng and X. Dong, Int. J. Hydrogen Energy, 2022 DOI:10.1016/j.ijhydene.2022.06.205.
- B. Sharma, A. Sharma and J.-S. Kim, Sens. Actuators, B, 2018, 262, 758–770 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Renewable Sustainable Energy Rev., 2016, 57, 850–866 CrossRef CAS.
- A. Zhong, A. Sun, B. Shen, H. Yu, Y. Zhou, Y. Liu, Y. Xie, J. Luo, D. Zhang and P. Fan, Int. J. Hydrogen Energy, 2022, 47, 2050–2058 CrossRef CAS.
- J. A. Okolie, B. R. Patra, A. Mukherjee, S. Nanda, A. K. Dalai and J. A. Kozinski, Int. J. Hydrogen Energy, 2021, 46, 8885–8905 CrossRef CAS.
- X. Bévenot, A. Trouillet, C. Veillas, H. Gagnaire and M. Clément, Sens. Actuators, B, 2000, 67, 57–67 CrossRef.
- D. Berndt, J. Muggli, F. Wittwer, C. Langer, S. Heinrich, T. Knittel and R. Schreiner, Sens. Actuators, A, 2020, 305, 111670 CrossRef CAS.
- X. Meng, M. Bi, Q. Xiao and W. Gao, Sens. Actuators, B, 2022, 359, 131612 CrossRef CAS.
- X.-T. Yin, S.-S. Wu, D. Dastan, S. Nie, Y. Liu, Z.-G. Li, Y.-W. Zhou, J. Li, A. Faik, K. Shan, Z. Shi, M. A. Tarighat and X.-G. Ma, Surf. Interfaces, 2021, 25, 101190 CrossRef CAS.
- S. Zhu, Q. Tian, G. Wu, W. Bian, N. Sun, X. Wang, C. Li, Y. Zhang, H. Dou, C. Gong, X. Dong, J. Sun, Y. An, Q. Jing and B. Liu, Int. J. Hydrogen Energy, 2022, 47, 17821–17834 CrossRef CAS.
- N. Van Duy, N. X. Thai, T. M. Ngoc, D. Thi Thanh Le, C. M. Hung, H. Nguyen, M. Tonezzer, N. Van Hieu and N. D. Hoa, Sens. Actuators, B, 2022, 351, 130979 CrossRef CAS.
- T. Sahoo and P. Kale, Adv. Mater. Interfaces, 2021, 8, 2100649 CrossRef CAS.
- G. Korotcenkov, S. D. Han and J. R. Stetter, Chem. Rev., 2009, 109, 1402–1433 CrossRef CAS PubMed.
- J. Ou, M. H. Yaacob, J. L. Campbell, K. Kalantar-zadeh and W. Wlodarski, Procedia Eng., 2010, 5, 1204–1207 CrossRef CAS.
- I. Constantinoiu and C. Viespe, Nanomaterials, 2020, 10, 760 CrossRef CAS PubMed.
- S. S. Kalanur, Y.-A. Lee and H. Seo, RSC Adv., 2015, 5, 9028–9034 RSC.
- Y. K. Kim, S. H. Hwang, S. M. Jeong, K. Y. Son and S. K. Lim, Talanta, 2018, 188, 356–364 CrossRef CAS PubMed.
- Y. Shi, H. Xu, T. Liu, S. Zeb, Y. Nie, Y. Zhao, C. Qin and X. Jiang, Adv. Mater., 2021, 2, 1530–1569 RSC.
- A. Mirzaei, S. G. Leonardi and G. Neri, Ceram. Int., 2016, 42, 15119–15141 CrossRef CAS.
- A. Mirzaei and G. Neri, Sens. Actuators, B, 2016, 237, 749–775 CrossRef CAS.
- A. Mirzaei, S. S. Kim and H. W. Kim, J. Hazard. Mater., 2018, 357, 314–331 CrossRef CAS PubMed.
- A. Mirzaei, J.-H. Kim, H. W. Kim and S. S. Kim, J. Mater. Chem. C, 2018, 6, 4342–4370 RSC.
- P ist der Anmelder, Germany Pat., DE2016388A1, 1971 Search PubMed.
- H.-J. Kim and J.-H. Lee, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS.
- S. Das and V. Jayaraman, Prog. Mater. Sci., 2014, 66, 112–255 CrossRef CAS.
- N. Yamazoe, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS.
- N. Yamazoe and K. Shimanoe, in Science and Technology of Chemiresistor Gas Sensors, Nova Science Publishers, Inc., 2007, pp. 1–31 Search PubMed.
