Solution-processed high efficiency OLED harnessing a thermally cross-linked hole-transporting layer and exciplex-forming emission layer†
Meng-Ju
Tsai
a,
Wei-Lun
Huang
b,
Li-Ming
Chen
 a,
Guo-Lun
Ruan
b,
Dian
Luo
a,
Guo-Lun
Ruan
b,
Dian
Luo
 c,
Zong-Liang
Tseng
*b and
Ken-Tsung
Wong
c,
Zong-Liang
Tseng
*b and
Ken-Tsung
Wong
 *ad
*ad
aDepartment of Chemistry, National Taiwan University, Taipei, 10617, Taiwan. E-mail: kenwong@ntu.edu.tw
bDepartment of Electronic Engineering, Ming Chi University of Technology, New Taipei City 243303, Taiwan. E-mail: zltseng@mail.mcut.edu.tw
cCollege of Photonics, National Yang Ming Chiao Tung University, Tainan, 71150, Taiwan
dInstitute of Atomic and Molecular Science, Academia Sinica, Taipei, 10617, Taiwan
First published on 13th December 2022
Abstract
A new dicarbazole-based donor, BCz3Ph, for exciplex formation was synthesized and characterized. The new green (PL λmax = 527 nm) exciplex BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend with a photoluminescence quantum yield (PLQY) of 43% and thermally activated delayed fluorescence (TADF) character was utilized as the emitting layer (EML) of solution-processed OLED devices. To facilitate hole injection into the EML, a new cross-linkable monomer, BCzC4Sy, adopting a dicarbazole core linked to a styrene group by a butyl chain was designed to realize a solvent resistant hole-transporting layer (HTL) after thermal polymerization. The flexible butyl (C4) bridge accounts for a lower polymerization temperature as compared to that of the methyl (C1)-bridged counterpart, BCzC1Sy, resulting in the formation of amorphous films with better solvent resistance as well as smoother morphology. The choice of dicarbazole as the HTL core not only suppresses the HTL-to-EML energy barrier, but also prevents the emission color variation stemming from the possible exciplex emission at the HTL/EML interface. The best OLED device with EQEmax = 9.2%, CEmax = 27.94 cd A−1 and PEmax = 28.7 lm W−1 was obtained with a thermally polymerized BCzC4Sy film as the HTL. The device achieved a maximum luminance up to 22
1) blend with a photoluminescence quantum yield (PLQY) of 43% and thermally activated delayed fluorescence (TADF) character was utilized as the emitting layer (EML) of solution-processed OLED devices. To facilitate hole injection into the EML, a new cross-linkable monomer, BCzC4Sy, adopting a dicarbazole core linked to a styrene group by a butyl chain was designed to realize a solvent resistant hole-transporting layer (HTL) after thermal polymerization. The flexible butyl (C4) bridge accounts for a lower polymerization temperature as compared to that of the methyl (C1)-bridged counterpart, BCzC1Sy, resulting in the formation of amorphous films with better solvent resistance as well as smoother morphology. The choice of dicarbazole as the HTL core not only suppresses the HTL-to-EML energy barrier, but also prevents the emission color variation stemming from the possible exciplex emission at the HTL/EML interface. The best OLED device with EQEmax = 9.2%, CEmax = 27.94 cd A−1 and PEmax = 28.7 lm W−1 was obtained with a thermally polymerized BCzC4Sy film as the HTL. The device achieved a maximum luminance up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 together with a very low efficiency roll-off, retaining 97% efficiency at 1000 cd m−2 (EQE1000cd = 8.9%). More significantly, a 2 × 2 cm2 device fabricated by slot-die coating gave good color homogeneity and rather high brightness and promising efficiency (EQE 5.0%), manifesting the potential of employing a thermally cross-linkable HTM and exciplex-based EML to produce high efficiency solution-processed OLEDs.
000 cd m−2 together with a very low efficiency roll-off, retaining 97% efficiency at 1000 cd m−2 (EQE1000cd = 8.9%). More significantly, a 2 × 2 cm2 device fabricated by slot-die coating gave good color homogeneity and rather high brightness and promising efficiency (EQE 5.0%), manifesting the potential of employing a thermally cross-linkable HTM and exciplex-based EML to produce high efficiency solution-processed OLEDs.
Introduction
Organic light emitting diodes (OLEDs) have been utilized to develop new displays and further applied to lighting applications.1,2 In recent years, OLEDs employing organic emitters with thermally activated delayed fluorescence (TADF) that can achieve 100% internal quantum efficiency (IQE) have attracted a great deal of attention.3–5 The basic principle of TADF is to minimize the energy gap (ΔEST) between the singlet state and triplet state, which allows the triplet excitons to be up-converted to singlet excitons through a reverse intersystem crossing (RISC) process with the aid of environmental thermal energy, thereby increasing the efficiency of OLED devices. In order to achieve a small ΔEST, the molecule should possess an intramolecular charge transfer (CT) excited state with the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) distributed on the donor (D) and acceptor (A), respectively. The manipulation of the electronic coupling between D and A as well as the spatial overlap of the HOMO and LUMO can be rationally achieved through sophisticated molecular design, yet is often challenging regarding syntheses.6–9 Therefore, another approach that incorporates the exciplex-forming system formed by optical or electrical excitation of a physically blended D and A pair has emerged.10a–e Since the HOMO and LUMO of exciplex excitons are localized on D and A molecules, respectively, the exciplex-forming system possesses a small ΔEST and thus TADF properties. The efficient RISC of the exciplex-forming system makes it suitable for developing highly efficient OLED devices.11–16 In addition, the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A blends render the feasibility of preparing smooth films by solution processes given the stronger intermolecular interactions between D and A molecules, which might promote the efficiency of solution-processed exciplex-based OLEDs.17 For example, Tseng and coworkers demonstrated the use of a TAPC
A blends render the feasibility of preparing smooth films by solution processes given the stronger intermolecular interactions between D and A molecules, which might promote the efficiency of solution-processed exciplex-based OLEDs.17 For example, Tseng and coworkers demonstrated the use of a TAPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blend as the emitting layer (EML) with efficient green exciplex emission to fabricate a solution-processed device with EQEmax of 7.1%.18 Though solution-processed technologies for OLEDs are still under development, this promising method presents potential benefits over the mainstream physical vapor deposition, such as the exclusion of expensive vacuum equipment, effective material utilization efficiency, and scalable and uniform large-area film production on flexible substrates.19 Typically, solution processes involve the coating of functional material solutions onto (indium tin oxide) ITO-treated substrates, followed by drying the layer to obtain films. For instance, water-soluble PEDOT:PSS is most commonly used as the hole injection layer (HIL), given its non-miscibility with organic solvents, which can then be followed by continuous deposition of multilayer stacks. It is crucial to use tailor-made functional materials for the solution process, where the small molecule materials used must present good solubility to facilitate the coating process and film formation.20 Nevertheless, excessive solubility brings about the erosion of formerly deposited layers, which would give rise to uneven thickness or even pinholes in the films, leading to poor device efficiencies. Specifically, the hole-transporting layer (HTL) is deemed the most requisite layer to improve the anti-solvent property. To tackle this issue, several teams have designed small molecules with special functional groups to enhance the solubility for film fabrication, and are further polymerized with self-assembly,21 photochemical reactions,22 electrochemical reactions23,24 or cross-linking25,26 to produce polymers with strong resistance towards solvent erosion and intermixing with the other layers. Among cross-linking methods, photo cross-linking can be carried out at low temperatures and requires a short amount of time with the aid of initiators. However, the addition of initiators may also cause side reactions to occur, producing undesired impurities and thus affecting the efficiency of OLED devices. In contrast, despite the fact that thermal cross-linking requires higher temperatures, the absence of initiators would lead to fewer side-products and generate films with fewer defects. Among different functional groups, styrene,27–30 acrylate,31 phenylethynyl group,32 azide,33 trifluorovinylether,34 benzocyclobutene35 and silane36 have been widely used as thermally polymerizable functional groups. Specifically, styrene can generate radicals at relatively low temperatures (about 180 °C) and be further polymerized to form a polymer, which provides excellent anti-erosion properties and is suitable for serving as the thermal cross-linking functional group.
PO-T2T blend as the emitting layer (EML) with efficient green exciplex emission to fabricate a solution-processed device with EQEmax of 7.1%.18 Though solution-processed technologies for OLEDs are still under development, this promising method presents potential benefits over the mainstream physical vapor deposition, such as the exclusion of expensive vacuum equipment, effective material utilization efficiency, and scalable and uniform large-area film production on flexible substrates.19 Typically, solution processes involve the coating of functional material solutions onto (indium tin oxide) ITO-treated substrates, followed by drying the layer to obtain films. For instance, water-soluble PEDOT:PSS is most commonly used as the hole injection layer (HIL), given its non-miscibility with organic solvents, which can then be followed by continuous deposition of multilayer stacks. It is crucial to use tailor-made functional materials for the solution process, where the small molecule materials used must present good solubility to facilitate the coating process and film formation.20 Nevertheless, excessive solubility brings about the erosion of formerly deposited layers, which would give rise to uneven thickness or even pinholes in the films, leading to poor device efficiencies. Specifically, the hole-transporting layer (HTL) is deemed the most requisite layer to improve the anti-solvent property. To tackle this issue, several teams have designed small molecules with special functional groups to enhance the solubility for film fabrication, and are further polymerized with self-assembly,21 photochemical reactions,22 electrochemical reactions23,24 or cross-linking25,26 to produce polymers with strong resistance towards solvent erosion and intermixing with the other layers. Among cross-linking methods, photo cross-linking can be carried out at low temperatures and requires a short amount of time with the aid of initiators. However, the addition of initiators may also cause side reactions to occur, producing undesired impurities and thus affecting the efficiency of OLED devices. In contrast, despite the fact that thermal cross-linking requires higher temperatures, the absence of initiators would lead to fewer side-products and generate films with fewer defects. Among different functional groups, styrene,27–30 acrylate,31 phenylethynyl group,32 azide,33 trifluorovinylether,34 benzocyclobutene35 and silane36 have been widely used as thermally polymerizable functional groups. Specifically, styrene can generate radicals at relatively low temperatures (about 180 °C) and be further polymerized to form a polymer, which provides excellent anti-erosion properties and is suitable for serving as the thermal cross-linking functional group.
In this work, a new electron donor BCz3Ph was synthesized and characterized to intermix with a benchmark triazine-based acceptor PO-T2T37 to form a new exciplex-forming system, which gave a green emission with a decent photoluminescence quantum yield (PLQY) of 43%. For creating a smooth hole injection and transport, dicarbazole was chosen as the core to be further functionalized with a styrene group to afford a new thermally cross-linkable HTM. The choice of dicarbazole as the core of the HTL suppresses the energy barrier between the HTL and EML issue and is also beneficial for preventing the emission color pollution from the exciplex formation at the HTL/EML interface. In a previous study, Lee and coworkers reported a cross-linkable dicarbazole-centered HTM BCzMs (BCzC1Sy) (Scheme 1),38 which served as a suitable HTM for solution-processed phosphorescent devices due to its hole-transporting and anti-solvent erosion properties. In this work, the length of the alkyl chain between the dicarbazole core and styrene is increased to afford a new thermally cross-linkable molecule BCzC4Sy. The effects of the carbon chain lengths of the hole-transporting materials were found to strongly influence the temperature required for thermal polymerization, in which BCzC4Sy exhibits apparent thermal polymerization behavior at a temperature of 146 °C, which is lower than that of 192 °C of BCzC1Sy. More importantly, the BCzC4Sy film after thermal treatment exhibits excellent solvent erosion resistance. The OLED devices employing thermally cross-linked BCzC1Sy and BCzC4Sy films as the HTL and the solution-processed BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blend as the EML gave EQEmax of 7.2% and 9.2%, respectively, while maintaining a high efficiency of 7.1% and 8.9% at 1000 cd m−2. This work highlights the design of a new thermally cross-linkable HTL with the same core chromophore of the donor for exciplex formation. The new molecular monomer BCzC4Sy with a more flexible alkyl linkage can thermally polymerize to give a solvent-resistive film with superior properties compared to the BCzC1Sy counterpart, affording better performing solution-processed exciplex-based OLED devices with lower efficiency roll-off.
PO-T2T blend as the EML gave EQEmax of 7.2% and 9.2%, respectively, while maintaining a high efficiency of 7.1% and 8.9% at 1000 cd m−2. This work highlights the design of a new thermally cross-linkable HTL with the same core chromophore of the donor for exciplex formation. The new molecular monomer BCzC4Sy with a more flexible alkyl linkage can thermally polymerize to give a solvent-resistive film with superior properties compared to the BCzC1Sy counterpart, affording better performing solution-processed exciplex-based OLED devices with lower efficiency roll-off.
Results and discussion
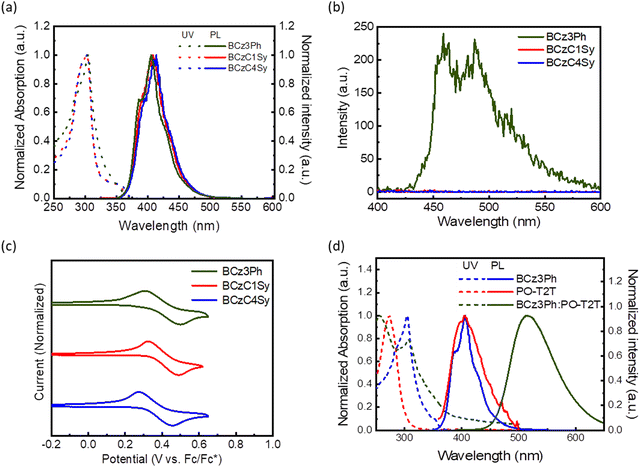
The molecular structures and synthetic pathways of BCz3Ph and BCzC4Sy are depicted in Scheme 1. The donor BCz3Ph for exploring the exciplex formation was prepared from 9H,9′H-3,3′-dicarbazole with 3-fluoro-1,1′-biphenyl via SNAr reaction. BCzC4Sy was synthesized from compound 9H,9′H-3,3′-dicarbazole via SN2 reaction with 1-(4-bromobutyl)-4-vinylbenzene. All new molecules were characterized with satisfactory spectroscopic data, and the experimental details are provided in the ESI.†The photophysical behaviors of BCz3Ph and BCzC4Sy as compared to those of the model counterpart BCzC1Sy were investigated by measuring the UV-vis absorption and photoluminescence (PL) spectra, as depicted in Fig. 1a, and the data are summarized in Table 1. In diluted toluene solution at room temperature, similar π–π* transition absorption peaks were observed for the absorption spectra of BCzC1Sy and BCzC4Sy, with a maximum absorption peak (λmax) centered around 303 nm together with weak absorptions around 330–360 nm corresponding to n–π* transition. It is noted that BCz3Ph equipped with an N-biphenyl substituent exhibits similar λmax to those of BCzC1Sy and BCzC4Sy, but less distinguished n–π* absorption. All three materials show similar emission spectra in toluene solution, and the PL maxima (PL λmax) are centered around 407–414 nm, and are assigned to the dicarbazole core. The phosphorescence spectrum of BCz3Ph in MeTHF was then acquired at 77 K, as depicted in Fig. 1b. The T1 energy level (ET) can then be estimated to be 2.90 eV from the onset of the phosphorescence spectrum. As a reference, the triplet energy level of BCzC1Sy was calculated to be 2.87 eV by density functional theory (DFT) and time-dependent DFT (TD-DFT) under the B3LYP/6-31G level.40 However, attempts to measure the phosphorescence spectra of BCzC1Sy and BCzC4Sy were not successful due to the appearance of styrene groups, which are an efficient triplet quencher.41a–c The transient photoluminescent (TRPL) spectra of BCz3Ph and BCzC4Sy shown in Fig. S1 (ESI†) exhibit a much longer lifetime of BCz3Ph as compared to that of BCzC4Sy, indicating the phosphorescence quenching process in the styrene-appended structure. Furthermore, the absorption and emission of BCzC1Sy and BCzC4Sy before and after cross-linking were examined as the results shown in Fig. S2 (ESI†). The absorptions remain intact, but the broadening of the emissions was observed after cross-linking, which might have arisen from the aggregation of the chromophore.
| Molecule | λ abs sol.a (nm) | λ PL sol. a (nm) | Optical Egb (eV) | E T (eV) | HOMO (eV) | LUMOe (eV) | T d (°C) | T cl (°C)g |
|---|---|---|---|---|---|---|---|---|
| a Measured in toluene at the concentration about 10−5 M. b Optical Eg is calculated from the onset of absorption. c Estimated from the onset of the Phos spectra at 77 K in MeTHF. d Calculated from potential vs. ferrocene/ferrocenium redox couple. e Calculated as the difference between the HOMO and the corresponding optical bandgap. f Reported value (ref. 38). g T cl is the peak temperature of cross-linking, determined by DSC. | ||||||||
| BCz3Ph | 305 | 406 | 3.34 | 2.90 | −5.22d | −1.88 | 454 | — |
| BCzC1Sy | 303 | 409 | 3.40 | — | −5.22d | −1.82 | 430f | 192 |
| BCzC4Sy | 303 | 414 | 3.40 | — | −5.18d | −1.78 | 453 | 146 |
Cyclic voltammetry (CV) was then implemented to investigate the electrochemical properties of these materials. The oxidation potential was measured and recorded with reference to the ferrocene/ferrocenium (Fc/Fc+) redox couple, as shown in Fig. 1c. A reversible oxidation potential at 0.34, 0.31 and 0.22 V was observed for BCz3Ph, BCzC1Sy and BCzC4Sy, respectively. Apparently, BCz3Ph exhibits a higher oxidation potential as compared to those of alkylated counterparts BCzC1Sy and BCzC4Sy due to the inductive effect of the biphenyl substituents. Also, it is noted that BCzC1Sy exhibits a slightly higher oxidation potential as compared to that of BCzC4Sy due to the shorter distance of styrene. This result indicates that the length of the alkyl chain has a slight effect on the oxidation behavior. With reference to the Fc/Fc+ redox couple, the HOMO energy levels are estimated as 5.22 eV (BCz3Ph), 5.22 eV (BCzC1Sy), and 5.18 eV (BCzC4Sy). The energy levels of BCzC1Sy and BCzC4Sy demonstrate that these molecules are suitable for serving as HTMs of OLED devices that employ the exciplex-forming system with BCz3Ph as the donor.
According to previous reports, BCzPh has been reported as a superior donor material for exciplex-forming systems when paired with triazine-centered acceptors.39 Herein, the new donor BCz3Ph with an additional phenyl group on the N-substituted peripherals can further enhance the morphological stability. A glass transition temperature (Tg = 140 °C) was identified by differential scanning calorimetry (DSC) as compared to that (60 °C) of BCzPh.40 In addition, BCz3Ph possesses comparable molecular weight with the triazine-based acceptors for better compatibility for film formation upon solution processes. To examine the possibility of forming a suitable exciplex blend, the donor BCz3Ph was mixed with a benchmark acceptor PO-T2T. The films were prepared by spin-coating 1.6 wt% solutions of BCz3Ph, PO-T2T and a BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture in chlorobenzene to give a thickness of the film of about 30 nm. As shown in Fig. 1d, the absorption spectrum of the BCz3Ph
1) mixture in chlorobenzene to give a thickness of the film of about 30 nm. As shown in Fig. 1d, the absorption spectrum of the BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend film can be deemed a linear combination of the individual donor and acceptor absorptions, indicating that no apparent ground state electronic interactions are present. On the other hand, compared with the emissions of BCz3Ph and PO-T2T, the emission of the BCz3Ph
1) blend film can be deemed a linear combination of the individual donor and acceptor absorptions, indicating that no apparent ground state electronic interactions are present. On the other hand, compared with the emissions of BCz3Ph and PO-T2T, the emission of the BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blend shows a distinctly red-shifted peak centered at 527 nm, which is a signature of exciplex formation in the blend. More importantly, the solution-processed BCz3Ph
PO-T2T blend shows a distinctly red-shifted peak centered at 527 nm, which is a signature of exciplex formation in the blend. More importantly, the solution-processed BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blend film exhibits a PLQY of 43%, indicating the efficient intermolecular charge transfer between BCz3Ph and PO-T2T. The phosphorescence spectrum of the exciplex-forming BCz3Ph
PO-T2T blend film exhibits a PLQY of 43%, indicating the efficient intermolecular charge transfer between BCz3Ph and PO-T2T. The phosphorescence spectrum of the exciplex-forming BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend is shown in Fig. S3 (ESI†). The overlapping peaks of the PL and Phos spectra indicate that the singlet state and triplet state of the exciplex are almost degenerate in energy, demonstrating the potential of efficient RISC. To verify the TADF character of the emission from the BCz3Ph
1) blend is shown in Fig. S3 (ESI†). The overlapping peaks of the PL and Phos spectra indicate that the singlet state and triplet state of the exciplex are almost degenerate in energy, demonstrating the potential of efficient RISC. To verify the TADF character of the emission from the BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend film, temperature-dependent (77–300 K) transient photoluminescence (TRPL) spectra were measured. As indicated in Fig. S4 (ESI†), the relaxation profiles of the BCz3Ph
1) blend film, temperature-dependent (77–300 K) transient photoluminescence (TRPL) spectra were measured. As indicated in Fig. S4 (ESI†), the relaxation profiles of the BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (2
PO-T2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) film emissions at different temperatures can be fitted with two-component exponential decays. The fitting data at 300 K are summarized in Table 2, while the data of different temperatures are summarized in Table S1 (ESI†). As indicated, the delayed fluorescence ratio of the exciplex-forming blend enhances along with the increase of the temperature, whereas the prompt fluorescence ratio slightly decreases as the temperature increases to 300 K. The two-component relaxation as well as the enhanced RISC rates at higher temperatures give rise to an increase in the delayed fluorescence ratio, which is a typical signature of materials with TADF character.
1) film emissions at different temperatures can be fitted with two-component exponential decays. The fitting data at 300 K are summarized in Table 2, while the data of different temperatures are summarized in Table S1 (ESI†). As indicated, the delayed fluorescence ratio of the exciplex-forming blend enhances along with the increase of the temperature, whereas the prompt fluorescence ratio slightly decreases as the temperature increases to 300 K. The two-component relaxation as well as the enhanced RISC rates at higher temperatures give rise to an increase in the delayed fluorescence ratio, which is a typical signature of materials with TADF character.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blend films
PO-T2T blend films
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio A ratio |
PLQYa (%) | λ PL/onset (nm) | TRPLb | |||
|---|---|---|---|---|---|---|
| A 1 | τ p (ns) | A 2 | τ d (μs) | |||
| a Measured with an integrating sphere (Hamamatsu C9920-02). b Measured under an ambient atmosphere (300 K), and the decay components were fitted with two exponential decay models as I(t) = A1exp(−t/τp) + A2exp(−t/τd), as shown in Fig. S4 (ESI). | ||||||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 | 533/455 | 0.02 | 58 | 0.98 | 2.59 |
The thermal stability of the carbazole-based materials was investigated with thermogravimetric analysis (TGA) under nitrogen. The TGA analysis indicates that BCz3Ph exhibits a higher decomposition temperature (Td = 454 °C, corresponding to 5 wt% loss) as compared to that (430 °C) of BCzPh.40BCzC4Sy exhibits good thermal stability with a Td of 453 °C, which is higher than that (430 °C)38 of BCzC1Sy, mainly attributed to its larger molecular weight. In addition, the feasible preparation of the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
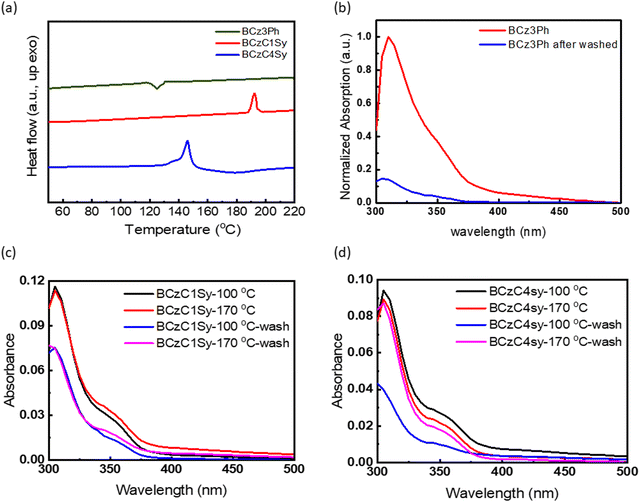
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A blended film reveals its suitability to serve as the EML of a solution-processed OLED device. For smooth hole injection to the EML, a solvent-resistant HTL is highly desired. To probe the thermal cross-linking temperatures of BCzC1Sy and BCzC4Sy, the materials were investigated by DSC at a heating rate of 10 °C min−1, and the results are shown in Fig. 2a. The cross-linkable materials BCzC1Sy and BCzC4Sy showed significant exothermic peaks at 192 °C and 142 °C, respectively, indicating that the cross-linking reactions occurred around these temperatures. The DSC profiles show a large difference between these two molecules. The exothermic peak of BCzC1Sy is about 50 °C higher as compared to that of BCzC4Sy. Apparently, the longer butyl (C4) chain of BCzC4Sy increases the flexibility and reactivity of the cross-linking styrene group, in turn facilitating the cross-linking reaction at a lower temperature.
A blended film reveals its suitability to serve as the EML of a solution-processed OLED device. For smooth hole injection to the EML, a solvent-resistant HTL is highly desired. To probe the thermal cross-linking temperatures of BCzC1Sy and BCzC4Sy, the materials were investigated by DSC at a heating rate of 10 °C min−1, and the results are shown in Fig. 2a. The cross-linkable materials BCzC1Sy and BCzC4Sy showed significant exothermic peaks at 192 °C and 142 °C, respectively, indicating that the cross-linking reactions occurred around these temperatures. The DSC profiles show a large difference between these two molecules. The exothermic peak of BCzC1Sy is about 50 °C higher as compared to that of BCzC4Sy. Apparently, the longer butyl (C4) chain of BCzC4Sy increases the flexibility and reactivity of the cross-linking styrene group, in turn facilitating the cross-linking reaction at a lower temperature.
In order to confirm the solvent resistance of these two thermally cross-linkable materials, an experiment was conducted to examine the solvent erosion effects via monitoring the difference in the absorption intensity of the thermally cross-linked films before and after the solvent treatment. Initially, the absorption intensities of the BCzC1Sy and BCzC4Sy films that have been thermally treated at 100 and 170 °C for thermal cross-linking and subsequently treated with chlorobenzene that was dripped onto the films and dried by spin-coating were measured. For comparison, the non-cross linkable BCz3Ph was also used as a model compound for examining the solvent resistance. As the spectra indicated in Fig. 2b, chlorobenzene erodes most of the BCz3Ph molecules, causing the intensity of its absorption to be significantly reduced. In contrast, the BCzC1Sy films have improved solvent resistance after thermal treatment at 100 and 170 °C as compared to that of the BCz3Ph film (Fig. 2c), despite displaying little temperature dependence. With the aid of the flexible C4 alkyl chain, the BCzC4Sy film after thermal cross-linking at 170 °C has a significant degree of resistance to solvent erosion as the absorption remains nearly unchanged under solvent washing (Fig. 2d). This result indicates that BCzC4Sy has perfect solvent erosion resistant after thermal cross-linking and can serve as a good HTL for solution-processed OLED devices. Furthermore, BCzC4Sy can be washed away prior to thermal cross-linking, which means that it has a great operating window and is thus more versatile in the fabrication of OLED devices.
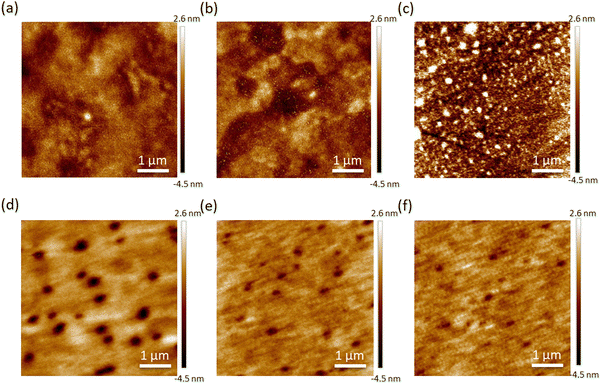
Atomic force microscopy (AFM) was further used to examine the integrity of the film morphology and provide insight towards the better solvent resistance of BCzC4Sy. Here, the films were measured before and after thermal cross-linking, along with rinsing with chlorobenzene, respectively. The results are shown in Fig. 3. The root mean square (RMS) value (0.68 nm) of BCzC1Sy shows that the film is quite smooth at the beginning, but it then slightly increases to 0.80 nm after thermal cross-linking. After rinsing with chlorobenzene, the RMS value was further deteriorated to 1.39 nm, which means that the film coating by the polymerized BCzC1Sy can’t resist solvent erosion very well. On the other hand, the RMS value (0.83 nm) of BCzC4Sy is larger than that of BCzC1Sy before thermal cross-linking, indicating that the surfaces of the films were relatively rough. By thermal cross-linking of BCzC4Sy, the RMS value was found to reduce to 0.56 nm. After further rinsing with chlorobenzene, the RMS value can still be maintained at 0.52 nm, demonstrating that the polymerized BCzC4Sy can indeed effectively resist solvent erosion after thermal cross-linking, and the surface morphology of the films can still be retained after solvent rinsing.
Device
To evaluate the potential of the cross-linkable materials as the HTLs for solution-processed OLEDs, devices were fabricated with the architecture configured as: ITO/PEDOT:PSS (40 nm)/BCzCnSy (n = 1 or 4; 40 nm)/BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T (in ratio 2
PO-T2T (in ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40 nm)/CN-T2T (60 nm)/LiF/Al. The device structure is shown in Fig. 4a. The BCzCnSy (n = 1 or 4) molecules possess a compatible HOMO energy level to the EML and high triplet energy, making them suitable for acting as the HTLs. CN-T2T was selected as the electron transporting layer (ETL) owing to its high triplet energy level (ET = 2.82 eV), high thermal stability (Td > 400 °C) and high electron mobility (μe ∼ 10−4 cm2 V−1 s−1 order).42 It is also crucial that the LUMO of CN-T2T is slightly higher than that of PO-T2T, such that the electron injection process across the interface is much more efficient.37Fig. 4b shows the energy levels of the HTMs, BCz3Ph, PO-T2T, and CN-T2T.
1, 40 nm)/CN-T2T (60 nm)/LiF/Al. The device structure is shown in Fig. 4a. The BCzCnSy (n = 1 or 4) molecules possess a compatible HOMO energy level to the EML and high triplet energy, making them suitable for acting as the HTLs. CN-T2T was selected as the electron transporting layer (ETL) owing to its high triplet energy level (ET = 2.82 eV), high thermal stability (Td > 400 °C) and high electron mobility (μe ∼ 10−4 cm2 V−1 s−1 order).42 It is also crucial that the LUMO of CN-T2T is slightly higher than that of PO-T2T, such that the electron injection process across the interface is much more efficient.37Fig. 4b shows the energy levels of the HTMs, BCz3Ph, PO-T2T, and CN-T2T.
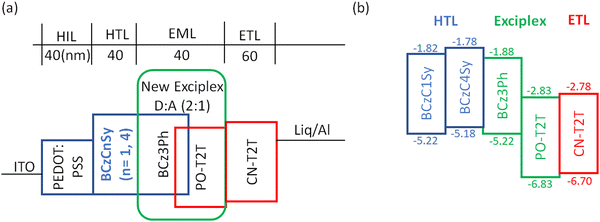 | ||
| Fig. 4 (a) Schematic OLED structure, and (b) energy levels of the HTLs, donor, acceptor and ETL used in this study. | ||
First, the thermally cross-linked (170 °C) BCzC1Sy film was utilized for screening the suitable D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio for giving the best EML. After examining the device characteristics (Fig. S5 and Table S2, ESI†), BCz3Ph
A ratio for giving the best EML. After examining the device characteristics (Fig. S5 and Table S2, ESI†), BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T = 2
PO-T2T = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
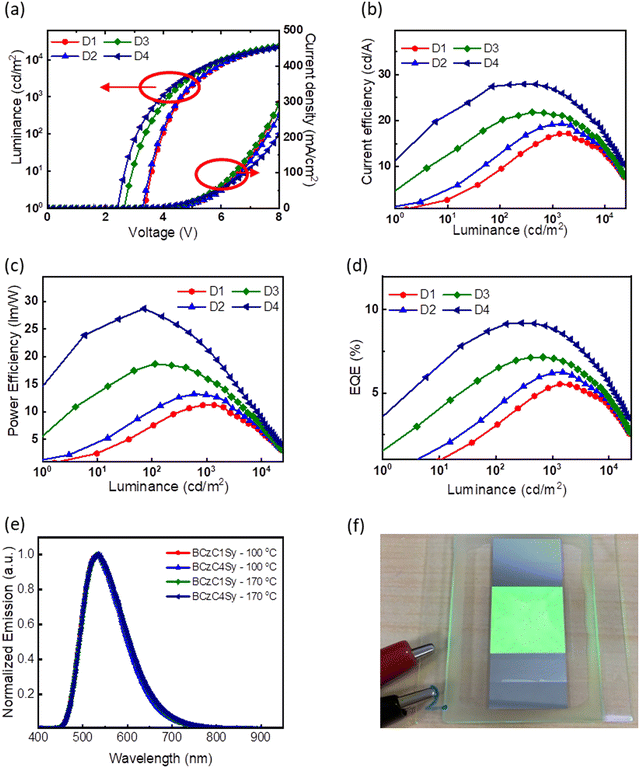
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PLQY = 43%) was selected as the EML owing to its pure exciplex emission. Therefore, four devices (the combination of two HTLs and two curing temperatures) were fabricated. All devices show green EL emission with wavelength peaks between 531 and 535 nm and the CIE coordinate of approximately (0.36, 0.55). The device characteristics are summarized in Table 3. Fig. 5a shows the current density–voltage–luminance (J–V–L) characteristics of the devices. The D1 and D2 devices employing BCzC1Sy and BCzC4Sy films annealed at 100 °C as the HTLs exhibit the same Von of 3.3 V, whereas the Von of the D3 and D4 devices employing the BCzC1Sy and BCzC4Sy films annealed at 170 °C as the HTLs are 2.7 and 2.5 V, respectively. The Von is drastically reduced when the annealing temperature is raised to 170 °C, which is attributed to the more sufficient cross-linking polymerization at a higher temperature, resulting in a smoother film morphology for better hole injection and transport. As indicated in Fig. 5b–d, the devices utilizing these cross-linkable materials as HTLs demonstrate decent EQEs of over 5%. More importantly, after thermal cross-linking at 170 °C, the EQEs of the devices are significantly increased to 7.2% and 9.2% for devices D3 and D4, respectively. It is noteworthy that the EQEs of the D3 and D4 devices remain at 7.1% and 8.9% at 1000 cd m−2, respectively, indicating the reduced efficiency roll-off and device stability. Furthermore, the D4 device exhibited the lowest Von and excellent efficiencies of 9.2% (EQE), 27.94 cd A−1 (CE) and 28.7 lm W−1 (PE), which is the best performance among the reported solution-processed exciplex-based OLEDs. Generally, ηEQE = ηout × β × γ × ΦPL,43 where β is the exciton generation factor induced from photons, γ is the carrier balance factor of the ratio between holes and electrons, and ΦPL is the PLQY. Given that the PLQY of the exciplex is 43%, ηout is assumed to be 20%, and the theoretical EQE can be deduced to be 9.8%, which is very close to that of the D4 device. This indicates that the D4 device based on thermally cross-linked BCzC4Sy film is well optimized to extract the maximum efficiency of the EML. The film integrity of the BCzC4Sy film achieved by the thermal cross-linking process is speculated to lead to the high EQE of the device. It has been reported that the cross-linked molecules would achieve the most thermodynamically stable molecular arrangement through slight writhing after film formation.44 Due to the longer side chain of BCzC4Sy, it is endowed with the highest degree of freedom to attain the most stable and complete polymer film, leading to superior solvent resistance and better hole-transporting capability under the same thermal curing conditions. This is the reason why the D4 device achieves the highest EQE, CE, PE and lower Von.
1 (PLQY = 43%) was selected as the EML owing to its pure exciplex emission. Therefore, four devices (the combination of two HTLs and two curing temperatures) were fabricated. All devices show green EL emission with wavelength peaks between 531 and 535 nm and the CIE coordinate of approximately (0.36, 0.55). The device characteristics are summarized in Table 3. Fig. 5a shows the current density–voltage–luminance (J–V–L) characteristics of the devices. The D1 and D2 devices employing BCzC1Sy and BCzC4Sy films annealed at 100 °C as the HTLs exhibit the same Von of 3.3 V, whereas the Von of the D3 and D4 devices employing the BCzC1Sy and BCzC4Sy films annealed at 170 °C as the HTLs are 2.7 and 2.5 V, respectively. The Von is drastically reduced when the annealing temperature is raised to 170 °C, which is attributed to the more sufficient cross-linking polymerization at a higher temperature, resulting in a smoother film morphology for better hole injection and transport. As indicated in Fig. 5b–d, the devices utilizing these cross-linkable materials as HTLs demonstrate decent EQEs of over 5%. More importantly, after thermal cross-linking at 170 °C, the EQEs of the devices are significantly increased to 7.2% and 9.2% for devices D3 and D4, respectively. It is noteworthy that the EQEs of the D3 and D4 devices remain at 7.1% and 8.9% at 1000 cd m−2, respectively, indicating the reduced efficiency roll-off and device stability. Furthermore, the D4 device exhibited the lowest Von and excellent efficiencies of 9.2% (EQE), 27.94 cd A−1 (CE) and 28.7 lm W−1 (PE), which is the best performance among the reported solution-processed exciplex-based OLEDs. Generally, ηEQE = ηout × β × γ × ΦPL,43 where β is the exciton generation factor induced from photons, γ is the carrier balance factor of the ratio between holes and electrons, and ΦPL is the PLQY. Given that the PLQY of the exciplex is 43%, ηout is assumed to be 20%, and the theoretical EQE can be deduced to be 9.8%, which is very close to that of the D4 device. This indicates that the D4 device based on thermally cross-linked BCzC4Sy film is well optimized to extract the maximum efficiency of the EML. The film integrity of the BCzC4Sy film achieved by the thermal cross-linking process is speculated to lead to the high EQE of the device. It has been reported that the cross-linked molecules would achieve the most thermodynamically stable molecular arrangement through slight writhing after film formation.44 Due to the longer side chain of BCzC4Sy, it is endowed with the highest degree of freedom to attain the most stable and complete polymer film, leading to superior solvent resistance and better hole-transporting capability under the same thermal curing conditions. This is the reason why the D4 device achieves the highest EQE, CE, PE and lower Von.
| Device | HTL | Annealing Temp. (°C) | EL λmax (nm) | V on (V) | EQEmax/CEmax/PEmax (%/cd A−1/lm W−1) | At 1000 cd m−2 (%/cd A−1/lm W−1) b | L max (cd m−2) | CIE (x, y) | |
|---|---|---|---|---|---|---|---|---|---|
| a Turn-on voltage at which emission became detectable. b The values of EQE, CE, PE and driving voltages of the device at 1000 cd m−2. | |||||||||
| D1 | BCzC1Sy | 100 | 531 | 3.3 | 5.53/17.24/11.22 | 5.46/16.75/11.21 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 904 |
(0.36, 0.55) | |
| D2 | BCzC4Sy | 100 | 533 | 3.3 | 6.25/19.33/13.18 | 6.21/19.14/13.07 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 222 |
(0.36, 0.55) | |
| D3 | BCzC1Sy | 170 | 531 | 2.7 | 7.15/21.82/18.68 | 7.07/21.26/15.90 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 287 287 |
(0.37, 0.55) | |
| D4 | BCzC4Sy | 170 | 531 | 2.5 | 9.21/27.94/28.7 | 8.89/26.87/21.65 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 137 |
(0.37, 0.55) | |
To avoid HTLs forming an exciplex with PO-T2T for giving different emission color at the HTL/EML interface, dicarbazole was selected as the core of the HTM and the donor of the exciplex. In order to verify this idea, bilayer-type films were made with the PO-T2T coating on top of the bare BCzC4Sy film or thermally cross-linked BCzC4Sy film. The photoluminescence spectra of these bilayer films are shown in Fig. S6 (ESI†) as compared to that of the film fabricated with the exciplex-forming blend BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T. Despite its weaker emission intensity, BCzC4Sy can indeed form the same exciplex emission as BCz3Ph with PO-T2T, confirming that the common dicarbazole core of BCz3Ph and BCzC4Sy is crucial to circumvent the pollution of the desired exciplex emission from unwanted interfacial interactions.
PO-T2T. Despite its weaker emission intensity, BCzC4Sy can indeed form the same exciplex emission as BCz3Ph with PO-T2T, confirming that the common dicarbazole core of BCz3Ph and BCzC4Sy is crucial to circumvent the pollution of the desired exciplex emission from unwanted interfacial interactions.
In addition, it is of great significance to demonstrate a large-area device fabricated under ambient environments, as it is beneficial to practical mass production and potential lighting applications. Slot-die coating is considered to be a well-suited means of achieving extremely uniform large area films among various solution-process technologies, due to the simple relationship between the flow rate of the solution, wet-film coating thickness, and speed of the coated substrate relative to the head. Besides, slot-die coating can be easily integrated into scaled-up processes, including sheet-to-sheet deposition and roll-to-roll coating systems. While the desired thickness of the film is on the scale of nanometers, the thickness of the as-coated wet film is on the scale of micrometers,45 containing mass solvent residues. It is thus crucial that the under layer, in this case the HTM, presents superior solvent resistance. Herein, a large-area 2 × 2 cm2 device (device D5) adopting the device structure of D4 with the thermally cross-linked BCzC4Sy film as the HTL was prepared in ambient atmosphere employing the slot-die coating technique, as shown in Fig. 5f. The flow rates and concentrations of solution, and the speeds of the coated head for each layer are listed in Table S3 (ESI†). The results of device D5 are shown in Fig. S7 and Table S4 (ESI†). The EQEs and Lmax remain at 5.0% and 20686 cd m−2, respectively, which demonstrates high stability of the solution-processed BCzC4Sy film as the HTL and BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO-T2T blended system as the EML in air. The bright and uniform green emission (Fig. 5f) without visible pinholes confirms the high solvent and air resistance of the thermally cross-linked BCzC4Sy film. It is noteworthy that the solvent resistance is a major limit of the slot-die coating method, it is evident that with the incorporation of a thermally cross-linkable material BCzC4Sy, a large-area OLED device with good efficiencies can be accomplished without using expensive vacuum deposition and extra operations with intricate exclusion of humidity and oxygen.
PO-T2T blended system as the EML in air. The bright and uniform green emission (Fig. 5f) without visible pinholes confirms the high solvent and air resistance of the thermally cross-linked BCzC4Sy film. It is noteworthy that the solvent resistance is a major limit of the slot-die coating method, it is evident that with the incorporation of a thermally cross-linkable material BCzC4Sy, a large-area OLED device with good efficiencies can be accomplished without using expensive vacuum deposition and extra operations with intricate exclusion of humidity and oxygen.
Conclusion
A new green exciplex-forming system comprising a BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POT2T (2
POT2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend with PL λmax of 527 nm and a high PLQY of 43% was identified to show TADF properties and exploited as the emitting layer of solution-processed OLEDs. Simultaneously, a new thermal cross-linkable hole-transporting monomer BCzC4Sy bearing a dicarbazole core with two butyl chain-linked styrenes was designed and synthesized. As compared to the reported model monomer BCzC1Sy, the effects of the linker length between styrene and dicarbazole on the thermal curing behavior and physical properties of the molecules and thermally polymerized films are examined. BCzC4Sy was found to undergo thermal polymerization at a lower temperature (142 °C) than that (192 °C) of BCzC1Sy due to the more flexible C4-linker. The absorptions of these thermally treated HTM films at different temperatures before and after chlorobenzene rinsing indicate that the thermally polymerized BCzC4Sy film can exhibit better solvent resistance. In addition, the AFM analyses found that the RMS difference of the BCzC4Sy film upon chlorobenzene rinsing was limited, indicating that the film had high integrity. Among the OLED devices with thermally cross-linked BCzC1Sy and BCzC4Sy films as HTLs, device D4 employing the BCzC4Sy film as the HTL and BCz3Ph
1) blend with PL λmax of 527 nm and a high PLQY of 43% was identified to show TADF properties and exploited as the emitting layer of solution-processed OLEDs. Simultaneously, a new thermal cross-linkable hole-transporting monomer BCzC4Sy bearing a dicarbazole core with two butyl chain-linked styrenes was designed and synthesized. As compared to the reported model monomer BCzC1Sy, the effects of the linker length between styrene and dicarbazole on the thermal curing behavior and physical properties of the molecules and thermally polymerized films are examined. BCzC4Sy was found to undergo thermal polymerization at a lower temperature (142 °C) than that (192 °C) of BCzC1Sy due to the more flexible C4-linker. The absorptions of these thermally treated HTM films at different temperatures before and after chlorobenzene rinsing indicate that the thermally polymerized BCzC4Sy film can exhibit better solvent resistance. In addition, the AFM analyses found that the RMS difference of the BCzC4Sy film upon chlorobenzene rinsing was limited, indicating that the film had high integrity. Among the OLED devices with thermally cross-linked BCzC1Sy and BCzC4Sy films as HTLs, device D4 employing the BCzC4Sy film as the HTL and BCz3Ph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POT2T (2
POT2T (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend as the EML gave the best EQEmax of 9.2%, CEmax = 27.94 cd A−1 and PEmax = 28.7 lm W−1, and a maximum luminance of over 22
1) blend as the EML gave the best EQEmax of 9.2%, CEmax = 27.94 cd A−1 and PEmax = 28.7 lm W−1, and a maximum luminance of over 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. This device also exhibited a low efficiency roll-off, retaining 97% of the maximum EQE at 1000 cd A−1. Our results demonstrate the successful use of thermal cross-linkable materials to achieve a good solvent resistant HTL with a compatible HOMO energy level to the EML for hole injection and suppressing the complexity of possible exciplex emission from the HTL/EML interface. More significantly, a 2 × 2 cm2 device adopting the D4 device configuration fabricated by the slot-die coating technique was realized with rather good device efficiency, which provides vast potential in the large-scale fabrication of solution-processed OLED devices for lighting applications in the future.
000 cd m−2. This device also exhibited a low efficiency roll-off, retaining 97% of the maximum EQE at 1000 cd A−1. Our results demonstrate the successful use of thermal cross-linkable materials to achieve a good solvent resistant HTL with a compatible HOMO energy level to the EML for hole injection and suppressing the complexity of possible exciplex emission from the HTL/EML interface. More significantly, a 2 × 2 cm2 device adopting the D4 device configuration fabricated by the slot-die coating technique was realized with rather good device efficiency, which provides vast potential in the large-scale fabrication of solution-processed OLED devices for lighting applications in the future.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge financial support from the National Science and Technology Council, Taiwan (Grant No. NSTC 107-2113-M-002-019-MY3, 10-2113-M-002-021 and 111-2221-E-131-031), and the mass spectrometry technical research services from NTU Consortia of Key Technologies.References
- B. Geffroy, P. I. Roy and C. Prat, Organic Light-Emitting Diode (OLED) Technology: Materials, Devices and Display Technologies, Polym. Int., 2006, 55, 572–582 CrossRef CAS.
- S. R. Forrest, The path to ubiquitous and low-cost organic electronic appliances on plastic, Nature, 2004, 428, 911–918 CrossRef CAS PubMed.
- M. Ikai and S. Tokito, Highly efficient phosphorescence from organic light-emitting devices with an exciton-block layer, Appl. Phys. Lett., 2001, 79, 156–158 CrossRef CAS.
- H. Kaji, H. Suzuki, T. Fukushima, K. Shizu, K. Suzuki, S. Kubo, T. Komino, H. Oiwa, F. Suzuki, A. Wakamiya, Y. Murata and C. Adachi, Purely organic electroluminescent material realizing 100% conversion from electricity to light, Nat. Commun., 2015, 6, 8476 CrossRef CAS PubMed.
- K.-H. Kim and J.-J. Kim, Origin and Control of Orientation of Phosphorescent and TADF Dyes for High-Efficiency OLEDs, Adv. Mater., 2018, 30, 1705600 CrossRef PubMed.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes, Appl. Phys. Lett., 2011, 98, 083302 CrossRef.
- K. Kawasumi, T. Wu, T. Zhu, H. S. Chae, T. V. Voorhis, M. A. Baldo and T. M. Swager, Thermally activated delayed fluorescence materials based on homoconjugation effect of donor–acceptor triptycenes, J. Am. Chem. Soc., 2015, 137, 11908–11911 CrossRef CAS PubMed.
- T.-L. Wu, M.-J. Huang, C.-C. Lin, P.-Y. Huang, T.-Y. Chou, R.-W. Cheng, H.-W. Lin, R.-S. Liu and C.-H. Cheng, Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off, Nat. Photonics, 2018, 12, 235–240 CrossRef CAS.
- W. Zeng, H.-Y. Lai, W.-K. Lee, M. Jiao, Y.-J. Shiu, C. Zhong, S. Gong, T. Zhou, G. Xie, M. Sarma, K.-T. Wong, C.-C. Wu and C. Yang, External quantum efficiency for orange–red organic light emitting diodes by employing thermally activated delayed fluorescence emitters composed of 1,8-naphthalimideacridine hybrids, Adv. Mater., 2018, 30, 1704961 CrossRef PubMed.
- (a) X.-K. Liu, Z. Chen, C.-J. Zheng, M. Chen, W. Liu, X.-H. Zhang and C.-S. Lee, Nearly 100% triplet harvesting in conventional fluorescent dopant-based organic light-emitting devices through energy transfer from exciplex, Adv. Mater., 2015, 27, 2025–2030 CrossRef CAS PubMed; (b) M. Zhang, C.-J. Zheng, H. Lin and S.-L. Tao, Thermally activated delayed fluorescence exciplexemitters for high-performance organiclight-emitting diodes, Mater. Horiz., 2021, 8, 401 RSC; (c) J. Guo, Y. Zhen, H. Dong and W. Hu, Recent progress on organic exciplex materialswith different donor–acceptor contacting modesfor luminescent applications, J. Mater. Chem. C, 2021, 9, 16843–16858 RSC; (d) M. Sarma and K.-T. Wong, Exciplex: An Intermolecular Charge-Transfer Approach for TADF, ACS Appl. Mater. Interfaces, 2018, 10(23), 19279–19304 CrossRef CAS PubMed; (e) M. Sarma, L.-M. Chen, Y.-S. Chen and K.-T. Wong, Exciplexes in OLEDs: Principles and promises, Mater. Sci. Eng., R, 2022, 150, 1006 CrossRef.
- M. Zhang, C.-J. Zheng, H. Lin and S.-L. Tao, Thermally activated delayed fluorescence exciplex emitters for highperformance organic light-emitting diodes, Mater. Horiz., 2021, 8, 401–425 RSC.
- M. Zhang, C.-J. Zheng, K. Wang, Y.-Z. Shi, D.-Q. Wang, X. Li, H. Lin, S.-L. Tao and X.-H. Zhang, Hydrogen-Bond-Assisted Exciplex Emitters Realizing Improved Efficiencies and Stabilities in Organic Light Emitting Diodes, Adv. Funct. Mater., 2021, 31, 2010100 CrossRef CAS.
- K. Goushi and C. Adachi, Efficient organic light-emitting diodes through up-conversion from triplet to singlet excited states of exciplexes, Appl. Phys. Lett., 2012, 101, 023306 CrossRef.
- Y.-C. Hu, Z.-L. Lin, T.-C. Huang, J.-W. Lee, W.-C. Wei, T.-Y. Ko, C.-Y. Lo, D.-G. Chen, P.-T. Chou, W.-Y. Hung and K.-T. Wong, New exciplex systems composed of triazatruxene donors and N-heteroarene-cored acceptors, Mater. Chem. Front., 2020, 4, 2029–2039 RSC.
- L.-M. Chen, I.-H. Lin, Y.-C. You, W.-C. Wei, M.-J. Tsai, W.-Y. Hung and K.-T. Wong, Substitution effect on carbazole-centered donors for tuning exciplex systems as cohost for highly efficient yellow and red OLEDs, Mater. Chem. Front., 2021, 5, 5044–5054 RSC.
- V. Jankus, C.-J. Chiang, F. Dias and A. P. Monkman, Deep blue exciplex organic light-emitting diodes with enhanced efficiency; p-type or e-type triplet conversion to singlet excitons?, Adv. Mater., 2013, 25, 1455–1459 CrossRef CAS PubMed.
- Y. J. Cho, S. Taylor and H. Aziz, Increased Electromer Formation and Charge Trapping in Solution- Processed versus Vacuum-Deposited Small Molecule Host Materials of Organic Light-Emitting Devices, ACS Appl. Mater. Interfaces, 2017, 9, 40564–40572 CrossRef CAS PubMed.
- Z.-L. Tseng, W.-L. Huang, T.-H. Yeh, Y.-X. Xu and C.-H. Chiang, Thermally Activated Delayed Fluorescence in CommerciallyAvailable Materials for Solution-Process Exciplex OLEDs, Polymers, 2021, 13, 1668 CrossRef CAS PubMed.
- K. Tong, X. Liu, F. Zhao, D. Chen and Q. Pei, Efficient Light Extraction of Organic Light-Emitting Diodes on a Fully Solution-Processed Flexible Substrate, Adv. Opt. Mater., 2017, 5, 1700307 CrossRef.
- T. Chiba, Y.-J. Pu and J. Kido, Solution-Processed White Phosphorescent Tandem Organic Light-Emitting Devices, Adv. Mater., 2015, 27, 4681–4687 CrossRef CAS PubMed.
- Q. Huang, G. Evmenenko, P. Dutta and T. J. Marks, Molecularly “Engineered” Anode Adsorbates for Probing OLED Interfacial Structure-Charge Injection/Luminance Relationships: Large, Structure-Dependent Effects, J. Am. Chem. Soc., 2003, 125, 14704–14705 CrossRef CAS PubMed.
- W. Hua, Z. Liu, L. Duan, G. Dong, Y. Qiu, B. Zhang, D. Cui, X. Tao, N. Cheng and Y. Liu, Deep-blue electroluminescence from nondopedand doped organic light-emitting diodes (OLEDs)based on a new monoaza[6]helicene, RSC Adv., 2015, 5, 75–84 RSC.
- M. Zhao, H. Zhang, C. Gu and Y. Ma, Electrochemical polymerization: an emergingapproach for fabricating high-quality luminescentfilms and super-resolution OLEDs, J. Mater. Chem. C, 2020, 8, 5310 RSC.
- C. Liu, H. Luo, G. Shi, J. Yang, Z. Chic and Y. Ma, Luminescent network film depositedelectrochemically from a carbazole functionalizedAIE molecule and its application for OLEDs, J. Mater. Chem. C, 2015, 3, 3752 RSC.
- N. Aizawa, Y.-J. Pu, T. Chiba, S. Kawata, H. Sasabe and J. Kido, Instant Low-Temperature Cross-Linking of Poly(N-vinylcarbazole) for Solution-Processed Multilayer Blue Phosphorescent Organic Light-Emitting Devices, Adv. Mater., 2014, 26, 7543–7546 CrossRef CAS PubMed.
- J. Wang, H. Liu, S. Wu, Y. Jia, H. Yu, X. Li and S. Wang, Chemically doped hole transporting materials with low cross-linking temperature and high mobility for solution-processed green/red PHOLEDs, Chem. Eng. J., 2020, 391, 12347 Search PubMed.
- W. Jiang, X. Ban, M. Ye, Y. Sun, L. Duan and Y. Qiu, A high triplet energy small molecule based thermally cross-linkable hole-transporting material for solution-processed multilayer blue electrophosphorescent devices, J. Mater. Chem. C, 2015, 3, 243 RSC.
- N. Aizawa, Y.-J. Pu, H. Sasabe and J. Kido, Thermally cross-linkable host materials for enabling solution-processed multilayer stacks in organic light-emitting devices, Org. Electron., 2013, 14, 1614 CrossRef CAS.
- W.-Y. Hung, C.-Y. Lin, T.-L. Cheng, S.-W. Yang, A. Chaskar, G.-L. Fan, K.-T. Wong, T.-C. Chao and M.-R. Tseng, A new thermally crosslinkable hole injection material for OLEDs, Org. Electron., 2012, 13, 2508 CrossRef CAS.
- J.-H. Jou, T.-H. Li, S. Kumar, C.-C. An, A. Agrawal, S.-Z. Chen, P.-H. Fang, G. Krucaite, S. Grigalevicius, J. Grazulevicius and C.-F. Sung, Enabling high-efficiency organic light-emitting diodes with a cross-linkable electron confining hole transporting material, Org. Electron., 2015, 24, 254 CrossRef CAS.
- N. Du, R. Tian, J. Peng, Q. Mei and M. Lu, Cross-linked alq3-containing polymers with improved electroluminescence efficiency used for OLEDs, Macromol. Rapid Commun., 2006, 27, 412–417 CrossRef CAS.
- B. G. Kang, H. Kang, N. G. Kang, C. L. Lee, K. Lee and J.-S. Lee, Thermally crosslinkable hole transporting polymer synthesized by living anionic polymerization for effective electron blocking and reduction of exciton quenching in multilayer polymer light emitting diodes, Polym. Chem., 2013, 4, 969–977 RSC.
- R. Pötzsch and B. Voit, Thermal and photochemical crosslinking of hyperbranched polyphenylene with organic azides, Macromol. Rapid Commun., 2012, 33, 635–639 CrossRef PubMed.
- Y. H. Niu, M. S. Liu, J. W. Ka and A. K. Y. Jen, Thermally crosslinked hole-transporting layers for cascade hole-injection and effective electron-blocking/exciton-confinement in phosphorescent polymer light-emitting diodes, Appl. Phys. Lett., 2006, 88, 93505 CrossRef.
- B. Ma, F. Lauterwasser, L. Deng, C. S. Zonte, B. J. Kim, J. M. J. Fréchet, C. Borek and M. E. Thompson, New thermally cross-linkable polymer and its application as a holetransporting layer for solution processed multilayer organic light emitting diodes, Chem. Mater., 2007, 19, 4827–4832 CrossRef CAS.
- S. Lee, Y.-Y. Lyu and S.-H. Lee, The use of cross-linkable interlayers to improve device performances in blue polymer light-emitting diodes, Synth. Met., 2006, 156, 1004–1009 CrossRef CAS.
- M. Sarma and K.-T. Wong, Exciplex: An Intermolecular Charge-Transfer Approach for TADF, ACS Appl. Mater. Interfaces, 2018, 10, 19279–19304 CrossRef CAS PubMed.
- S. Ameen, J. Lee, H. Han, M. C. Suh and C. Lee, Curing temperature reduction and performance improvement of solution-processable holetransporting materials for phosphorescent OLEDs by manipulation of cross-linking functionalities and core structures, RSC Adv., 2016, 6, 33212–33220 RSC.
- M. Wang, Y.-H. Huang, K.-S. Lin, T.-H. Yeh, J. Duan, T.-Y. Ko, S.-W. Liu, K.-T. Wong and B. Hu, Revealing the Cooperative Relationship between Spin, Energy, and Polarization Parameters toward Developing High-Efficiency Exciplex Light-Emitting Diodes, Adv. Mater., 2019, 31, 1904114 CrossRef CAS PubMed.
- H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y.-J. Pu and J. Kido, 3,3′-Bicarbazole-Based Host Materials for High-Efficiency Blue Phosphorescent OLEDs with Extremely Low Driving Voltage, Adv. Mater., 2012, 24, 3212–3217 CrossRef CAS PubMed.
- (a) R. E. Rebbert and P. Ausloos, Quenching of the Triplet State of Acetone and Biacetyl by various Unsaturated Hydrocarbons, J. Am. Chem. Soc., 1965, 87, 24 Search PubMed; (b) R. Bonneau and B. Herran, Structure and Properties of the Triplet State of Styrene Derivatives Studied by Laser-Flash-Photolysis, Laser Chem., 1984, 4, 151–170 CrossRef CAS; (c) D. Koyama and A. J. Orr-Ewing, Triplet State formation and quenching dynamics of 2-mercaptobenzothiazole in solution, Phys. Chem. Chem. Phys., 2016, 18, 26224 RSC.
- D. K. Dubey, S. S. Swayamprabha, R. A. K. Yadav, D. Tavgenieneb, D. Volyniuk, S. Grigalevicius and J.-H. Joua, A thermally cross-linkable hole-transporting small-molecule for efficient solution-processed organic light emitting diodes, Org. Electron., 2019, 73, 94–101 CrossRef CAS.
- T. Miwa, S. Kubo, K. Shizu, T. Komino, C. Adachi and H. Kaji, Blue organic light-emitting diodes realizing external quantum efficiency over 25% using thermally activated delayed fluorescence emitters, Sci. Rep., 2017, 7, 284 CrossRef PubMed.
- Y. Wada, H. Nakagawa, S. Matsumoto, Y. Wakisaka and H. Kaji, Organic light emitters exhibiting very fast reverse intersystem crossing, Nat. Photonics, 2020, 14, 643–664 CrossRef CAS.
- M. Schmitt, P. Scharfer and W. Schabel, Slot die coating of lithium-ion battery electrodes: investigationson edge effect issues for stripe and pattern coatings, J. Coat. Technol. Res., 2014, 11, 57–63 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc04638e |
| This journal is © The Royal Society of Chemistry 2023 |