Electrochemical biosensors based on saliva electrolytes for rapid detection and diagnosis
Jiayi
Liu
 a,
Yufei
Tang
ab,
Yuhua
Cheng
c,
Wei
Huang
a,
Yufei
Tang
ab,
Yuhua
Cheng
c,
Wei
Huang
 *c and
Lin
Xiang
*c and
Lin
Xiang
 *ad
*ad
aState Key Laboratory of Oral Diseases & National Clinical Research Centre for Oral Diseases, West China Hospital of Stomatology, Sichuan University, No 14th, 3rd section, Renmin South Road, Chengdu, 610041, China. E-mail: dentistxiang@126.com
bDepartment of Orthodontics, West China Hospital of Stomatology, Sichuan University, No 14th, 3rd section, Renmin South Road, Chengdu, 610041, China
cSchool of Automation Engineering, University of Electronic Science and Technology of China, Chengdu, 611731, China. E-mail: whuang@uestc.edu.cn
dDepartment of Oral Implantology, West China Hospital of Stomatology, Sichuan University, No 14th, 3rd section, Renmin South Road, Chengdu, 610041, China
First published on 15th November 2022
Abstract
In recent years, electrochemical biosensors (ECBSs) have shown significant potential for real-time disease diagnosis and in situ physical condition monitoring. As a multi-constituent oral fluid comprising various disease signaling biomarkers, saliva has drawn much attention in the field of point-of-care (POC) testing. In particular, during the outbreak of the COVID-19 pandemic, ECBSs which hold the simplicity of a single-step assay compared with the multi-step assay of traditional testing methods are expected to relieve the human and economic burden caused by the massive and long-term sample testing process. Noteworthily, ECBSs for the detection of SARS-CoV-2 in saliva have already been developed and may replace current testing methods. Furthermore, the detection scope has expanded from routine indices such as sugar and uric acid to abnormal biomarkers for early-stage disease detection and drug level monitoring, which further facilitated the evolution of ECBSs in the last 5 years. This review is divided into several main sections. First, we discussed the latest advancements and representative research on ECBSs for saliva testing. Then, we focused on a novel kind of ECBS, organic electrochemical transistors (OECTs), which hold great advantages of high sensitivity and signal-to-noise ratio and on-site detection. Finally, application of ECBSs with integrated portable platforms in oral cavities, which lead to powerful auxiliary testing means for telemedicine, has also been discussed.
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused the clinical disease named coronavirus disease 2019 (COVID-19) has led to a worldwide pandemic lasting for years. Because the incubation period (1 to 14 days, usually 3 to 7 days) of SARS-CoV-2 varies from person to person and asymptomatic infected persons are also sources of infection, epidemic prevention and control are significantly challenging. Moreover, current detection process still needs multiple steps, which are carried out by professionals via bench-type instruments, and require massive manpower and material resources. Therefore, on-site and real-time diagnosis strategies with high accuracy are urgently needed to support virus testing, optimize the treatment plan, and end the spread of SARS-CoV-2.Due to the completion of the isolation, culture, and gene sequencing of the novel coronavirus, the diagnosis of COVID-19 is mainly divided into genetic diagnosis and immunological diagnosis. Genetic diagnosis is mostly via reverse transcription-polymerase chain reaction (RT-PCR) detection, which targets the open reading frame 1ab (ORF1ab) and nucleocapsid protein N in the novel coronavirus genome. However, the results can be false because nucleic acid detection is easily affected.1 Falsely negative test results have appeared in many laboratories,2 resulting in inconsistencies between the nucleic acid test results and imagological examinations. The reasons behind this are numerous, including preanalytical error (substandard specimen quality, premature or late collection of specimens, inappropriate storage/transport, and/or handling of specimens) and analytical error (virus mutation which leads to failure of recognition between primers and the mutated sequence).3 Immunological diagnosis methods, mainly including enzyme-linked immunosorbent assay (ELISA) and colloidal gold lateral flow immunoassays (LFIA), typically show an advantage over genetic ones in detection accuracy. This is because these assays target the presence of antigens, a more convincing proof, by the antigen–antibody binding reaction. Due to a higher positive rate, this assay is considered more reliable in early clinical screening and asymptomatic diagnosis.4 Since it is the antibodies that chemically combine with the viral antigen and initiate the immune response, the remaining levels of antibodies in serum can reflect the health state of the patient and aid the detection of asymptomatic infection.5 Furthermore, the antibody levels of the crowds can also facilitate the evaluation of infection and immunity rates in epidemiological investigations.6 However, the technical procedure of ELISA is quite complicated, making it difficult to simultaneously achieve portability and high-throughput detection (automated equipment with the capability of rapidly testing millions of samples at the molecular level). On the other hand, although LFIA is easy to operate and portable, it can still lead to misdiagnosis as the assay demands high-quality samples, which are not easy to maintain.
It is validated that SARS-CoV-2 antigens exist in saliva and can be detected,7 and best of all, saliva can outdo serum when it comes to non-invasive sample collection and test conduction. However, since the level of antibodies in human saliva can be several orders lower than that in serum, higher analytical sensitivity and lower limit of detection (LOD) are expected. Thus, there is a clinical need to further reduce the LOD as much as possible while maintaining high detection accuracy. New strategies that can simplify the collection and preservation process to minimize pre-analytical errors and increase the sensitivity and specificity of the test, are highly expected. Among these emerging diagnostic strategies, electrochemical biosensors (ECBSs) stand out for rapid developments toward point-of-care (POC) diagnostics and large population screening, owing to their advantages in simplicity, portability, and high accuracy. Therefore, ECBSs can be used not only for infectious disease diagnosis (such as COVID-19), but also for that of non-infectious diseases (such as diabetes, drug intoxication, and cancer).
ECBSs that transduce biological responses and binding events into an electrical readout have made significant progress in recent years.8 In a typical ECBS, there are three electrodes, a working electrode (WE, an electrode for sensing), a reference electrode (RE, an ideal non-polarizable electrode that maintains a known potential), and a counter electrode (CE, a polarizable electrode which contacts the electrolytic solution to apply a current to WE). During operation, the device is immersed in the targeting electrolyte where the targeting analyst(s) exist(s). Thereupon, WE serves as a transducer, which produces voltametric, potentiometric, conductometric, and/or impedimetric signals when voltage is applied. Over the years, different materials have been used for electrodes, such as metals, ceramics, conductive carbon, polymers, etc. For instance, gold (Au) is among the most common electrodes owing to its high conductivity, stability, and ability to form a self-assembly monolayer (SAM) with thiol groups.9–11 Indium tin oxide (ITO), silicon, and carbon polystyrene are also utilized as working electrodes in ECBSs. Due to the low cost and good electrical conductivity of ITO, it has been widely used for functional electrodes with antibody modification through SAM.12,13 Meanwhile, a wide range of biomolecules such as enzymes, antibodies, nucleic acids, and their complexes have been modified onto WE as biorecognition elements to enhance sensing properties.14 Consequently, when applying a certain potential on WE, a specific electrochemical reaction initiates on WE depending on both the material(s) modified on WE and the analyst in the electrolyte. Due to the unique sensing mechanism and compatibility with various functional modifications on WE, ECBSs hold many advantages, which include real-time detection, good selectivity, low cost, miniaturization, etc.
To better immobilize biorecognition elements on the electrode, different kinds of nanomaterials to form binding layers (such as SAMs) have been developed.15 Specifically, significant progress has been made for advanced nanomaterials with optimized morphologies, dimensions, surface charges, and physicochemical properties. These nanomaterials can not only help with the immobilization of bioaffinity elements, but also facilitate reactions with target analytes. Nowadays, common types of nanocomposites are carbon, metal, metal oxides, or polymer based nanomaterials.15 For carbon-based nanomaterials, they typically perform as substrates for surface functionalization. Moreover, since carbon-based nanomaterials also hold high surface-to-volume ratios, high electrical conductivity, and good mechanical strength, lower LODs and higher sensitivities can be obtained.16 On the other hand, metal-based nanomaterials are normally employed as catalytic elements covering the electrode surface, which could facilitate the catalytic reactions. Through careful design of the surface and localized environments using capping agents and supramolecular interactions, the sizes, shapes, and facets of the metal-based nanomaterials can be controlled to achieve various customized nanoparticles. Therefore, the interactions between the metal nanomaterials and the targeted molecules are highly tunable by changing their morphology, composition, and structure.17 Consequently, selectivity towards specific analytes can be achieved by engineering their size, chemical composition, and adsorption strength. A variety of metal oxide nanoparticles have been adopted in ECBSs, including titanium oxide (TiO2), copper oxide (CuO), nickel oxide (NiO), etc.18 In terms of polymer nanomaterials, dendrimers, conducting polymers (CPs), and molecular-imprinted polymers are most common in ECBSs. In particular, active biomolecules can be easily immobilized on polymers without losing their biological activity, which helps to produce efficient conducting interfaces.19
Moreover, the application of various bio-nanomaterials greatly prompts the development of ECBSs due to the highly selective catalytic and recognition properties of these biomaterials, including enzymes, antibodies, antigens, and nucleic acids. Specifically, due to the advancement in synthesis, functionalization, and integration of bio-nanomaterial, ideal sensitivity and selectivity can be easily achieved.15 Examples and details about biomaterial-based ECBSs for the detection of biomolecules are further discussed in this review.
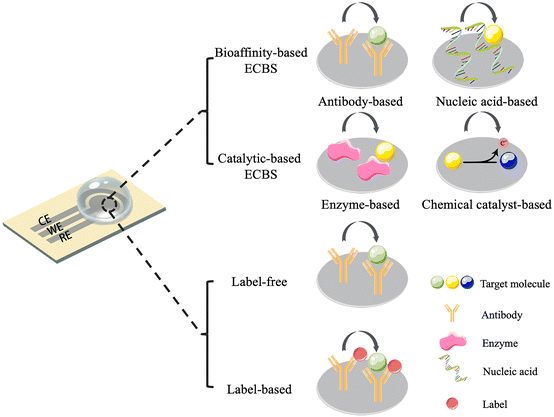
Based on the differences in biorecognition elements, ECBSs can be classified into bioaffinity-based biosensors and catalytic-based biosensors (Fig. 1). The former can be subdivided into nucleic acid-based ECBSs and antibody-based ECBSs. The main biorecognition elements used in nucleic acid-based ECBSs are aptamers, a kind of functional oligonucleotides that can capture specific targets with affinities. In addition to aptamer-based ECBSs, double-stranded DNAs and RNAs are also immobilized as biorecognition probes (biorecognition probes indicated in this review are functionalized WEs). While the catalytic-based biosensors are mainly about enzyme-based ECBSs or chemical catalyst-based ECBSs. As for catalyst-based ECBSs, both direct and indirect methods can be applied. The direct methods are mainly about the detection of reduction and oxidation processes using amperometry sensing. The indirect methods usually aim at common by-products of enzymatic reactions, such as H2O2, H2S, etc., to estimate the concentration of analytes.
On the other hand, depending on whether a label is attached, ECBSs can be classified into label-based and label-free biosensors (Fig. 1). Labels, which are normally redox reporter molecules, are directly attached to biorecognition elements or through a secondary binding step. Although label-free ECBSs are not drawing much attention now, it is believed that label-free ECBSs have a shorter response time and a simpler preparation process.
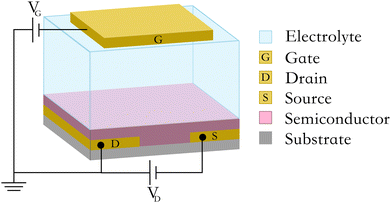
In recent years, organic electrochemical transistors (OECTs), an advanced type of ECBSs, have shown great potential in disease diagnosis. Typical bioaffinity-based ECBSs simply transduce target bio-signals to electrical signals (current or voltage variations), which are usually proportional to the amount of analyte–bioreceptor interactions.20 Consequently, signal outputs from these typical ECBSs are not amplified and show a relatively low signal-to-noise ratio. OECTs combine the advantages of both ECBS and transistors and have become a research highlight due to high transconductance (>mS), low driving voltage, flexibility, and biocompatibility. In particular, due to high transconductance (gm), OECTs hold the capability of sensing and amplification simultaneously. Therefore, OECT-based biosensors possess the merits of high sensitivity, low cost and easy fabrication, and are suitable for multiplexing and high-throughput detection of biomarkers.21 A typical OECT (Fig. 2) is composed of a gate, a source, and a drain electrode, with an organic semiconductor channel connecting the source and the drain, and an electrolyte medium connecting the gate and the channel. With ion-permeable conjugated polymers utilized as the channel, analytes would affect the injection process of ions from the electrolyte to the channel by modulating the effective gate bias amplitude.22–25 It should be stressed that the volumetric coupling between ionic and electronic charges in the channel improves the quality of recorded signals and makes OECTs powerful voltage amplifiers.26 As a result, even a few binding events at the gate electrode can lead to significant modulations in the channel current.27–29 Hence, thanks to the efficient on-site amplification of input signals, OECTs can miniaturize their size and reveal low-noise recording, making this type of device especially suitable for POC applications.28
Besides the amperometry method used by OECTs, which measured changes in the conductivity of the source-drain channel, there are many other electrochemical methods that belong to the ECBS category. These methods can be classified as potentiometry, amperometry, conductometry, and impedimetry. Detailed discussions of these electrochemical methods have been provided in other reviews.12,13,30 Here we briefly discussed some methods that are commonly used in recent years.