- Y. Masuda, Sens. Actuators, B, 2022, 364, 131876 CrossRef CAS.
- S. Sub Kim, H. Gil Na, H. Woo Kim, V. Kulish and P. Wu, Sci. Rep., 2015, 5, 10723 CrossRef PubMed.
- Y. J. Kwon, S. Y. Kang, P. Wu, Y. Peng, S. S. Kim and H. W. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 13646–13658 CrossRef CAS PubMed.
- D. Meng, D. Liu, G. Wang, Y. Shen, X. San, M. Li and F. Meng, Sens. Actuators, B, 2018, 273, 418–428 CrossRef CAS.
- X. Lian, Y. Li, J. Zhu, Y. Zou, D. An and Q. Wang, Mater. Sci. Semicond. Process., 2019, 101, 198–205 CrossRef CAS.
- G. Li, Z. Cheng, Q. Xiang, L. Yan, X. Wang and J. Xu, Sens. Actuators, B, 2019, 283, 590–601 CrossRef CAS.
- Y. Luo, C. Zhang, B. Zheng, X. Geng and M. Debliquy, Int. J. Hydrogen Energy, 2017, 42, 20386–20397 CrossRef CAS.
- T. Xu, M. P. Zach, Z. L. Xiao, D. Rosenmann, U. Welp, W. K. Kwok and G. W. Crabtree, Appl. Phys. Lett., 2005, 86, 203104 CrossRef.
- A. Mirzaei, H. R. Yousefi, F. Falsafi, M. Bonyani, J.-H. Lee, J.-H. Kim, H. W. Kim and S. S. Kim, Int. J. Hydrogen Energy, 2019, 44, 20552–20571 CrossRef CAS.
- N. Yamazoe, Sens. Actuators, B, 1991, 5, 7–19 CrossRef CAS.
- O. Lupan, V. Postica, F. Labat, I. Ciofini, T. Pauporté and R. Adelung, Sens. Actuators, B, 2018, 254, 1259–1270 CrossRef CAS.
- M. Weber, J. H. Kim, J. H. Lee, J. Y. Kim, I. Iatsunskyi, E. Coy, M. Drobek, A. Julbe, M. Bechelany and S. S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 34765–34773 CrossRef CAS PubMed.
- C. T. Campbell, Surf. Sci. Rep., 1997, 27, 1–111 CrossRef CAS.
- S. J. Tauster, S. C. Fung and R. L. Garten, J. Am. Chem. Soc., 2002, 100, 170–175 CrossRef.
- L. Assaud, N. Brazeau, M. K. S. Barr, M. Hanbücken, S. Ntais, E. A. Baranova and L. Santinacci, ACS Appl. Mater. Interfaces, 2015, 7, 24533–24542 CrossRef CAS PubMed.
- M. K. S. Barr, L. Assaud, N. Brazeau, M. Hanbücken, S. Ntais, L. Santinacci and E. A. Baranova, J. Phys. Chem. C, 2017, 121, 17727–17736 CrossRef CAS.
- S. Sayegh, J.-H. Lee, D.-H. Yang, M. Weber, I. Iatsunskyi, E. Coy, A. Razzouk, S. S. Kim and M. Bechelany, Sens. Actuators, B, 2021, 344, 130302 CrossRef CAS.
- M. Weber, J. Y. Kim, J. H. Lee, J. H. Kim, I. Iatsunskyi, E. Coy, P. Miele, M. Bechelany and S. S. Kim, J. Mater. Chem. A, 2019, 7, 8107–8116 RSC.
- X. Xiao, H. Wang, W. Bao, P. Urbankowski, L. Yang, Y. Yang, K. Maleski, L. Cui, S. J. L. Billinge, G. Wang and Y. Gogotsi, Adv. Mater., 2019, 31, e1902393 CrossRef PubMed.
- H. C. M. Knoops, L. Baggetto, E. Langereis, M. C. M. van de Sanden, J. H. Klootwijk, F. Roozeboom, R. A. H. Niessen, P. H. L. Notten and W. M. M. Kessels, J. Electrochem. Soc., 2008, 155, G287–G294 CrossRef CAS.
- M.-D. Cheng, T. Luoh, C.-T. Su, T.-H. Yang, K.-C. Chen and C.-Y. Lu, Thin Solid Films, 2010, 518, 2285–2289 CrossRef CAS.
- A. Shearrow, G. Koolstra, S. J. Whiteley, N. Earnest, P. S. Barry, F. J. Heremans, D. D. Awschalom, E. Shirokoff and D. I. Schuster, Appl. Phys. Lett., 2018, 113, 212601 CrossRef.
