Open Access Article
Open Access ArticleInterfacial biosilica coating of chitosan gel using fusion silicatein to fabricate robust hybrid material for biomolecular applications†
Kasun
Godigamuwa
,
Kazunori
Nakashima
 *,
Sota
Tsujitani
,
Ryo
Naota
,
Ilham
Maulidin
and
Satoru
Kawasaki
*,
Sota
Tsujitani
,
Ryo
Naota
,
Ilham
Maulidin
and
Satoru
Kawasaki
Division of Sustainable Resources Engineering, Faculty of Engineering, Hokkaido University, Kita 13, Nishi 8, Kita-Ku, Sapporo 060-8628, Japan. E-mail: k.naka@eng.hokudai.ac.jp
First published on 8th February 2023
Abstract
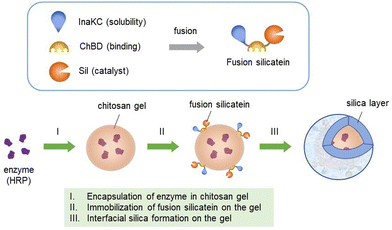
An enzyme-encapsulated silica-based hybrid material was developed using a chitosan gel. Fusion silicatein (InaKC-ChBD-Sil), silicatein fused with a soluble tag and chitin-binding domain, was employed as an interfacial catalyst to form silica on a chitosan gel matrix under physiological conditions, and horseradish peroxidase was immobilised on the hybrid material. Silica formation on the gel was verified via fluorescence microscopy using a designed fusion protein called TBP-mCherry, a fluorescent protein fused with a silica-binding peptide. We report a chitosan gel–silica hybrid material capable of encapsulating enzymes for biomedical and environmental applications.
Immobilisation significantly improves the stability of biomolecules under harsh and variable reaction conditions and enhances their reusability, thus yielding high-purity products.1,2 Hydrogels are three-dimensional networks of polymers that are considered promising matrix biomaterials for immobilising bio-related substances such as enzymes and cells because of their mechanical properties, biocompatibility, and responsiveness.3 Biomolecules immobilised in hydrogels can be released in response to external stimuli, such as pH, temperature, light, and magnetic fields.4 Polysaccharide-based natural hydrogels, including alginate, carrageenan, and chitosan, have been developed for biomedical applications owing to their biodegradability, non-toxicity, and low cost.5–7 Chitosan, a derivative of chitin, dissolves in a solution below pH 6.3 and forms a hydrogel at higher pH.8–10 Chitosan gel can be applied in various applications including controlled drug release, scaffold material formation in tissue, cell growth, and regenerative medicine due to its biocompatibility.5
Although hydrogels are intrinsically soft and weak materials, their properties can be changed by introducing robust inorganic materials on their surfaces.11 The concept of inorganic–organic hybrid materials can provide dual properties, such as dry/wet, soft/hard, and hydrophobic/hydrophilic and thus have significant potential for use in biomedicine, electronics, energy generation, and wastewater treatment.12–14 Furthermore, fabricating inorganic–organic hybrid materials incorporating biological substances such as proteins, enzymes, and live cells could promote the development of next-generation materials.15
Among the inorganic matrices used in hybrid materials, silica is a conventional and reliable inorganic polymer because of its mechanical and chemical stability, controllable permeability via changing thickness and/or porosity, optical transparency, and biocompatibility.16–18 However, there are difficulties in silica formation on hydrogels incorporating biomolecules which arise due to two primary reasons: i) damage to biomolecules in the silica synthesis process and ii) insufficient silica formation on hydrogels. Enzymes and cells are damaged by harsh reaction conditions during silica synthesis, such as high temperatures or extreme pH conditions.19
To overcome these problems, silicatein, a silica-polymerising enzyme,20 can be used to synthesise a silica layer on hydrogel materials under physiological conditions. The biosilica synthesized by silicatein is amorphous and has a porous structure containing nano-silica aggregates.21 The biocompatibility of biosilica synthesised by silicatein has been confirmed by many studies which are related to biomedical applications such as drug delivery, bone formation, immobilization of biomolecules and tissue engineering.22–25 Furthermore, silicatein can be fused with polysaccharide-binding proteins, which exhibit a high affinity for polysaccharides, to be immobilised on the surface of hydrogels for highly efficient interfacial biocatalysts.