Potentiometry refers to a method where an electrical potential is measured in response to an applied current. An advantage of this approach is the suitability for label-free measurements.12,13,31 Voltammetry refers to a method where a current is measured in response to an applied electrical potential. Commonly used voltametric methods are chronoamperometry (CA), cyclic voltammetry (CV), square wave voltammetry (SWV), and differential pulse voltammetry (DPV). In CA, the electrical potential at the working electrode is applied in steps and varied with time.32 In CV, the electrical potential is swept in both the forward and reverse directions in partial cycles, full cycles, or a series of cycles.33 In SWV, a symmetric square-wave pulse is superimposed on a staircase potential waveform. The forward pulse of the waveform coheres with the staircase step.34 In DPV, the electrical potential is scanned with a series of fixed amplitude pulses and superimposed on a changing base potential.35 For all of these voltametric methods, the resulting current is measured as a function of time. Impedimetric methods refer to methods where the response to periodic applied current or potential waveforms at either a fixed frequency or over a range of frequencies is measured. In electrochemical impedance spectroscopy (EIS), the impedance and phase angle of the system are measured as a function of the frequency of the applied electrical potential. EIS is a versatile electrochemical method, which can be carried out as a faradaic/non-faradaic process, allowing for the study of biorecognition events at the electrode surface.13 Single Frequency Impedance (SFI) is a commonly used EIS technique where a single frequency is utilized as an excited signal instead of a wide frequency range. By using this technique, the interaction between antibody and antigen can be monitored easily. Unlike standard EIS, SFI measures electrochemical impedance at one frequency periodically.36 Electrochemical methods used in different studies are listed in Tables 1–3.
| Application | WE material | Target | Classification | Electrochemical probe | Electrochemical method | Linear region | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: SPCE: screen-printed carbon electrode; EIS: electrochemical impedance spectroscopy; DPV: differential pulse voltammetry; SPEAu: screen-printed gold electrode; CA: chronoamperometry; PEC: photo-electrochemical; SFI: single frequency impedance; reRNA: reporter RNA; WHTS: Wuhan variant; UKTS: UK variant trimeric spike protein; INTS: Indian variant trimeric protein; CV: cyclic voltammetry; SWV: square wave voltammetry; SARS-CoV-2 NP: SARS-CoV-2 nucleocapsid protein; MAMP: methamphetamine; GCE: glassy carbon electrode; Δ9-THC: delta-9-tetrahydrocannabinol; PB: Prussian-blue; METH: methamphetamine; DEVD: Asp-Glu-Val-Asp; GFP: green fluorescent protein; IgG: immunoglobulin G. | ||||||||
| Cancer diagnosis | SPCE | P53 | Antibody-based sensor | Ab-p53 | EIS | — | 0.16 Ucell per mL | 88 (2022) |
| ITO | SP17 | Antibody-based sensor | Ab-SP17 | DPV | 100–5000 pg mL−1 | 70.07 pg mL−1 | 89 (2022) | |
| PtNi | H2S | Catalytic-based sensor | PtNi alloy nanoparticles | Amperometry | 0.0125–1031 μM | 0.004 μM | 63 (2022) | |
| SPCE | CA125 | Antibody-based sensor | Ab-CA125 | DPV | 5–100 U | 1.18 U mL−1 | 90 (2021) | |
| Ag | Cyfra 21.1 | Antibody-based sensor | Ab-Cyfra21.1 | DPV | 0.0025–10 ng mL−1 | 0.0025 ng mL−1 | 57 (2021) | |
| SPEAu | TNF-α | Antibody-based sensor | Ab-TNF-α/TNF-α/Ab-TNF-α-HRP | CA | 1–15 pg mL−1 | 0.3 pg mL−1 | 91 (2019) | |
| Au | TNF-α | Antibody-based sensor | Ab-TNF-α-HRP | CA | 1–30 pg mL−1 | 1 pg mL−1 | 32 (2018) | |
| Au | PSA | Antibody-based sensor | Ab-PSA | PEC | 0.1 pg mL−1–100 ng mL−1 | 40 fg mL−1 | 59 (2018) | |
| ITO | IL-1β | Antibody-based sensor | Ab-IL-1β | SFI | 0.025–3 pg mL−1 | 7.5 fg mL−1 | 36 (2018) | |
| ITO | IL-8 | Antibody-based sensor | Ab-IL-8 | SFI | 0.02–3 pg mL−1 | 6 fg mL−1 | 61 (2018) | |
| ITO | TNF-α | Antibody-based sensor | Ab-TNF-α | EIS | 0.01–2 pg mL−1 | 3.7 fg mL−1 | 60 (2017) | |
| Au | CD59 | Antibody-based sensor | Ab-CD59 | EIS | 1–1000 fg mL−1 | 0.38 fg mL−1 | 56 (2016) | |
| Pathogenic virus detection | Graphene | SARS-CoV-2 spike protein | Antibody-based sensor | Ab-SARS-CoV-2 CR 3022 | DPV | 0.1 pg mL−1–500 ng mL−1 | 2.0 ng mL−1 | 69 (2022) |
| Au | SARS-CoV-2 spike protein | Nucleic acid-based sensor | reRNA tagged with MB and biotin | DPV | 1.0 × 10−1–1.0 × 105 fg mL−1 | 4.4 × 10−2 and 8.1 × 10−2 fg mL−1 for ORF and S genes | 35 (2022) | |
| Au | SARS-CoV-2 RNA and immunoglobulins | Enzyme-based sensor and Antibody-based sensor | Biotinylated ssDNA and Ag-SARS-CoV-2 | CV | — | 0.8 viral RNA copies μl−1 for SARS-CoV-2 RNA | 92 (2022) | |
| SPCE | SARS-CoV-2 spike protein | Antibody-based sensor | Ab-SARS-CoV-2 S1/S1/Ab-SARS-CoV-2 S1/HRP-IgG | CA | 0.5–3 ng mL−1 | 0.13 ng mL−1 | 93 (2022) | |
| SPCE | SARS-CoV-2 NP | Antibody-based sensor | Ab-N-protein | EIS | 0.01–100 ng mL−1 | 6 pg mL−1 | 94 (2022) | |
| Au | SARS-CoV-2 spike protein | Nucleic acid-based sensor | DSA1N5-SH | EIS | 4 fM–4.4 pM | 1, 2.8 and 3.6 fM for WHTS, UKTS and INTS | 71 (2021) | |
| GCE | SARS-CoV-2 NP | Antibody-based sensor | SARS-CoV-2-Ab2/SARS-CoV-2 NP/BSA/c-SARS-CoV-2-Ab1/Bi2WO6/Bi2S3/GCE | DPV | 0.01–1.00 pg mL−1 | 3.00 fg mL−1 | 95 (2021) | |
| GCE | SARS-CoV-2 spike protein | Antibody-based sensor | Ab-SARS-CoV-2 S1 | CV | 1 ag mL−1–10 fg mL−1 | 1 ag mL−1 | 33 (2021) | |
| Au | SARS-CoV-2 | Antibody-based sensor | DNA/Ab-SARS-CoV-2 S1 | CA | — | 4 × 103 viral particles mL−1 | 96 (2021) | |
| Au | SARS-CoV-2 N-gene | Nucleic acid-based sensor | Thiol-modified antisense oligonucleotides | Potentiometry | 585.4–5.854 × 107 copies μL−1 | 6.9 copies μL−1 | 97 (2020) | |
| Drug level monitoring | Au | MAMP | Nucleic acid-based sensor | 38-base aptamer sequence | SWV | 0.02–20 μM | 20 nM | 34 (2022) |
| GCE | Doxorubicin | Catalytic-based sensor | UiO-66-NH2 | DPV | 0.1 μM–75 μM | 0.051 μM | 98 (2022) | |
| Pt | Δ9-THC | Antibody-based sensor | Ab-THC | Amperometry | 0.1 nM to 5 μM | 0.1 nM | 87 (2021) | |
| PB carbon | Alcohol and Δ9-THC | Enzyme-based sensor | AOx | Amperometry and SWV | 0.2–1 mM and 1–6 μM | 0.2 mM and 0.5 μM | 99 (2020) | |
| GCE | METH | Antibody-based sensor | Ab-METH | DPV | 10–100 μg mL−1 | 13.07 μg mL−1 | 81 (2016) | |
| WE material | Target | Classification | Electrochemical probe | Linear region | LOD | Ref. |
|---|---|---|---|---|---|---|
| Abbreviations: Δ9-THC: delta-9-tetrahydrocannabinol; DEVD: Asp-Glu-Val-Asp; GFP: green fluorescent protein; IgG: immunoglobulin G. | ||||||
| Au | SARS-CoV-2 spike protein | Antibody-based sensor | Alpaca-derived nanobody Ty1 | 30 aM–300 nM | 100 aM | 103 (2022) |
| Pt | Δ9-THC | Antibody-based sensor | Ab-THC antibody | 0.1 nM to 5 μM | 0.1 nM | 87 (2021) |
| Au | caspase-3 | Antibody-based sensor | DEVD peptide | 0.1–100 pM | 0.1 pM | 114 (2021) |
| Au | SARS-CoV-2 and GFP | Antibody-based sensor | Anti-GFP nanobody, SARS-CoV nanobody, and MERS nanobody | — | 23 fM, 1.5 fM for GFP and SARS-CoV-2 | 8 (2021) |
| Au | SARS-CoV-2 IgG | Antibody-based sensor | Spike protein | 10 fM–100 nM | 1 fM | 6 (2021) |
| Au | C-reactive protein | Antibody-based sensor | Anti-CRP protein | — | 13 ± 4 proteins | 115 (2019) |
| Au | Glucose, lactate, and cholesterol | Enzyme-based sensor | Glucose oxidase, lactate oxidase, and cholesterol oxidase | 0.02–1 × 10−3 M, 0.1–2 × 10−3 M and 0.01–0.7 × 10−3 M for glucose, lactate and cholesterol | 10 μM, 50 μM, and 10 μM for glucose, lactate, and cholesterol | 102 (2016) |
| Pt | Uric acid | Enzyme-based sensor | Enzyme uricase | 100 × 10−9–500 × 10−6 M | 30 × 10−9 M | 101 (2015) |
| Supporting platform | WE Materials | Targets | Electrochemical probe | Electrochemical method | Linear region | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: PB: Prussian blue; Δ9-THC: delta-9-tetrahydrocannabinol; AOx: alcohol oxidase; SWV: square wave voltammetry; CA: chronoamperometry; RF: radiofrequency; CV: cyclic voltammetry; AMPs: antimicrobial peptides; EIS: electrochemical impedance spectroscopy. | |||||||
| Ring | PB carbon | Alcohol and Δ9-THC | AOx | Amperometry and SWV | 0.2–1 mM and 1–6 μM for alcohol and Δ9-THC | 0.2 mM and 0.5 μM for alcohol and Δ9-THC | 99 (2020) |
| Pacifier | PB carbon | Glucose | Glucose oxidase | CA | 0.1–1.4 mM | 0.04 mM | 139 (2019) |
| Tooth enamel | Au | PH and temperature | Hydrogel-based interlayers | EIS | 3–5 and 27–45 °C for PH and temperature | — | 135 (2018) |
| Palate mucosa | Pd | Sodium | Sodium membrane | Potentiometry | 10−4–1 M | 10−4 M | 138 (2018) |
| Mouthguard | Pt | Glucose | Glucose oxidase | CV | 5–1000 μmol L−1 | 1 μmol L−1 | 133 (2016) |
| Mouthguard | PB carbon | Uric acid | Uricase | CA | 0–1 mM | — | 132 (2015) |
| Mouthguard | PB carbon | Lactate | Lactate oxidase | CA | 0.1–1.0 mM | 0.050 mM | 131 (2014) |
| Tooth enamel | Au | H. pylori | AMPs | EIS | 100–106 bacterial cells | ∼100 cells | 134 (2012) |
As novel materials, functionalization strategies, and electrochemical techniques are continuously advancing, ECBSs for saliva testing are growing rapidly, especially for clinical use such as cancer diagnosis, pathogenic virus detection, and drug level monitoring. It is worth noting that, a biosensor consists of a transducer and a biorecognition element. While, most existing reviews about ECBSs mainly focus on the former part, with detailed discussions on electrode materials, fabrication methods,37–40 and electrochemical techniques,41 a few reviews focused on the classification of typical biorecognition elements,12,13,42–44 wearable ECBS platforms for sweat, tear, urine, and saliva.45,46 However, a comprehensive review that critically concludes and evaluates the emerging ECBSs based on saliva electrolytes for rapid detection and diagnosis, including their similarities and differences in the fabrication process and clinical applications, is still missing.
In this review, we focus on recent advancements in ECBSs for saliva testing or oral cavity application along with detailed technical discussions. Particularly, this review not only focuses on ECBS design for various conventional metabolite molecules but also underlines novel device structures and integrated systems that have potential in the prognosis of diseases and continuous monitoring of patient health conditions through saliva-based sensing. Finally, we also provide views on performance optimization and application scope expansion of ECBSs for future work from biological and clinical points of view.
