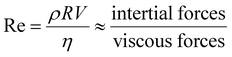Recent advancements in Mg-based micromotors for biomedical and environmental applications
Yue
Wang
 a,
Boyu
Qin
*e,
Sihan
Gao
a,
Xuanchun
Wang
a,
Hongyue
Zhang
*b and
Zhiguang
Wu
a,
Boyu
Qin
*e,
Sihan
Gao
a,
Xuanchun
Wang
a,
Hongyue
Zhang
*b and
Zhiguang
Wu
 *acd
*acd
aSchool of Medicine and Health, Harbin Institute of Technology, Harbin 150001, China. E-mail: zhiguangwu@hit.edu.cn
bLaboratory for Space Environment and Physical Sciences, Harbin Institute of Technology, Harbin 150001, China. E-mail: hongyuezhang@hit.edu.cn
cState Key Laboratory of Robotics and System, Harbin Institute of Technology, Harbin 150001, China
dKey Laboratory of Microsystems and Microstructures Manufacturing (Ministry of Education), Harbin Institute of Technology, Harbin 150001, China
eDepartment of Oncology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing 100039, China. E-mail: qpm2017@126.com
First published on 21st November 2023
Abstract
Synthetic micro/nanomotors have attracted considerable attention due to their promising potential in the field of biomedicine. Despite their great potential, major micromotors require chemical fuels or complex devices to generate external physical fields for propulsion. Therefore, for future practical medical and environmental applications, Mg-based micromotors that exhibit water-powered movement and thus eliminate the need for toxic fuels, and that display optimal biocompatibility and biodegradability, are attracting attention. In this review, we summarized the recent microarchitectural design of Mg-based micromotors for biomedical applications. We also highlight the mechanism for realizing their water-powered motility. Furthermore, recent biomedical and environmental applications of Mg-based micromotors are introduced. We envision that advanced Mg-based micromotors will have a profound impact in biomedicine.
1. Introduction
With the advancements in micro- and nano-technologies, micro/nanomotors that can convert their own energy or energy from the local environment into mechanical energy1–3 and perform movements under the control of external fields have been proposed.4–8 Micromotors can perform various tasks that are difficult for macroscale robots to complete, such as minimally invasive surgery,9 targeted drug delivery,10,11 bioimaging,12,13 biosensing,14,15 and cell transportation,16–18 and they therefore have great application prospects in the field of biomedicine. Due to biological barriers preventing the transport of therapeutics, in conventional drug delivery, drug molecules are distributed freely throughout the body, and these molecules generally do not precisely reach the desired location. Therefore, the efficiency of drug delivery methods needs to be improved.19 An improvement in the efficiency can be realized through the use of micro/nanomotors. Various drugs can be loaded on micromotors for active delivery, and Mg can react with the local environment to produce active H2 that can remove hydroxyl free radicals in the organism, further improving the therapeutic effect.20,21 In addition, the self-stirring effect generated by the self-driven micromotors in a fluid also promotes bacteria removal from that environment22 and improves detection efficiency in biosensing applications.23Thus, because of the versatility of micromotors, they are promising for applications in several fields, including the biomedical field. Therefore, the development of micromotors with biocompatibility, degradability, and motility is of considerable importance. Fig. 1 is a summary of the structure, motion, and related applications of Mg-based micromotors.
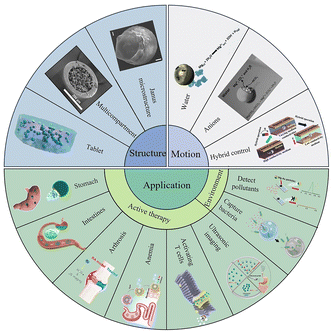 | ||
| Fig. 1 Schematic summary of the structure, movement, and applications of Mg-based micromotors. Janus microstructure. Reproduced with permission. Copyright 2020, Elsevier Ltd. All rights reserved.24 Multicompartment. Reproduced with permission. Copyright 2023, The Authors. Small published by Wiley-VCH GmbH.25 Tablet. Reproduced with permission. Copyright 2022, American Chemical Society.26 Water. Reproduced with permission. Copyright 2017, American Chemical Society.22 Anions. Reproduced with permission. Copyright 2013, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.27 Hybrid control. Reproduced with permission. Copyright 2022, American Chemical Society28 Stomach. Reproduced with permission. Copyright 2017, Springer Nature.29 Intestines. Reproduced with permission. Copyright 2022, American Chemical Society.26 Arthrosis. Reproduced with permission. Copyright 2021, American Chemical Society.21 Anemia. Reproduced with permission. Copyright 2019, American Chemical Society.30 Activating T cells. Reproduced with permission. Copyright 2020, Elsevier Ltd. All rights reserved.31 Ultrasonic imaging. Reproduced with permission. Copyright 2021, Elsevier Ltd. All rights reserved.32 Capture bacteria. Reproduced with permission. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.33 Detect pollutants. Reproduced with permission. Copyright 2016, American Chemical Society.34 | ||
However, in practical applications, micro/nanomotors have many limitations in terms of their microscopic characteristics, especially the Reynolds number (Re) of micro-/nanomotors. The motility of micromotors depends on the radius of micromotors (R), the density of fluids (ρ), the speed of micromotors (V), and the viscosity of the fluid (η); Re is given by
Early chemically driven micromotors were propelled by the decomposition of H2O2 with noble metals (such as Pt) to produce O2 that drove micromotors by the recoil force generated by O2 bubbles. As early as 2002, catalytically propelled motors on a centimetre scale were realized.37 In 2004, Sen et al. proposed a rod-shaped Pt/Au/Ni nanomotor in which the Pt segment can catalyse the generation of O2 bubbles from H2O2 in the solution and propel micromotors.38 In addition, Jänis et al. synthesized poly(3,4-ethylenedioxythiophene) (PEDOT)/MnO2 tubular micromotors through template-assisted deposition. The catalytic activity and rate when MnO2 acted as the catalyst were higher to a certain extent than that when Pt was the catalyst.39 With the further development of micro- and nano-technologies, Janus micromotors with various physical and chemical properties, such as optical, magnetic, and acoustic properties, have been developed to achieve functional diversity and collaboration. For example, Cai and Ren et al. reported a photo-responsive bismuth iodide (BiOI) micromotor whose motion can be controlled by its response to light at different wavelengths.40 For ultrasound-driven micromotors, mainly using the piezoelectric effect of materials, Iino et al. found that the asymmetrical micromotor can create different sound pressures using ultrasound to make the motor rotate.41 Magnetic and electric fields also regulate the speed and direction of micromotors with magnetic or conductive materials. However, these micromotors usually require an external control platform, and the operation process is complicated. Chemically driven micromotors are propelled by the medium or carried by the “fuel” itself, relying on the chemical gradient of the “fuel” to achieve propulsion, and the locally available chemical energy is converted into a driving force. This has the advantages of simplicity and high speed.
Although H2O2 as a “fuel” can effectively propel micromotors, this method is not suitable for a wide range of applications in vivo due to the strong oxidation and biological toxicity of H2O2.27 In order to avoid the side effects of H2O2in vivo, Mano et al. proposed a bioelectrochemically driven micromotor that is driven by the reaction between glucose and O2, and showed that the energy generated in the bioelectrochemical reaction can be directly converted into the mechanical power for propulsion.42 Wilson et al. successfully prepared a biocompatible enzyme-driven Janus micromotor based on hollow mesoporous silica nanoparticles (HMSNPs). The micromotor is powered by a biocatalytic reaction of three different enzyme/fuel combinations (catalase/H2O2, urease/urea, and GOx/glucose). These micromotors can be loaded with both hydrophilic and hydrophobic drugs and are promising for drug delivery applications.43 However, few suitable enzymes have been identified and the performance of these micromotors is limited by the local environment; finally, researchers have designed Janus micromotors with water as the fuel.44,45
Mg is an essential trace element and the main intracellular earth-metal cation that plays an important role in mental function. For example, Mg is important for maintaining human cardiovascular health46 and for promoting the regeneration of human nerve and bone cells.47 Moreover, Mg can significantly improve the HDL-cholesterol levels of patients with diabetes,48 improve the health of patients with non-alcoholic fatty liver disease,49 and reduce the risk of strokes.50 Studies reported that insulin can enhance cellular Mg uptake among patients with diabetes, and Mg intake is correlated to the degree of hyperglycaemia.51 A person deficient in Mg will present with health issues such as cardiometabolic disease,52 moderate tension-type headaches,53 and alcohol-related liver injury.54 Currently, Mg is widely used in bone scaffolds in the body.55 Mg is not only crucial for bodily functions but also has a very high reactivity: micrometre-scale Mg can easily react with water to produce Mg(OH)2 and H2. The generated H2 bubbles can propel micromotors. The Mg(OH)2 can be etched by salt ions in a physiological environment, to carry out continuous bubble-driven motion. The reaction rate of Mg can also be increased by heating or by introducing metals such as Al, Zn, Sr, and Mn, to increase the current corrosion. Furthermore, the motion of Mg-based micromotors can be regulated via reactions of Mg between solution and other ions so that Mg-based micromotors can complete various transportation tasks in complex fluid environments.56
In this paper, in order to clarify the development and potential applications of Mg-based micromotors, the unique properties of Mg-based micromotors, a variety of preparation methods, and various motility types are discussed. In addition, the use of Mg-based micromotors for in vivo therapy and environmental remediation and biosensing is introduced. Finally, the potential applications and existing challenges of Mg-based micromotors are discussed.
2. Architectonics for Mg-based micromotors
The chemically propelled micro/nanomotors must have an asymmetrical structure to create the local asymmetry field to offer the driving force. Researchers have proposed Janus micromotors composed of asymmetric materials that have an asymmetric structure.69 Janus micromotors have two different regions, one that is anisotropic in composition and another with a large specific surface area. These micromotors exhibit not only a sufficient number of modification sites but also a single-direction propulsion force.362.1 Janus microstructure
The micromotors with the Janus structure have a unique design that combines two different components in a single unit. Thus, Mg-based Janus micromotors exhibit a combination of different properties that impart new analytical capabilities to the micromotors. To fabricate the Janus structure of Mg-based micromotors, Tu et al. dispersed Mg microspheres on a glass sheet and then coated the surface of the Mg microspheres with poly(lactic-co-glycolic acid) (PLGA) containing doxorubicin hydrochloride (DOX). This was followed by drying, and then, the Mg/PLGA@DOX Janus micromotors were lightly scratched off the glass sheet and collected (Fig. 2a).24 The bottom-up multilayer modification method is one of the most commonly used strategies for preparing Mg-based Janus micromotors. By employing this strategy, simple and easily modified substances are imparted with multifunctional layers, such as a temperature-responsive layer, a pH-responsive layer, and a layer of loaded drugs.30,31,57 Mg microparticles were dispersed onto glass slides or polymethyl methacrylate (PMMA) to form a monolayer, and then, the metal layer was deposited, thereby modifying the polymer. Because part of Mg is blocked by glass or PMMA, the metal layer and the polymer cannot be attached to this part, resulting in an asymmetric structure. If Mg microspheres are dispersed on the PMMA, the micromotors can be collected by dissolving the PMMA film.58,59 Wang et al. and Zhang et al. coated red blood cell (RBC) membranes on the surface of Mg-based micromotors to realize micromotors with great biocompatibility and the ability to simulate cell motion. A layer of Mg microparticles was dispersed on a parafilm, and then, Au and alginate (ALG) were deposited on the surface of Mg. Finally, RBC membranes were coated onto the AuNP/ALG-covered surface.57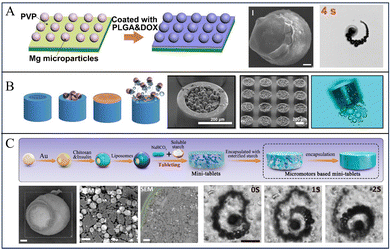 | ||
| Fig. 2 Various structures of Mg-based micromotors. (A) Mg-based Janus sphere micromotors. Reproduced with permission. Copyright 2020, Elsevier Ltd. All rights reserved.24 (B) Mg-based micromotors for intestinal tract targeted nesting inside microcontainers. Reproduced with permission. Copyright 2023, The Authors. Small published by Wiley-VCH GmbH.25 (C) An oral Mg-based micromotor tablet. Reproduced with permission. Copyright 2022, American Chemical Society.26 | ||
The Janus structure can also be prepared using asymmetric evaporation of a water-in-oil (w/o) emulsion. For example, the PABA-modified GODs were dissolved in an aqueous solution containing surfactants, and then, Pt and Fe3O4 nanoparticles were dissolved in chloroform; the resulting two solutions were evenly mixed to obtain a w/o emulsion. Because the w/o interface energy reduces spontaneously when the solution evaporates, the asymmetric deposition of nanoparticles was realized.60 Due to its simple preparation and easy multi-functional modification, the Janus structure is the most common form for bubble-ejection.
2.2 Multicompartment structure
To improve the penetration capability and cargo protection, one strategy is to nest Mg-based micromotors into multicompartment microdevices. For example, Maric et al. proposed a cascade device that loads Mg-based micromotors (Fig. 2b). The cascade devices were prepared by dispersing Mg microspheres on top of a 2-inch single-side polished silicon wafer, and by then depositing an Au layer by ion sputtering deposition. Subsequently, paracetamol was dissolved in ethanol and mixed with Mg/Au micromotors for 18 h to coat the micromotors with the drug and lid. To prepare multicompartment containers, a nanoscale layer of Ti and Au was deposited on a 4-inch silicon substrate by physical vapor deposition to promote the detachment of a single microcontainer after micromotor loading. The substrate was cut into millimetre-scale chips. This was followed by filling of the gap of the microcontainer with polydimethylsiloxane (PDMS) to prevent micromotors from being loaded into the gap, and then, the micromotors were loaded onto each chip. Finally, PDMS was carefully removed with tweezers to obtain the microscale containers full of micromotors.25Wang and Zhang et al. prepared tubular Mg-based micromotors using a polycarbonate (PC) film by using the template-assisted deposition technique. First, a layer of Au was deposited by sputtering on one side of the PC film as the working electrode, and then, PEDOT/Au microtubules were deposited on the film by electrochemical deposition. Next, 1–5 μm Mg spheres were loaded into the microtubules by vacuum infiltration. Finally, the PC film was dissolved with methylene chloride to obtain tubular micromotors. Furthermore, to protect the micromotors from reaction before reaching the intestine, the surface of the micromotors were coated with an enteric coating. The resultant micromotors can deliver drugs precisely via dissolution of the enteric coating to activate the propulsion and prolong the retention time of the drug in the tissue.61 Moreover, they also used a similar method to prepare Zn-based multicompartment micromotors.62
2.3 Tablet
To improve the usage of Mg-based micromotors for clinical translation, Mg-based micromotors were incorporated into a tablet for oral administration. For instance, Wang and Zhang et al. reported a microstirring pill for oral drug delivery: first, a single layer of TiO2 was deposited on Mg microspheres by sputtering. Then, lactose and maltose were mixed with drugs and micromotors. Finally, the resulting mixture was mixed with 75% ethanol solution to form a paste that was pressed to form the tablets. The Mg-based-micromotor tablet delivery system displays a high efficiency in drug delivery via oral administration. The investigation in both murine and porcine models illustrate that this tablet enhances the bioavailability of drug payloads in vivo.63 Subsequently, to improve the bioavailability and reduce the blood sugar levels of patients with diabetes, a similar method was used to prepare self-stirring tablets loaded with metformin.64Liu, Peng, and Tu et al. adopted the hydrophobic modification of natural starch by acetic anhydride, washed and removed pyridine and unreacted acetic anhydride to obtain esterified starch, and then dried and pulverized the esterified starch; then, they prepared insulin-loaded Mg/Au-CHI Janus micromotors by bottom-up deposition and coating with a Mg monolayer. Finally, esterified starch and micromotors were mixed and then pressed into tablets (Fig. 2c).26 These tablets with a self-stirring capability display improved drug delivery efficiency and enhanced drug absorption after gastrointestinal delivery in vivo.
3. Motion
The chemical propulsion of micromotors usually requires an asymmetric field, and this type of propulsion can be categorized as self-electrophoresis, self-diffusiophoresis, and bubble-ejection.65 Self-electrophoresis is led by uneven proton distribution that reduces or oxidizes around the micromotors, and self-diffusiophoresis enables motility through an asymmetric concentration gradient field. However, self-electrophoresis and self-diffusiophoresis can usually be driven well in salt-rich environments, because it is limited by ion concentrations. Because of the low solubility of the gas in solution and the surface tension in liquid, when micromotors react with the local environment to produce gas, a succession of bubbles are generated at the opening of the micromotors. Bubble-ejection micromotors can thus generate microjets when bubbles grow and collapse at the opening and propel micromotors by the recoil force from microjets.65,66 The details regarding the propulsion of Mg-based micromotors are given in Table 1. The displacement of each micromotor is the superposition of the radius of each bubble, so the size and frequency of bubbles are the key factors affecting the speed of the micromotor.| Material | Size | Medium | Speed | Ref. |
|---|---|---|---|---|
| Therapy | ||||
| Mg, Au | ∼27 | Simulated gastric fluid | ∼90 | 68 |
| Mg, Au, PEDOT | Length 15, diameter 5 | Gastric and intestinal fluid | ∼60 | 61 |
| Mg, Au | ∼20 | Gastric fluid | ∼60 | 67 |
| Mg, TiO2, PLGA, Fe, Se | ∼20 | Simulated intestinal fluid | ∼119 | 30 |
| Mg, Au, RBC, ALG | ∼20 | 0.08 M NaCl | ∼165 | 57 |
| Mg, TiO2, RBC, CHI | ∼20 | Intestinal fluid | ∼150 | 69 |
| Mg, TiO2 | ∼25 | Simulated gastric fluid, 1.2% Triton X-100, 37 °C | ∼350 | 64 |
| Mg, TiO2 | ∼25 | Simulated intestinal fluid, 1.2% Triton X-100, 37 °C | ∼180 | 64 |
| Mg, Au, CHI, liposomes | ∼25 | Simulated intestinal solution, mucin | ∼80 | 26 |
| Mg, Au, crosslinked SU-8 | Diameter 300; height 240 | 2 M NaCl, 0.34% Triton X-100 | ∼17 | 25 |
| Mg, Au | ∼24 | 2 M NaCl, 0.34% Triton X-100 | ∼55 | 25 |
| Mg, PLGA, ALG, CHI, anti-CD3 | ∼20 | 0.5 M NaHCO3, 0.2 wt% SDS | ∼42 | 31 |
| Mg, Au, TiO2, PLGA, CHI | ∼20 | PBS (pH = 5) | ∼27 | 73 |
| Mg, PLGA | ∼27 | Simulated body fluid | ∼57 | 24 |
| Mg, PLGA, HA hydrogel | ∼21 | Simulate synovial fluid | ∼40 | 21 |
| Mg, PLGA | ∼24 | Artificial cerebrospinal fluid | ∼51 | 20 |
| Mg, TiO2 | ∼20 | Simulated gastric fluid | ∼190 | 72 |
| Mg, TiO2 | ∼10 | Simulated intestinal fluid | ∼62 | 72 |
| Mg, TiO2, macrophage | ∼20 | Simulated gastric fluid | ∼136 | 72 |
| Mg, TiO2, macrophage | ∼10 | Simulated intestinal fluid | ∼19 | 72 |
| Environmental remediation | ||||
| Mg, Au, Ti, Ni | ∼30 | 0.3 M NaCl | ∼90 | 45 |
| Mg, Au, Ti, Ni | ∼30 | 3 M NaCl | ∼300 | 45 |
| Mg, Au, TiO2 | ∼20 | Seawater (0.54 M NaCl) | ∼110 | 74 |
| Mg, Au, PLGA, ALG, CHI | ∼20 | Drinking water | ∼36 | 75 |
| Mg, PLGA, ALG, CHI | ∼20 | Seawater | ∼72 | 75 |
| Mg, Ag, Fe, Au | ∼15 | Water pH 6, 2% Tween-20 | ∼12 | 22 |
| Biosensing | ||||
| Mg, Au | ∼20 | Milk | ∼108 | 34 |
| Mg, Au | ∼20 | Water | ∼296 | 34 |
| Mg, Au | ∼20 | Whiskey | ∼223 | 34 |
| Mg, Au | ∼20 | Serum | ∼40 | 34 |
| Mg, Pt | ∼30 | HS/PBS-FcMeOH, 1 mM glucose | ∼80 | 76 |
| Mg, Fe3O4 | ∼25 | PBS | ∼53 | 58 |
| Mg, Fe3O4 | ∼25 | Siluted serum | ∼30 | 58 |
| Mg, Fe3O4 | ∼25 | Diluted blood | ∼28 | 58 |
The progress of Mg-based micromotors is expressed as follows:
| Motility: Mg + 2H+ = Mg2+ + H2↑ |
| Form passivation: Mg2+ + 2OH− = Mg(OH)2↓ |
| Etch passivation: Mg(OH)2↓ + 2HCO3− = Mg2+ + 2CO32− + 2H2O |
Guan et al. reported the motion of Mg-based micromotors via a Mg–water reaction. In water, the reaction of Mg with water generates Mg(OH)2 and H2, and Mg(OH)2 on the exposed Mg surface impedes the further progress of the reaction, so the rapid removal of this passivation layer is necessary. Therefore, Guan et al. dissolved Mg(OH)2 in an aqueous solution of sodium NaHCO3 because HCO3− reacts with insoluble Mg(OH)2 to form soluble CO32−, so Mg can react with water and generate H2 bubbles. Subsequently, they proposed the motion of Mg-based micromotors in simulated body fluid (SBF) and blood plasma. They considered using anions (such as Cl− and HCO3−) in blood plasma to improve the motion of micromotors, and found that the pit corrosion induced by Cl− and the buffering effect of SBF and blood plasma increased the speed of micromotors (Fig. 3a).27,44 Moreover, Wang et al. also reported that the motion of Mg-based micromotors depended on the Cl− concentration, so increasing the Cl− concentration could effectively improve the speed of micromotors. For example, when the concentration of NaCl in the medium was increased from 0.3 M to 3 M, the speed of micromotors could be increased from 90 μm s−1 to 300 μm s−1.45 To further stabilize the generated bubbles in order to improve the driving efficiency, a minority surfactant, such as SDS,31 Triton X-100,64 and Tween-20,22 is added to the reaction medium. Finally, thermal energy can also improve the motion of micromotors: as the temperature rises, the rate of bubble generation increases, thus increasing the speed of the micromotors.
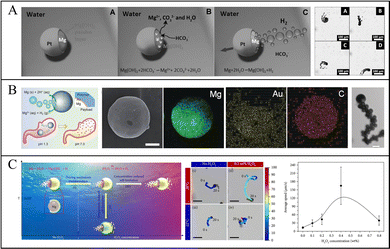 | ||
| Fig. 3 Motion of Mg-based micromotors. (A) Etched Mg(OH)2 on the surface of Mg-based Janus micromotors by HCO3−. Reproduced with permission. Copyright 2013, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.27 (B) Acid-responsive Mg-based micromotors moving in simulated gastric fluid. Reproduced with permission. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.67 (C) Mg-based Janus micromotors moving in response to variations in temperature and H2O2 concentration. Reproduced with permission. Copyright 2020, Kang Xiong et al.70 | ||
Physiological conditions, especially the gastric acid environment that has abundant H+ and anions, can effectively improve the motion of micromotors. Further study of the motion of micromotors in biological fluids showed that Mg-based micromotors exhibit efficient motion in simulated gastric fluid (Fig. 3b)67,68 and simulated intestinal fluid.26,69 Wang and Zhang et al. studied the speed of micromotors in simulated gastric and simulated intestinal fluids, and the speed of the micromotors was as high as 350 μm s−1 in simulated gastric fluid with a low pH and decreased to 180 μm s−1 in simulated intestinal fluid with higher pH.64
However, the control of the direction of micromotors is difficult: a single-drive can no longer propel the micromotor to complete complicated tasks. To control the motility of micromotors, field-controlled particles were induced based on chemically driven micromotors. Wang et al. loaded micromotors with magnetic nanoparticles by ion beam sputtering to control the direction of the micromotors by using an external magnetic field. They deposited a Ni layer on the micromotors so the micromotors could move to the target area and be recovered through magnetic field control.45 This method not only controls the micromotor in the complex environment, but also improves the motility of the micromotor after the fuel is exhausted. Later, Mei et al. also designed a tubular micromotor with a catalytic Pt layer and a magnetic Ni layer; the micromotor can be continuously propelled by H2 bubbles generated by the reaction of the Pt layer and H2O2, and the direction of micromotors can be controlled by using a magnetic field.71 The size of the micromotor can also affect the speed: Mei et al. also demonstrated that when the size of micromotors range from 10 to 15 μm, the average speed of micromotors was ∼190 μm s−1, and when the size was 20–25 μm, the average speed was ∼63 μm s−1 in the simulated gastric fluid.72
Guan et al. proposed micromotor-movement control using variations in the temperature and H2O2 concentration (Fig. 3c). In a solution containing NaHCO3, Mg reacts with water to generate H2, and Mg(OH)2 on the surface can be continuously etched by HCO3−. When H2O2 is added to the solution, the catalytic reaction of H2O2 and Pt is more likely to occur than the reaction between Mg and H2O. Therefore, the driving mode of Mg-based micromotors will change, the H2O2 decomposition reaction is the main reaction, effectively extending the driving life of the micromotor. The temperature-sensitive poly(N-isopropyl acrylamide) (PNIPAM) hydrogel layers deposited on Mg-based micromotors exist in a state of hydrophilic expansion when the temperature is lower than the lower critical solution temperature (LCST), so H2O2 has a high diffusion constant. When the temperature is lowered to the point where the concentration of H2O2 penetrating PNIPAM is high enough to generate bubbles, bubbles will be generated on both sides of the micromotors, so the speed of the micromotors will reduce and they may even hover in place.70
Enzyme-driven chemical drive methods are also important. Unlike traditional fuels, enzymes are biocompatible, making enzyme-powered micromotors valuable in the biomedical field.77 Wu's group designed a tubular DNA assembly enzyme-powered micro-motor capable of reaching a movement speed exceeding 450 μm s−1 through an enzymatic reaction at a concentration of 5% wt H2O2.78 Sánchez's group designed a porous micromotor built from a metal–organic framework (MOF) and powered by enzymatic reactions. Catalase was loaded in the porous structure of the MOF, and H2O2 was used as fuel to power the micromotor through the O2 bubbles generated by the enzymatic reactions.79 To further enhance the motility of enzyme-powered micromotors, Sánchez's group discovered that commonly used enzymes were not pure enough. After purifying the enzyme, they found that the micromotor loaded with purified urease achieved about 2.5 times the speed of the motor loaded with unpurified urease.80
4. Biocompatibility
Mg-based micromotors have great biocompatibility: other common metal micromotors usually do not degrade adequately in the body, but because Mg-based micromotors are driven by Mg as the fuel, the Mg core will be exhausted and retained in the body in the form of ions. Mg is a common mineral in the human body, and the Mg2+ concentration is the second largest of cations in cells. Moreover, Mg2+ is a cofactor and is indispensable for energy and nucleic acid synthesis. Mg2+ can bind electrostatically to negatively charged groups in membranes, proteins, and nucleic acids.51 Additionally, Mg has been approved by the U.S. Food and Drug Administration (FDA) for in vivo use,81 and it is widely used in biological implant applications.82The polymers deposited on the surface of Mg-based micromotors, such as PLGA, PVP, and Eudragit L100-55, also exhibit good degradability and biocompatibility in the body.
Wang and Zhang et al. investigated gastrointestinal drug delivery in mice; the gastrointestinal tract of mice was observed with hematoxylin and eosin (H&E) staining after 24 h. The results were compared with those for a control group, and no significant changes in the epithelial structure of the gastrointestinal tract and no signs of inflammation were found on the mucosa and submucosa.61
Similarly, Peng and Tu et al. performed H&E staining of the gastrointestinal tissues of mice 1 day and 7 days after drug delivery. A comparison of the results for the control group and of the group administered the micromotors showed that the intestinal tissue structure did not change significantly one day after administration, and the colonic mucosa remained smooth without any damage after continuous administration for 7 days.
Thus, the Mg-based micromotors have good biocompatibility and biosafety.26
5. Applications
Mg-based micromotors exhibit a wide range of biomedical applications, including the transportation of drugs, antigens, and cells, which play important roles in therapy. Moreover, micromotors can effectively enhance the fluid mass transfer process in the environment, accelerating environmental remediation and biosensing.5.1. Active therapy
Conventional passive treatment methods lack precision and controllability. Due to their motility and ease of functionalization, micromotors enable drug delivery to the target area and then release drugs, thereby improving the retention period in the body.29,30,57 In addition, the H2 generated during the driving process of Mg-based micromotors can also be used as an active substance for hydrogen therapy.20,21,24,83 Thus, by combining hydrogen therapy,73 Mg-based micromotors provide an optimal pathway for active therapy in vivo.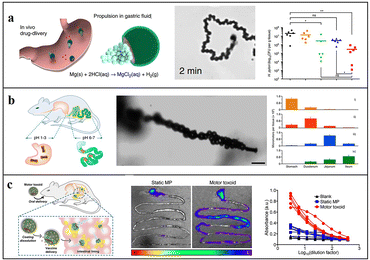 | ||
| Fig. 4 Application of Mg-based micromotors for active targeted delivery. (A) Clarithromycin-loaded Mg-based micromotor for treating Helicobacter pylori infection. Reproduced with permission. Copyright 2017, Springer Nature.29 (B) Tubular Mg-based micromotors with pH-responsive retention in the gastrointestinal tract. Reproduced with permission. Copyright 2016, American Chemical Society.61 (C) Mg-based micromotors used for active antigen delivery to the gastrointestinal tract in order to induce mucosal immunity. Reproduced with permission. Copyright 2019, American Chemical Society.69 | ||
To prevent the drug from being released prematurely in the stomach, Wang and Zhang et al. introduced an enteric coating on the surface of the micromotor so that the motor can smoothly pass through the stomach and then into the intestine: they deposited PEDOT/Au microtubules on a porous carbonate membrane, and then, loaded Mg into the tubules, thus preparing tubular Mg-based micromotors (Fig. 4b). Enteric Mg micromotors are 25% less likely to stay in the stomach compared with uncoated micromotors. Their data verified that both the enteric coating and self-driving feature are crucial for accurate delivery and drug retention.61 They also found that the micromotor can neutralize the stomach acid and adjust the gastric pH to neutral in less than 20 minutes. Compared with the traditional method, Mg-based micromotors can temporarily change the local environment without affecting the other functions of the local environment. The effect of traditional inhibitors is eliminated, and the release of drug loads can be triggered by the active regulation of the local environment.67 A similar method in which Fe and Se are loaded on self-driven Mg-based micromotors coated with an enteric membrane can also enhance the delivery and absorption of minerals in the intestine, to treat anaemia.30
Although micromotors have many applications in biotherapy, most materials used are metals, which have poor degradability in vivo and inadequate biocompatibility. To improve the biocompatibility of micromotors, Wang and Zhang et al. proposed Mg-based Janus micromotors with cell membrane functionalization. These are composed of Mg, RBC membranes, Au nanoparticles, and ALG to mimic the movement of natural cells. These micromotors can attract, capture, and neutralize toxins in vivo through the cell simulation function of RBCs.57 Subsequently, they used RBCs to effectively fix and neutralize the toxin, and loaded them on Mg-based micromotors, then coated chitosan and enteric-soluble polymer on the outermost layer (Fig. 4c). In response to changes in the gastrointestinal pH value, micromotors can effectively deliver the toxin to the intestine. Compared with traditional vaccine methods, the active delivery of oral vaccines can greatly improve immunogenicity, antigenicity, and safety, and it can be widely used for the safe load delivery of a variety of antigens by regulating the composition of the load.69
The biocompatibility, degradability, and safety of micromotors in vivo can be significantly improved by using cell membranes and biocompatible materials such as PLGA, CHI, and ALG, thus potentially enabling further applications of micromotors in vivo. However, the problem of how real-time monitoring of micromotors can be realized persists. To solve this, Wu and Li et al. proposed an intestinal micromotor combined with photoacoustic computed tomography (PACT). Mg-based Janus micromotors were encapsulated in a capsule and released drugs in the intestine through near-infrared light irradiation. Then, PACT was used for real-time monitoring. These micromotors can not only be monitored in real-time but also prolong the retention time of the drug in the intestine because they are self-driven.84
For the convenience for oral administration, the Mg-based micromotors were integrated into tablets. Wang and Zhang et al. prepared Mg-based micromotor tablets through the bottom-up multilayer modification of metformin, lactose, and maltose. These tablets exhibit a self-stirring capability because of the motility of the micromotors, and this improves the interaction of the tablet with local biological fluids and enables accelerated drug transport and release. Experimental results show that the rate of reduction in the postprandial blood sugar levels realized by using self-stirring metformin tablets was higher than that achieved via the traditional delivery of metformin.64 To improve the resistance of oral insulin to the harsh digestive environment, Liu, Peng, and Tu et al. proposed the oral delivery of insulin system via tablets of insulin-loaded Mg micromotors and auxiliary materials with subsequent esterified by starch encapsulation; by using these tablets, blood sugar control for more than 5 h was realized.26 To increase the speed of micromotors and enhance the penetration of micromotors into the gastrointestinal mucous membrane of organisms, Maric et al. proposed a cascading device that nested Mg-based Janus micromotors in a microcontainer for drug loading and transportation. The advantage of this microcontainer is that it can provide the best conditions for spraying the micromotors into the mucus layer that is not readily penetrated by the microcontainer. In addition, the rate of propulsion of micromotors enclosed within the microcontainers through the mucous membrane was faster than that of single Janus micromotors.25
The micromotor described above only exhibits rapid self-driving in a single-component, unvarying, uniform medium, but biological environments are usually complex with considerable differences in the local pH and the ions contained in different tissue parts. Therefore, in these complex environments, a single propulsion driver may be insufficient for the micromotor to complete necessary tasks, and the Mg-based micromotor can be stopped when the Mg core is almost consumed. To solve this problem, Wang and Yossifon et al. proposed a triple-engine hybrid micromotor with electric and magnetic fields. Under the conditions of a low pH, the fluid conductivity is high, and the micromotors are mainly chemically driven; on the other hand, they are mainly electrically driven in the medium with a low conductivity and the transition area is dominated by magnetic rolling. At a low electric field frequency, the motor can capture cells at the opening of Janus micromotors. In addition, the drug contained in the micromotor shell can be delivered to these captured cells, and with the increase in the electric field frequency, the captured cells can be released. The single-cell-capture capability of these micromotors enables local electroporation and transfection of drugs and genes.28
In addition to drug delivery, micromotors can be used in immunotherapy. Peng and Tu et al. proposed a PLGA/ALG/CHI-coated Mg-based micromotor system with an anti-CD3 load for antigen presentation; this can trigger Ca2+ channels to activate T cells and further trigger immune signals to clear pathogens and malignant cells.31 To enhance the effect of tumour immunotherapy, Wang and Zhang et al. prepared a Mg-based micromotor coated with a bacterial outer membrane and used the interaction of Mg-based micromotors with water in solid tumours to destroy the surrounding tumour tissue. The bacterial outer membrane vesicles carry a series of immune-stimulating molecules, which can cause the biological immune response. This method combines the destruction of tumour tissue with biological stimulation and effectively alleviates the effect of cancer.73
Guan et al. adopted a modified Stöber method to coat Mg-based Janus micromotors with mesoporous SiO2 nanoshells and adjusted the thickness of the nanoshell by adjusting the proportion of tetraethyl orthosilicate (TEOS) to control the H2 release rate (Fig. 5a). Under physiological conditions, micromotors can produce H2 efficiently for a long time to remove ˙OH and protect cells from oxidative damage, and cells treated with the micromotors show a higher activity. Moreover, the SiO2 shell can be easily modified with other substances, and this opens up new avenues for potential applications involving intelligent H2 reservoirs.85 Liu, Hu, Peng, and Tu et al. treated tumour cells with Mg-based micromotors loaded with DOX. The motility characteristics of the micromotors indicated a higher drug delivery efficiency, and the locally generated H2 effectively improved the sensitivity of tumour cells toward chemotherapy drugs.24 They also prepared Mg-based Janus micromotors by an asymmetric coating of Mg with hyaluronic acid (HA) hydrogel and PLGA, and then injected micromotors into the knee-joint under ultrasound guidance (Fig. 5b). The H2 produced by the reaction of the Mg core with the local environment can not only propel micromotors but also decrease the ROS content in cells and clear inflammation. This method has shown remarkable therapeutic effects in treating arthritis damage and inhibiting arthritis.21 These micromotors can also solve stoke, injecting micromotors into the lateral ventricle of middle cerebral artery occlusion (MCAO) rats, by using the self-drive of the micromotors and the generation of active H2, the infarct volume in the brain of the rats was effectively reduced. By using this method the spatial learning and memory ability of the rats were greatly improved20 (Fig. 5c).
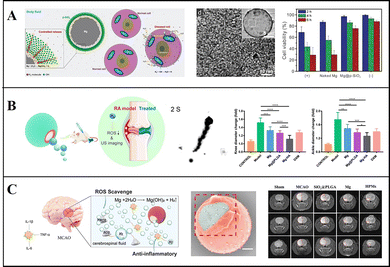 | ||
| Fig. 5 Mg-based micromotors for hydrogen therapy. (A) Mg-based Janus micromotors for removal of hydroxyl free radicals (˙OH). Reproduced with permission. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.85 (B) Mg-based Janus micromotors used in hydrogen therapy for treating rheumatoid arthritis. Reproduced with permission. Copyright 2021, American Chemical Society.21 (C) Mg-based Janus micromotors used in hydrogen therapy for treating acute ischemic stroke. Reproduced with permission. Copyright 2021, Wiley-VCH GmbH.20 | ||
In addition to the treatment of diseases, the bubbles continuously generated by micromotors under physiological conditions can be employed as contrast enhancers under ultrasound guidance. Li and Li et al. coated the surface of the Mg-based micromotor with Ni and controlled the micromotor to reach the target environment by using a magnetic field. Under the influence of the ionic environment, the micromotor was chemically driven and generated numerous bubbles. The bubbles gathered under ultrasound guidance, which can effectively enhance the contrast of ultrasonic imaging and imaging effect. This method has great application potential in the field of medical imaging.32
5.2 Environmental remediation
Mg-based Janus micromotors can be readily modified, so they can also be widely employed in environmental remediation. Through modification of the surface of micromotors by using bactericidal substances and chemical attractants, bacteria can be captured and eliminated with the self-driven micromotors, to enhance the local environmental mass transfer and to improve the speed and efficiency of sterilization.Wang et al. proposed a Mg/Ti/Ni/Au Janus micromotor that is coated with Ti, Ni, and Au metal layers on a Mg monolayer, and uses galvanic corrosion and pitting corrosion in seawater to react Mg(OH)2 on the surface of micromotors, so as to promote the reaction between Mg and water producing bubbles for promotion, which can eliminate the requirement for external fuel. The Ni layer on the surface of the micromotor can be used for magnetic guidance, and through the control of an external magnetic field, the micromotor can approach, capture, and transport motor oil droplets in seawater, thus realizing environmental remediation.45 In addition to carrying pollutants out of the environment, they remove insoluble matters by modifying the photocatalyst and sterilizing through the ROS generated from Mg. They coated the Mg monolayer with TiO2 containing Au nanoparticles to prepare Mg-based Janus micromotors, which can be self-driven to complete environmental remediation (Fig. 6a). The TiO2 coating can remove hard-to-decompose bis(4-nitrophenyl)phosphate chemical warfare agents under the action of photocatalysis. ROS generated by the interaction of the Mg core with water can destroy Bacillus anthracis spores and thus display a bactericidal effect.74 Dong et al. also killed Escherichia coli in the environment by using Mg-based Janus micromotors modified with Ag nanoparticles and found that the bactericidal effect of this method was nine times that of static methods.86 Moreover, Wang et al. assembled a monolayer of meso-2,3-dimercaptosuccinic acid (DMSA) on the surface of Janus micromotors, and used these to effectively chelate heavy metal ions from polluted water media. The Zn, Cd, and Pb removal rates using these micromotors reached 100%.87
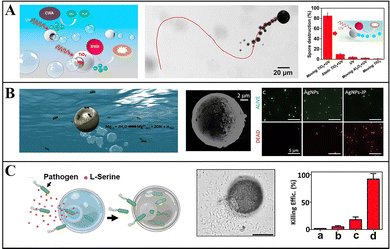 | ||
| Fig. 6 Mg-based micromotors for environmental remediation. (A) Mg-based Janus micromotors for photocatalytic degradation of Bacillus globigii spores. Reproduced with permission. Copyright 2014, American Chemical Society.74 (B) AgNP-modified Mg-based Janus micromotors to kill bacteria by releasing Ag ions. Reproduced with permission. Copyright 2017, American Chemical Society.22 (C) L-Serine-modified Mg-based Janus micromotors for attraction, trapping, and destruction of pathogens. Reproduced with permission. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.33 | ||
To improve the biodegradability and recyclability of micromotors for water sterilization, Wang et al. proposed a simple and environmentally friendly method of coating Mg micromotors with highly biodegradable CHI, ALG, and PLGA. Because of the motility characteristics of micromotors, they can almost completely kill bacteria within 10 min.75 Sánchez et al. synthesized Mg/Au/Fe Janus micromotors modified with Ag nanoparticles, in which the magnetic Fe layer is used for magnetic guidance and recovery of micromotors (Fig. 6b). When micromotors are in contact with water, H2 bubbles are generated and drive the movement of micromotors, increasing the contact opportunity between modified Ag ions and bacteria. This method involving these micromotors has a higher sterilization efficiency than the static method and can effectively kill more than 80% of E. coli.22
To further improve the efficiency of micromotors in capturing and killing bacteria, Wang et al. proposed an onion-like multifunctional microtrap with Mg as the self-driven core and with Ag nanoparticles and the inducer L-serine, to realize the attraction, capture, and destruction of pathogens (Fig. 6c). This micromotor enhances the accumulation and capture of E. coli within the trap through the chemical inducer L-serine and their killing via the release of Ag nanoparticles.33 These studies on the use of micromotors for environmental remediation and water purification present an economical and environmentally friendly solution.
5.3 Biosensing
Conventional detection methods require complex sample processing, and due to the limitation of detection methods, such as the mass transfer of liquid samples being slow and uneven, result in a long detection time and low sensitivity. With the motility of micromotors and the generation of bubbles, the mass transfer of liquid samples can be greatly improved, so the liquid samples can become more uniform, thereby improving the detection accuracy. Furthermore, micromotors can directly convert some substances that are difficult to detect, such as non-electrically active substances, into electrochemically active substances and then detect them via electrochemical detection.Wang et al. reported micromotor-assisted printable sensor strips that enable efficient electrochemical measurements: they deposited Ni and Au on a Mg monolayer by ion beam sputtering deposition to prepare Mg/Ni/Au Janus micromotors that quickly convert undetectable paraoxon into detectable electroactive p-nitrophenol. The Ni layer can fix micromotors on the sensing strip by magnetically constraining them, thus preventing the micromotors from moving to the electrode region. Through the interaction of Mg and water, bubbles are generated and enable effective fluid transport, and the fluid near the anchor points is significantly improved.23 Subsequently, Jurado-Sánchez and Escarpa et al. deposited an Au layer on Mg micromotors to prepare Mg/Au Janus micromotors that are capable of efficient degradation and detection of persistent organic pollutants in biological samples. The self-stirring improves the analysis signal, and the non-electroactive diphenyl phthalate (DPP) was quickly converted into electroactive phenol by the hydroxyl which was generated by the reaction of Mg with water, and the electrical signal was detected on the electrode (Fig. 7a). Thus, DPP can be directly detected in food and biological samples without any sample handling, and carbohydrates and dopamine can be detected with different electrodes.34
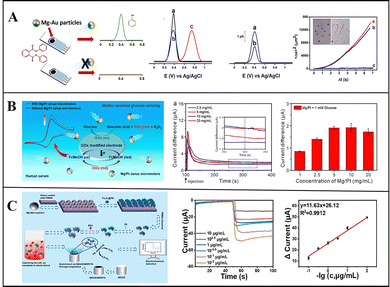 | ||
| Fig. 7 Mg-based Janus micromotors in biosensing. (A) Mg-based Janus micromotors for the detection of organic pollutants in food and biological samples. Reproduced with permission. Copyright 2016,34 American Chemical Society. (B) Mg-based Janus micromotors used for the detection of glucose in human serum. Reproduced with permission. Copyright 2019, American Chemical Society.76 (C) Mg-based Janus micromotors for electrochemical sensing of oxidized low-density lipoprotein (Ox-LDL) in human blood. Reproduced with permission. Copyright 2022 Elsevier B.V. All rights reserved.58 | ||
The fast self-driving speed of micromotors can enhance the mass transfer of fluids. To improve the speed and accuracy of biosensing, Guan et al. used Mg/Pt Janus micromotors for the electrochemical detection of glucose in human serum (Fig. 7b). They found that the current increases with the number of micromotors, and this method involving micromotors is faster and more accurate than static detection is, confirming its great application prospects in biosensing.76 Furthermore, Mou and Zhou et al. prepared Mg–Fe3O4@PB@Ab@BSA Janus micromotors to detect oxidized low-density lipoprotein (Ox-LDL) in human blood by electrochemical sensing. After the Mg cores are depleted, Fe3O4 can capture, collect, and detect Ox-LDL on a magnetic glass electrode modified with multi-walled carbon nanotubes. It can detect the Ox-LDL in a whole blood solution at the range from 1 × 10−2 to 10 μg mL−1, with high sensitivity, good reproducibility, and a linear response (Fig. 7c).58 Wan and Shen et al. prepared aldehyde-functionalized Mg-based Janus micromotors using PMMA as the substrate and an aldehyde–amine condensation reaction to modify Fe3O4/polydopamine/anti-epithelial cell adhesion molecule (EpCAM). Tumour cells were also captured and detected by magnetic glass electrodes. This work presents a reliable method for the early detection of cancer and prompt initiation of its treatment.59
6. Conclusions
This review presents a comprehensive overview of the progress in research on Mg-based micromotors in recent years. The preparation and motion control of various Mg-based micromotors have been discussed. These Mg-based micromotors are suitable for therapy, biosensing, environmental remediation, and other applications, and these methods exhibit lower costs, simple procedures, and high efficiencies. Mg-based micromotors are expected to be used as drug carriers for tumour treatment, especially stomach tumours because the gastric acid environment is extremely favourable for driving the micromotors, and rapid analysis and detection of biological samples by using micromotors is also expected. Furthermore, in sewage treatment plants, micromotors can replace traditional chemical agents to eliminate and recover bacteria and harmful substances. The advantages and disadvantages of Mg-based micromotors in various applications are summarized in Table 2.| Advantage | Disadvantage | |
|---|---|---|
| Biomedical | High drug delivery efficiency; | Difficult for precise direction control; |
| Prolong the retention time of drugs in the body; | ||
| Provide active substances for auxiliary hydrogen therapy hydrogen therapy; | ||
| Analytical | Simple operation process; | Limited life; |
| Low cost; | ||
| Fast detection speed and high precision; | ||
| Environment remediation | High precision; | Limited life; |
| Good self-degradability; | ||
| Recyclable |
Despite their various advantages and capabilities, Mg-based micromotors still need to be improved for practical applications. First, the lifetime of Mg-based micromotors is limited: when the Mg core is exhausted, the micromotor is no longer self-driven. Although H2 produced in the process of micromotor driving is beneficial to the human body, when the amount produced in the body is very high, adverse side effects may be experienced, so the amount of Mg-based micromotors cannot exceed a certain limit. Finally, direction control has always been a difficult challenge for chemically driven micromotors because factors including external field control and the introduction of magnetic particles and light-responsive particles are usually needed. Therefore, a further study of motility, biocompatibility and degradability becomes necessary. These challenges limit the practical and commercial applications of Mg-based micromotors. However, with continuous innovations in materials and technologies, Mg-based micromotors are expected to become important for diverse biomedical and environmental applications.
Author contributions
Y. W. summarized wrote the manuscript. B. Q. supervised the studies. S. G. and X. W. collected and organized relevant literature and materials. H. Z. checked the manuscript. Z. W. conceived the project. All authors reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21972035 and 52375565), the Interdisciplinary Research Foundation of HIT (IR2021112), and the State Key Laboratory of Robotics (2019-O02).Notes and references
- C. Zhou, N. J. Suematsu, Y. Peng, Q. Wang, X. Chen, Y. Gao and W. Wang, ACS Nano, 2020, 14, 5360–5370 CrossRef CAS PubMed.
- F. Mou, Q. Xie, J. Liu, S. Che, L. Bahmane, M. You and J. Guan, Natl. Sci. Rev., 2021, 8, nwab066 CrossRef CAS PubMed.
- F. Wong and A. Sen, ACS Nano, 2016, 10, 7172–7179 CrossRef CAS PubMed.
- Y. Hou, H. Wang, R. Fu, X. Wang, J. Yu, S. Zhang, Q. Huang, Y. Sun and T. Fukuda, Lab Chip, 2023, 23, 848–868 RSC.
- H. Zhang, Z. Li, C. Gao, X. Fan, Y. Pang, T. Li, Z. Wu, H. Xie and Q. He, Sci Robot, 2021, 6, eaaz9519 CrossRef PubMed.
- Y. Gao, F. Wei, Y. Chao and L. Yao, Biomed. Microdevices, 2021, 23, 52 CrossRef PubMed.
- L. Ren, W. Wang and T. E. Mallouk, Acc. Chem. Res., 2018, 51, 1948–1956 CrossRef CAS PubMed.
- L. Xu, D. Gong, N. Celi, J. Xu, D. Zhang and J. Cai, Appl. Surf. Sci., 2022, 579, 152165 CrossRef CAS.
- W. Xi, A. A. Solovev, A. N. Ananth, D. H. Gracias, S. Sanchez and O. G. Schmidt, Nanoscale, 2013, 5, 1294–1297 RSC.
- M. Luo, Y. Feng, T. Wang and J. Guan, Adv. Funct. Mater., 2018, 28, 1706100 CrossRef.
- R. Lin, W. Yu, X. Chen and H. Gao, Adv. Healthcare Mater., 2021, 10, e2001212 CrossRef PubMed.
- Q. Wang, K. F. Chan, K. Schweizer, X. Du, D. Jin, S. C. H. Yu, B. J. Nelson and L. Zhang, Sci. Adv., 2021, 7, eabe5914 CrossRef CAS PubMed.
- D. Vilela, U. Cossio, J. Parmar, A. M. Martinez-Villacorta, V. Gomez-Vallejo, J. Llop and S. Sanchez, ACS Nano, 2018, 12, 1220–1227 CrossRef CAS PubMed.
- Q. Wang, T. Shi, M. Wan, J. Wei, F. Wang and C. Mao, J. Mater. Chem. B, 2021, 9, 283–294 RSC.
- Y. Hu, W. Liu and Y. Sun, Adv. Funct. Mater., 2021, 32, 2109181 CrossRef.
- M. Amouzadeh Tabrizi, M. Shamsipur, R. Saber and S. Sarkar, Biosens. Bioelectron., 2018, 110, 141–146 CrossRef CAS PubMed.
- M. S. Draz, N. K. Lakshminaraasimulu, S. Krishnakumar, D. Battalapalli, A. Vasan, M. K. Kanakasabapathy, A. Sreeram, S. Kallakuri, P. Thirumalaraju, Y. Li, S. Hua, X. G. Yu, D. R. Kuritzkes and H. Shafiee, ACS Nano, 2018, 12, 5709–5718 CrossRef CAS PubMed.
- L. Zhao, Y. Liu, S. Xie, P. Ran, J. Wei, Q. Liu and X. Li, Chem. Eng. J., 2020, 382, 123041 CrossRef CAS.
- E. Blanco, H. Shen and M. Ferrari, Nat. Biotechnol., 2015, 33, 941–951 CrossRef CAS PubMed.
- S. Wang, K. Liu, Q. Zhou, C. Xu, J. Gao, Z. Wang, F. Wang, B. Chen, Y. Ye, J. Ou, J. Jiang, D. A. Wilson, S. Liu, F. Peng and Y. Tu, Adv. Funct. Mater., 2021, 31, 2009475 CrossRef CAS.
- C. Xu, S. Wang, H. Wang, K. Liu, S. Zhang, B. Chen, H. Liu, F. Tong, F. Peng, Y. Tu and Y. Li, Nano Lett., 2021, 21, 1982–1991 CrossRef CAS PubMed.
- D. Vilela, M. M. Stanton, J. Parmar and S. Sanchez, ACS Appl. Mater. Interfaces, 2017, 9, 22093–22100 CrossRef CAS PubMed.
- S. Cinti, G. Valdes-Ramirez, W. Gao, J. Li, G. Palleschi and J. Wang, Chem. Commun., 2015, 51, 8668–8671 RSC.
- K. Liu, J. Ou, S. Wang, J. Gao, L. Liu, Y. Ye, D. A. Wilson, Y. Hu, F. Peng and Y. Tu, Appl. Mater. Today, 2020, 20, 100694 CrossRef.
- T. Maric, V. Adamakis, Z. Zhang, C. Milian-Guimera, L. H. E. Thamdrup, E. Stamate, M. Ghavami and A. Boisen, Small, 2023, 19, e2206330 CrossRef PubMed.
- K. Liu, Q. Liu, J. Yang, C. Xie, S. Wang, F. Tong, J. Gao, L. Liu, Y. Ye, B. Chen, X. Cai, Z. Liu, Z. Li, F. Peng and Y. Tu, ACS Nano, 2023, 17, 300–311 CrossRef CAS PubMed.
- F. Mou, C. Chen, H. Ma, Y. Yin, Q. Wu and J. Guan, Angew. Chem., Int. Ed., 2013, 52, 7208–7212 CrossRef CAS PubMed.
- S. S. Das, S. Erez, E. Karshalev, Y. Wu, J. Wang and G. Yossifon, ACS Appl. Mater. Interfaces, 2022, 14, 30290–30298 CrossRef CAS PubMed.
- B. E. de Avila, P. Angsantikul, J. Li, M. Angel Lopez-Ramirez, D. E. Ramirez-Herrera, S. Thamphiwatana, C. Chen, J. Delezuk, R. Samakapiruk, V. Ramez, M. Obonyo, L. Zhang and J. Wang, Nat. Commun., 2017, 8, 272 CrossRef PubMed.
- E. Karshalev, Y. Zhang, B. Esteban-Fernandez de Avila, M. Beltran-Gastelum, Y. Chen, R. Mundaca-Uribe, F. Zhang, B. Nguyen, Y. Tong, R. H. Fang, L. Zhang and J. Wang, Nano Lett., 2019, 19, 7816–7826 CrossRef CAS PubMed.
- Z. Wang, S. Wang, K. Liu, D. Fu, Y. Ye, J. Gao, L. Liu, D. A. Wilson, Y. Tu and F. Peng, Appl. Mater. Today, 2020, 21, 100839 CrossRef.
- Y. Feng, X. Chang, H. Liu, Y. Hu, T. Li and L. Li, Appl. Mater. Today, 2021, 23, 101026 CrossRef.
- F. Soto, D. Kupor, M. A. Lopez-Ramirez, F. Wei, E. Karshalev, S. Tang, F. Tehrani and J. Wang, Angew. Chem., Int. Ed., 2020, 59, 3480–3485 CrossRef CAS PubMed.
- D. Rojas, B. Jurado-Sanchez and A. Escarpa, Anal. Chem., 2016, 88, 4153–4160 CrossRef CAS PubMed.
- C. Chen, E. Karshalev, J. Guan and J. Wang, Small, 2018, 14, e1704252 CrossRef PubMed.
- A. M. Pourrahimi and M. Pumera, Nanoscale, 2018, 10, 16398–16415 RSC.
- R. F. Ismagilov, A. Schwartz, N. Bowden and G. M. Whitesides, Angew. Chem., Int. Ed., 2002, 41, 652–654 CrossRef CAS.
- T. R. Kline, W. F. Paxton, T. E. Mallouk and A. Sen, Angew. Chem. Int. Ed. Engl., 2005, 44, 744–746 CrossRef CAS PubMed.
- M. Safdar, O. M. Wani and J. Janis, ACS Appl. Mater. Interfaces, 2015, 7, 25580–25585 CrossRef CAS PubMed.
- R. Dong, Y. Hu, Y. Wu, W. Gao, B. Ren, Q. Wang and Y. Cai, J. Am. Chem. Soc., 2017, 139, 1722–1725 CrossRef CAS PubMed.
- A. Iino, K. Suzuki, M. Kasuga, M. Suzuki and T. Yamanaka, Ultrasonics, 2000, 38, 54–59 CrossRef PubMed.
- N. Mano and A. Heller, J. Am. Chem. Soc., 2005, 127, 11574–11575 CrossRef CAS PubMed.
- B. J. Toebes, L. K. E. A. Abdelmohsen and D. A. Wilson, Polym. Chem., 2018, 9, 3190–3194 RSC.
- F. Mou, C. Chen, Q. Zhong, Y. Yin, H. Ma and J. Guan, ACS Appl. Mater. Interfaces, 2014, 6, 9897–9903 CrossRef CAS PubMed.
- W. Gao, X. Feng, A. Pei, Y. Gu, J. Li and J. Wang, Nanoscale, 2013, 5, 4696–4700 RSC.
- S. Cambray, M. Ibarz, M. Bermudez-Lopez, M. Marti-Antonio, M. Bozic, E. Fernandez and J. M. Valdivielso, Nutrients, 2020, 12, 1–12 CrossRef PubMed.
- B. A. Ryan and C. S. Kovacs, Semin Fetal Neonatal Med, 2020, 25, 101062 CrossRef PubMed.
- R. Salehidoost, G. Taghipour Boroujeni, A. Feizi, A. Aminorroaya and M. Amini, Sci. Rep., 2022, 12, 18209 CrossRef CAS PubMed.
- C. Yang, S. Wu, Y. Lan, S. Chen, D. Zhang, Y. Wang, Y. Sun, W. Liao and L. Wang, Biol. Trace Elem. Res., 2023, 201, 4625–4636 CrossRef CAS PubMed.
- Z. L. Nie, Z. M. Wang, B. Zhou, Z. P. Tang and S. K. Wang, Nutr Metab Cardiovasc Dis, 2013, 23, 169–176 CrossRef CAS PubMed.
- N. E. Saris, E. Mervaala, H. Karppanen, J. A. Khawaja and A. Lewenstam, Clin. Chim. Acta, 2000, 294, 1–26 CrossRef CAS PubMed.
- R. Fritzen, A. Davies, M. Veenhuizen, M. Campbell, S. J. Pitt, R. A. Ajjan and A. J. Stewart, Nutrients, 2023, 15, 2355 CrossRef CAS PubMed.
- J. A. Maier, G. Pickering, E. Giacomoni, A. Cazzaniga and P. Pellegrino, Nutrients, 2020, 12, 2660 CrossRef CAS PubMed.
- R. Nicoll, K. Gerasimidis and E. Forrest, Alcohol Alcohol, 2022, 57, 275–282 CrossRef CAS PubMed.
- Y. Yang, C. He, E. Dianyu, W. Yang, F. Qi, D. Xie, L. Shen, S. Peng and C. Shuai, Mater. Des., 2020, 185, 108259 CrossRef CAS.
- N. T. Kirkland and N. Birbilis, Magnesium Biomaterials, 2014, ch. 4, pp. 73–94 DOI:10.1007/978-3-319-02123-2_4.
- Z. G. Wu, J. X. Li, B. E. F. de Avila, T. L. Li, W. W. Gao, Q. He, L. F. Zhang and J. Wang, Adv. Funct. Mater., 2015, 25, 7497–7501 CrossRef.
- D. Fang, S. Tang, Z. Wu, C. Chen, M. Wan, C. Mao and M. Zhou, Biosens. Bioelectron., 2022, 217, 114682 CrossRef CAS PubMed.
- Q. Chen, W. Guo, D. Fang, T. Li, L. Chen, C. Mao, M. Wan and J. Shen, Sens. Actuators, B, 2023, 390, 133933 CrossRef CAS.
- B. Jurado-Sanchez, M. Pacheco, J. Rojo and A. Escarpa, Angew. Chem., Int. Ed., 2017, 56, 6957–6961 CrossRef CAS PubMed.
- J. Li, S. Thamphiwatana, W. Liu, B. Esteban-Fernandez de Avila, P. Angsantikul, E. Sandraz, J. Wang, T. Xu, F. Soto, V. Ramez, X. Wang, W. Gao, L. Zhang and J. Wang, ACS Nano, 2016, 10, 9536–9542 CrossRef CAS PubMed.
- B. Esteban-Fernandez de Avila, M. A. Lopez-Ramirez, R. Mundaca-Uribe, X. Wei, D. E. Ramirez-Herrera, E. Karshalev, B. Nguyen, R. H. Fang, L. Zhang and J. Wang, Adv. Mater., 2020, 32, e2000091 CrossRef PubMed.
- R. Mundaca-Uribe, E. Karshalev, B. Esteban-Fernandez de Avila, X. Wei, B. Nguyen, I. Litvan, R. H. Fang, L. Zhang and J. Wang, Adv. Sci., 2021, 8, 2100389 CrossRef CAS PubMed.
- R. Mundaca-Uribe, M. Holay, A. Abbas, N. Askarinam, J. S. Sage-Sepulveda, L. Kubiatowicz, R. H. Fang, L. Zhang and J. Wang, ACS Nano, 2023, 17, 9272–9279 CrossRef CAS PubMed.
- G. Zhao and M. Pumera, Nanoscale, 2014, 6, 11177–11180 RSC.
- L. Cai, D. Xu, Z. Zhang, N. Li and Y. Zhao, Research, 2023, 6, 0044 CrossRef CAS PubMed.
- J. Li, P. Angsantikul, W. Liu, B. Esteban-Fernández de Ávila, S. Thamphiwatana, M. Xu, E. Sandraz, X. Wang, J. Delezuk, W. Gao, L. Zhang and J. Wang, Angew. Chem., Int. Ed., 2017, 56, 2156–2161 CrossRef CAS PubMed.
- Q. Song, X. Ding, Y. Liu, W. Liu, J. Li, B. Wang and Z. Gu, Appl. Mater. Today, 2023, 31, 101779 CrossRef.
- X. Wei, M. Beltran-Gastelum, E. Karshalev, B. Esteban-Fernandez de Avila, J. Zhou, D. Ran, P. Angsantikul, R. H. Fang, J. Wang and L. Zhang, Nano Lett., 2019, 19, 1914–1921 CrossRef CAS PubMed.
- K. Xiong, L. Xu, J. Lin, F. Mou and J. Guan, Research, 2020, 2020, 1–12 CrossRef PubMed.
- A. A. Solovev, Y. Mei, E. Bermudez Urena, G. Huang and O. G. Schmidt, Small, 2009, 5, 1688–1692 CrossRef CAS PubMed.
- F. Zhang, R. Mundaca-Uribe, H. Gong, B. Esteban-Fernandez de Avila, M. Beltran-Gastelum, E. Karshalev, A. Nourhani, Y. Tong, B. Nguyen, M. Gallot, Y. Zhang, L. Zhang and J. Wang, Adv. Mater., 2019, 31, e1901828 CrossRef PubMed.
- J. Zhou, E. Karshalev, R. Mundaca-Uribe, B. Esteban-Fernandez de Avila, N. Krishnan, C. Xiao, C. J. Ventura, H. Gong, Q. Zhang, W. Gao, R. H. Fang, J. Wang and L. Zhang, Adv. Mater., 2021, 33, e2103505 CrossRef PubMed.
- J. Li, V. V. Singh, S. Sattayasamitsathit, J. Orozco, K. Kaufmann, R. Dong, W. Gao, B. Jurado-Sanchez, Y. Fedorak and J. Wang, ACS Nano, 2014, 8, 11118–11125 CrossRef CAS PubMed.
- J. A. Delezuk, D. E. Ramirez-Herrera, B. Esteban-Fernandez de Avila and J. Wang, Nanoscale, 2017, 9, 2195–2200 RSC.
- L. Kong, N. Rohaizad, M. Z. M. Nasir, J. Guan and M. Pumera, Anal. Chem., 2019, 91, 5660–5666 CrossRef CAS PubMed.
- H. Yuan, X. Liu, L. Wang and X. Ma, Bioactive Mater., 2021, 6, 1727–1749 CrossRef CAS PubMed.
- S. Fu, X. Zhang, Y. Xie, J. Wu and H. Ju, Nanoscale, 2017, 9, 9026–9033 RSC.
- Y. Yang, X. Arqué, T. Patiño, V. Guillerm, P.-R. Blersch, J. Pérez-Carvajal, I. Imaz, D. Maspoch and S. Sánchez, J. Am. Chem. Soc., 2020, 142, 20962–20967 CrossRef CAS PubMed.
- M. Valles, S. Pujals, L. Albertazzi and S. Sánchez, ACS Nano, 2022, 16, 5615–5626 CrossRef CAS PubMed.
- B. R. E. Smith, C. M. Njardarson and T. Jon, J. Med. Chem., 2014, 57, 9764–9773 CrossRef CAS PubMed.
- N. Sezer, Z. Evis, S. M. Kayhan, A. Tahmasebifar and M. Koç, J. Mag. Alloys, 2018, 6, 23–43 CrossRef CAS.
- D. Ramanathan, L. Huang, T. Wilson and W. Boling, Med. Gas Res., 2023, 13, 94–98 CrossRef CAS PubMed.
- Z. Wu, L. Li, Y. Yang, P. Hu, Y. Li, S. Y. Yang, L. V. Wang and W. Gao, Sci Robot, 2019, 4, eaax0613 CrossRef PubMed.
- L. Kong, C. Chen, F. Mou, Y. Feng, M. You, Y. Yin and J. Guan, Part. Part. Syst. Charact., 2019, 36, 1800424 CrossRef.
- Y. Ge, M. Liu, L. Liu, Y. Sun, H. Zhang and B. Dong, Nanomicro Lett, 2016, 8, 157–164 Search PubMed.
- D. A. Uygun, B. Jurado-Sánchez, M. Uygun and J. Wang, Environ. Sci.: Nano, 2016, 3, 559–566 RSC.
| This journal is © The Royal Society of Chemistry 2023 |