In the present study, we demonstrated a novel approach for fabricating a hybrid silica-coated chitosan gel incorporating enzymes. To immobilise silicatein on the chitosan gel, we employed a chitin-binding module (ChBD),26 which is known to bind chitosan gel. Although silicatein is an aggregative enzyme in water, we successfully solved this problem by introducing highly soluble proteins such as ProS221 and InaKC.27 Using this strategy, we constructed a triple-fusion silicatein composed of InaKC, ChBD, and silicatein to fabricate a silica-chitosan hydrogel incorporating horseradish peroxidase (HRP) (Scheme 1). The fusion silicatein, InaKC-ChBD-Sil, was expected to function as an interfacial biocatalyst for silica synthesis on the gel and act as a promising binding molecule between silica and hydrogels. The function of each component in InaKC-ChBD-Sil has been evaluated previously. InaKC enhanced the solubility of silicatein. ChBD and Sil were responsible for the binding fusion silicatein on chitin or chitosan, and silica polymerisation, respectively.27 The silica polymerisation activity of InaKC-ChBD-Sil was 9.5 μM min−1.27
Silicatein derived from Suberites domuncula,28 chitin-binding domain (ChBD) found in chitinase A1 from Bacillus circulans WL-1,29 and the soluble protein tag InaKC27 derived from Pseudomonas syringae30 were used to construct fusion proteins. InaKC was found to be a highly hydrophilic protein, and the fusion silicatein was stably soluble in water after protein refolding (Fig. S1, ESI†).
Chitosan gels can be formed in different shapes, such as sheets31 and spheres.32 Because spherical gel beads are used in many applications, the formation of spherical chitosan gel beads has been well researched. After optimising the chitosan percentage (3%) for the formation of chitosan gel beads, chitosan dissolved in acetic acid was added dropwise to the NaOH solution. The average weight of the beads was 6 mg. HRP-immobilized chitosan gel was obtained by dissolving HRP in a chitosan solution followed by neutralisation.
Silicateins have been successfully immobilised on materials via microcontact printing or chemical bonding to prepare coating materials using silica or titania.33 However, the direct immobilisation of silicatein on material surfaces or chemical bonding results in the loss of its enzymatic activity.34 Previously, our research group successfully demonstrated the fabrication of silica on chitin using ChBD-fused silicatein.27 In some studies, different tags, such as Cys-tag and GST-tags, have been studied to immobilise silicatein on materials in a milder way.33,34 However, surface modification is required for these tags. Because chitosan is a derivative of chitin, ChBD was used as an affinity tag, considering the possible interaction between ChBD and chitosan gel, to immobilise silicatein on the gel surface as an interfacial catalyst to fabricate a chitosan gel–silica hybrid in this study. Adsorption tests showed that the fusion silicatein readily adsorbed on the chitosan gel and yielded almost 100% adsorption after 90 min (Fig. S2, ESI†). Subsequently, the fusion silicatein adsorbed on the chitosan gel beads was subjected to silica formation using tetraethyl orthosilicate (TEOS, 100 mM) as the precursor at 25 °C and pH 7.4. As a control, chitosan gel was treated with TEOS solution under the same conditions without fusion silicatein adsorption.
The silica formed on the chitosan gel beads was analysed using SEM-EDS and fluorescence microscopy. To detect silica formed on chitosan gel beads using SEM-EDS, the beads were freeze-dried after washing with ethanol and ultrapure water. Fig. 1 shows the SEM images and elements present on the chitosan gel beads subjected to silica formation with and without silicatein fusion. Small particles were deposited on the chitosan gel in the presence of fusion silicatein (Fig. 1B), while a smooth surface was observed in the absence of fusion silicatein (Fig. 1A). Elemental analysis of the gels showed a uniform distribution of silicon (Si) on the chitosan gel with fusion silicatein, indicating that SiO2 was adequately formed and coated the gel. In contrast, negligible amounts of Si were found on the gel in the absence of fusion silicatein. These results suggest that the fusion silicatein catalysed silica formation on chitosan gel. Cracks on the gel prepared with fusion silicatein occurred during the freeze-drying process because the gel samples shrank after removing the water, which likely occurred due to the remaining silica on the gel.