- J.-Z. Kong, P. Xu, Y.-Q. Cao, A.-D. Li, Q.-Z. Wang and F. Zhou, Surf. Coat. Technol., 2020, 381, 125108 CrossRef CAS.
- Z. Pan, Y. Xiao, Z. Fu, G. Zhan, S. Wu, C. Xiao, G. Hu and Z. Wei, J. Mater. Chem. A, 2014, 2, 13966–13975 RSC.
- S. Liu, W. Qi, S. Adimi, H. Guo, B. Weng, J. P. Attfield and M. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 7238–7247 CrossRef CAS PubMed.
- A. Kolmakov, D. O. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
- X. Wang, N. Aroonyadet, Y. Zhang, M. Mecklenburg, X. Fang, H. Chen, E. Goo and C. Zhou, Nano Lett., 2014, 14, 3014–3022 CrossRef CAS PubMed.
- M. Leskelä and M. Ritala, Thin Solid Films, 2002, 409, 138–146 CrossRef.
- R. W. Johnson, A. Hultqvist and S. F. Bent, Mater. Today, 2014, 17, 236–246 CrossRef CAS.
- A. S. Asundi, J. A. Raiford and S. F. Bent, ACS Energy Lett., 2019, 4, 908–925 CrossRef CAS.
- C. Marichy, M. Bechelany and N. Pinna, Adv. Mater., 2012, 24, 1017–1032 CrossRef CAS PubMed.
- C. H. Ahn, S. G. Cho, H. J. Lee, K. H. Park and S. H. Jeong, Met. Mater. Int., 2001, 7, 621–625 CrossRef CAS.
- J.-S. Min, Y.-W. Son, W.-G. Kang, S.-S. Chun and S.-W. Kang, Jpn. J. Appl. Phys., 1998, 37, 4999–5004 CrossRef CAS.
- J.-W. Lim, J.-S. Park and S.-W. Kang, J. Appl. Phys., 2000, 87, 4632–4634 CrossRef CAS.
- L. Assaud, K. Pitzschel, M. Hanbücken and L. Santinacci, ECS J. Solid State Sci. Technol., 2014, 3, P253–P258 CrossRef CAS.
- C. Badie, H. Tissot, B. Sciacca, M. K. Barr, J. Bachmann, C. Vallée, G. Gautier, T. Defforge, V. Astie, J.-M. Decams, M. Bechelany and L. Santinacci, J. Vac. Sci. Technol., A, 2022, 41, 032401 CrossRef.
- L. Assaud, E. Monyoncho, K. Pitzschel, A. Allagui, M. Petit, M. Hanbücken, E. A. Baranova and L. Santinacci, Beilstein J. Nanotechnol., 2014, 5, 162–172 CrossRef PubMed.
- M. Weber, C. Lamboux, B. Navarra, P. Miele, S. Zanna, M. E. Dufond, L. Santinacci and M. Bechelany, Nanomaterials, 2018, 8 CAS.
- A. J. Mackus, M. J. Weber, N. F. Thissen, D. Garcia-Alonso, R. H. Vervuurt, S. Assali, A. A. Bol, M. A. Verheijen and W. M. Kessels, Nanotechnology, 2016, 27, 034001 CrossRef PubMed.
- F. S. Fedorov, D. Settipani, M. E. Melandsø Buan, J. Sainio, F. S. M. Ali, D. Ilatovskii, T. Kallio and A. G. Nasibulin, ChemElectroChem, 2020, 7, 2651–2659 CrossRef CAS.
- J. W. Elam, A. Zinovev, C. Y. Han, H. H. Wang, U. Welp, J. N. Hryn and M. J. Pellin, Thin Solid Films, 2006, 515, 1664–1673 CrossRef CAS.
- P. C. Stair, J. Chem. Phys., 2008, 128, 182507 CrossRef PubMed.
- D. N. Goldstein and S. M. George, Thin Solid Films, 2011, 519, 5339–5347 CrossRef CAS.
- H. Feng, J. A. Libera, P. C. Stair, J. T. Miller and J. W. Elam, ACS Catal., 2011, 1, 665–673 CrossRef CAS.
- M. Weber, P. Collot, H. El Gaddari, S. Tingry, M. Bechelany and Y. Holade, ChemElectroChem, 2018, 5, 743–747 CrossRef CAS.
- T. L. H. Doan, J.-Y. Kim, J.-H. Lee, L. H. T. Nguyen, Y. T. Dang, K.-B. T. Bui, A. T. T. Pham, A. Mirzaei, T. B. Phan and S. S. Kim, Sens. Actuators, B, 2021, 348, 130684 CrossRef CAS.
- T. L. H. Doan, J.-Y. Kim, J.-H. Lee, L. H. T. Nguyen, H. T. T. Nguyen, A. T. T. Pham, T. B. Nguyen Le, A. Mirzaei, T. B. Phan and S. S. Kim, Sens. Actuators, B, 2021, 349, 130741 CrossRef CAS.
- D. B. Williams and C. B. Carter, Transmission Electron Microscopy, Springer, New York, NY, 2009 Search PubMed.
- D. Wanger, W. M. Riggs, L. E. Davis and J. F. Moulder, Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979 Search PubMed.
- N. C. Saha and H. G. Tompkins, J. Appl. Phys., 1992, 72, 3072–3079 CrossRef CAS.
- M. A. Al-Ghouti and D. A. Da'ana, J. Hazard. Mater., 2020, 393, 122383 CrossRef CAS PubMed.
- M. S. Choi, M. Y. Kim, A. Mirzaei, H.-S. Kim, S.-i. Kim, S.-H. Baek, D. Won Chun, C. Jin and K. Hyoung Lee, Appl. Surf. Sci., 2021, 568, 150910 CrossRef.
- V. Brynzari, G. Korotchenkov and S. Dmitriev, Sens. Actuators, B, 1999, 61, 143–153 CrossRef CAS.
- V. T. Nguyen, V. C. Nguyen, V. D. Nguyen, S. H. Hoang, N. Hugo, D. H. Nguyen and V. H. Nguyen, J. Hazard. Mater., 2016, 301, 433–442 CrossRef CAS PubMed.
- J.-H. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Sens. Actuators, B, 2019, 285, 358–367 CrossRef CAS.
- K. Nguyen, C. M. Hung, T. M. Ngoc, D. T. Thanh Le, D. H. Nguyen, D. Nguyen Van and H. Nguyen Van, Sens. Actuators, B, 2017, 253, 156–163 CrossRef CAS.
- A. Umar, H. Y. Ammar, R. Kumar, T. Almas, A. A. Ibrahim, M. S. AlAssiri, M. Abaker and S. Baskoutas, Int. J. Hydrogen Energy, 2020, 45, 26388–26401 CrossRef CAS.
- X. T. Yin, J. Li, Q. Wang, D. Dastan, Z. C. Shi, N. Alharbi, H. Garmestani, X. M. Tan, Y. Liu and X. G. Ma, Langmuir, 2021, 37, 13548–13558 CrossRef CAS PubMed.
- P. G. Choi, N. Izu, N. Shirahata and Y. Masuda, ACS Omega, 2018, 3, 14592–14596 CrossRef CAS PubMed.
- X. Zhang, J. Sun, K. Tang, H. Wang, T. Chen, K. Jiang, T. Zhou, H. Quan and R. Guo, Microsyst. Nanoeng., 2022, 8, 67 CrossRef CAS PubMed.
- E. Lee, Y. S. Yoon and D. J. Kim, ACS Sens., 2018, 3, 2045–2060 CrossRef CAS PubMed.
- G. Y. Chai, O. Lupan, E. V. Rusu, G. I. Stratan, V. V. Ursaki, V. Şontea, H. Khallaf and L. Chow, Sens. Actuators, A, 2012, 176, 64–71 CrossRef CAS.
- Y. Zhang, J. Xu, Q. Xiang, H. Li, Q. Pan and P. Xu, J. Phys. Chem. C, 2009, 113, 3430–3435 CrossRef CAS.
- Sunaina, K. K. Yadav, Ankush, S. K. Guchhait, K. Sood, S. K. Mehta, A. K. Ganguli and M. Jha, Sep. Purif. Technol., 2020, 242, 116835 CrossRef CAS.
- F. Fillot, T. Morel, S. Minoret, I. Matko, S. Maîtrejean, B. Guillaumot, B. Chenevier and T. Billon, Microelectron. Eng., 2005, 82, 248–253 CrossRef CAS.
- S. Dhall, M. Kumar, M. Bhatnagar and B. R. Mehta, Int. J. Hydrogen Energy, 2018, 43, 17921–17927 CrossRef CAS.
- J.-H. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Sens. Actuators, B, 2019, 297, 126693 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Schematic representation of the SnO2 NWs synthesis. ALD mechanisms for TiN and Pd depositions. EDS analysis of the SnO2 NWs after TiN and Pd depositions. I–V curves of composite materials. Transient resistance curves and response plots of synthesized materials. Response and recovery times of Pd/TiN (1 nm)/SnO2 NWs. See DOI: https://doi.org/10.1039/d3ta00020f |
| ‡ Co-first authors. |
| This journal is © The Royal Society of Chemistry 2023 |