2 Advancements in biomarker detection in saliva by ECBSs
Saliva, an easy-accessible biofluid with a lot of vital biomarkers, has been identified to be an important specimen in POC to achieve non-invasive disease diagnosis and continuous health/treatment monitoring in recent years.47 However, concentrations of various biomarkers in saliva can be as low as fM, and vary by approximately 10 orders of magnitude (from fM to mM level).48,49 Therefore, it is challenging to develop biosensors that cover both the LOD and full concentration scale.50 ECBSs are well-known sensors with low LOD and a wide sensing range, which make them appropriate biomarker sensors for saliva.51 In the following sections, we will discuss in detail the latest developments and representative examples of continuous physiological monitoring and rapid detection of biomarkers in saliva via ECBSs. These research progresses not only provide state-of-the-art results for the detection of various metabolites but also are applicable for the diagnosis of some systemic diseases and also for drug level monitoring. A comprehensive summarization of ECBSs for saliva testing is also provided in Table 1, where materials, targeting analytes, sensing performance, along with other important parameters of ECBSs, are included.2.1 Cancer-related biomarker detection
Since saliva is extensively supplied by blood, molecules such as DNAs, RNAs, proteins, metabolites, and microbiota, which are present in blood, can also be found in saliva.52 The prognosis of cancer and continuous monitoring of cancer patients’ physiological conditions are of supreme importance for effective treatment and enhanced patient survival rate. Recently, development in advanced surface functionalization techniques and high-throughput electrochemical sensor fabrication processes has put the designation of on-site detection devices at the forefront of creating alternatives to conventional laboratory tests. The most general biorecognition element for electrochemical detection of cancer cells is antibodies,53 where the combination of antigens and antibodies leads to the transduction of current or voltage signals in ECBSs.For example, tumor necrosis factor-α (TNF-α) has complex and powerful effects on both inducing apoptosis in cancer cells and supporting cancer cell growth, and has drawn wide attention in cancer-related studies.54,55 ECBS for TNF-α cytokine detection in human saliva was thus designed via immobilizing TNF-α antibody (Ab-TNF-α) and a secondary antibody, anti-TNF-α labeled with horseradish peroxidase (HRP) (antibody conjugate HRP), on gold WE (Fig. 3a and Table 1). The use of a secondary antibody was investigated using tetramethyl benzidine (TMB) as an electrochemical substrate. The sandwich-type detection strategy was employed for TNF-α detection through the labeled antibody Ab-TNF-α-HRP activity in a TMB solution. Then, chronoamperometric (CA) analysis was performed for each TNF-α antigen concentration level.32 Specifically, the anti-TNF-α antibody and the secondary antibody were immobilized onto gold electrodes through 4-carboxymethylaniline (CMA), where CMA provides carboxylic acid groups for the antibody to attach. Compared with DNA probes commonly labelled by redox reporters like methylene blue (MB) and ferrocene (Fc), the antibody was rarely labelled. HRP label used here is believed to amplify the signal effectively which originates from the increased current result from the sandwich-type detection. Although the detection process was reported to last over 30 min because of the time-consuming incubation process in the TNF-α-HRP solution, the strategy showed an excellent linear relation between current amplitude and TNF-α concentration in the range of 1–30 pg mL−1 (Fig. 3b).32
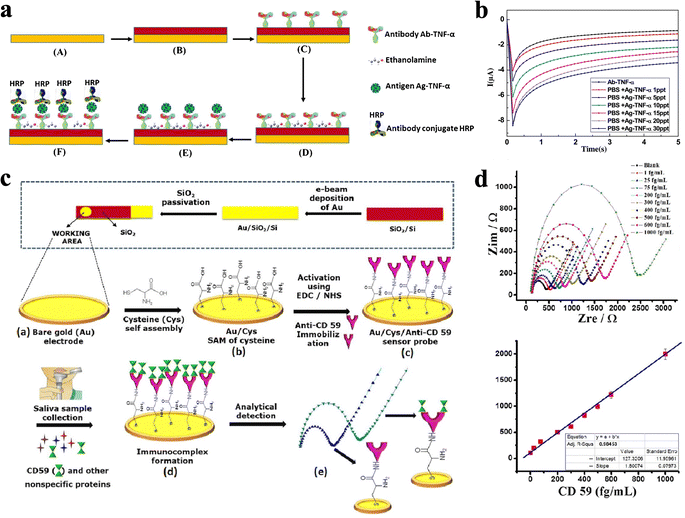 | ||
| Fig. 3 (a) Schematic illustration of the preparation process, where the WE surface was functionalized through incubation with Ab-TNF-α and Ag-TNF-α solution. (b) CA measurements of Ag-TNF-α within the range of 1–30 pg mL−1.32 Copyright 2016, Elsevier. (c) Schematic representation of oral cancer biosensor fabrication and the detection principle, where Au surface was functionalized by L-cysteine (Cys) and anti-CD59 antibodies. (d) Calibration plots based on the signal obtained from the EIS data between 1 and 1000 fg mL−1 CD59 concentrations.56 Copyright 2018, Wiley. | ||
Unlike TNF-α, which acts as a pleiotropic cytokine and plays a dual function in the development of cancer. Cluster of differentiation 59 (CD59) was validated as one of the most important and clinically relevant biomarkers in the early stage of oral cancer. Choudhary et al.56 developed a label-free impedimetric biosensor to diagnose oral cancer by immobilizing CD59 antibodies on gold WE through L-cysteine SAM (Fig. 3c and Table 1). The developed immunosensor is successfully applied for the direct detection of CD59 in clinically relevant human saliva samples. Such ECBS was applied for the detection of CD59 in clinically relevant human saliva with a detection range of 1–1000 fg mL−1. Moreover, the developed biosensor can detect CD59 in the mixed sample solution and shows low LOD values of 0.38 fg mL−1 and 1.46 fg mL−1 in standard buffer and untreated human saliva samples (Fig. 3d).56
Serum cytokeratin fragment 21.1 (Cyfra 21.1), known as a promising biomarker in oral cancer detection, can also be monitored using an antibody-based biosensor. Tofighi et al.57 established a paper-based biosensor by using silver nano-ink as the electrode materials, which adopts Ab-Cyfra 21.1 for the binding of Cyfra 21.1 (Fig. 4a). This ECBS reaches a low LOD of 0.0025 ng mL−1 and linearity in the range of 0.0025–12.5 ng mL−1 (Fig. 4b and Table 1). Note, the utilization of paper as the substrate was believed to hold the merits of biocompatibility, porous nature, flatness, and presence of capillary forces.58 Due to the porosity of the paper substrate, it also works for antibody stabilization and can provide portable analytical tools with high detection sensitivity.
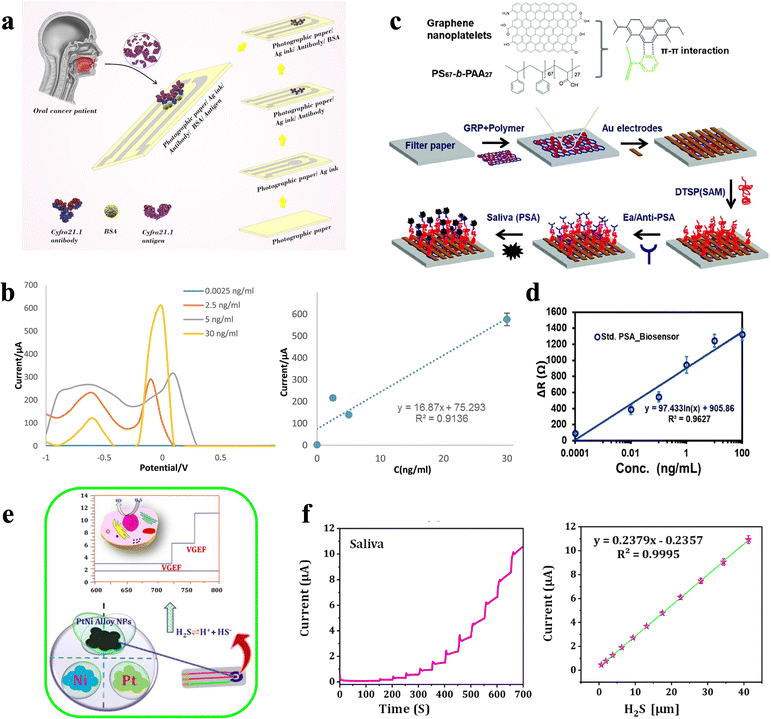 | ||
| Fig. 4 (a) Schematic illustration of a paper-based immunosensor for the detection of Cyfra21.1 biomarker. (b) Differential pulse voltammetry (DPVs) of paper-based immunosensor in various concentrations of Cyfra21.1. and the calibration curve of the data.57 Copyright 2021, Wiley. (c) Schematic illustration of the fabrication process of paper-based ECBS for PSA, where graphene-polymer was spin coated on filter paper followed by deposition of the Au electrode and immobilization of Ab-PSA. (d) Calibrated range for PSA solution from 0.1 pg mL−1 to 100 ng mL−1 and an LOD of 40 fg mL−1.59 Copyright 2022, Royal Society of Chemistry. (e) Schematic representation of PtNi alloy NP formation and electrochemical detection of H2S in a human breast cancer cell. (f) PtNi sensor showing a good linear relation and decent recovery values with an average recovery of 98.8% in saliva.63 Copyright 2022, MDPI. | ||
Moreover, Khan et al.59 developed a novel paper-based ECBS which utilized the Ab-prostate-specific antigen (PSA) as binding elements to detect prostate-specific antigen (PSA) in saliva samples (Fig. 4c). PSA is a common biomarker for the early detection of prostate cancer and can be used for the monitoring of therapeutic response. It has been validated that saliva PSA is directly correlated with blood PSA, which makes PSA a valuable alternative to invasive detection in the serum and whole blood. The performance of this ECBS is measured using impedance methods, which shows a linear range from 0.1 pg mL−1–100 ng mL−1 with a low LOD of 40 fg mL−1 for PSA detection (Fig. 4d and Table 1). This well-designed biosensor, which holds multilayer structure, including filter paper and graphene-polymer nanocomposite, facilitates the absorption of the fluid component in saliva samples.
Aydın et al. utilized functionalized ITO as WE for cancer biomarker detection and showed successful detection of TNF-α,60 interleukin-8 (IL-8),61 interleukin-1β (IL-1β)36 in saliva, all of which can aid early-stage cancer diagnosis, and have been validated to be closely related to their levels in serum (Table 1). Notably, they claimed that they were the first to apply 6-phosphonohexanoic acid (PHA) to form SAM on WE, which offers a larger surface area for the immobilization of antibodies. Consequently, the detection ranges of these ECBS with functional ITO WEs, reach physiological limits of 3.7 fg mL−1, 6 fg mL−1, and 7.5 fg mL−1 for TNF-α, IL-8, and IL-1β, respectively.
Note, for the antibody-modified electrodes, the choice of antibody is vital. If feasible, antibodies used as biorecognition should be monoclonal, whose dissociation constants with partner proteins can be less than 10 nM.62 Even though it might not be necessary to reach such low LOD to screen specific target protein, low LOD makes it possible for the collected sample to be diluted immensely, which could reduce antibody cross-reactivity and decrease nonspecific binding interferences. Furthermore, when target protein levels are well over the limit of detection, the high sensitivity can be exchanged for a shorter testing time in certain cases.62 Meanwhile, an indirect detection strategy, which instead of targets on the biomarkers themselves (including secreted protein, enzyme, and nucleic acid), focuses on the sensing of their corresponding metabolites, such as metabolic inorganic matter, has been developed. For instance, hydrogen peroxide (H2O2) and hydrogen sulfide (H2S) are two typical indirect targets, as they are by-products in many reduction reactions that relate to cancer cells. Panda et al.63 constructed an ECBS for hydrogen sulfide (H2S) sensing with PtNi alloy nanoparticles (PtNi NPs) on WE as electrocatalytic elements (Fig. 4e and Table 1). This ECBS could monitor H2S released by human breast cancer cells in situ and quantify H2S in saliva and other samples. With good biocompatibility, this device could be cultured with human breast cancer cells and reach a sensitivity of 0.323 μA μM−1 cm−2. Using amperometry measurements, the PtNi sensor showed a good linear relation and decent recovery values with an average recovery of 98.8% in saliva (Fig. 4f).63
2.2 Pathogenic virus detection
The capability of sensors to detect infectious viruses rapidly and directly in saliva could effectively reduce test costs, allow a more efficient diagnostic process, and track the progression of diseases. In bioaffinity-based biosensors, probes decorated with nucleic acid or protein, function as alternatives to antibodies for the recognition of viruses sensitively and selectively by matching specific virus characteristic proteins. For instance, for SARS-CoV-2, its spike protein, which sticks out and forms like a crown on the surface of the spherical virus, is widely regarded as a prime target.64–68 Typically, a bioaffinity-based biosensor induces conformational change due to biorecognition between the virus and the probe and leads to voltage/current variation of the device. For redox reporter-modified probes, protein level variation also promotes a distance shift between the redox reporter and the gold surface, which then produces quantitative electrochemical signals. Due to the quick response time (less than a few seconds), and a single-step sensing process, these sensors are expected to be used in massive and fast detection.As one of the major structural proteins, SARS-CoV-2 spike (S) protein, comprising receptor binding domain (RBD), S1, and S2 protein as subunits, are common targets for virus detection. Jaewiaroenwattana et al.69 fabricated a paper-based ECBS for BD spike protein detection of SARS-CoV-2 in swabs and saliva samples using DPV (Fig. 5a). Monoclonal antibody (mAb) CR3022, a kind of recombinant antibody which was created using plant transient expression system, was used to specifically binding RBD, and reached a low LOD of 2.0 fg mL−1 and a linear relationship from 0.1 pg mL−1 to 500 ng mL−1. Moreover, the application of a paper substrate provides the biosensor with foldability which allows multilayer designs. For this paper-based biosensor, the designed device comprises two parts, one contains the system with three electrodes while the other is folded and attached with an absorbent pad in order to utilize the wicking ability (Fig. 5b).
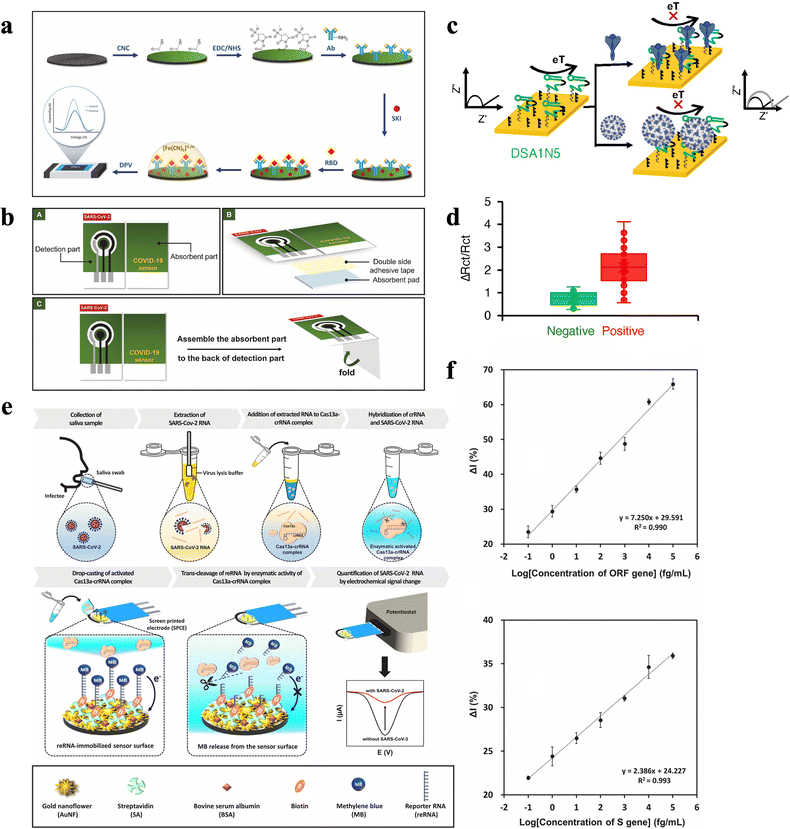 | ||
| Fig. 5 (a) Process of mAb CR3022 covalent immobilization onto the electrode surface for electrochemical detection of SARS-CoV-2 RBD. (b) Illustration showing the design and operation of the paper-based immunosensor.69 Copyright 2022, Elsevier. (c) Schematic of the electrochemical assay for the detection of SARS-CoV-2 using a spike protein aptamer. The electrochemical biosensor, based on a high-affinity dimeric DNA aptamer for the SARS-CoV-2 spike protein, was designed to detect the wild-type and Alpha and Delta variant viruses. (d) Signal change measured on the Cov-eChip using 36 clinically-obtained COVID-19 positive (red) and 37 negative (green) patient saliva samples.71 Copyright 2021, Wiley. (e) Schematic illustration of the electrochemical biosensor utilized with the CRISPR/Cas13a for SARS-CoV-2 detection, where the activated Cas13a–crRNA complex is subsequently loaded onto the sensor surface for cleaving the RNA immobilized on the electrode. (f) Current variation of the constructed sensor under different concentrations of ORF and S genes, respectively.35 Copyright 2021, Elsevier. | ||
Moreover, due to the fast emergence of various variants of the SARS-CoV-2 spike protein, the corresponding adjustment on the recognition elements needs to be applied accordingly.70 In this case, ECBSs with specifically designed probes are capable of recognizing different variants of the spike proteins simultaneously. For instance, Zhang et al.71 utilized a novel dimeric DNA (DSA1N5) as the recognition element on WE, which can specifically recognize pseudotyped lentiviruses expressing either wildtype or alpha trimeric spike proteins (Fig. 5c and Table 1). Hence, such a sensor can detect the wildtype SARS-CoV-2 virus as well as the alpha and delta variants in patients’ saliva samples. To be specific, this ECBS can detect 1000 viral particles per mL in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diluted saliva within 10 min without any further sample processing. Moreover, the evaluation of 36 positive and 37 negative patient saliva samples produces a clinical sensitivity of 80.5% and a specificity of 100%, which is the first reported rapid test that can detect multi-emerging variants of SARS-CoV-2 (Fig. 5d).71
1 diluted saliva within 10 min without any further sample processing. Moreover, the evaluation of 36 positive and 37 negative patient saliva samples produces a clinical sensitivity of 80.5% and a specificity of 100%, which is the first reported rapid test that can detect multi-emerging variants of SARS-CoV-2 (Fig. 5d).71
Moreover, short nucleotide sequences in viruses can also act as targets in the detection of many diseases, mainly depending on if they can be recognized by relevant nucleic acids. It is validated that short nucleotide sequences, such as RNA, single-stranded DNA, and double-stranded DNA, consisting of saliva or other biofluids can reflex physiological changes in humans and play promising roles in early diagnosis and intervention.72 However, the concentration of virus nucleic acids is lower than pM level, which makes direct detection via traditional assays extremely difficult.62 Moreover, traditional ways of nucleic acid detection always require complex and multifarious processes (such as PCR, which includes amplifying and copying of small segments of DNA, and demands a qualified laboratory technical staff, high-quality laboratory instruments, and fine chemical reagents). Consequently, ECBSs with ultra-high sensitivity are expected to provide amplification-free detections via adopting a nucleic acid probe. Heo et al.35 developed an ECBS that targets the SARS-CoV-2 RNA by using the Cas13a-crRNA complex (an enzyme and nucleic acid complex) modified WE via the combination of a specific sequence of SARS-CoV-2 RNA, spike protein (S) genes and an open reading frame (ORF) (Fig. 5e and Table 1). Consequently, the LODs of S genes and ORF are as low as 8.1 × 10−2 fg mL−1 and 4.4 × 10−2 fg mL−1, respectively (Fig. 5f). It thus demonstrated a process for the detection of ultra-low concentrations of SARS-CoV-2 that is free of amplification, and provided new insights in nucleic acid detection.35
Moreover, as nonimmunogenic substances, nucleic acid probes show advantages of high stability, ease of preparation, and non-toxicity in bio-analytical assays.73 However, to determine the aptamers’ capability of binding specific target molecules, systematic evolution of ligands by exponential enrichment (SELEX, an in vitro approach) is required. This approach serves as an iterative screening process that selects nucleic acid molecules from a large pool of oligonucleotides of variant sequences, followed by multiple rounds of enrichment of bound oligonucleotides.74Via the selecting assay, aptamers are then optimized for higher affinity and selectivity with specific target molecules, which can cover an extensive range, from tiny molecules such as drugs and ions to whole cells such as cancer cells and bacteria.75
2.3 Drug level evaluation
Non-invasive analysis methods, such as the analysis of saliva, may promote home-based therapeutic drug monitoring (TDM) service and simplify the TDM process. Such methods could be beneficial for some special populations such as infants and seniors, who have a high risk of infection. Besides, compared with urine, sweat, and other non-invasive samples, saliva also has the advantages of good privacy, on-site collection, and simple collection procedures.76–78 In recent years, physiologists have revealed that drug levels between saliva and blood show good linearity in most circumstances.79,80 Thereupon, with the establishment of a correction factor, saliva may serve as an alternative to blood or plasma in drug level evaluation. In recent years, ECBSs were also adopted for the evaluation of drug levels in saliva and showed high selectivity and sensitivity.For instance, Demir et al.81 established a new platform to monitor methamphetamine (METH) in saliva by using an ECBS based on antibody functionalized WE (Fig. 6a and Table 1). It is demonstrated that an ECBS, in which METH antibody was immobilized on a fluorescent-labeled polypeptide, acts as a matrix through glutaraldehyde as a linker for the electrochemical analysis. Good linearity in the detection range of 10–100 μg mL−1 and an LOD of 13.07 μg mL−1 are achieved (Fig. 6b).81 To test the selectivity of the biosensor, possible interferants (including chlorpromazine, diazepam, and bupropion HCl) were applied and did not create significant changes in the current signals obtained by differential pulse voltammetry. Spike and recovery measurements were also conducted, where a good recovery value of around 93% in saliva was obtained.81
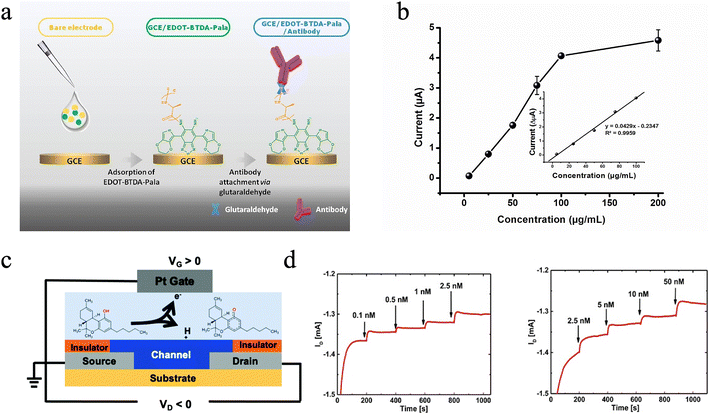 | ||
| Fig. 6 (a) Schematic of the electrochemical biosensor working electrode for the analysis of methamphetamine (METH), where a fluorescent-labeled polypeptide (EDOT-BTDA-Pala) is employed as a glassy carbon electrode (GCE) covering the host, to immobilize the METH-selective antibody. (b) Calibration curve of the ECBS with different METH concentrations generated by the DPV method.81 Copyright 2016, Elsevier. (c) Schematic of the OECT-based Δ9-THC sensor, where a platinum gate coupled with an aerosol jet printed OECT is constructed and can detect Δ9-THC concentration via its oxidation reaction at the gate. (d) Drain current response of the OECT showing repeatable results when exposed to different concentrations of Δ9-THC.87 Copyright 2014, Royal Society of Chemistry. | ||
Other than bioaffinity-based ECBSs for drug level evaluation, catalytic biosensors which utilize redox reaction between the probe and the target were also investigated. For instance, cannabis, which possesses strong neurotoxicity, remained elusive in terms of its effective and quantitative detection. Therefore, on-site detection of cannabis quantities in the human body is of great importance for public safety and health. Δ9-Tetrahydrocannabinol (Δ9-THC), a psychoactive ingredient in cannabis products, was chosen as the target biomolecular.82 In blood, the dwell time of Δ9-THC is transitory after consumption,83,84 while its level in saliva provides a more accurate indication of cannabis consumption with a concentration of 3.18–1039.88 nM.85,86 Consequently, Majak et al.87 develop an ECBS for Δ9-THC detection based on an organic electrochemical transistor (OECT), which shows LOD values of only 0.1 nM and 1 nM, in diluted deionized water and synthetic saliva buffer, respectively (Fig. 6c and Table 1). Furthermore, decent selectivity and repeatability are achieved through the selective oxidation reaction at the gate. For example, different concentrations of methanol as interference only resulted in a transient spike in drain current due to the oxidation of methanol at the Pt gate, while did not affect Δ9-THC measurement. Different devices were tested with incremental Δ9-THC concentrations, and all show repeatable sensing results (Fig. 6d). It is noteworthy that the sensitive gate electrode based on platinum was not functionalized, and the detection of Δ9-THC concentration relied on the self-initiated oxidation reaction at the platinum gate.87
3 Organic electrochemical transistors (OECTs) for biomarker detection in saliva
Due to the low concentration of vital biomarkers in biofluids (typically lower than fM) in nature, effective detection requires sensors with ultra-high sensitivity and low LOD.100 Thanks to the efficient on-site amplification capability of input signals, OECTs hold the merit of miniaturization and low-noise recording, which makes them especially suitable for POC applications. Therefore, many studies have shown that OECTs can be successfully utilized as outstanding enzyme biosensors for non-invasive detection in saliva. For instance, Liao et al. develop flexible OECTs for uric acid (UA) detection (Fig. 7a and Table 2). The sensitivity and selectivity of the sensors can be simultaneously enhanced by co-modifying the gate electrodes with charged bilayer polymer films, where graphene oxide (GO) acted as the matrix for effective enzyme immobilization. GO provides functional groups which can react with the amine groups of the protein enzyme, leading to better enzyme immobilization via the chemical covalent bonding. Notably, such sensors are successfully utilized for non-invasive UA detection, with an LOD of 10 × 10−9 M and good linearity in a wide range of UA levels (100 × 10−9 M to 500 × 10−6 M), which covers the levels of UA in human saliva (Fig. 7b).101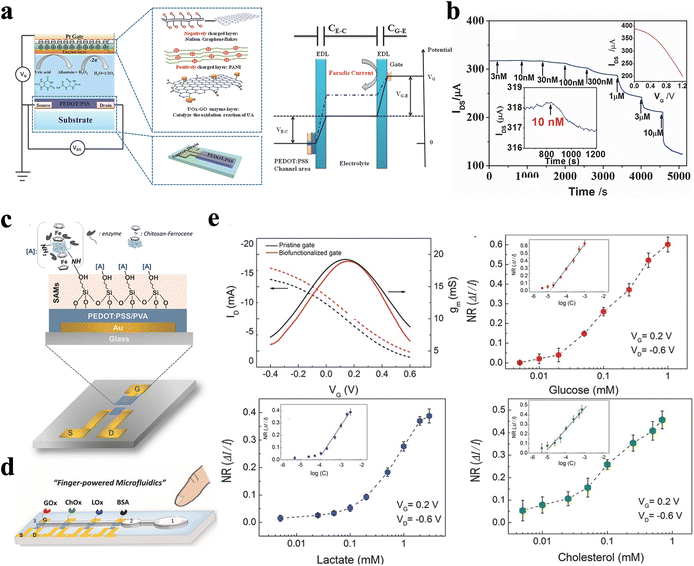 | ||
| Fig. 7 (a) Schematic diagram of an OECT with a UOx-GO/PANI/Nafion-graphene/Pt gate electrode, and (b) current responses of the OECT to the additions of UA.101 Copyright 2014, Wiley. (c) Schematic diagram of the OECT device structure and an illustration of the generic biofunctionalization (functionalized with CS-Fc/GOx complex) scheme at the gate electrode. (d) Schematic illustration of the biosensing multiplatform. (e) Transfer characteristics and normalized calibration curves derived from the chronoamperometric response of the OECTs after successive additions of increasing concentrations of glucose, lactate, and cholesterol, respectively.102 Copyright 2014, Wiley. | ||
Pappa et al.102 also develop a multiplexed biosensor composed of an OECT microarray, which shows the capability for glucose, lactate, and cholesterol sensing in real saliva samples, simultaneously (Fig. 7c, d and Table 2). A novel biofunctionalization method is applied, which provides selectivity towards specific metabolites as well as minimization of any background interference. Specifically, the conducting polymer, composed of PEDOT:PSS and polyvinyl alcohol (PVA), provides free hydroxyl groups on the surface for covalent attachment of a heterobifunctional silane (3-glycidoxypropyltrimethoxysilane, GOPS). Then, different proteins, including glucose oxidase, lactate oxidase, and cholesterol oxidase for the detection of glucose, lactate, and cholesterol, respectively, are then immobilized on each gate electrode of the four OECTs, with bovine serum albumin (BSA) immobilized on the fourth electrode as the control. Such OECTs show low LOD values of 10 μM, 50 μM, and 10 μM for glucose, lactate, and cholesterol, respectively (Fig. 7d). Moreover, to avoid crosstalk, each OECT sensor in microarray holds individual source and drain electrodes. Such a strategy sets an applicable model for other enzymatic biosensors where multi-analysis exists in targeting liquid.102
Furthermore, OECTs hold the capability for effectively sensing various biomolecules, such as proteins, metabolites, nucleic acids, cells, and, especially, viruses, where detection of viruses in saliva has becoming the global spotlight.6,21,25,104–113 Consequently, Guo et al.8 developed a nanobody-functionalized OECT with a modular architecture for on-site quantification of specific proteins, such as green fluorescent protein and SARS-CoV-2 spike protein, in complex body fluids (Fig. 8a and Table 2). As shown in Fig. 8a, a synthetic SpyTag peptide is immobilized on the 1,6-hexanedithiol (HDT) monolayer to form a chem-SAM. The nanobody–SpyCatcher fusion protein then attaches itself to this chemical layer through the autocatalytic formation of a covalent SpyCatcher–SpyTag bond to form the bio-SAM. Notably, the nanobody domain defines sensor specificity and can be reprogrammed to detect any protein antigen using the corresponding nanobody. When using the anti-GFP nanobody, the sensor can respond to a GFP concentration as low as 4.7 aM with a dynamic range spanning ten orders of magnitude. When using the SARS-CoV nanobody, the sensor can detect SARS-CoV-2 S1 at 4.7 aM with a 30% change in the normalized response (Fig. 8b).8 Liu et al.6 further demonstrated OECT-based biosensors for the detection of SARS-CoV-2 IgG (Fig. 8c), which show ultralow IgG protein LOD values of 1 fM in aqueous solutions and 10 fM in saliva, respectively (Fig. 8d and Table 2). Such approaches offer advantages for rapid screening of massive samples, especially for urgent needs during a pandemic outbreak. Furthermore, voltage pulses applied to the gate during incubation were reported to reduce 50% of the incubation time, making the fabrication process even more efficient. Additionally, to enable faster detection, voltage pulses are applied on the gate electrode of the OECT to accelerate binding between the antibody and antigen. Benefiting from the label-free approach and rapid antigen–antibody reaction, the whole test can be conducted within 5 min.6
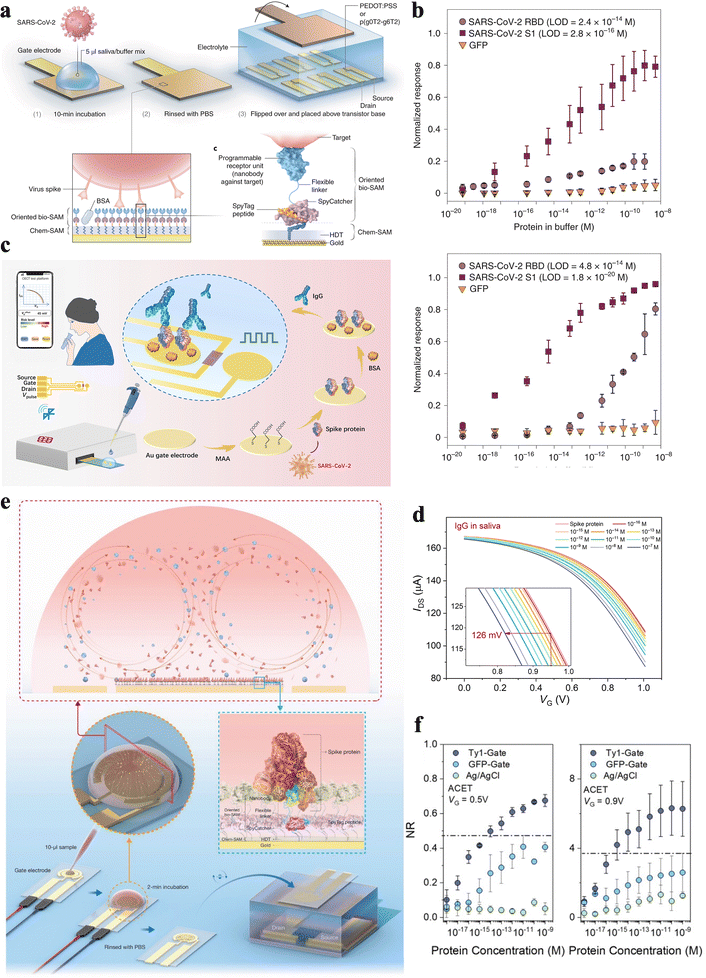 | ||
| Fig. 8 (a) Schematic diagram of an OECT with Chem-SAM and bio-SAM assembled on the gate electrode surface. (b) Normalized response of SARS-CoV nanobody-functionalized OECTs to SARS-CoV-2 RBD or S1 and GFP.8 Copyright 2021, Nature Publishing Group. (c) Scheme of a portable SARS-CoV-2 IgG sensing system and the gate modification process, where the Au gate surface is modified with biomolecules. (d) Transfer characteristics of the OECT after incubation of SARS-CoV-2 IgG in saliva with different concentrations in sequence.6 Copyright 2021, American Association for the Advancement of Science. (e) Schematic and operation steps of the ACET enhanced nanobody-OECT biosensor. (f) The response of the ACET-assisted n-type OECT sensor to various concentrations of SARS-CoV-2 S1 in saliva.103 Copyright 2022, Royal Society of Chemistry. | ||
To accelerate the incubation process for the capture of target molecules via biorecognition elements, Koklu et al.103 integrated alternating current electrothermal flow (ACET) with OECT for SARS-CoV-2 spike protein to shorten the detection time (Fig. 8e). The ACET integrated OECTs exhibit better selectivity and sensitivity than the equivalent biosensor without ACET facilitation. This OECT shows a low LOD of 100 aM with a linear region from 30 aM to 300 nM (Fig. 8f and Table 2). Note, ACET enables the biosensor to detect SARS-CoV-2 spike protein within only 2 min of sample exposure, which greatly supports its application in clinical use. The results demonstrate that ACET integration substantially decreases the detection speed while increasing the sensitivity and selectivity of the OECT-based sensor.