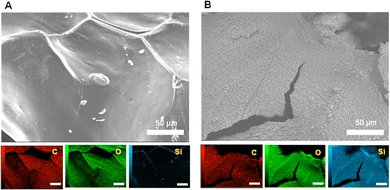 | ||
| Fig. 1 SEM-EDS analysis of chitosan gel subjected to silica formation without (A), and with (B) adsorption of InaKC-ChBD-Sil. Size bars indicate 50 μm. | ||
Although SEM analysis provides important information on the material surface, the material should be vacuum-dried before analysis, which causes morphological changes in wet hydrogels. To evaluate the surface of gel particles under wet conditions, we developed a method for directly detecting silica formed on chitosan beads without freeze-drying using a fluorescence microscope. In the present approach, a titanium-binding peptide (TBP, amino acid sequence: RKLPDAPGMHTW) was used because it is known to bind to silica and titania.35 The red fluorescent protein mCherry fused with TBP (TBP-mCherry) was prepared to detect silica on the gel using fluorescent microscopy (Fig. S3; ESI†). The silica-binding fluorescence protein TBP-mCherry was added to glass beads to confirm its silica-binding ability along with a control experiment using unmodified mCherry. Although no fluorescence was detected in the glass beads treated with unmodified mCherry, strong red fluorescence was observed in those treated with TBP-mCherry (Fig. S4, ESI†). This indicates that mCherry does not bind to silica by itself while TBP-mCherry adequately binds to silica to allow its detection by fluorescence microscopy. Therefore, TBP-mCherry was used to detect silica on chitosan gel beads under wet conditions. Chitosan gel beads subjected to silica formation with or without fusion silicatein were treated with TBP-mCherry after washing with ethanol and Tris/HCl buffer. Higher fluorescence was observed in chitosan gel beads with fusion silicatein than in those without fusion silicatein (Fig. S5, ESI†). However, fluorescent images of the bead surface were obscure, so we obtained cross-sections to better evaluate silica formation at the interface. The resulting chitosan gel beads were dipped in freshly prepared agar solution and allowed to solidify. Cross-sections of the gel beads were obtained by cutting the immobilised chitosan gel beads in agar gel media (Fig. S6, ESI†). The fluorescence images of the cross sections of the materials are shown in Fig. 2. In these images, fluorescence was detected at the boundary between the chitosan and agar gels. A higher fluorescence intensity was detected at the surface of the chitosan gel beads prepared with fusion silicatein. This observation revealed adequate formation of silica by the fusion silicatein on chitosan gel beads. The amounts of silica formed on the chitosan gel beads with and without adsorption of fusion silicatein were quantified using the molybdenum blue colourimetric method.15 Amounts were 394 and 76 μg of silica per chitosan gel bead with and without adsorption of fusion silicatein, respectively. Chitosan gel–silica hybrid materials have been prepared using sol–gel processes for the application of heavy metal adsorption36 and the development of biosensors.37 In some studies, magnetic properties have been obtained by immobilizing Fe3O4 magnetic nanoparticles in a hydrogel to fabricate drug delivery systems.38 This approach has great potential for the functionalization of bio-based hydrogels.
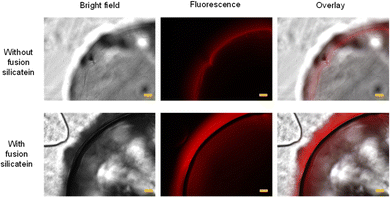 | ||
| Fig. 2 Microscopic images of cross sections of chitosan gel beads treated with TBP-mCherry. Size bars indicate 100 μm. | ||
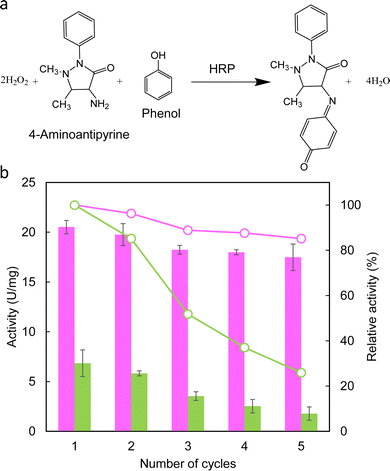
The activity of biomolecules incorporated into hydrogels is another important factor influencing the applicability of biohybrid materials. The enzymatic activity of HRP incorporated into the chitosan gel was examined using the catalytic reaction shown in Fig. 3a. Immediately after HRP was immobilised in the chitosan gel matrix, its enzymatic activity was 40 U mg−1. Then, the HRP-incorporated chitosan gel beads were subjected to silica formation with and without fusion silicatein, and the activity of HRP in the gel was investigated using substrate solution as described in the ESI† (Cycle 1). The gels were recovered and used for subsequent reactions to check enzyme activity in Cycles 2–5. Fig. 3b shows the enzymatic activities and relative activities of HRP in chitosan gels after silica formation with and without fusion silicatein in each cycle. HRP activities of the gels were reduced with and without fusion silicatein compared to that of the intact chitosan gel (40 U mg−1). However, the activity was much higher in the gel with fusion silicatein (∼21 U mg−1) than that without fusion silicatein (∼7 U mg−1). As a control, only the chitosan gel beads were used and they did not show an enzymatic activity. The coverage of the gel with silica formed by fusion silicatein was considered to suppress the release of the trapped enzyme. The relative activity of the gel with fusion silicatein was maintained at 85% even after five cycles, while it decreased to 26% in the gel without fusion silicatein. This result suggested that the fabrication of the silica layer on chitosan gel provided more stability than that without silica. Immobilisation of enzymes such as lipase,39 alcohol dehydrogenase,40 HRP,41 and trypsin42 in a chitosan gel matrix can ensure a wide range of working pH and thermostability. Optimizing the chitosan gel with silica will ensure improved enzyme trapping and stabilisation. The production of glucosamine residues as a result of enzymatic hydrolysis can be used to explain the biodegradability of chitosan at the molecular level.43 Chitinase from Pyrococcus furiosus was used to investigate the biodegradation of chitosan gel beads. The concentrations of N-acetyl glucosamine were significantly higher in chitosan gel treated with chitinase than in the controls confirming the biodegradability of chitosan gel beads (Fig. S7, ESI†).
Using this approach, agrochemical degradation enzymes could be immobilized in the chitosan gel–silica hybrid materials while protecting their enzymatic activities to use as a sustainable method to improve the quality of soil polluted with agrochemicals such as herbicides and pesticides. As a carrier system for vaccine delivery, chitosan gel microspheres have been studied as a promising carrier system for mucosal vaccination, especially via the oral and nasal route to induce enhanced immune responses.44 Similar systems can be further developed to increase stability by coating with silica.
In conclusion, fusion silicatein was adsorbed on chitosan gel and successfully catalysed silica formation on chitosan gel beads. The HRP enzyme was successfully immobilised in a chitosan gel–silica hybrid material. The chitosan gel–silica hybrid material ensured HRP stability. This strategy could be used to develop new materials for biomedical and environmental applications. Furthermore, TBP-mCherry can be used as a fluorescent tag to detect silica formed on chitosan gel using fluorescence microscopy. Interestingly, silicatein has other biocatalytic activities towards titania and Au nanocrystals.45,46 A novel method of enzyme immobilisation in silica-coated hydrogels under mild conditions would be a powerful tool for fabricating next-generation hybrid materials that can be applied in a wide range of research and industrial areas.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the ENEOS TonenGeneral Research/Development Encouragement & Scholarship Foundation and JSPS KAKENHI grant numbers JP18H03395 and JP21H03627. K. Godigamuwa would like to thank the Uehara Memorial Foundation for financial support.Notes and references
- Y. Zhang, J. Ge and Z. Liu, ACS Catal., 2015, 5, 4503–4513 CrossRef.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- F. Ullah, M. B. H. Othman, F. Javed, Z. Ahmad and H. M. Akil, Mater. Sci. Eng., C, 2015, 57, 414–433 CrossRef CAS PubMed.
