Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: The role of ion solvation in lithium mediated nitrogen reduction
O.
Westhead
ab,
M.
Spry
a,
A.
Bagger
cd,
Z.
Shen
a,
H.
Yadegari
a,
S.
Favero
d,
R.
Tort
d,
M.
Titirici
de,
M. P.
Ryan
ae,
R.
Jervis
ef,
Y.
Katayama
g,
A.
Aguadero
aeh,
A.
Regoutz
i,
A.
Grimaud
*bjk and
I. E. L.
Stephens
*ae
aDepartment of Materials, Imperial College London, UK. E-mail: i.stephens@imperial.ac.uk
bSolid-State Chemistry and Energy Laboratory, UMR8260, CNRS, Collège de France, France. E-mail: alexis.grimaud@bc.edu
cDepartment of Chemistry, University of Copenhagen, Denmark
dDepartment of Chemical Engineering, Imperial College London, UK
eThe Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot, OX11 0RA, UK
fElectrochemical Innovation Lab, Department of Chemical Engineering, University College London, UK
gSANKEN, Osaka University, Japan
hInstituto de Ciencia de Materiales de Madrid ICMM-CSIC, Spain
iDepartment of Chemistry, University College London, UK
jRéseau sur le Stockage Electrochimique de l'Energie (RS2E), CNRS FR 3459, 80039 Amiens Cedex 1, France
kDepartment of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA, USA
First published on 13th January 2023
Abstract
Correction for ‘The role of ion solvation in lithium mediated nitrogen reduction’ by O. Westhead et al., J. Mater. Chem. A, 2023, https://doi.org/10.1039/D2TA07686A.
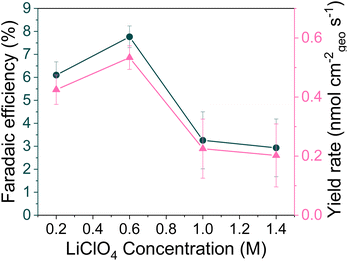
The authors regret an error in their calculation of the yield rate in Fig. 1b. Due to an error with the unit conversion the peak yield rate at 0.6 M LiClO4 was incorrectly given as 60 ± 3 nmol cm−2 s−1 (n = 3). The corrected yield rate is 0.53 ± 0.04 nmol cm−2 s−1 (n = 3) and the corrected version of Fig. 1b is provided herein.
An independent expert reviewed the data provided by the authors and concluded that it does not change the discussion or conclusions presented in the article.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |