Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protein cohabitation: long-term immunoglobulin G storage at room temperature†
Pankaj
Bharmoria
 *acd,
Saik Ann
Ooi
*acd,
Saik Ann
Ooi
 a,
Andrea
Cellini
a,
Andrea
Cellini
 a,
Daniel
Tietze
a,
Daniel
Tietze
 a,
Michal
Maj
a,
Michal
Maj
 b,
Kasper
Moth-Poulsen
b,
Kasper
Moth-Poulsen
 *cdef and
Alesia A.
Tietze
*cdef and
Alesia A.
Tietze
 *a
*a
aDepartment of Chemistry and Molecular Biology, University of Gothenburg, Kemigården 10, 412 96, Gothenburg, Sweden. E-mail: alesia.a.tietze@gu.se
bDepartment of Chemistry – Ångström Laboratory, Physical Chemistry, Lägerhyddsvägen 1, 751 20 Uppsala, Sweden
cDepartment of Chemistry and Chemical Engineering, Chalmers University of Technology, Kemivägen 4, 412 96, Gothenburg, Sweden. E-mail: kasper.moth-poulsen@chalmers.se
dThe Institute of Materials Science of Barcelona, ICMAB-CSIC, Bellaterra, 08193, Barcelona, Spain. E-mail: pbharmoria@icmab.es
eCatalan Institution for Research & Advanced Studies, ICREA, Pg. Lluís Companys 23, Barcelona, Spain
fDepartment of Chemical Engineering, Universitat Politècnica de Catalunya, EEBE, Eduard Maristany 10–14, 08019 Barcelona, Spain
First published on 23rd May 2023
Abstract
Long-term functional storage of therapeutic proteins at room temperature has been an eternal challenge. Inspired by the cellular cooperativity of proteins, we have taken a step forward to address this challenge by cohabitating Immunoglobulin G (IgG1) with a food protein gelatin in the solid-state at room temperature. Interestingly, IgG1 remained functionally active for a record 14 months revealed from the western-blot assay. Further quantification by HP-LC analysis showed 100% structural integrity of IgG1 with no degradation in the gelatin matrix during this period. The developed formulation has a direct application in oral medical nutrition therapy to cure gastrointestinal microbial infections. Also the strategy provides a robust energy economic alternative to the protein engineering methods for long-term functional storage of therapeutic proteins at room temperature.
10th Anniversary StatementWe are proud to be part of the success of Journal of Materials Chemistry B in the field of materials science and congratulate it on its 10th anniversary. As for all jubilarians, we wish the journal and editorial team a lucky hand to wisely select manuscripts to further strengthen the journal's international reputation. |
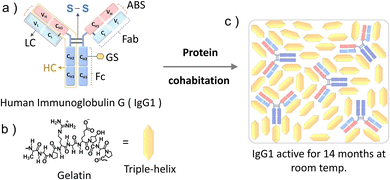
Immunoglobulin G (IgG1) is a 150 kDa antibody that is a major contributor to the humoral immunity of humans.1a,b IgG1 is produced as a monomer by plasma B cells in the lymph, followed by secretion into the blood and extracellular fluid where it acts against microbial infections (viruses, bacteria, and fungi).2 IgG1 has a Y shape structure comprising a pair of identical heavy and small chains linked to each other by disulfide bonds (Fig. 1a). Each end of the two arms of IgG1 contain antigen-binding sites at the variable domain of both the heavy (VH) and light chain (VL) and hence IgG1 has a dual antigen-binding site to counter pathogens (Fig. 1a).3a,b
Other than their biological role in neutralizing pathogens,1–3 oral immunoglobulin formulations have attracted a lot of interest in oral medical nutrition therapy.4 Historically, the plasma formulation of immunoglobulin has been part of animal feed to tackle intestinal microbial disorders.4a,5 However, in the past 50 years, successful human trials of the oral formulations of immunoglobulins with human and bovine serums (lyophilized powder or liquid) against gastrointestinal microbial infections have also been performed.6a–n These studies are mainly focused on the pharmacokinetics of IgG in the gastrointestinal tract of healthy and microbially infected people of various age groups.6a–n
In addition to the in vivo pharmacokinetics of IgG formulations, their poor ex vivo stability has been a key challenge for long-term storage and transportation.7 This is due to the time-dependent structural alterations at room temperature, which ultimately affect their functional activity.8 Being a globular protein, immunoglobulins are susceptible to time-dependent aggregation-induced unfolding in an aqueous medium at room temperature and hence are stored at around −70 °C (for long-term usages) and 4 °C (for short-term usages).9a,b The time-dependent circular dichroism spectra of the aqueous solution of IgG1 at room temperature showing alterations in the secondary structure are shown in Fig. S1 (ESI†). This is due to the small Gibbs free energy of unfolding of globular proteins at room temperature (ΔGU = 5–15 kcal mol−1).10a,b In addition to this they are also prone to digestion by proteases.7 Attempts have been made to address such issues in liquid formulations by adding excipients such as sugars,11 polyols,12 peptides,13 buffers,14a–c and bacteriostatic agents.15 For example, enhanced folding stability and reduced aggregation of IgG1 have been reported in 0.5 M sucrose.11 A >5 °C enhancement in thermal stability of IgG1-kappa (1 mg mL−1 or 50 mg mL−1) against aggregation has been reported at pH. 4.5 in aqueous solution of 20% glucose, 20% sorbitol, and 5% glucose + 10% sorbitol.12 Enhanced time-dependent thermal stability (at 55 °C for 7 days) of the IgG1 monomer has been reported in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous formulation with lysine dendron L6 peptide.13 Buffers,14a–c on the other hand, can just stabilize proteins at 4 °C for a day or a few weeks. Recently Kuzman et al.16 have developed a long-term stability prediction method using Arrhenius-based kinetics for antibody storage in solution at 5 °C for up to three years.16 Schaefer et al.17 studied the mechanism of the kinetic stability of IgG in the presence of various salts (NaClO4, NaCl, and Na2SO4), and osmolytes (Arginine, betaine, sarcosine, TMAO, sorbitol, sucrose, and trehalose) and concluded that the kinetic stability of IgGs depends, in a complex manner, both on the intrinsic properties of an IgG, particularly the stability of the Fabs with respect to the other domains, and on the nature of interactions between the protein and additives.17 Beyond aqueous formulations, enhanced stability of antibody/antibody fragments using chemical engineering through covalent conjugation18a–c or ionic liquefaction19 has also been reported. However, the maximum aqueous stability of such conjugates has been reported to be 8 months at 4 °C in phosphate buffer solution.18a Hence the low shelf-life of the aqueous formulations of immunoglobulins at room temperature or even at 4 °C remains a key challenge. That is why they are commercially sold as lyophilized powders in combination with cryoprotectants and need to be stored at −20 °C for long-term stability.15,20a–c But the multiple freeze–thaw cycles of lyophilized antibodies can affect their structure and performance during long-term storage and use.21 Hence, there is a need to develop strategies for long-term functional storage of antibodies in a solid-state at room temperature. An interesting example of work in this direction is IgG1 and IgM dried on cellulose filter paper that have been reported to remain functionally stable for a month at room temperature22 and for more than a year at −20 °C.23 But the long-term functional stability at room temperature in the solid-state remains a key challenge that we are addressing through this work.
1 aqueous formulation with lysine dendron L6 peptide.13 Buffers,14a–c on the other hand, can just stabilize proteins at 4 °C for a day or a few weeks. Recently Kuzman et al.16 have developed a long-term stability prediction method using Arrhenius-based kinetics for antibody storage in solution at 5 °C for up to three years.16 Schaefer et al.17 studied the mechanism of the kinetic stability of IgG in the presence of various salts (NaClO4, NaCl, and Na2SO4), and osmolytes (Arginine, betaine, sarcosine, TMAO, sorbitol, sucrose, and trehalose) and concluded that the kinetic stability of IgGs depends, in a complex manner, both on the intrinsic properties of an IgG, particularly the stability of the Fabs with respect to the other domains, and on the nature of interactions between the protein and additives.17 Beyond aqueous formulations, enhanced stability of antibody/antibody fragments using chemical engineering through covalent conjugation18a–c or ionic liquefaction19 has also been reported. However, the maximum aqueous stability of such conjugates has been reported to be 8 months at 4 °C in phosphate buffer solution.18a Hence the low shelf-life of the aqueous formulations of immunoglobulins at room temperature or even at 4 °C remains a key challenge. That is why they are commercially sold as lyophilized powders in combination with cryoprotectants and need to be stored at −20 °C for long-term stability.15,20a–c But the multiple freeze–thaw cycles of lyophilized antibodies can affect their structure and performance during long-term storage and use.21 Hence, there is a need to develop strategies for long-term functional storage of antibodies in a solid-state at room temperature. An interesting example of work in this direction is IgG1 and IgM dried on cellulose filter paper that have been reported to remain functionally stable for a month at room temperature22 and for more than a year at −20 °C.23 But the long-term functional stability at room temperature in the solid-state remains a key challenge that we are addressing through this work.
Herein, inspired by the biological cooperativity of proteins to remain stable and execute a specific function24a,b we are proposing the protein cohabitation10a phenomenon for long-term functional storage of IgG1 in the solid matrix of a food protein gelatin (Fig. 1). Gelatin is a commercial food protein25 that is a hydrolyzed form of collagen. Upon dehydration of the aqueous solution of gelatin, it undergoes interchain cross-linking26 resulting in the formation of a stable three-dimensional semi-crystalline structure comprising crystalline triple-helices and amorphous inter-helix regions.27 Due to its long-term room temperature stability in the solid-state27 and food-grade properties, gelatin is a suitable cohabitation partner for IgG1 when considering its application in medical nutrition therapy.4a–c Interestingly IgG1 remained functionally active with the retention of its original structure even after 14 months of cohabitation with gelatin in the solid-state at room temperature. Hence through this proof-of-concept demonstration we have overcome a key challenge of poor long-term stability of protein therapeutics at room temperature by developing a cheap and robust fabrication approach resulting in a biocompatible solid-state formulation. The model structure of IgG1 and molecular structure of gelatin and illustration of IgG1-gelatin protein cohabitation is presented in Fig. 1a–c. The IgG1-gelatin cohabitation films were prepared by air drying an aqueous solution of IgG1-gelatin in 0.1 M NaCl (see Method section and Fig. S2 (ESI†) for the detailed procedure). The final solid formulation contains 1.2 wt% of IgG1 and 98.8 wt% of gelatin.
The prepared IgG1-gelatin solid formulation was then allowed to cohabitate at room temperature for 14 months. After 14 months of cohabitation, the IgG1-gelatin formulation was redissolved in phosphate buffer pH 7.4 and characterized using circular dichroism (CD) spectroscopy,28 and SDS-PAGE29 for secondary structure, molecular weight analysis, and hyperfine liquid chromatography (HPLC) and western blot (WB) assay30 to confirm the structural stability and functional activity of the incubated IgG1 in comparison to the freshly prepared unformulated lgG1 sample.
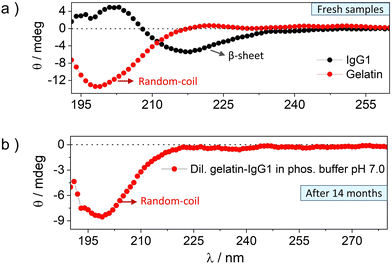
The CD spectra of fresh and 14 months incubated gelatin-IgG1 samples are shown in Fig. 2 and the corresponding HT [V] plots demonstrating the accuracy of the data are shown in Fig. S3 (ESI†). The CD spectrum of fresh IgG1 (Fig. 2a, black symbol) shows a fingerprint β-sheet conformation with a negative absorption band at an ellipticity minimum of 217 nm (−θ = 217 nm) and a positive absorption band at an ellipticity maximum of 202 nm (+θ = 202 nm).28 Whereas the CD spectrum of fresh gelatin (Fig. 2a, red symbol) shows a fingerprint random coil conformation with a negative absorption band at the ellipticity minimum of 198 nm (−θ = 198 nm).31
The CD spectrum of IgG1-gelatin cohabitated for 14 months in dilute solution is shown in Fig. 2b. Due to the mixed protein solution with very low IgG1 concentration, we could not see the IgG1 bands in low concentration solution. But we observed the random coil band corresponding to gelatin (Fig. 2b). This data confirms the stability of the secondary structure of gelatin during cohabitation. Since the post-incubation CD spectrum of IgG1 and gelatin were recorded in their mutual presence, we tried to find the IgG1 bands by increasing the concentration of IgG1-gelatin solution (Fig. S4, ESI†). We found a red shifted band showing positive and negative ellipticity just like IgG1, but it could also be due to the triple-helix structure of gelatin at high concentration that also shows similar spectra. The combined spectrum of IgG1-gelatin shows saturation of the gelatin peak due to high concentration confirmed from HT[V] data (Fig. S5, ESI†). Therefore, CD spectra could not confirm the post-incubation secondary structural stability of IgG1 in its mixture with gelatin due to spectral interference. Hence, we used SDS-PAGE to analyse the structural integrity of IgG1.
The structural integrity of IgG1 during cohabitation has been confirmed by comparing its molecular weights with the fresh IgG1 using SDS-PAGE (see Methods section of the ESI† for the detailed experimental procedure).29 IgG1 has a molecular weight of 150 kDa, corresponding to 50 kDa for a pair of heavy chains and 25 kDa for a pair of light chains (Fig. 3a).1a,b The IgG1 in gelatin solution cohabitated for 14 months in the solid-state shows a band at 150 kDa in SDS-PAGE (Fig. 3b), which is the same as that of the fresh IgG1 solution. Hence, the SDS-PAGE images confirm the retention of the structural integrity of IgG1 without any fragmentation of its various domains during cohabitation.
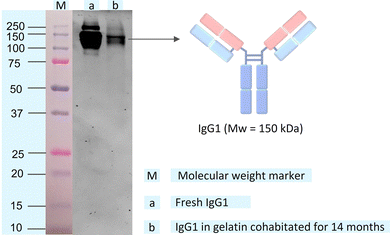 | ||
| Fig. 3 SDS-PAGE gel image of (a) fresh IgG1 solution and (b) IgG1 in gelatin solution after 14 months of solid-state cohabitation at room temperature in the solid-state. The protein ladder or molecular weight marker (M) on the gel with IgG1 bands is shown in Fig. S6 (ESI†). | ||
To quantify the % of IgG1 retaining its structural integrity after 14 months, we performed HPLC analysis. Therefore, the 14 month old sample was cut into two halves (named sample I and sample II), dissolved in buffer (phosphate, 10 mM, pH 7.2) and then analysed separately.
So far, we were able to separate the gelatin from IgG1, yielding a peak at 2.5 and 3.2 min, which corresponds to gelatin and IgG1, respectively (Fig. 4, yellow line and Fig. S7, ESI†). To quantify IgG1 in the 14 month-old sample, both samples were spiked with two different amounts of an IgG1 reference solution. The original IgG1 content of the gelatin-IgG1 sample was calculated from the average increase of the integral of the IgG1 peak at 3.2 min (Fig. 4, red and violet lines) after addition of the IgG1 reference (Table S1, ESI†). The experimentally determined IgG1 content was slightly higher (3.12 mg ± 0.8 mg and 5.2 mg ± 1.2 mg) than the original IgG1 content (2.4 mg) in the sample. However, it should be noted that the IgG1-gelatin samples were still much more viscous than a normal aqueous sample (although the samples were handled at 40 to 45 °C), which might partly explain the high error and discrepancy compared to the original IgG1 content. Nevertheless, the reference IgG1 coelutes with the IgG1, which was stored for 14 months in the gelatin suggesting that the IgG1 is still intact and structurally preserved.
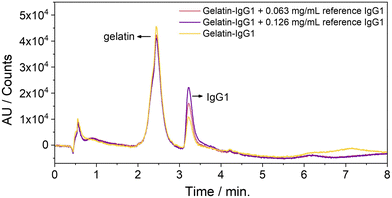 | ||
| Fig. 4 HP-LC-chromatograms of IgG1 stored in gelatin for 14 months at room temperature (yellow line) and spiked with two different amounts of reference IgG1. | ||
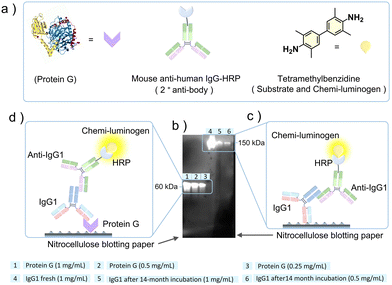
After ascertaining the structural integrity of IgG1 after 14 months of cohabitation in gelatin, we analyzed its functional activity using a western blot assay (see Methods section of the ESI† for the detailed experimental procedure).30 For the western blot assay, we used recombinant protein G for IgG1 binding as the primary antibody (fresh or 14 months incubated), and mouse anti-human IgG1-horseradish peroxidase (anti-IgG1-HRP) conjugate as a secondary antibody for binding with the primary antibody. Protein G is a ∼60 kDa cell surface protein expressed in group G of Streptococcal bacteria.32 It is a type III Fc receptor that has a high binding affinity for the Fc fragment of IgG1 compared to the Fab fragment.33 The mouse anti-human IgG1-HRP conjugate is a secondary antibody where anti-IgG1 is conjugated with the enzyme horseradish peroxidase (HRP). It primarily binds to the Fc fragment of the heavy chain of IgG1.34 The HRP conjugated to the anti-IgG1 catalyzes the oxidation of colorless tetramethylbenzidine (TMB) to colored tetramethylbenzidine diimine (TMBD) that acts as a signal of anti-IgG1 binding to IgG1.35
Fig. 5a shows the ribbon and model structures of the protein G and mouse anti-human IgG-HRP conjugate and molecular structure of tetramethylbenzidine. The western blot assay was performed at different concentrations of protein G (0.25 mg mL−1 to 1 mg mL−1) and 14 months incubated IgG1 (0.5 mg mL−1 and 1 mg mL−1) and a single concentration of fresh IgG1 (1 mg mL−1), as shown in Fig. 5b. To confirm the binding ability of the 14 months incubated IgG1 with protein G, we also did a control experiment where fresh IgG1 was used as the primary antibody (Fig. S8, ESI†). The detection of chemiluminescence signals in samples 1 to 6 on the nitrocellulose blotted paper (Fig. 5b) confirmed the functional activity of both Fc and Fab fragments of the IgG1 incubated with gelatin for 14 months at room temperature.
This is because chemiluminescence signals could be developed only if anti-IgG1-HRP binds to IgG1, which catalyzes the oxidation of TMB to TMBD. The anti-IgG1-HRP either binds to IgG1 attached directly to the nitrocellulose paper (Fig. 5c) or through protein G attached to the nitrocellulose paper (Fig. 5d). We detected strong chemiluminescence signals for the protein G mediated binding (Fig. 5b, 1–3 and Fig. 5d). This could be due to the poor transfer of high molecular weight IgG1 from SDS-PAGE gel to nitrocellulose paper during blotting, which is further masked by the presence of the high concentration of gelatin. This is a common issue with the high molecular weight proteins during nitrocellulose paper blotting. However, the obtained signals in sample 5, and 6 (Fig. 5b and c) are quite clear to ascertain the IgG1 binding to anti-IgG1-HRP.
Conclusion
In conclusion we have developed a simple method of solid-state protein cohabitation to address an outstanding issue of poor long-term functional storage of antibodies at room temperature. The IgG1 cohabitated in the solid-state with a food protein gelatin for 14 months at room temperature retained both structural and functional stability confirmed by SDS-PAGE, HP-LC, and western blot assay. The IgG1-gelatin solid-state formulation has a direct application in oral medical nutrition therapy for treating gastrointestinal microbial infections. Moreover, the developed approach gives new directions for long-term room temperature stability of protein/peptide therapeutics and other commercial globular proteins, thus providing a robust energy economic alternative to the protein engineering methods.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Marie Skłodowska-Curie Actions – European Commission post-doctoral grant (NIRLAMS, Grant agreement ID: 844972, PB), The La-Caixa Foundation (ID: 100010434, PB), the Knut and Alice Wallenberg Foundation via the Wallenberg Centre for Molecular and Translational Medicine (AT), and Swedish Research Council (2020-04299) (AT), Catalan Institute of Advanced Studies (ICREA) (KMP) are gratefully acknowledged for financial support. Hanna Zhdanova is acknowledged for assistance during SDS-PAGE.References
- (a) C. Black, Immunol. Cell Biol., 1997, 75, 65 CrossRef CAS PubMed; (b) L. L. Lu, T. J. Suscovich, S. M. Fortune and G. Alter, Nat. Rev. Immunol., 2018, 18, 46 CrossRef CAS PubMed.
- L. Mesin, J. Ersching and G. D. Victora, Immunity, 2016, 45, 471 CrossRef CAS PubMed.
- (a) H. W. Schroeder Jr and L. Cavacini, J. Allergy Clin. Immunol., 2010, 125, S41 CrossRef PubMed; (b) C. A. Janeway Jr, P. Travers, M. Walport and M. J. Shlomchik, The structure of a typical antibody molecule. In Immunobiology: The Immune System in Health and Disease, Garland Science, 5th edn, 2001 Search PubMed.
- (a) V. S. Jasion and B. P. Burnett, Nutr. J., 2015, 14, 22 CrossRef PubMed; (b) N. Roos, S. Mahe, R. Benamouzig, H. Sick, J. Rautureau and D. Tome, Nutr. J., 1995, 125, 1238 CAS; (c) H. Donabedian, Nutr. J., 2006, 5, 21 CrossRef PubMed; (d) A. C. Brown and R. D. A. Valiere, Nutr. Clin. Care, 2004, 7, 56 Search PubMed.
- (a) R. Mehra, P. Marnila and H. Korhonen, Int. Dairy J., 2006, 16, 1262 CrossRef CAS PubMed; (b) D. DuBourdieu, in Colostrum Antibodies, Egg Antibodies and Monoclonal Antibodies Providing Passive Immunity for Animals, Nutraceuticals in Veterinary Medicine, ed. R. Gupta, A. Srivastava, R. Lall, Springer, Cham, 2019, p. 245, DOI:10.1007/978-3-030-04624-8_18; (c) C. J. Hedegaard, M. L. Strube, M. B. Hansen, B. K. Lindved, A. Lihme, M. Boye and P. M. H. Heegaard, PLoS One, 2016, 11, e0147373 CrossRef PubMed.
- (a) R. M. Zinkernagel, H. Hilpert and H. Gerber, Experientia, 1972, 28, 741 CrossRef PubMed; (b) P. M. Blum, D. L. Phelps, B. J. Ank, H. J. Krantman and E. R. Stiehm, Pediatr. Res., 1981, 15, 1256 CrossRef CAS PubMed; (c) M. M. Eibl, H. M. Wolf, H. Furnkranz and A. Rosenkranz, N. Engl. J. Med., 1988, 319, 1 CrossRef CAS PubMed; (d) H. Hilpert, H. Brussow, C. Mietens, J. Sidoti, L. Lerner and H. Werchau, J. Infect. Dis., 1987, 156, 158 CrossRef CAS PubMed; (e) G. A. Losonsky, J. P. Johnson, J. A. Winkelstein and R. H. Yolken, J. Clin. Invest., 1985, 76, 2362 CrossRef CAS PubMed; (f) C. P. Kelly, S. Chetham, S. Keates, E. F. Bostwick, A. M. Roush, I. Castagliuolo, J. T. LaMont and C. Pothoulakis, Antimicrob. Agents Chemother., 1997, 41, 236 CrossRef CAS PubMed; (g) M. Warny, A. Fatimi, E. F. Bostwick, D. C. Laine, F. Lebel, J. T. LaMont, C. Pothoulakis and C. P. Kelly, Gut, 1999, 44, 212 CrossRef CAS PubMed; (h) R. E. McClead Jr, T. Butler and G. H. Rabbani, Am. J. Med., 1988, 85, 811 CrossRef CAS PubMed; (i) E. A. Copelan, T. P. Bechtel, J. P. Klein, J. L. Klein, P. Tutschka, N. Kapoor, N. C. Featheringham and B. R. Avalos, Bone Marrow Transplant., 1994, 13, 87 CAS; (j) A. K. Bogstedt, L. Hammarstrom and A. K. Robertson, Antimicrob. Agents Chemother., 1997, 41, 2320 CrossRef CAS PubMed; (k) R. Lissner, P. A. Thurmann, G. Merz and H. Karch, Int. J. Clin. Pharmacol. Ther., 1998, 36, 239 CAS; (l) J. Pacyna, K. Siwek, S. J. Terry, E. S. Roberton, R. B. Johnson and G. P. Davidson, J. Pediatr. Gastroenterol. Nutr., 2001, 32, 162 CrossRef CAS PubMed; (m) M. Wu, X. Wang, L. Sun and Z. Chen, Nutr. Metab., 2022, 22, 1 CAS; (n) H. Ma, C. O’Fagain and R. O’Kennedy, Biochimie, 2020, 177, 213 CrossRef CAS PubMed.
- T. Laptoš and J. Omersel, Exp. Ther. Med., 2018, 15, 3161 Search PubMed.
- V. Joshi, T. Shivach, V. Kumar, N. Yadav and A. Rathore, Biotechnol. J., 2014, 9, 1195 CrossRef CAS PubMed.
- (a) A. Siew and M. Brown, Biopharm Int., 2015, 28, 40 Search PubMed; (b) M. M. Gromiha, J. An, H. Kono, M. Oobatake, H. Uedaira and A. Sarai, Nucleic Acids Res., 1999, 27, 286 CrossRef CAS PubMed.
- (a) P. Bharmoria, S. F. H. Correia, M. Martins, M. A. Hernandez-RodRíguez, S. P. M. Ventura, R. A. S. Ferreira, L. D. Carlos and J. A. P. Coutinho, J. Phys. Chem. Lett., 2020, 11, 6249 CrossRef CAS PubMed; (b) P. Manikwar, R. Majumdar, J. M. Hickey, S. V. Thakkar, H. S. Samra, H. A. Sathish, S. M. Bishop, C. R. Middaugh, D. D. Weis and D. B. Volkin, J. Pharmacol. Sci., 2013, 102, 2136 CrossRef CAS PubMed.
- A. Bhambhani, J. M. Kissmann, S. B. Joshi, D. B. Volkin, R. S. Kashi and C. R. Middaugh, J. Pharmacol. Sci., 2012, 101, 1120–1135 CrossRef CAS PubMed.
- R. Bansal, S. Dhawan, S. Chattopadhyay, G. P. Maurya, V. Haridas and A. S. Rathore, Bioconjugate Chem., 2017, 28, 2549–2559 CrossRef CAS PubMed.
- R. J. Kubiak, N. Lee, Y. Zhu, W. R. Franch, S. V. Levitskaya, S. R. Krishnan, V. Abraham, P. F. Akufongwe, C. J. Larkin and W. I. White, J. Immunol. Res., 2016, 1485615 Search PubMed.
- (a) Y. Yazdani, S. Mohammadi, M. Yousefi and F. Shokri, Avicenna J. Med. Biotechnol., 2015, 7, 145 Search PubMed; (b) B. K. Chavez, C. D. Agarabi, E. K. Read, M. T. Boyne, M. A. Khan and K. A. Brorson, BioMed Res. Int., 2016, 2074149 Search PubMed; (c) J. Cini, A. Nagi and M. Taddei, Buffer Formulations for Enhanced Antibody Stability, US Pat., 2016/0235845 A1, 2013 Search PubMed.
- C. ÓFágáin and K. Colliton, in Storage and lyophilization of pure proteins, Protein chromatography methods and protocols, ed. D. Walls, S. Loughran, Humana Press-Springer Nature, NY, 2nd edn, 2017, p. 159 Search PubMed.
- D. Kuzman, M. Bunc, M. Ravnik, F. Reiter, L. Žagar and M. Bončina, Sci. Rep., 2021, 11, 1 CrossRef PubMed.
- J. V. Schaefer, E. Sedlák, F. Kast, M. Nemergut and A. Plückthun, MABS, 2018, 10, 607 CrossRef CAS PubMed.
- (a) A. Maruani, M. E. B. Smith, E. Miranda, K. A. Chester, V. Chudasama and S. Caddick, Nat. Commun., 2015, 6, 6645 CrossRef CAS PubMed; (b) E. R. Johnson, H. Qi, R. J. Borgmeyer, K. R. Kessler and L. D. Zeng, Stable pH Optimized Formulation of a Modified Antibody, WO 2004/019861 A2, 2002 Search PubMed; (c) C. Roque, A. Sheung, N. Rahman and S. F. Ausar, Mol. Pharm., 2015, 12, 562 CrossRef CAS PubMed.
- J. M. Slocik, P. B. Dennis, Z. Kuang, A. Pelton and R. R. Naik, Commun. Mater., 2021, 2, 118 CrossRef CAS.
- (a) A. L. Daugherty and R. J. Mrsny, Adv. Drug Delivery Rev., 2006, 58, 686 CrossRef CAS PubMed; (b) J. Park, K. Nagapudi, C. Vergara, R. Ramachander, J. S. Laurence and S. Krishnan, Pharm. Res., 2013, 30, 968 CrossRef CAS PubMed; (c) S. Telikepalli, O. S. Kumru, J. H. Kim, S. B. Joshi, K. B. O'Berry, A. W. Blake-Haskins, M. D. Perkins, C. R. Middaugh and D. B. Volkin, J. Pharm. Sci., 2015, 104, 495 CrossRef CAS PubMed.
- J. Horna, S. Jenab, A. Aksanb and W. Friess, Eur. J. Pharm. Biopharm., 2019, 134, 185 CrossRef PubMed.
- E. N. De-Gaspari, A. A. Ribeiro-Filho and W. D. Zollinger, Braz. J. Med. Biol. Res., 1994, 27, 2889–2893 CAS.
- A. S. Ferraz, E. F. Belo, L. M. Coutinho, A. P. Oliveira, A. M. Carmo, D. L. Franco, T. Ferreira, A. Y. Yto, M. S. Machado, M. C. Scola and E. De Gaspari, BMC Infect. Dis., 2008, 8, 30 CrossRef PubMed.
- (a) A. Levitzki and J. Schlessinger, Biochemistry, 1974, 13, 214 CrossRef PubMed; (b) C. M. Porter and B. G. Miller, Bioorg. Chem., 2012, 43, 44 CrossRef CAS PubMed.
- K. B. Djagny, Z. Wang and S. Xu, Crit. Rev. Food Sci. Nutr., 2001, 41, 481 CrossRef CAS PubMed.
- A. V. Tobolskv, Nature, 1967, 215, 509–510 Search PubMed.
- P. Bharmoria, S. Hisamitsu, Y. Sasaki, T. S. Kang, M. Morikawa, B. Joarder, K. Moth-Poulsen, H. Bildirir, A. Mårtensson, N. Yanai and N. Kimizuka, J. Mater. Chem. C, 2021, 9, 11655–11661 RSC.
- A. Janda and A. Casadevall, Mol. Immunol., 2010, 47, 1421–1425 CrossRef CAS PubMed.
- T. L. Kirley and A. B. Norman, Biochem. Biophys. Res. Commun., 2018, 503, 944 CrossRef CAS PubMed.
- U. Mårtensson, A. G. Sjöholm, G. Sturfelt, L. Truedsson and A.-B. Laurell, Immunol., 1992, 35, 735 Search PubMed.
- P. Manavalan and W. C. Johnson, Nature, 1983, 305, 831 CrossRef CAS.
- U. Sjobring, L. Bjorck and W. Kastern, J. Biol. Chem., 1991, 266, 399 CrossRef CAS.
- G. C. Stone, U. Sjöbring, L. Eljörck, J. Sjoquist, C. V. Barber and F. A. Nardella, J. Immunol., 1989, 143, 565 CrossRef CAS.
- Z. He, L. Jiang, T. Zhang, M. Zhou, D. Wu, T. Yuan, Y. Yuan and Y. Cheng, Int. J. Mol. Med., 2018, 42, 2269 Search PubMed.
- L. S. A. Busa, T. Komatsu, S. Mohammadi, M. Maeki, A. Ishida, H. Tani and M. Tokeshi, Anal. Sci., 2016, 32, 815 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb00161j |
| This journal is © The Royal Society of Chemistry 2023 |