- A. Bordbar-Khiabani and M. Gasik, Int. J. Mol. Sci., 2022, 23, 3665 CrossRef CAS PubMed.
- M. C. G. Pellá, M. K. Lima-Tenório, E. T. Tenório-Neto, M. R. Guilherme, E. C. Muniz and A. F. Rubira, Carbohydr. Polym., 2018, 196, 233–245 CrossRef PubMed.
- M. K. Lima-Tenório, E. T. Tenório-Neto, M. R. Guilherme, F. P. Garcia, C. V. Nakamura, E. A. G. Pineda and A. F. Rubira, Chem. Eng. J., 2015, 259, 620–629 CrossRef.
- P. A. G. Soares, A. I. Bourbon, A. A. Vicente, C. A. S. Andrade, W. Barros, M. T. S. Correia, A. Pessoa and M. G. Carneiro-Da-Cunha, Mater. Sci. Eng., C, 2014, 42, 219–226 CrossRef CAS PubMed.
- P. P. Luan, Y. J. Jiang, S. P. Zhang, J. Gao, Z. G. Su, G. H. Ma and Y. F. Zhang, J. Biosci. Bioeng., 2014, 118, 575–582 CrossRef CAS PubMed.
- M. Arteche Pujana, L. Pérez-Álvarez, L. C. Cesteros Iturbe and I. Katime, Carbohydr. Polym., 2013, 94, 836–842 CrossRef CAS PubMed.
- J. Xu, L. Ma, Y. Liu, F. Xu, J. Nie and G. Ma, Int. J. Biol. Macromol., 2012, 50, 438–443 CrossRef CAS PubMed.
- C. Weinberger, D. Kuckling and M. Tiemann, Gels, 2018, 4, 83 CrossRef CAS PubMed.
- C. Sanchez, B. Julián, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559–3592 RSC.
- R. Aversa, R. V. V. Petrescu, A. Apicella and F. I. T. Petrescu, Am. J. Biochem. Biotechnol., 2017, 13, 34–41 CrossRef CAS.
- W. Y. Cai, Q. Xu, X. N. Zhao, J. J. Zhu and H. Y. Chen, Chem. Mater., 2006, 18, 279–284 CrossRef CAS.
- K. Godigamuwa, K. Nakashima, J. Okamoto and S. Kawasaki, Biomacromolecules, 2020, 21, 2922–2928 CrossRef CAS PubMed.
- T. Asefa and Z. Tao, Chem. Res. Toxicol., 2012, 25, 2265–2284 Search PubMed.
- B. G. Trewyn, S. Giri, I. I. Slowing and V. S. Y. Lin, Chem. Commun., 2007, 3236–3245 RSC.
- H. Yan, C. Teh, S. Sreejith, L. Zhu, A. Kwok, W. Fang, X. Ma, K. T. Nguyen, V. Korzh and Y. Zhao, Angew. Chem., Int. Ed., 2012, 51, 8373–8377 CrossRef CAS PubMed.
- A. Rai and C. C. Perry, Langmuir, 2010, 26, 4152–4159 CrossRef CAS PubMed.
- K. Shimizu, J. Cha, G. D. Stucky and D. E. Morse, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6234–6238 CrossRef CAS PubMed.
- H. Oguri, K. Nakashima, K. Godigamuwa, J. Okamoto, Y. Takeda, F. Okazaki, M. Sakono and S. Kawasaki, J. Biosci. Bioeng., 2022, 133, 222–228 CrossRef CAS PubMed.
- X. Wang, H. C. Schröder, M. Wiens, H. Ushijima and W. E. Müller, Curr. Opin. Biotechnol, 2012, 23, 570–578 CrossRef CAS PubMed.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- L. Tan, X. Yu, P. Wan and K. Yang, J. Mater. Sci. Technol., 2013, 29, 503–513 CrossRef CAS.
- N. Shadjou and M. Hasanzadeh, Mater. Sci. Eng., C, 2015, 55, 401–409 CrossRef CAS PubMed.