Overall, the outstanding properties of OECTs greatly promote the shift in molecular detection from complex, multi-step, tightly controlled laboratory assays to straightforward, single-step, more manageable POC tests.48 In the meantime, numerous efforts have been put into the stretchability of OECTs to improve the mechanical reliability for in vivo sensing.29,116–121 It is noteworthy that, as local amplifiers themselves, OECTs possess smaller device dimensions compared to typical ECBSs, which enables broader applicability in vivo. For instance, studies have already exhibited OECT sensors for various applications, including electrocardiograph (ECG), electrooculography (EOG), electroencephalography (EEG), and other in vivo scenarios.122–125 However, label-free OECT biosensors that are commercially available, are still missing,126,127 as issues still exist in terms of complex sensor designs, the necessity for carefully regulated conditions, and reliance on off-the-shelf antibodies and their random chemical immobilization in all orientations.128 Future innovation of OECT-based portable sensors relies on combined effort from engineers, chemists, biologists, and clinicians with improvement in fabrication techniques, advanced materials, biorecognition molecules, and integration strategies.
4 Portable and wearable biosensing systems based on ECBSs for biomarker detection in saliva
Notably, portable sensing systems, which combine ECBSs with processing and communication units, have emerged in recent years.40,129 Moreover, accompanied by the development of advanced ECBSs, portable and wearable sensing systems can not only track physical exercise activity based on bioelectrical sensing but also extend to tracking other biomarkers based on biochemical sensing. As a result, next-generation portable and wearable biosensing systems hold the capability to conduct prognostic judgment and illness monitoring. Moreover, the implementation of ECBSs in POC systems can further enhance the portability, test efficiency, sensitivity, and accuracy for continuous analyte monitoring. A sensing system targeting saliva is more convenient as saliva is more accessible when compared with systems based on sweat, tears, and urine (collection of these biofluids is more intensive and time-consuming).130 Note, that most studies on biosensing systems focus on epidermal wearable biosensors or ocular wearable biosensors rather than on portable/wearable biosensors for saliva, even though saliva biosensors have shown great potential in infectious disease diagnosis, dental research, and even some systemic disease prevention.45 In this section, we focus on the recent development of portable and wearable biosensing systems based on ECBSs that are used for on-the-spot monitoring of specific biomarkers in saliva and discuss in detail the pros and cons of these systems.4.1 ECBS systems on denture platforms and mouthguards
Due to the potential biofouling effect caused by rich salivary protein content and the extremely low concentration of some target biomarkers, the designation of wearable oral cavity biosensors faces many restrictions and obstacles. Despite these challenges, in-mouth biosensing platforms can offer an appealing and painless route for in situ monitoring of various chemical information in saliva. Oral wearable platforms require the incorporation of biosensors and electronic interfaces into an orally mounted unit, such as a mouthguard or denture-based platform.One of the earliest attempts at oral sensors was based on a partial denture platform in the 1960s.33 Due to the comparatively large size of the device, the replacement of several teeth was required and subject to risks associated with potential leakage of the internal sensor solution. Kim et al.131 developed a mouthguard-based salivary metabolite ECBS by utilizing screen-printed enzymatic WE (Fig. 9a and Table 3). The response of the sensor was examined using unstimulated human saliva spiked with 0.1–0.5 mM of lactate. As indicated by the well-defined chronoamperograms, the resulting calibration plot exhibits good linearity (slope: 0.202 μA mM−1; correlation coefficient (R2): 0.988) in 0.1 M PBS. The endogenous lactate level can thus be estimated to be 0.010 mM, which is in the normal range of human saliva at rest without stimulation (Fig. 9b and Table 3).131
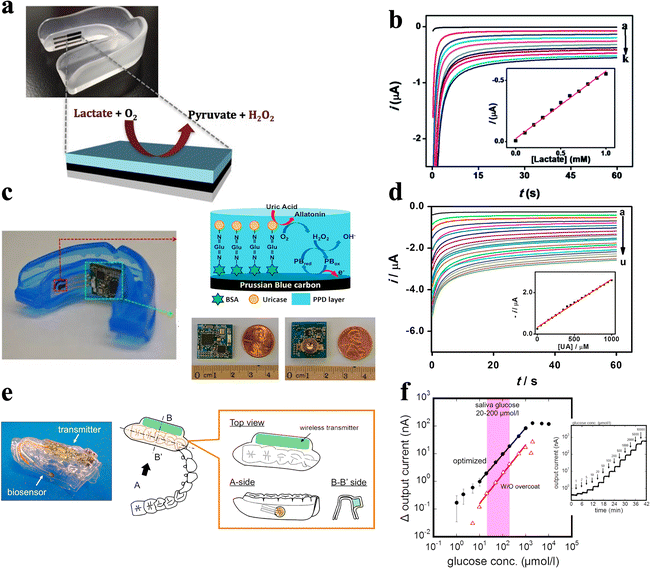 | ||
| Fig. 9 ECBS based on denture platforms and mouthguards. (a) A wearable salivary lactate sensor based on a printable enzymatic electrode on a mouthguard. (b) Chronoamperograms obtained for increasing lactate concentration from 0.1 mM to 1 mM in PBS.131 Copyright 2014, Royal Society of Chemistry. (c) An instrumented mouthguard for non-invasive monitoring of salivary uric acid levels. (d) Chronoamperograms obtained for increasing UA concentration from 50 μM to 1 mM.132 Copyright 2015, Elsevier. (e) A mouthguard biosensor for non-invasive monitoring of saliva glucose. (f) Calibration curves of the optimized glucose sensor on PETG.133 Copyright 2016, Elsevier. | ||
Since then, biosensors attached to mouthguards have expanded their sensing scope to other large molecules whose concentration in saliva has shown a good correlation with those in blood. For instance, Kim et al.132 developed an instrumented mouthguard capable of non-invasively monitoring salivary uric acid levels (Fig. 9c and Table 3). The system based on a uricase-modified screen-printed electrode has been integrated onto a mouthguard along with anatomically-miniaturized instrumentation electronics, which combine a potentiostat, microcontroller, and Bluetooth low energy (BLE) transceiver. The mouthguard biosensor system covers the whole uric acid concentration ranges for both healthy people and hyperuricemia patients. The corresponding current response is proportional to the uric acid concentration, leading to good linearity (slope: 2.32 mA mM−1; R2: 0.998) (Fig. 9d and Table 3). Therefore, the wireless mouthguard biosensor system can monitor uric acid levels for POC applications and can be readily expanded to other analytes, thus enabling attractive wearable monitoring systems for diverse health and fitness applications.132
Similarly, Arakawa et al.133 developed detachable “Cavitas sensors” that can be applied to the human oral cavity for non-invasive monitoring of saliva glucose (Fig. 9e and Table 3). The Pt working electrode in this sensor is coated with a glucose oxidase (GOD) membrane, while all the electrodes are fabricated on the surface of the polyethylene terephthalate glycol (PETG) mouthguard. This glucose sensor is seamlessly integrated with a wireless measurement system and can detect glucose in the range of 10–1000 mmol L−1 in artificial saliva composed of salts and proteins (Fig. 9f and Table 3). This range encompasses that of glucose concentrations in human saliva. Moreover, stable and long-term real-time monitoring longer than 5 hours with the telemetry system is achieved.133
4.2 ECBS systems attached to oral cavity structures
In the meantime, sensors can also be directly attached to natural structures in the oral cavity. For instance, Mannoor et al.134 designed a saliva-based ECBS for bacteria sensing with single-cell level sensitivity via modifying SAM of antimicrobial peptides (AMP) onto graphene WE (Fig. 10a and Table 3). Notably, graphene nano-sensors with water-soluble silk thin-film substrate are integrated, which can be attached directly to the enamel. This strategy provided a new tool for further research on remote content monitoring and detection in saliva.134 While Tseng et al.135 illustrated an approach to reduce the footprint of an ECBS to the millimeter-scale, thus extending the practical adoptability of such sensors (Fig. 10b and Table 3). Intriguingly, the sensor can be designed to fit onto a single human tooth for the detection of biofluids. In fact, in vivo sensing capability towards alcohol content, salinity, sugars, pH, and temperature is integrated via the introduction of hydrogel interlayers with high effective permittivity. For instance, the sensor establishes a large frequency shift due to the volume phase transition of the hydrogel induced by temperature or pH (Fig. 10b). Then, the response of the sensor is collected by using a mobile reader, composed of a portable vector network analyzer (VNA) attached to a tablet or cell phone.135 Moreover, silk film, serving as an intermediary in the integration process, can be dissolved in water and lead to the transference of metallic electrodes and other circuit components onto the biosurface. In normal cases, the circuit is attached to tissues by capillary adsorption.136,137 However, as a calcified tissue, the surface of the enamel lacks capillaries, leading to less desirable adhesion strength only by van der Waals forces. Therefore, long-term stability of the sensors applied in oral cavity still needs further investigation.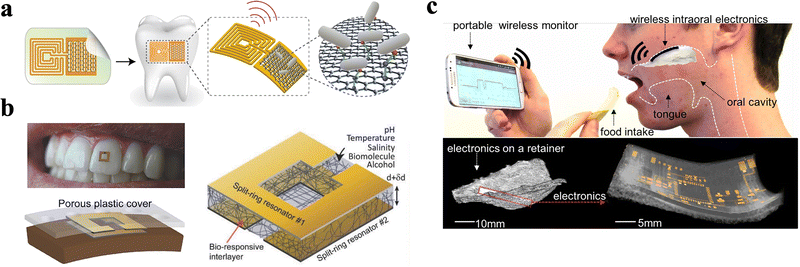 | ||
| Fig. 10 ECBS based on oral cavity structures. (a) A graphene nano-sensor integrated onto a tooth for remote respiration monitoring and bacteria detection in saliva.134 Copyright 2016, Nature Publishing Group. (b) A millimeter scale sensor adhered to tooth enamel for in vivo monitoring of ingested fluids.135 Copyright 2018, Wiley. (c) A soft electronic platform along with miniaturized sensors laminated on the palate. The low-profile, intraoral electronics can offer continuous real-time monitoring of sodium intake to actively manage human health and wellness.138 Copyright 2018, PNAS. | ||
Lee et al.138 designed an ECBS-based sensing system that can be attached to the palate, where specifically, the biosensor directly adhered to the mucous membrane (Fig. 10c and Table 3). The testing platform is a stretchable hybrid electronic system that has a small volume with a total mass of only 1.5 g. By embedding a low-profile, monopole ceramic antenna that reliably provides a high frequency (∼2.4 GHz), long-range (>10 m) wireless monitoring of sodium intake was effectively realized. The ECBS use potentiometry to measure the difference in potential between ion-selective sodium electrodes, consisting of working and reference electrodes. The potential at ion-selective electrode is selective in its response to species of interest due to the presence of an ion-selective membrane. The ion-selective sodium electrodes, demonstrate successful detection of sodium ion concentration down to 10−4 M. Moreover, benefiting from the application of a highly porous membrane, this platform demonstrated low thermal loading on the tissue than common elastomers. The mechanical reliability of the membrane circuit platform was confirmed through mechanical modeling and experimental validation, indicating that such a platform can be bent or stretched, which is more suitable for soft tissues like oral mucosa.138
Though requirements for biocompatibility and biological comfort for this class of platform are high, their application ranges are still wide as the oral mucosa is where secretory ducts of salivary glands are distributed, leading to a more complete contact between saliva and the sensor.
4.3 ECBS systems based on other supporting platforms
Designs that rely on platforms outside the oral cavity also show high feasibility and applicability. For instance, Mishra et al.99 fabricated a ring-based sensor platform that contained an embedded electronic board and allowed wireless data transmission for the detection of Δ9-tetrahydrocannabinol (THC) and alcohol in saliva (Fig. 11a and Table 3). The dual drug-of-abuse sensing ring cap contains a voltametric THC sensor and an amperometry alcohol biosensor, which offer simultaneous detection of the two substances with no apparent cross talk. Such a sensing system shows low LOD values of 0.5 μM and 0.2 mM for THC and alcohol, respectively (Fig. 11b and Table 3). Moreover, the specific design of the spring-loaded pins used to connect the printed circuit allows rapid replacement of the sensing end after each saliva assay. Therefore, the portable THC/alcohol ring sensor holds considerable promise for roadside screening and has the potential to be extended to detect other major drugs of abuse.99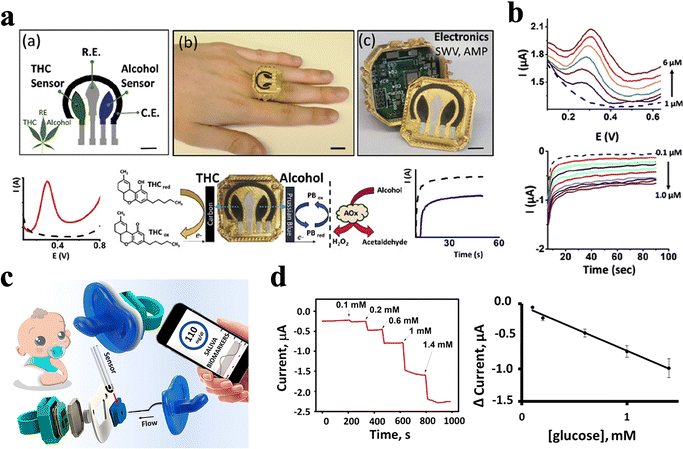 | ||
| Fig. 11 ECBS based on other platforms. (a) A wearable ring sensor platform for the detection of salivary THC and alcohol. The disposable sensing-electrode ring cap can be easily replaced after each saliva assay. (b) SWV for the oxidation of THC in PBS and amperometry response obtained for increasing alcohol concentration from 0.1 to 1 μM.99 Copyright 2020, Elsevier. (c) An electrochemical sensor integrated onto a pacifier. The wearable device integrated saliva sampling with electrochemical sensing, along with miniaturized wireless electronics on a single pacifier platform toward non-invasive chemical monitoring of the infant's saliva. (d) In vitro test for two type I diabetes saliva (a and b) before and after a meal.139 Copyright 2019, ACS Publications. | ||
More interestingly, García-Carmona et al.139 integrated a biosensor with a pacifier for the monitoring of infant saliva in real-time (Fig. 11c and Table 3), which offered a simple way to detect infants’ saliva in a non-invasive and nonharmful way. The mouth movements on the pacifier lead to efficient saliva pumping and promote unidirectional flow from the mouth to the electrochemical sensing chamber, which locates outside of the oral cavity. Not only for newborns, but this kind of system is also suitable for adults. The electrochemical sensing chamber contains an enzymatic ECBS, which is designed for glucose detection and is tested on diabetic adults. Testing results show a linear range between 0.1 and 1.4 mM, which reveal a good correlation to that in their blood levels, demonstrating accurate and excellent sensing performance of such pacifier-based sensor (Fig. 11d and Table 3).139
The above two sensing systems can also avoid problems associated with long-term exposure to harmful devices/chemicals inside the mouth. For instance, biofouling and reversibility are avoided as these sensors are disposable,139 and foreign body sensations can be effectively reduced. Hence, these kinds of biosensors may have wider applications where round-the-clock monitoring is not needed and regular check for body state is enough.
Consequently, the achievements in portable and wearable biosensing systems have smoothed the path to modern wearable biosensors for non-invasive biomonitoring applications in a variety of healthcare scenarios. However, it is noteworthy that, a common drawback among these devices is the storage stability in a biofluid environment. Theoretically, except for ECBSs that directly use an electrode as an oxidation–reduction interface, sensors based on nucleic acid probes or proteins cannot be stored in in vivo environments. To prevent proteins from denaturation, modified electrodes need to be preserved at 4 °C under dry conditions. Therefore, a more feasible and practical way is to make the sensor as a removable part of the portable system and to be installed only when conducting tests. In this way, these devices would be able to meet home medical monitoring requirements. Furthermore, for chronic disease patients who need regular medical care, wearable devices seem to be particularly important during a pandemic. It is expected that coupling telemedicine with wearables would decrease the number of physical visits while keeping close medical monitoring.
5 Discussion
The importance of saliva as a readily available and continuously renovated biofluid has been stressed to stimulate further research on the detection of salivary biomarkers140 whose timely sensing can effectively improve the life quality of patients and their survival rate. However, limitations and disadvantages still exist in ECBSs for saliva detection. Specifically, detection range is typically limited due to the lack of appropriate bio-recognition agents. Functionalization process and materials can be refined to alleviate cross-sensitivity of other compounds. Meanwhile, POC application is not easy to achieve, as accessory reagents are still required in the detection process to amplify electrochemical signals. In this section, discussions and perspectives on the aspects that need further improvement for ECBSs are presented.5.1 Design of bio-recognition agents
Bio-recognition element plays a critical role on the performance of ECBSs. The use of just nucleic acid as a probe has drawn tons of attention at the early stage of relevant research, then it has been classified into oligonucleotides, single-stranded DNAs, and DNA pyramids after decades of research. However, as analytes that can be recognized by nucleic acid probe are limited, this kind of probe has drawn less attention recently. Meanwhile, due to the relatively low electrical conductivity, DNA probes depend on redox reporters and are not suitable for label-free detection. Furthermore, larger macromolecules, such as antibodies, are hard to detect since they dramatically increase steric hindrance which can affect the molecules’ ability to bond or even prevent them from targeting the desired bond site in the reaction. This decreases the baseline current to a level where target binding events are difficult to discern. However, with a stable chemical property, the nucleic acid probe is easier to preserve and has advantages over protein probes in continuous monitoring.Regarding the design of antibody-based sensors, antibody immobilization is crucial to the sensor's performance. Antigens and antibodies are usually protein molecules with isoelectric points of pH 3–5 and pH 5–6, respectively. In a neutral or weakly alkaline environment, the surface is negatively charged. An appropriate concentration of electrolytes will make them lose part of the negative charge and combine with each other. In most cases, antibodies were directly immobilized via a chemical adsorption technique, in which antibodies are covalently linked to SAM on the surface of electrodes.141 While to achieve even lower LOD and shorter detection time, scientists have designed different recognition structures. For instance, Guo et al.8 used nanobodies, which are made from the antigen-binding domain (VHH) of an unusual class of heavy-chain-only antibodies found in camelids, to construct a recognition probe with a smaller size and an easier programming process. Meanwhile, the SpyTag/SpyCatcher protein conjugation system was used to connect the nanobodies to the 1,6-hexanedithiol (HDT) SAM. It is noteworthy that this strategy managed to immobilize the nanobody probe in a precisely defined orientation at a very high physical density.8 Das et al.142 designed an inverted molecular pendulum as the recognition element with DNA as the pendulum rod and antibody as the pendulum bob. Electric field-mediated transport of such a sensor was measured using the electron-transfer kinetics of an attached reporter molecule. Instead of measuring the voltage difference or the current, this strategy measures the time required for the MP to reach the electrode surface.142 This pattern was also tested using different proteins with varying charges, sizes, and molecular weights. All proteins were detectable with significant currents above unbound sensors, providing an example of larger protein detection. Compared with antibody or enzyme decorated probes, DNAs or smaller proteins decorated electrodes can reach a much higher functionalization density, because the size of the antibody was the main limitation. A higher immobilization density not only contributes to better sensitivity but also a longer guarantee period as a larger number of antibodies are likely to survive.
5.2 Strategies for increased binding specificity
It is validated that adding BSA to the functionalization protocol and target-binding buffer can increase the sensitivity and selectivity. While the mechanism lying behind has not been clarified yet. This kind of albumin is widely used in biochemistry tests, including western blot, PCR, ELISA, immunohistochemistry, etc., where it acts as an enzyme stabilizer and protein blocker. Similarly, in this scenario, BSA is added to the functionalization process mainly due to its ability to block unspecific binding sites. Specifically, BSA attaches to possible binding sites on SAM layers or antibody probes to deactivate carboxyl or sulfhydryl ends.36 However, Guo et al.8 showed a different perspective where BSA is more likely to function by capturing contaminating proteins in solution rather than blocking the binding sites, where they used fluorescence to label BSA and detected no fluorescence on the surface after immobilization. Though this is a qualitative experiment, it still proved that BSA acted as a capturer instead of a blocker. Either way, adding BSA has been proven to promote sensor performance. Other than BSA, ethanolamine has also been reported to deactivate the functionalization layer by blocking the carboxylic acid groups.32 Theoretically, proteins, that can seal all unbound sites without replacing and recognizing the antibody probe, can be used as blocking agents, although the effects are not guaranteed. Based on blocking buffers applied in biochemistry tests, skim milk, serum, gelatin, polyvinyl pyrrolidone (PVP) and Tween-20 can all be potential candidates. Though investigations on different blocking proteins have not been conducted yet, BSA can be considered more suitable based on its performance in combination with a wide range of substances, from metal ions to fatty acids. Such characteristic also indirectly supports Guo's hypothesis. In addition, investigations have also demonstrated that BSA can also slow the degradation of antibodies by increasing the protein concentration in the solution.5.3 Choice of test samples
Similar to ELISA, bioaffinity-based sensors also depend on the antigen–antibody binding process for analyte quantification. Hence, the matrix effect should also be taken into account. The direct use of ECBSs for saliva testing can be challenging due to the presence of other proteins, nucleic acids, and even external substances that have the potential to interfere with the analysis.62 In most cases, artificial saliva or real saliva is the only biofluid that has been analyzed to clinically validate the developed biosensor. However, only in a few studies, the device performance was evaluated in both buffer solution and real saliva,32 to estimate the matrix effect of a real biofluid as opposed to standard solution.143For instance, a graphene-based fully integrated portable nano-sensing system for IL-6 is constructed and tested in 1 × PBS and gargle solution with LOD values of 10.5 and 11.8 pM mL−1, respectively. These results are still slightly different from the LOD in real human saliva of 12.2 pM mL−1.144 Ortega et al.145 developed a novel biomolecule-free ECBS for THC and tested it in both simulated and real saliva. The LOD in saliva (1.6 ng mL−1) was slightly higher than that in PBS (1.1 ng mL−1), along with a range of detection between 1.6 and 10 ng mL−1. This performance is attributed to the interfering components present in the real saliva, which increase viscosity, affect diffusion, prevent the combination process, and cause unwanted adsorptions on the electrode surface.
It should be noted that the viscosity of human saliva is about 1.3 times higher than that of water, affecting the analytes' diffusion and the reaction rates on the electrodes. Artificial saliva manages to keep up with real saliva in terms of pH while failing to simulate complex organic components, especially the enzymes, proteins, non-protein nitrogenous substances, and cellular components in real saliva, let alone food debris and microbes. Therefore, artificial saliva has fewer interfering factors and lower viscosity. Since synthetic saliva lacks the viscosity of authentic saliva, the extrapolation of the results from simulated saliva to natural saliva is not recommended. Hence, calibration curves for sensors in real saliva should be performed instead of in simulated saliva.146
Furthermore, most real saliva used in tests is diluted, centrifuged, or filtered, to deal with the matrix effect. For example, the LOD of CD59 in real centrifuged and diluted saliva was 0.84 (±0.04) fg mL−1, while in untreated clinical saliva the LOD was 1.46 (±0.05) fg mL−1, which was slightly higher.56 Such kind of disparity of LOD was probably due to the complex matrix effect caused by the biological and chemical components present in human saliva. However, LOD of some biosensors was only tested in buffer solution, without being conducted in real or artificial saliva samples.63,81 Therefore, the direct use of ECBSs for real saliva testing is still challenging.
6 Summary
Future wearable biosensors are expected to become more streamlined, not only on denture and mouth guard textiles but also on other accessories that may blend further into a wearer's daily life,45 such as rings, watches,147 pacifiers,139 or some carry-home devices. The ultimate goal of this type of device is to be reliable in clinical use as an alternative to invasive biomedical devices and gold standard blood assays. Therefore, quantitative research that defines the abnormal range of biomarkers in different kinds of biofluids is required. Yet, additional research on concentrations correlativity between saliva and other physiological fluid is demanded to validate the clinical value of saliva as a diagnostic biofluid. Only through specific studies on technical and biological variability of salivary biomarkers, will we be able to control potential systemic and random errors to implement their assessment in clinical research. However, the absence of disease-specific markers and well-designed clinical trials that can validate values of absolute sensitivity and specificity imposes restrictions on the applicability of advanced biosensors in clinical use.143 Therefore, future advances, breakthroughs, and growth will require close multidisciplinary collaboration among the engineering, scientific and medical communities.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 82170997, and 62273073), the National Key R&D Program of China (No. 2022YFE0134800), Sichuan Science and Technology Program (No. 2022NSFSC0877), Project of Chengdu Science and Technology Bureau (No. 2021-YF05-02054-SN), and Research Funding from West China School/Hospital of Stomatology Sichuan University (No. RCDWJS2020-6). W. Huang also thanks the financial support of the UESTC Excellent Young Scholar Project.References
- P. S. Wikramaratna, R. S. Paton, M. Ghafari and J. Lourenço, Eurosurveillance, 2020, 25, 2000568 CrossRef CAS PubMed.
- I. Arevalo-Rodriguez, D. Buitrago-Garcia, D. Simancas-Racines, P. Zambrano-Achig, R. Del Campo, A. Ciapponi, O. Sued, L. Martinez-García, A. W. Rutjes, N. Low, P. M. Bossuyt, J. A. Perez-Molina and J. Zamora, PLoS One, 2020, 15, e0242958 CrossRef CAS PubMed.
- R. Rahbari, N. Moradi and M. Abdi, Clin. Chim. Acta, 2021, 516, 1–7 CrossRef CAS PubMed.
- Z. Li, Y. Yi, X. Luo, N. Xiong, Y. Liu, S. Li, R. Sun, Y. Wang, B. Hu, W. Chen, Y. Zhang, J. Wang, B. Huang, Y. Lin, J. Yang, W. Cai, X. Wang, J. Cheng, Z. Chen, K. Sun, W. Pan, Z. Zhan, L. Chen and F. Ye, J. Med. Virol., 2020, 92, 1518–1524 CrossRef CAS PubMed.
- Y. Galipeau, M. Greig, G. Liu, M. Driedger and M. A. Langlois, Front. Immunol., 2020, 11, 610688 CrossRef CAS PubMed.
- H. Liu, A. Yang, J. Song, N. Wang, P. Lam, Y. Li, H. K. Law and F. Yan, Sci. Adv., 2021, 7, eabg8387 CrossRef CAS PubMed.
- L. Chen, J. Zhao, J. Peng, X. Li, X. Deng, Z. Geng, Z. Shen, F. Guo, Q. Zhang, Y. Jin, L. Wang and S. Wang, Cell Proliferation, 2020, 53, e12923 CAS.
- K. Guo, S. Wustoni, A. Koklu, E. Díaz-Galicia, M. Moser, A. Hama, A. A. Alqahtani, A. N. Ahmad, F. S. Alhamlan, M. Shuaib, A. Pain, I. McCulloch, S. T. Arold, R. Grünberg and S. Inal, Nat. Biomed. Eng., 2021, 5, 666–677 CrossRef CAS PubMed.
- L. Lu, B. Liu, S. Li, W. Zhang and G. Xie, Colloids Surf., B, 2011, 86, 339–344 CrossRef CAS PubMed.
- Y. Xue, X. Li, H. Li and W. Zhang, Nat. Commun., 2014, 5, 4348 CrossRef CAS PubMed.
- J. Yoon, H. Y. Cho, M. Shin, H. K. Choi, T. Lee and J. W. Choi, J. Mater. Chem. B, 2020, 8, 7303–7318 RSC.
- S. W. Loo and T. S. Pui, Sensors, 2020, 20, 1854 CrossRef CAS PubMed.
- E. Cesewski and B. N. Johnson, Biosens. Bioelectron., 2020, 159, 112214 CrossRef CAS PubMed.
- D. Grieshaber, R. MacKenzie, J. Vörös and E. Reimhult, Sensors, 2008, 8, 1400–1458 CrossRef CAS PubMed.
- G. Maduraiveeran, M. Sasidharan and V. Ganesan, Biosens. Bioelectron., 2018, 103, 113–129 CrossRef CAS PubMed.
- N. L. Teradal and R. Jelinek, Adv. Healthcare Mater., 2017, 6, 1700574 CrossRef PubMed.
- B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901–986 CrossRef CAS PubMed.
- L. Lan, Y. Yao, J. Ping and Y. Ying, Biosens. Bioelectron., 2017, 91, 504–514 CrossRef CAS PubMed.
- C. G. Qian, Y. L. Chen, P. J. Feng, X. Z. Xiao, M. Dong, J. C. Yu, Q. Y. Hu, Q. D. Shen and Z. Gu, Acta Pharmacol. Sin., 2017, 38, 764–781 CrossRef CAS PubMed.
- M. Hasanzadeh, N. Shadjou, A. Mokhtarzadeh and M. Ramezani, Mater. Sci. Eng., C, 2016, 68, 482–493 CrossRef CAS PubMed.
- D. Khodagholy, J. Rivnay, M. Sessolo, M. Gurfinkel, P. Leleux, L. H. Jimison, E. Stavrinidou, T. Herve, S. Sanaur, R. M. Owens and G. G. Malliaras, Nat. Commun., 2013, 4, 2133 CrossRef PubMed.
- H. S. White, G. P. Kittlesen and M. S. Wrighton, J. Am. Chem. Soc., 1984, 106, 5375–5377 CrossRef CAS.
- E. Stavrinidou, P. Leleux, H. Rajaona, D. Khodagholy, J. Rivnay, M. Lindau, S. Sanaur and G. G. Malliaras, Adv. Mater., 2013, 25, 4488–4493 CrossRef CAS PubMed.
- X. Ji and P. K. Chan, Methods Mol. Biol., 2017, 1572, 205–216 CrossRef CAS PubMed.
- H. Tang, F. Yan, P. Lin, J. B. Xu and H. L. W. Chan, Adv. Funct. Mater., 2011, 21, 2264–2272 CrossRef CAS.
- R. B. Rashid, X. Ji and J. Rivnay, Biosens. Bioelectron., 2021, 190, 113461 CrossRef CAS PubMed.
- S. Inal, J. Rivnay, A. O. Suiu, G. G. Malliaras and I. McCulloch, Acc. Chem. Res., 2018, 51, 1368–1376 CrossRef CAS PubMed.
- J. Rivnay, S. Inal, A. Salleo, R. M. Owens, M. Berggren and G. G. Malliaras, Nat. Rev. Mater., 2018, 3, 17086 CrossRef CAS.
- D. Ohayon and S. Inal, Adv. Mater., 2020, 32, e2001439 CrossRef PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2nd edn, 2000 Search PubMed.
- P. Batista Deroco, J. D. F. Giarola, D. Wachholz Júnior, G. Arantes Lorga and L. Tatsuo Kubota, in Comprehensive Analytical Chemistry, ed. A. Merkoçi, Elsevier, 2020, vol. 89, pp. 91–137 Search PubMed.
- L. Barhoumi, A. Baraket, F. G. Bellagambi, G. S. Karanasiou, M. B. Ali, D. I. Fotiadis, J. Bausells, N. Zine, M. Sigaud and A. Errachid, Sens. Actuators, B, 2018, 266, 477–484 CrossRef CAS.
- L. Liv, G. Çoban, N. Nakiboğlu and T. Kocagöz, Biosens. Bioelectron., 2021, 192, 113497 CrossRef CAS PubMed.
- Y. Xie, S. Wu, Z. Chen, J. Jiang and J. Sun, Anal. Chim. Acta, 2022, 1207, 339742 CrossRef CAS PubMed.
- W. Heo, K. Lee, S. Park, K. A. Hyun and H. I. Jung, Biosens. Bioelectron., 2022, 201, 113960 CrossRef CAS PubMed.
- E. B. Aydın and M. K. Sezgintürk, Anal. Chim. Acta, 2018, 1039, 41–50 CrossRef PubMed.
- A. Sanati, M. Jalali, K. Raeissi, F. Karimzadeh, M. Kharaziha, S. S. Mahshid and S. Mahshid, Mikrochim. Acta, 2019, 186, 773 CrossRef CAS PubMed.
- G. Maduraiveeran, M. Sasidharan and V. Ganesan, Biosens. Bioelectron., 2018, 103, 113–129 CrossRef CAS PubMed.
- M. Gutiérrez-Capitán, A. Baldi and C. Fernández-Sánchez, Sensors, 2020, 20, 967 CrossRef PubMed.
- F. Meng, A. Aihaiti, X. Li, W. Zhang, Y. Qin, N. Zhu and M. Zhang, Biosens. Bioelectron., 2022, 203, 114031 CrossRef CAS PubMed.
- A. Singh, A. Sharma, A. Ahmed, A. K. Sundramoorthy, H. Furukawa, S. Arya and A. Khosla, Biosensors, 2021, 11, 336 CrossRef CAS PubMed.
- M. H. Hassan, C. Vyas, B. Grieve and P. Bartolo, Sensors, 2021, 21, 4672 CrossRef CAS PubMed.
- L. Tang, S. J. Chang, C. J. Chen and J. T. Liu, Sensors, 2020, 20, 6925 CrossRef CAS PubMed.
- F. Alam, S. RoyChoudhury, A. H. Jalal, Y. Umasankar, S. Forouzanfar, N. Akter, S. Bhansali and N. Pala, Biosens. Bioelectron., 2018, 117, 818–829 CrossRef CAS PubMed.
- J. Kim, A. S. Campbell, B. E. de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- N. G. Costa, J. C. Antunes, A. J. Paleo and A. M. Rocha, Biosensors, 2022, 12, 252 CrossRef CAS PubMed.
- T. Pfaffe, J. Cooper-White, P. Beyerlein, K. Kostner and C. Punyadeera, Clin. Chem., 2011, 57, 675–687 CrossRef CAS PubMed.
- A. Clifford, J. Das, H. Yousefi, A. Mahmud, J. B. Chen and S. O. Kelley, J. Am. Chem. Soc., 2021, 143, 5281–5294 CrossRef CAS PubMed.
- Z. Sun, Y. Jiang and M. Stenzel, SmartMat, 2021, 2, 127–130 CrossRef CAS.
- J. R. Sempionatto, V. R. Montiel, E. Vargas, H. Teymourian and J. Wang, ACS Sens., 2021, 6, 1745–1760 CrossRef CAS PubMed.
- Y. Li, B. Cui, S. Zhang, B. Li, J. Li, S. Liu and Q. Zhao, Small, 2022, 18, e2107413 CrossRef PubMed.
- X. Wang, K. E. Kaczor-Urbanowicz and D. T. Wong, Med. Oncol., 2017, 34, 7 CrossRef PubMed.
- A. Eftekhari, M. Hasanzadeh, S. Sharifi, S. M. Dizaj, R. Khalilov and E. Ahmadian, Int. J. Biol. Macromol., 2019, 124, 1246–1255 CrossRef CAS PubMed.
- G. D. Kalliolias and L. B. Ivashkiv, Nat. Rev. Rheumatol., 2016, 12, 49–62 CrossRef CAS PubMed.
- S. P. Sasi, X. Yan, H. Enderling, D. Park, H. Y. Gilbert, C. Curry, C. Coleman, L. Hlatky, G. Qin, R. Kishore and D. A. Goukassian, Oncogene, 2012, 31, 4117–4127 CrossRef CAS PubMed.
- M. Choudhary, P. Yadav, A. Singh, S. Kaur and J. Ramirez-Vick, Electroanal, 2016, 28, 2565–2574 CrossRef CAS.
- F. B. Tofighi, A. Saadati, H. Kholafazad-kordasht, F. Farshchi, M. Hasanzadeh and M. Samiei, J. Mol. Recognit., 2021, 34, e2932 CrossRef CAS PubMed.
- O. Amor-Gutiérrez, E. Costa-Rama and M. T. Fernández-Abedul, Sensors, 2022, 22, 6232 CrossRef PubMed.
- M. S. Khan, K. Dighe, Z. Wang, I. Srivastava, E. Daza, A. S. Schwartz-Dual, J. Ghannam, S. K. Misra and D. Pan, Analyst, 2018, 143, 1094–1103 RSC.
- E. B. Aydın, M. Aydın and M. K. Sezgintürk, Biosens. Bioelectron., 2017, 97, 169–176 CrossRef PubMed.
- E. B. Aydın and M. K. Sezgintürk, Anal. Biochem., 2018, 554, 44–52 CrossRef PubMed.
- J. F. Rusling and R. J. Förster, Biomedicines, 2021, 9, 702 CrossRef CAS PubMed.
- A. K. Panda, M. Keerthi, R. Sakthivel, U. Dhawan, X. Liu and R. J. Chung, Nanomaterials, 2022, 12, 258 CrossRef CAS PubMed.
- A. Idili, C. Parolo, R. Alvarez-Diduk and A. Merkoçi, ACS Sens., 2021, 6, 3093–3101 CrossRef CAS PubMed.
- M. D. T. Torres, W. R. de Araujo, L. F. de Lima, A. L. Ferreira and C. de la Fuente-Nunez, Matter, 2021, 4, 2403–2416 CrossRef CAS PubMed.
- B. S. Vadlamani, T. Uppal, S. C. Verma and M. Misra, Sensors, 2020, 20, 5871 CrossRef CAS PubMed.
- L. Liv, G. Çoban, N. Nakiboğlu and T. Kocagöz, Biosens. Bioelectron., 2021, 192, 113497 CrossRef CAS PubMed.
- H. Yousefi, A. Mahmud, D. Chang, J. Das, S. Gomis, J. B. Chen, H. Wang, T. Been, L. Yip, E. Coomes, Z. Li, S. Mubareka, A. McGeer, N. Christie, S. Gray-Owen, A. Cochrane, J. M. Rini, E. H. Sargent and S. O. Kelley, J. Am. Chem. Soc., 2021, 143, 1722–1727 CrossRef CAS PubMed.
- J. Jaewjaroenwattana, W. Phoolcharoen, E. Pasomsub, P. Teengam and O. Chailapakul, Talanta, 2023, 251, 123783 CrossRef CAS PubMed.
- A. Triveri, S. A. Serapian, F. Marchetti, F. Doria, S. Pavoni, F. Cinquini, E. Moroni, A. Rasola, F. Frigerio and G. Colombo, J. Chem. Inf. Model., 2021, 61, 4687–4700 CrossRef CAS PubMed.
- Z. Zhang, R. Pandey, J. Li, J. Gu, D. White, H. D. Stacey, J. C. Ang, C. J. Steinberg, A. Capretta, C. D. M. Filipe, K. Mossman, C. Balion, M. S. Miller, B. J. Salena, D. Yamamura, L. Soleymani, J. D. Brennan and Y. Li, Angew. Chem., Int. Ed., 2021, 60, 24266–24274 CrossRef CAS PubMed.
- T. Ouyang, Z. Liu, Z. Han and Q. Ge, Anal. Chem., 2019, 91, 3179–3186 CrossRef CAS PubMed.
- S. Tombelli, M. Minunni and M. Mascini, Biosens. Bioelectron., 2005, 20, 2424–2434 CrossRef CAS PubMed.
- F. Yang, W. Z. Ouma, W. Li, A. I. Doseff and E. Grotewold, in Methods in Enzymology, ed. S. E. O'Connor, Academic Press, 2016, vol. 576, pp. 251–304 Search PubMed.
- M. Hasanzadeh, A. Zargami, H. N. Baghban, A. Mokhtarzadeh, N. Shadjou and S. Mahboob, Int. J. Biol. Macromol., 2018, 116, 735–743 CrossRef CAS PubMed.
- R. ter Heine, J. H. Beijnen and A. D. Huitema, Bioanalysis, 2009, 1, 1329–1338 CrossRef CAS PubMed.
- H. C. Pandya, N. Spooner and H. Mulla, Bioanalysis, 2011, 3, 779–786 CrossRef CAS PubMed.
- M. D. Kruizinga, F. E. Stuurman, G. J. A. Driessen, A. F. Cohen, K. R. Bergmann and M. J. van Esdonk, Ther. Drug Monit., 2021, 43, 546–554 CrossRef CAS PubMed.
- V. Franco, G. Gatti, I. Mazzucchelli, R. Marchiselli, C. Fattore, P. Rota, C. A. Galimberti, G. Capovilla, F. Beccaria, V. De Giorgis, C. Johannessen Landmark and E. Perucca, Epilepsia, 2020, 61, e79–e84 CrossRef CAS PubMed.
- A. Zygula, P. Kosinski, A. Zwierzchowska, M. Sochacka, P. Wroczynski, M. Makarewicz-Wujec, B. Pietrzak, M. Wielgos, M. Rzentala and J. Giebultowicz, Diabetes Res. Clin. Pract., 2019, 148, 72–80 CrossRef CAS PubMed.
- B. Demir, T. Yilmaz, E. Guler, Z. P. Gumus, H. Akbulut, E. Aldemir, H. Coskunol, D. G. Colak, I. Cianga, S. Yamada, S. Timur, T. Endo and Y. Yagci, Talanta, 2016, 161, 789–796 CrossRef CAS PubMed.