- T. H. K. Nawarathna, K. Nakashima, T. Kawabe, W. Mwandira, K. Kurumisawa and S. Kawasaki, ACS Sustainable Chem. Eng., 2021, 9, 11493–11502 CrossRef CAS.
- K. Godigamuwa, K. Nakashima, S. Tsujitani and S. Kawasaki, Bioprocess Biosyst. Eng., 2021, 44, 1883–1890 CrossRef CAS PubMed.
- W. E. G. Müller, S. Engel, X. Wang, S. E. Wolf, W. Tremel, N. L. Thakur, A. Krasko, M. Divekar and H. C. Schröder, Biomaterials, 2008, 29, 771–779 CrossRef PubMed.
- M. Hashimoto, T. Ikegami, S. Seino, N. Ohuchi, H. Fukada, J. Sugiyama, M. Shirakawa and T. Watanabe, J. Bacteriol., 2000, 182, 3045–3054 CrossRef CAS PubMed.
- Y. Xu, Q. Liu, L. Zhou, Z. Yang and Y. Zhang, Mar. Biotechnol., 2008, 10, 701–708 CrossRef CAS PubMed.
- A. Fiamingo, A. Montembault, S.-E. Boitard, H. Naemetalla, O. Agbulut, T. Delair, S. P. Campana-Filho, P. Menasché and L. David, Biomacromolecules, 2016, 17, 1662–1672 CrossRef CAS PubMed.
- B. Qu and Y. Luo, Int. J. Biol. Macromol., 2020, 152, 437–448 CrossRef CAS PubMed.
- M. Wiens, T. Link, T. A. Elkhooly, S. Isbert and W. E. G. Müller, Chem. Commun., 2012, 48, 11331–11333 RSC.
- M. R. Ki, K. B. Yeo and S. P. Pack, Bioprocess Biosyst. Eng., 2013, 36, 643–648 CrossRef CAS PubMed.
- K.-I. Sano, H. Sasaki and K. Shiba, Langmuir, 2005, 21, 3090–3095 CrossRef CAS PubMed.
- H. Zhao, J. Xu, W. Lan, T. Wang and G. Luo, Chem. Eng. J., 2013, 229, 82–89 CrossRef CAS.
- W. Li, R. Yuan, Y. Chai, L. Zhou, S. Chen and N. Li, J. Biochem. Biophys. Methods, 2008, 70, 830–837 CrossRef CAS PubMed.
- D. Zhang, P. Sun, P. Li, A. Xue, X. Zhang, H. Zhang and X. Jin, Biomaterials, 2013, 34, 10258–10266 CrossRef CAS PubMed.
- H. Yagar and U. Balkan, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 864–870 CrossRef CAS PubMed.
- A. K. Dwamena, S. H. Woo and C. S. Kim, Biotechnol. Lett., 2020, 42, 845–852 CrossRef CAS PubMed.
- M. Bilal, T. Rasheed, Y. Zhao and H. M. N. Iqbal, Int. J. Biol. Macromol., 2019, 124, 742–749 CrossRef CAS PubMed.
- A. Manrich, C. M. A. Galvão, C. D. F. Jesus, R. C. Giordano and R. L. C. Giordano, Int. J. Biol. Macromol., 2008, 43, 54–61 CrossRef CAS PubMed.
- A. Kalidason, K. Saito, Y. Nanbu, H. Sasaki, R. Ohsumi, A. Kanazawa and T. Kuroiwa, J. Chem. Eng. Jpn., 2022, 55, 61–70 CrossRef CAS.
- M. A. Islam, J. Firdous, Y. J. Choi, C. H. Yun and C. S. Cho, Int. J. Nanomed., 2012, 7, 6077–6093 CAS.
- M. N. Tahir, M. Eberhardt, H. A. Therese, U. Kolb, P. Theato, W. E. G. Müller, H. C. Schröder and W. Tremel, Angew. Chem., Int. Ed., 2006, 45, 4803–4809 CrossRef CAS PubMed.
- P. Curnow, P. H. Bessette, D. Kisailus, M. M. Murr, P. S. Daugherty and D. E. Morse, J. Am. Chem. Soc., 2005, 127, 15749–15755 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tb02581g |
| This journal is © The Royal Society of Chemistry 2023 |