- M. Klimuntowski, M. M. Alam, G. Singh and M. M. R. Howlader, ACS Sens., 2020, 5, 620–636 CrossRef CAS PubMed.
- A. R. Williams, E. V. Nunes, A. Bisaga, F. R. Levin and M. Olfson, Am. J. Drug Alcohol Abuse, 2019, 45, 1–10 CrossRef PubMed.
- F. Grotenhermen, Clin. Pharmacokinet., 2003, 42, 327–360 CrossRef CAS PubMed.
- S. Anizan, G. Milman, N. Desrosiers, A. J. Barnes, D. A. Gorelick and M. A. Huestis, Anal. Bioanal. Chem., 2013, 405, 8451–8461 CrossRef CAS PubMed.
- M. S. Odell, M. Y. Frei, D. Gerostamoulos, M. Chu and D. I. Lubman, Forensic Sci. Int., 2015, 249, 173–180 CrossRef CAS PubMed.
- D. Majak, J. Fan, S. Kang and M. Gupta, J. Mater. Chem. B, 2021, 9, 2107–2117 RSC.
- T. J. Bondancia, A. C. Soares, M. Popolin-Neto, N. O. Gomes, P. A. Raymundo-Pereira, H. S. Barud, S. A. S. Machado, S. J. L. Ribeiro, M. E. Melendez, A. L. Carvalho, R. M. Reis, F. V. Paulovich and O. N. Oliveira, Biomater. Adv., 2022, 134, 112676 CrossRef PubMed.
- A. K. Yadav, P. Gulati, R. Sharma, A. Thakkar and P. R. Solanki, Talanta, 2022, 243, 123376 CrossRef CAS PubMed.
- B. Öndeş, S. Evli, M. Uygun and D. Aktaş Uygun, Biosens. Bioelectron., 2021, 191, 113454 CrossRef PubMed.
- L. Barhoumi, F. G. Bellagambi, F. M. Vivaldi, A. Baraket, Y. Clément, N. Zine, M. Ben Ali, A. Elaissari and A. Errachid, Sensors, 2019, 19, 692 CrossRef.
- D. Najjar, J. Rainbow, S. Sharma Timilsina, P. Jolly, H. de Puig, M. Yafia, N. Durr, H. Sallum, G. Alter, J. Z. Li, X. G. Yu, D. R. Walt, J. A. Paradiso, P. Estrela, J. J. Collins and D. E. Ingber, Nat. Biomed. Eng., 2022, 6, 968–978 CrossRef CAS.
- A. Erdem, H. Senturk, E. Yildiz and M. Maral, Talanta, 2022, 244, 123422 CrossRef CAS PubMed.
- C.-C. Wu, Y.-H. Chiang and H.-Y. Chiang, Biosensors, 2022, 12, 265 CrossRef CAS PubMed.
- C. Karaman, B. B. Yola, O. Karaman, N. Atar, İ. Polat and M. L. Yola, Mikrochim. Acta, 2021, 188, 425 CrossRef CAS PubMed.
- H. Yousefi, A. Mahmud, D. Chang, J. Das, S. Gomis, J. B. Chen, H. Wang, T. Been, L. Yip, E. Coomes, Z. Li, S. Mubareka, A. McGeer, N. Christie, S. Gray-Owen, A. Cochrane, J. M. Rini, E. H. Sargent and S. O. Kelley, J. Am. Chem. Soc., 2021, 143, 1722–1727 CrossRef CAS PubMed.
- M. Alafeef, K. Dighe, P. Moitra and D. Pan, ACS Nano, 2020, 14, 17028–17045 CrossRef CAS PubMed.
- S. Rong, L. Zou, L. Meng, X. Yang, J. Dai, M. Wu, R. Qiu, Y. Tian, X. Feng, X. Ren, L. Jia, L. Jiang, Y. Hang, H. Ma and H. Pan, Anal. Chim. Acta, 2022, 1196, 339545 CrossRef CAS PubMed.
- R. K. Mishra, J. R. Sempionatto, Z. Li, C. Brown, N. M. Galdino, R. Shah, S. Liu, L. J. Hubble, K. Bagot, S. Tapert and J. Wang, Talanta, 2020, 211, 120757 CrossRef CAS PubMed.
- S. Upasham, A. Tanak, B. Jagannath and S. Prasad, Sci. Rep., 2018, 8, 16745 CrossRef PubMed.
- C. Liao, C. Mak, M. Zhang, H. L. Chan and F. Yan, Adv. Mater., 2015, 27, 676–681 CrossRef CAS PubMed.
- A. M. Pappa, V. F. Curto, M. Braendlein, X. Strakosas, M. J. Donahue, M. Fiocchi, G. G. Malliaras and R. M. Owens, Adv. Healthcare Mater., 2016, 5, 2295–2302 CrossRef CAS PubMed.
- A. Koklu, S. Wustoni, K. Guo, R. Silva, L. Salvigni, A. Hama, E. Diaz-Galicia, M. Moser, A. Marks, I. McCulloch, R. Grünberg, S. T. Arold and S. Inal, Adv. Mater., 2022, 34, 2202972 CrossRef CAS.
- D. A. Bernards, G. G. Malliaras, G. E. S. Toombes and S. M. Gruner, Appl. Phys. Lett., 2006, 89, 053505 CrossRef.
- G. Tarabella, F. M. Mohammadi, N. Coppede, F. Barbero, S. Iannotta, C. Santato and F. Cicoira, Chem. Sci., 2013, 4, 1395–1409 RSC.
- P. Lin, F. Yan and H. L. Chan, ACS Appl. Mater. Interfaces, 2010, 2, 1637–1641 CrossRef CAS PubMed.
- D. Khodagholy, V. F. Curto, K. J. Fraser, M. Gurfinkel, R. Byrne, D. Diamond, G. G. Malliaras, F. Benito-Lopez and R. M. Owens, J. Mater. Chem., 2012, 22, 4440–4443 RSC.
- D. A. Bernards, D. J. Macaya, M. Nikolou, J. A. DeFranco, S. Takamatsu and G. G. Malliaras, J. Mater. Chem., 2008, 18, 116–120 RSC.
- H. Tang, P. Lin, H. L. Chan and F. Yan, Biosens. Bioelectron., 2011, 26, 4559–4563 CrossRef CAS.
- C. Z. Liao, M. Zhang, L. Y. Niu, Z. J. Zheng and F. Yan, J. Mater. Chem. B, 2014, 2, 191–200 RSC.
- R. X. He, M. Zhang, F. Tan, P. H. M. Leung, X. Z. Zhao, H. L. W. Chan, M. Yang and F. Yan, J. Mater. Chem., 2012, 22, 22072–22076 RSC.
- M. Kanungo, D. N. Srivastava, A. Kumar and A. Q. Contractor, Chem. Commun., 2002, 680–681 RSC.
- P. Lin, F. Yan, J. Yu, H. L. Chan and M. Yang, Adv. Mater., 2010, 22, 3655–3660 CrossRef CAS PubMed.
- J. Yu, A. Yang, N. Wang, H. Ling, J. Song, X. Chen, Y. Lian, Z. Zhang, F. Yan and M. Gu, Nanoscale, 2021, 13, 2868–2874 RSC.
- E. Macchia, K. Manoli, B. Holzer, C. Di Franco, R. A. Picca, N. Cioffi, G. Scamarcio, G. Palazzo and L. Torsi, Anal. Bioanal. Chem., 2019, 411, 4899–4908 CrossRef CAS PubMed.
- A. Spanu, L. Martines and A. Bonfiglio, Lab Chip, 2021, 21, 795–820 RSC.
- S. G. Higgins, A. Lo Fiego, I. Patrick, A. Creamer and M. M. Stevens, Adv. Mater. Technol., 2020, 5, 2000384 CrossRef CAS.
- R. A. Picca, K. Manoli, E. Macchia, L. Sarcina, C. Di Franco, N. Cioffi, D. Blasi, R. Osterbacka, F. Torricelli, G. Scamarcio and L. Torsi, Adv. Funct. Mater., 2020, 30, 1904513 CrossRef CAS.
- S. Keene and Y. van de Burgt, Nat. Mater., 2020, 19, 584–586 CrossRef CAS PubMed.
- B. Sun, G. D. Zhou, T. Guo, Y. N. Zhou and Y. M. A. Wu, Nano Energy, 2020, 75, 104938 CrossRef CAS.
- C. Cea, G. D. Spyropoulos, P. Jastrzebska-Perfect, J. J. Ferrero, J. N. Gelinas and D. Khodagholy, Nat. Mater., 2020, 19, 679–686 CrossRef CAS PubMed.
- A. Nawaz, Q. Liu, W. L. Leong, K. E. Fairfull-Smith and P. Sonar, Adv. Mater., 2021, 33, e2101874 CrossRef PubMed.
- Y. Yao, W. Huang, J. Chen, G. Wang, H. Chen, X. Zhuang, Y. Ying, J. Ping, T. J. Marks and A. Facchetti, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2111790118 CrossRef CAS.
- J. Chen, W. Huang, D. Zheng, Z. Xie, X. Zhuang, D. Zhao, Y. Chen, N. Su, H. Chen, R. M. Pankow, Z. Gao, J. Yu, X. Guo, Y. Cheng, J. Strzalka, X. Yu, T. J. Marks and A. Facchetti, Nat. Mater., 2022, 21, 564–571 CrossRef CAS PubMed.
- L. Huang, Z. Wang, J. Chen, B. Wang, Y. Chen, W. Huang, L. Chi, T. J. Marks and A. Facchetti, Adv. Mater., 2021, 33, e2007041 CrossRef PubMed.
- D. Gentili, P. D'Angelo, F. Militano, R. Mazzei, T. Poerio, M. Brucale, G. Tarabella, S. Bonetti, S. L. Marasso, M. Cocuzza, L. Giorno, S. Iannotta and M. Cavallini, J. Mater. Chem. B, 2018, 6, 5400–5406 RSC.
- E. Macchia, P. Romele, K. Manoli, M. Ghittorelli, M. Magliulo, Z. M. Kovács-Vajna, F. Torricelli and L. Torsi, Flexible Printed Electron., 2018, 3, 034002 CrossRef.
- A. K. Trilling, J. Beekwilder and H. Zuilhof, Analyst, 2013, 138, 1619–1627 RSC.
- J. Ma, L. Shen, Y. Jiang, H. Ma, F. Lv, J. Liu, Y. Su and N. Zhu, Anal. Chem., 2022, 94, 2333–2340 CrossRef CAS PubMed.
- M. Villiger, R. Stoop, T. Vetsch, E. Hohenauer, M. Pini, P. Clarys, F. Pereira and R. Clijsen, Eur. J. Clin. Nutr., 2018, 72, 69–76 CrossRef CAS PubMed.
- J. Kim, G. Valdés-Ramírez, A. J. Bandodkar, W. Jia, A. G. Martinez, J. Ramírez, P. Mercier and J. Wang, Analyst, 2014, 139, 1632–1636 RSC.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdés-Ramírez, T. R. Paixão, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- T. Arakawa, Y. Kuroki, H. Nitta, P. Chouhan, K. Toma, S. Sawada, S. Takeuchi, T. Sekita, K. Akiyoshi, S. Minakuchi and K. Mitsubayashi, Biosens. Bioelectron., 2016, 84, 106–111 CrossRef CAS PubMed.
- M. S. Mannoor, H. Tao, J. D. Clayton, A. Sengupta, D. L. Kaplan, R. R. Naik, N. Verma, F. G. Omenetto and M. C. McAlpine, Nat. Commun., 2012, 3, 763 CrossRef PubMed.
- P. Tseng, B. Napier, L. Garbarini, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2018, 30, e1703257 CrossRef.
- D. H. Kim, J. Viventi, J. J. Amsden, J. Xiao, L. Vigeland, Y. S. Kim, J. A. Blanco, B. Panilaitis, E. S. Frechette, D. Contreras, D. L. Kaplan, F. G. Omenetto, Y. Huang, K. C. Hwang, M. R. Zakin, B. Litt and J. A. Rogers, Nat. Mater., 2010, 9, 511–517 CrossRef CAS PubMed.
- D. H. Kim, N. Lu, R. Ma, Y. S. Kim, R. H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. I. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H. J. Chung, H. Keum, M. McCormick, P. Liu, Y. W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838–843 CrossRef CAS PubMed.
- Y. Lee, C. Howe, S. Mishra, S. Lee Dong, M. Mahmood, M. Piper, Y. Kim, K. Tieu, H.-S. Byun, P. Coffey James, M. Shayan, Y. Chun, M. Costanzo Richard and W.-H. Yeo, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5377–5382 CrossRef CAS PubMed.
- L. García-Carmona, A. Martín, J. R. Sempionatto, J. R. Moreto, M. C. González, J. Wang and A. Escarpa, Anal. Chem., 2019, 91, 13883–13891 CrossRef PubMed.
- C. Z. Zhang, X. Q. Cheng, J. Y. Li, P. Zhang, P. Yi, X. Xu and X. D. Zhou, Int. J. Oral Sci., 2016, 8, 133–137 CrossRef CAS.
- M. Hasanzadeh, S. Rahimi, E. Solhi, A. Mokhtarzadeh, N. Shadjou, J. Soleymani and S. Mahboob, Microchem. J., 2018, 143, 379–392 CrossRef CAS.
- J. Das, S. Gomis, J. B. Chen, H. Yousefi, S. Ahmed, A. Mahmud, W. Zhou, E. H. Sargent and S. O. Kelley, Nat. Chem., 2021, 13, 428–434 CrossRef CAS PubMed.
- R. Goldoni, A. Scolaro, E. Boccalari, C. Dolci, A. Scarano, F. Inchingolo, P. Ravazzani, P. Muti and G. Tartaglia, Biosensors, 2021, 11, 396 CrossRef CAS PubMed.
- Z. Hao, Y. Pan, W. Shao, Q. Lin and X. Zhao, Biosens. Bioelectron., 2019, 134, 16–23 CrossRef CAS PubMed.
- G. A. Ortega, S. R. Ahmed, S. K. Tuteja, S. Srinivasan and A. R. Rajabzadeh, Talanta, 2022, 236, 122863 CrossRef CAS PubMed.
- S. K. Islam, Y. P. Cheng, R. L. Birke, M. V. Cañamares, C. Muehlethaler and J. R. Lombardi, Chem. Phys., 2020, 536, 110812 CrossRef CAS.
- Y. Zhao, B. Wang, H. Hojaiji, Z. Wang, S. Lin, C. Yeung, H. Lin, P. Nguyen, K. Chiu, K. Salahi, X. Cheng, J. Tan, B. A. Cerrillos and S. Emaminejad, Sci. Adv., 2020, 6, eaaz0007 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |