Open Access Article
Open Access ArticleSubambient passive radiative cooling effects of barium sulfate and calcium carbonate paints under Malaysia's tropical climate
William Raphael
Joseph
a,
Jun Yeang
Tan
a,
Apurav Krishna
Koyande
a,
Ianatul
Khoiroh
 *a,
Jerry
Joynson
b and
Steve
Willis
b
*a,
Jerry
Joynson
b and
Steve
Willis
b
aDepartment of Chemical and Environmental Engineering, Faculty of Science and Engineering, University of Nottingham Malaysia, Jalan Broga, 43500 Semenyih, Selangor Darul Ehsan, Malaysia. E-mail: Ianatul.Khoiroh@nottingham.edu.my
bCquestr8 Sdn. Bhd., D1105, Menara Suezcap 1, KL Gateway, Gerbang Kerinchi Lestari No 2, Jalan Kerinchi, Kuala Lumpur, Malaysia
First published on 24th October 2023
Abstract
Global cooling requirements are increasing at an unprecedented rate due to rapid urbanization and population growth, further aggravating climate concerns. Passive radiative cooling is a unique phenomenon that can be utilized to reduce global cooling, energy consumption and alleviate the urban heat island effect. Paints can act as passive cooling devices that are able to reflect incoming sunlight and emit radiation in the atmospheric window (8–13 μm), where it propagates directly into deep space without any interference. In this work, we have successfully fabricated and tested two different types of cooling paints, consisting of BaSO4 and CaCO3 as their respective pigments under Malaysia's tropical climate. Different types of binders, solvents, and pigment concentrations were tested to obtain the most optimum cooling paint configuration. Field test results proved that both cooling paints were able to achieve remarkable subambient temperatures throughout the entire day, even under direct solar irradiation. The BaSO4 cooling paint was able to achieve subambient temperature reductions of up to −6.1 °C and a mean net cooling power of 71.0 W m−2 while the CaCO3 cooling paint achieved a maximum subambient temperature reduction of −6.0 °C and a mean net cooling power of 69.9 W m−2. Both paints were able to significantly outperform commercial white paint on a variety of different surfaces, in terms of cooling performance. The hindering effects of various climate conditions including humidity levels and local wind speeds on the overall cooling performance of both the paints were also investigated.
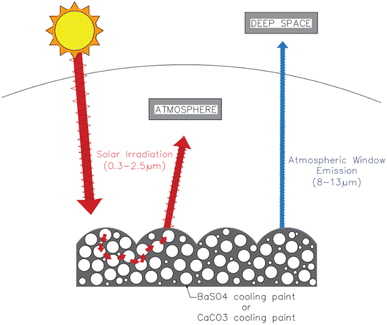
Environmental significanceAll materials absorb infrared radiation (IR) across a broad range of wavelengths, and often re-emit IR at other wavelengths. The IR emitted by solid bodies is generally absorbed by the air surrounding the body, such that the air acts as a blanket stopping the body from cooling appreciably. However, the gases in air do not absorb IR in the range 8 to 13 microns wavelength. Any IR in this range aimed at the sky can pass through the atmosphere and leave the Earth into outer space, thereby helping to cool the Earth. Some materials have been discovered that can emit appreciable amounts of IR in the range of 8 to 13 microns wavelength such as barium sulphate (BaSO4). |
1. Introduction
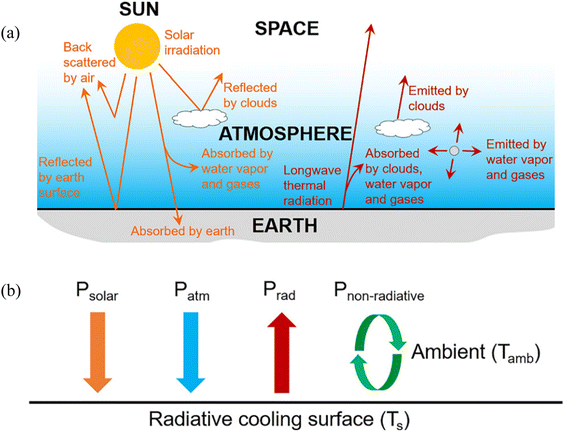
Environmental issues such as global warming and climate change have become among the most pressing challenges faced by the current generation. These issues can be directly linked to the emission of greenhouse gases (GHG) stemming from various anthropogenic activities.1 The sector that contributes the most by a significant amount is the energy sector, accounting for a staggering 73.2% of global GHG emissions.2 The Intergovernmental Panel on Climate Change (IPCC) reports that at the current state of climate policies, the global mean temperature will increase by 3.2 °C by the year 2100 causing numerous adverse effects leading to widespread loss and damage to both nature and people.3 Thus, there is an urgent need to significantly cut down on GHG emissions and explore alternatives that are able to achieve that goal.Cooling constitutes a large percentage of the energy consumption sector in both residential and commercial applications.4 Dong et al. estimated that just the cooling industry itself is responsible for over 10% of global GHG emissions and global cooling requirements are projected to at least double by 2050 due to rapid urbanization, population growth and the rise in global temperatures.5 Passive radiative cooling is a technology whereby heat is naturally dissipated directly into deep space via radiation. This is achieved by emitting the heat through the sky, mainly at the wavelength interval between 8 to 13 μm, which is also known as the “atmospheric window” or “sky window”. At this range, almost all terrestrial thermal radiation can propagate through the atmosphere without interference, as it is highly transparent and has a significantly high atmospheric transmittance as can be seen in Fig. 1. There also exists a narrower secondary atmospheric window, between 16 to 25 μm, which could potentially be utilized for additional cooling. Its passive nature means it does not consume any electricity and has enormous potential to be utilized for the cooling of buildings, solar cells, and thermal power plants among others.6
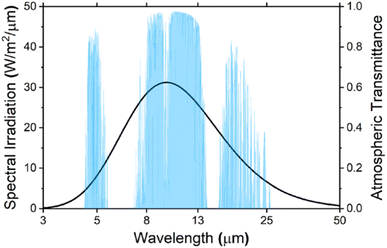 | ||
| Fig. 1 Spectral irradiation of a blackbody surface at a temperature of 300 K and the atmospheric transmittance in the mid- and far-infrared regions.6 | ||
Passive radiative cooling is not a new concept, in fact its applications can be traced up to several centuries back, but thorough systematic research about it had only begun in the 1960s.7 Early research work was only limited to night-time usage, as even a small proportion of daytime solar irradiation onto a surface would negate and counteract its outgoing cooling power. The main drawback is the intrinsically low-energy density of night-time cooling which significantly limits its practical applications and would not have a meaningful impact on modern-day cooling requirements. It has been reported that under perfect conditions, night-time radiative cooling can only provide between 40–80 W m−2 of cooling power.8 To achieve sufficient daytime cooling, the material must have specific properties such as an extremely low absorptivity in the highly intensive solar spectrum (0.3–2.5 μm), while at the same time possessing a high emissivity in the atmospheric window region (8–13 μm).9 With those highly stringent requirements needed to be met, it is unsurprising why there have been little development in daytime cooling technologies only until recently.
Latest developments in radiative cooling technologies have allowed for materials or surfaces to achieve subambient temperatures even when placed under direct sunlight during the day. This is a significant achievement as it could be harnessed to minimize the use of air conditioning units for the cooling of buildings or various structures, especially in regions with a hot and sunny climate. Several methods have been proven to successfully achieve this phenomenon including manufacturing microstructure metasurfaces, selective emitters, porous structures, and random particle distribution through coatings.10 However, most of those approaches involve complex multilayer structures that are hard to scale up and are expensive, limiting them from many applications.11 One approach of particular interest recently is the development of radiative cooling paints because of their high reproducibility, easily scalable nature, and abundance in our modern surroundings. Researchers have developed a plethora of different radiative cooling white paints that utilize different pigments such as BaSO4, CaCO3, Ca3(PO4), and MgO instead of TiO2, which is what is commonly used in commercial white paints.
In 2021, an “ultrawhite” paint with BaSO4 as its pigment was developed which has garnered global attention. It was proven to have a solar reflectance of 98.1% and was able to achieve temperatures of more than 4.5 °C below ambient temperature and a cooling power of 117 W m−2.11 A year prior to that, the same researchers also developed a cooling white paint using CaCO3 as the primary pigment. It showed promising results with a solar reflectance of 95.5%, reaching temperatures of more than 1.7 °C below ambient temperatures with a cooling power exceeding 37 W m−2.12Fig. 2 shows schematic diagram of how the radiative cooling paint works. For comparison, commercial white paints usually have a solar reflectance of between 80% to 90% and are unable to achieve subambient temperatures during the day under direct solar irradiation. Most experimental research have only been conducted in North America, where there are four seasons and a large variability of weather conditions. There has not been enough conclusive experimental works done to test the effects of these cooling paints at low latitude regions, particularly in Southeast Asia which has been theorized that passive radiative cooling can have a positive impact in Southeast Asia's tropical climate.13 Altamimi et al., explored daytime and nigh-time cooling with single layer films consisting of TiO2, BaSO4, and BaSO4/TiO2 microparticles embedded in PTFE/PVDF polymers.14 The BaSO4/TiO2 film, exposed to direct sunlight, effectively reduced surface temperatures by approximately 4–6 °C compared to ambient conditions. Furthermore, on a warm summer day, the BaSO4/TiO2/PVDF/PTFE film demonstrated an average radiative cooling power of approximately 50 W m−2 at 33% humidity and 46.5 W m−2 at 38% humidity. Atiganyanun et al., tested CaCO3-hollow SiO2 with PVC binder paint for passive radiative cooling in Thailand.15 The formulated paint performed well from 0900 to 1200, however, the ambient temperature was lower than the recorded temperatures from 1200 until 1700. The authors concluded that further investigation is required for effective passive radiative cooling.
In this work, we have conducted experimental tests to observe and quantify the effects of BaSO4 and CaCO3 cooling white paints under Malaysia's tropical climate. The climate in Malaysia can be characterized to be hot and humid with heavy tropical rains at certain points throughout the year. The mean annual temperature is 25.4 °C and there is relatively little seasonal variability in the temperature all year round. The mean humidity levels also range between 42% to 94%, varying from different places and months.16 The first section of the research paper focuses on obtaining the most effective paint configuration, testing between different paint binders, solvents, and pigment concentrations. Next, a 24 hours field test was conducted to observe trends of the temperature profile of the cooling white paints against commercial white paint and the ambient temperature. The net cooling power of the cooling paints was also evaluated and the effects of various factors such as humidity levels and wind speed on the cooling performance was studied. The overall aim of this research paper is to evaluate the cooling paints performance under real-world conditions in Malaysia and determine its feasibility of widespread adoption to reduce cooling requirements within the country.
2. Methods and materials
2.1. Materials and FE-SEM analysis
The pigments used for the cooling paints include barium sulfate, BaSO4 (ChemPur) and calcium carbonate, CaCO3 (Sirih Pinang). Both chemicals have a purity of 100% respectively. The binders used include acrylic resin (FCPG) and epoxy resin (EveryOneShop) while the solvents used were N,N-dimethylformamide (R&M Chemicals) and distilled water. Field emission scanning electron microscopy (FE-SEM) analysis was conducted on the BaSO4 and CaCO3 powders to characterize their particle size distribution. The BaSO4 powders had a particle size distribution of 404 ± 500 nm while the purchased CaCO3 powders had a particle size distribution of 2.3 ± 2 μm. Fig. 3 shows the respective SEM images of the BaSO4 and CaCO3 particles. Both powders have a relatively wide particle size distribution range, which has been proven to be beneficial to efficiently scatter the wavelengths within the solar spectrum while also significantly enhancing the overall solar reflectance as opposed to a uniform particle size distribution.11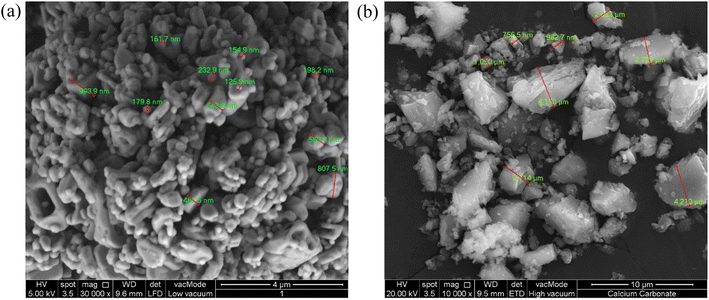 | ||
| Fig. 3 SEM images of (a) BaSO4 powder used in BaSO4 cooling paint. (b) CaCO3 powder using in CaCO3 cooling paint. | ||
2.2. Fabrication of cooling paints
To fabricate the BaSO4 cooling paint, the chosen solvent (dimethylformamide or water) was mixed with the BaSO4 particles in a beaker placed on a stirring hot plate. The mixture was stirred constantly and heated up to approximately 70 °C. Then, the chosen resin (acrylic or epoxy) was slowly added into the mixture. Next, the mixture was ultrasonicated for 15 minutes using a UP400S Hielscher Ultrasonic Probe Mixer. This was done to reduce particle agglomerations and to introduce air bubbles into the mixture. After ultrasonication, the mixture was left to stir and be heated under the same settings as previously for another 1 hour. The constant stirring at a higher temperature was to ensure that all the chemicals were mixed thoroughly. Finally, the mixture was left to dry overnight to remove any excess solvent within the paint. To fabricate the CaCO3 cooling paint, the same exact steps were conducted but substituting calcium carbonate for barium sulfate particles.2.3. Painting methods
In the initial experiments conducted to obtain the most effective paint configuration, all the fabricated paints were painted using a paint brush. Before painting, the paints were mixed for approximately 15 minutes to ensure a uniform texture. Then, a paint brush was used to apply the first coat on the cardboard, it was left to dry under the sun for 30 minutes and then painted with the second coat. Once the optimum paint configuration was determined for both the BaSO4 and CaCO3 cooling paints, a spray gun was used to spray paint the two paints on different surfaces for the field test. To prepare the paints before spraying, they were heated up and stirred thoroughly. Additional amounts of the chosen solvent were also added to dilute the paints, to make it less viscous and more sprayable. A Ford viscosity cup was used to determine the suitable paint viscosity before spraying. Each paint type was sprayed on for a total of 5 layers of coatings. This was done to ensure that the surface was completely covered with the paint coating and to achieve more efficient radiative cooling effects. The surfaces used for the field test include wood, porcelain tiles and zinc roofing sheets.2.4. Preliminary test setup
To determine the most optimum binder, solvent and concentration of the cooling paints, the fabricated paints were painted on a cardboard surface of approximately 20 cm × 20 cm and left to dry for a week. A sample with the chosen commercial white paint (Dulux Aura High Gloss) was also prepared and another sample which was not painted was also placed to act as a control. Then, the samples were placed under direct sunlight as can be seen in Fig. 4, and the temperatures of each sample was measured using two identical infrared thermometers (Pro'sKit MT-4606). Each test run was conducted for 1 hour, whereby the temperature measurements were manually recorded at an interval of 10 minutes. To minimize the uncertainty, each test run was repeated for 3 times on different days to ensure more reliable results. The ambient temperature was recorded using a mercury thermometer which was placed nearby the samples.2.5. Field test setup
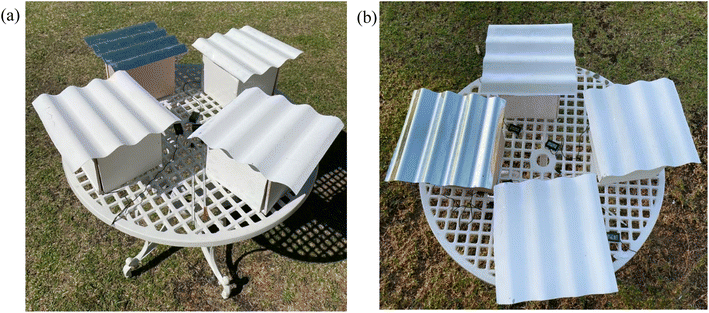
Four different field test setups were made to compare their different cooling performances throughout the day. A miniature house, consisting of four wooden planks as the walls, a porcelain tile as the base and a corrugated zinc sheet as the roof was created in each setup. The indoor house set up was a cube with length, width, and height of 20 cm and a roof with surface area of 31 cm × 31 cm (Fig. 5). The material for the roof was zinc, while the material for the walls was wood, and the floor inside the building was porcelain tiles. The inside temperature was measure using a laboratory digital thermometer with the sensor only in contact with the air, not any surfaces. One of the setups was left blank with no paint, another setup was painted with commercial white paint (Dulux Aura High Gloss), while the other two were painted with the BaSO4 cooling paint and CaCO3 cooling paint, respectively. Only the exteriors of the wooden planks were painted, as well as the top of both the porcelain tiles and zinc roofing. The field test was done to simulate real-world conditions of a building and to determine the efficiency even with the effects of surrounding factors such as convection from external wind speeds and conduction. | ||
| Fig. 5 Photographs of the painted wooden planks with (a) commercial white paint, (b) CaCO3 cooling paint, (c) BaSO4 cooling paint. | ||
The setups were left out in an unshaded area for a duration of 24 hours. The temperature measurements were recorded manually at an interval of 20 minutes. A temperature sensor was placed in each of the four houses, to record the inner surrounding temperature. Meanwhile, two identical infrared thermometers (Pro'sKit MT-4606) were used to simultaneously measure the temperatures of different surfaces around the house. One infrared thermometer was used to constantly measure the temperature of the house with the commercial white paint to act as the control, while the other was used to measure the other houses separately. To measure the ambient temperature, a mercury thermometer was placed beside the houses and exposed to the surrounding environment. Fig. 6 shows the field test setups from two different angles. Local wind speed, humidity levels, and solar irradiation data were obtained from an online meteorological database (SOLCAST).17
2.6. Basic principles of radiative cooling
To quantify the effectiveness of a radiative cooling material, the net cooling power must be obtained. It is defined as follows:| Pnet = Pradnet − Pnon-radiative, | (1) |
| Pradnet = Prad − Patm − Psolar, | (2) |
| Pradnet = (0.0079 × Ts2) + (1.27 × Ts) + 31.6 for daytime, | (3) |
| Pradnet = (0.0079 × Ts2) + (1.27 × Ts) + 69.9 for night-time, | (4) |
| Pnon-radiative = hA(Tamb − Ts), | (5) |
| h = (8.3 + 2.5Vwind), | (6) |
It can be seen from the equations that in order to calculate the net cooling power of a surface, several important measurements are needed which include Ts, Tamb, A, and Vwind. Hence, all these measurements were recorded during the field tests.
The figure of merit RC was also calculated for both cooling paints. It is used to fairly evaluate the cooling radiative performance of the paints, independent of the weather conditions. It is defined as:
| RC = εsky − r(1 − Rsolar), | (7) |
For the experimental results obtained, the standard deviation, σ was calculated to evaluate the variability within the measurements to determine its degree of reliability. The formula is as follows:
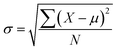 | (8) |
3. Results and discussion
3.1. Determining optimum binder (acrylic vs. epoxy)
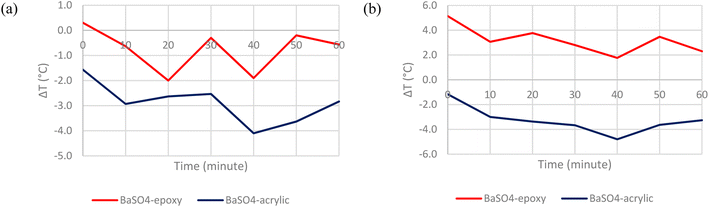
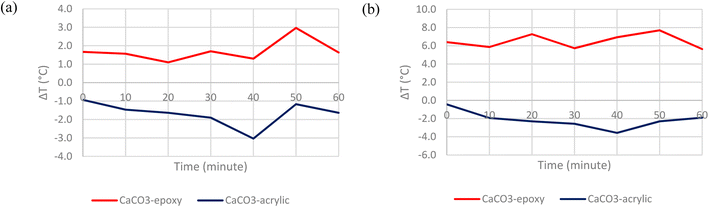
The first step in determining the optimum configuration of the cooling paint was to select a suitable binder. In this case, acrylic and epoxy resins were chosen as two possible candidates as they are among the most common types of binders used in commercial paints.18 Recent developments in radiative cooling paints have proven that acrylic resin work well as binders that aids in the overall cooling performance but not many studies have been done testing epoxy resin as the binder. When bonded with the pigment, the binder should contribute to the high emissivity in the atmospheric window.11Fig. 8 shows the temperature comparison between BaSO4-epoxy paint vs. BaSO4-acrylic paint when placed under direct solar irradiation. Fig. 9 shows the temperature comparison between CaCO3-epoxy paint versus CaCO3-acrylic paint when placed under direct solar irradiation.It is evident that for both pigments and both solvents, the acrylic resin has a significantly higher cooling capability than the epoxy resin. The acrylic-based paints can consistently achieve subambient surface temperatures even when placed directly under the Sun. The range of subambient cooling is between −0.3 °C to −4.8 °C. In comparison, the epoxy-based paints generally absorb the solar irradiation without emitting most of it back into the atmosphere, resulting in mostly above-ambient surface temperatures. This can be attributed to the fact that the acrylic matrix introduces vibrational resonance peaks in the IR region, which ensures a higher atmospheric window emissivity while the epoxy matrix does not.10 Hence, acrylic resin was deemed to be the more suitable binder and is chosen for the subsequent tests.
3.2. Determining optimum solvent (DMF vs. water)
The next step required was choosing the most suitable solvent for the cooling paints. In this experimental work, two different solvents were compared, namely dimethylformamide (DMF) and water. Previous research works into cooling paints have mostly used DMF as their preferred solvent, but water has also been suggested to be a potential alternative.12 The two solvents may also vary depending on the application. Organic solvents such as DMF are more suited to be used as the solvent for exterior paints due to their higher resistance to extreme weather conditions and strong odour. Meanwhile, water is more suitable as the solvent for interior paints as they have low levels of volatile organic compounds (VOCs) and have little to no smell.19Fig. 10 shows the temperature comparison between the paints created with DMF and water as the solvent when placed under direct solar irradiation.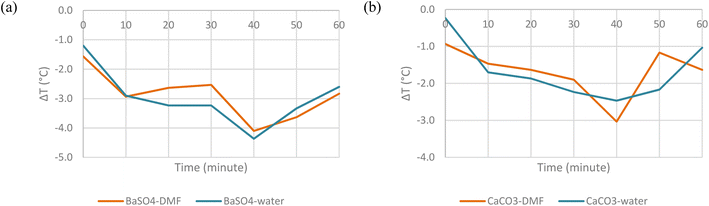 | ||
| Fig. 10 Surface temperature difference with ambient temperature of (a) BaSO4-DMF paint and BaSO4-water paint. (b) CaCO3-DMF paint and CaCO3-water paint. | ||
From the results obtained, it can be concluded that there is no clear difference between the cooling properties of the paints with different solvents. The average degree of subambient cooling for both BaSO4 paints is 3 °C while both CaCO3 paints is 1.7 °C. This is as expected as the solvent does not contribute in any way to the overall absorptivity or emissivity of the paint. Most, if not all of the solvent is usually evaporated once painted on the surface. Although there is no effect of the solvent on the cooling properties, DMF was still chosen as the preferred solvent due to its suitability to be painted on exterior surfaces as radiative cooling paint technologies work best under direct solar irradiation.
3.3. Determining optimum pigment concentration (50% vs. 60% vs. 70%)
The final step in finding the optimum cooling paint composition was to determine the most effective pigment concentration. The basis was set at 60% volume concentration, following what was reported by Li et al. with their BaSO4 and CaCO3 cooling paints. It is a much higher concentration than what is commonly used in commercial paints. This was done because a high pigment concentration is needed to overcome the low refractive index of the pigments themselves.11 A variation of ±10% relative to the basis concentration was adopted, resulting in a comparison between pigment volume concentrations of 50%, 60% and 70%. Fig. 11 shows the temperature comparison for both BaSO4 and CaCO3 cooling paints at different concentrations.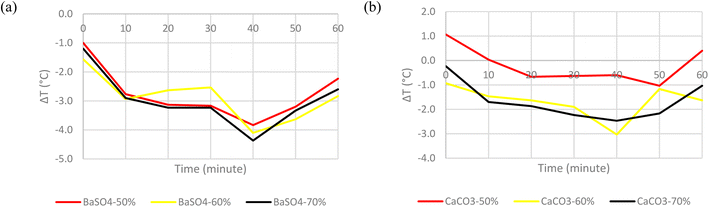 | ||
| Fig. 11 Surface temperature difference with ambient temperature of (a) BaSO4 cooling paint at three different concentrations. (b) CaCO3 cooling paint at three different concentrations. | ||
For BaSO4 cooling paints, the results indicated that there was not a significant difference between the degree of subambient cooling for the different concentrations. The mean temperature difference was only within ±0.2 °C for all three samples. Hence, to determine the most optimum concentration, the standard deviation of all the measurements was also considered. Eqn (8) was used to calculate the standard deviation values. The volume concentration of 60% was chosen as it had a good degree of subambient temperature reduction as well as a relatively low standard deviation. A summary of the results obtained from the three BaSO4 samples can be seen in Table 1.
| Volume concentration (%) | Mean temperature difference (°C) | Standard deviation |
|---|---|---|
| 50 | −2.8 | 1.105 |
| 60 | −2.9 | 1.112 |
| 70 | −3.0 | 1.546 |
The CaCO3 cooling paints on the other hand, showed a slightly different trend. The lower concentration of 50% showed a significantly lower degree of subambient cooling, with some of the measurements even being at above ambient temperatures. A possible explanation for this is because at 50% CaCO3 volume concentration, the texture of the paint sample fabricated was too runny and not suitable to be brushed on the cardboard surface, the trends for both 60% and 70% volume concentration are more comparable with one another with both samples recording the same mean temperature difference. Nevertheless, the volume concentration of 70% was deemed more suitable as it had a lower standard deviation. Table 2 shows a summary of the results obtained from the three CaCO3 samples.
| Volume concentration (%) | Mean temperature difference (°C) | Standard deviation |
|---|---|---|
| 50 | −0.2 | 1.648 |
| 60 | −1.7 | 1.516 |
| 70 | −1.7 | 1.190 |
From the previous tests, the optimum composition and configuration of the cooling paints was determined. They were then fabricated and used to proceed with the field test. A summary of the two different paint types can be seen in Table 3.
| Name | Pigment | Particle size (nm) | Binder | Solvent | Volume concentration (%) |
|---|---|---|---|---|---|
| BaSO4 cooling white paint | BaSO4 | 404 ± 500 | Acrylic resin | DMF | 60 |
| CaCO3 cooling white paint | CaCO3 | 2300 ± 2000 | Acrylic resin | DMF | 70 |
3.4. Performance of cooling paints on different surfaces
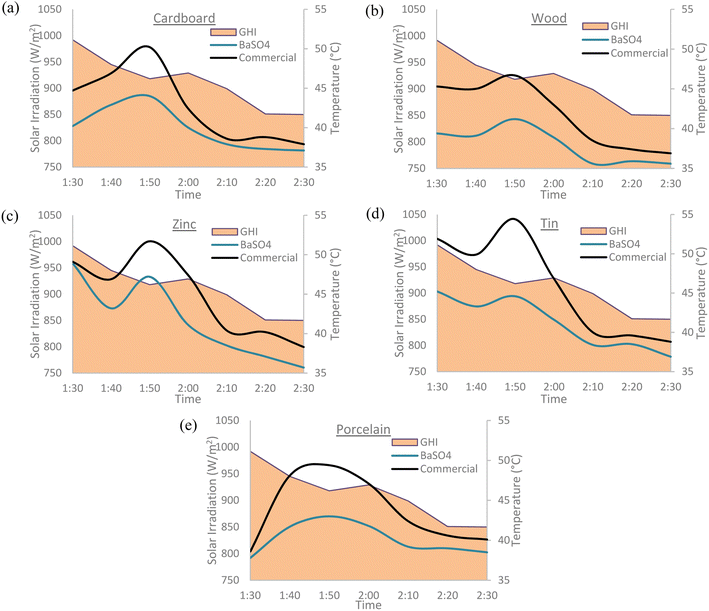
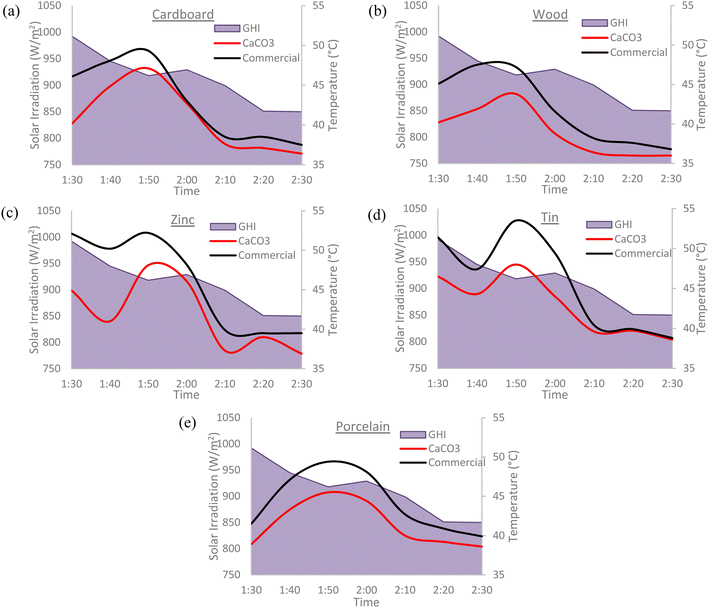
The cooling paints were tested against commercial white paint (Dulux Aura High Gloss) on several different types of surfaces including cardboard, wood, zinc, tin, and porcelain. Different surfaces were tested to determine which material would be the most suitable for cooling paints to be applied upon. The tests were conducted at times with peak solar irradiation during the day with all the surfaces being placed directly under the Sun. The results obtained showed that both cooling paints considerably outperformed the commercial white paint on every surface type with the BaSO4 cooling paint yielding the best results. The results are summarized in Table 4 and the trends can clearly be seen in Fig. 12 and 13. GHI denotes the global horizontal irradiance which is the value of total solar radiation incident on a horizontal surface. The GHI data was obtained from SOLCAST, an online meteorological database.| Surface type | Mean temperature difference (°C) | Minimum temperature reduction (°C) | Maximum temperature reduction (°C) | |||
|---|---|---|---|---|---|---|
| BaSO4 | CaCO3 | BaSO4 | CaCO3 | BaSO4 | CaCO3 | |
| Cardboard | −2.9 | −2.1 | −0.7 | −0.3 | −6.2 | −5.9 |
| Wood | −3.9 | −3.0 | −1.3 | −0.8 | −5.9 | −5.6 |
| Zinc | −3.2 | −4.0 | −0.2 | −0.5 | −6.3 | −9.2 |
| Tin | −4.7 | −2.9 | −1.1 | −0.2 | −9.8 | −5.5 |
| Porcelain | −3.6 | −2.8 | −0.8 | −1.3 | −6.4 | −3.9 |
It is evident that the cooling paints have a better performance on certain surface types more than others. However, it was not possible to conclude a definitive relationship between the surface type and the cooling paint performance. Further experimental work and investigation is required.
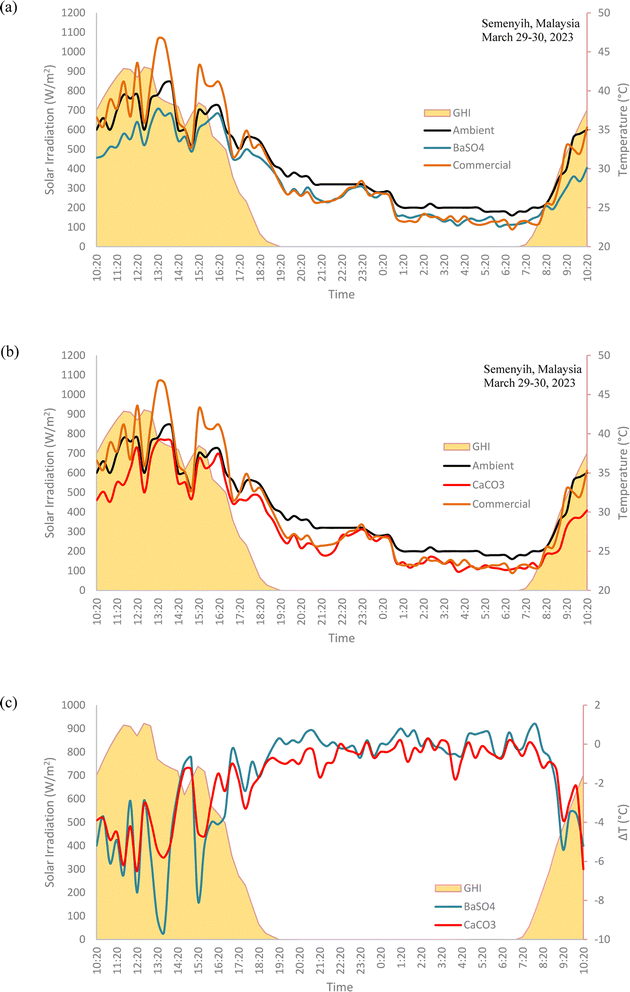
3.5. Field test results (comparison with ambient temperature)
The field test results, as shown in Fig. 14, show that both the BaSO4 and CaCO3 cooling paints displayed remarkable 24 hours long subambient temperatures, even when placed directly under the Sun during the day. In fact, the biggest subambient temperature difference occurs when the solar irradiation is at its highest. The range of subambient temperature difference for the BaSO4 cooling paint was between −0.2 °C to −6.1 °C while for the CaCO3 cooling paint, the range was between −0.2 °C to −6 °C. Throughout nighttime, there was relatively little subambient temperature difference, mainly due to the lack of solar irradiation and the high relative humidity levels. The performance of both cooling paints was comparable, with the BaSO4 paint having a mean daytime subambient temperature difference of −2.6 °C while the CaCO3 paint was −2.5 °C.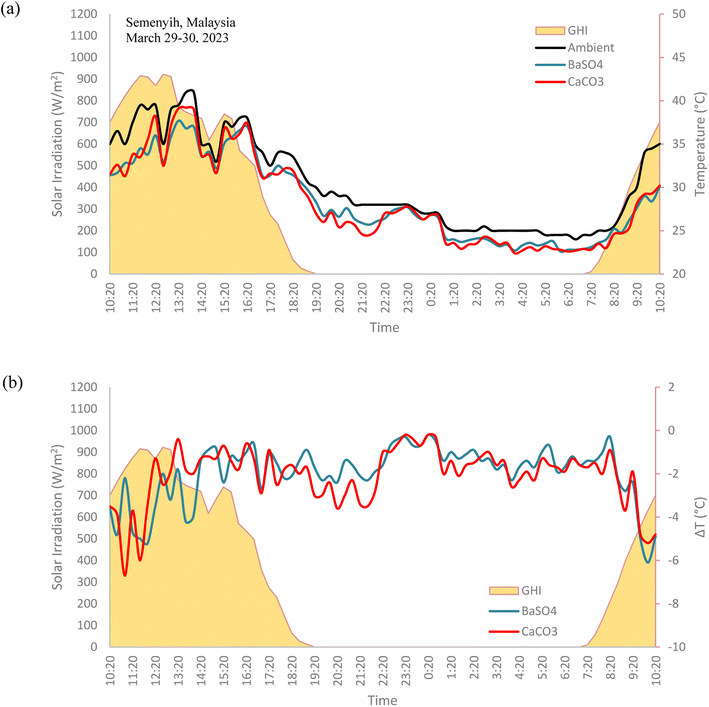 | ||
| Fig. 14 Field test results of (a) BaSO4 cooling paint, CaCO3 cooling paint and ambient temperatures. (b) Temperature difference with ambient for BaSO4 cooling paint and CaCO3 cooling paint. | ||
To consistently achieve subambient surface temperatures, it is essential to have a high solar reflectance and high emissivity in the atmospheric window. The high solar reflectance is usually contributed by the pigment particles while the high emissivity can come from either the pigment particles and/or the matrix (combination of pigment and binder). The remarkable performance of both cooling paints can be attributed to the properties of the respective pigments (i.e., refractive index, volume concentration, particle size and particle size distribution). For BaSO4 particles, the high electron band gap of ∼6 eV contributes to the reduced absorption in the Ultraviolet (UV) band while phonon resonance of the particles also occurs at 9 μm, which is within the atmospheric window.11 As for the CaCO3 particles, the electron band gap is also relatively high at around >5 eV which also reduces the UV absorption.12
A key challenge faced when utilizing both pigments in paints is their low refractive index which causes a decrease in the scattering effect when compared to commercial pigments such as TiO2.20 To overcome this, a relatively higher pigment volume concentration had to be adopted, namely 60% for BaSO4 and 70% for CaCO3. In comparison, commercial white paints usually have pigment volume concentrations ranging from 5–25%, depending on the specific type.21 A higher pigment volume concentration in turn also reduces the volume concentration of the binder. Which aids in reducing the overall absorption in the NIR region. Another method employed to overcome the low refractive index was to adopt a large particle size distribution. Based on the FE-SEM analysis, both pigments had a distribution of approximately ±100% of the mean particle size. This aids in increasing the efficiency of scattering the wavelengths in the solar spectrum. As mentioned previously, the presence of the acrylic matrix ensures that the cooling paints have a higher atmospheric window emissivity as it introduces resonance peaks in the IR region.10
3.6. Field test results (comparison with commercial paint)
When compared to commercial white paint (Dulux Aura High Gloss), both BaSO4 and CaCO3 cooling paints demonstrated significant cooling performance during the daytime when solar irradiation is present. The temperature difference with commercial paint reached up to −9.6 °C for BaSO4 cooling paint and up to −6.5 °C for CaCO3 cooling paint when a peak solar irradiation value of 922 W m−2 was observed. The mean daytime difference with commercial throughout the day was −2.9 °C for BaSO4 cooling paint and −2.7 °C for CaCO3 cooling paint. The temperature profiles were more comparable during night-time with little to no difference between the surface temperatures as can be seen in Fig. 15.The significant increase in commercial paint surface temperatures under solar irradiation can be attributed to the pigment (TiO2) having a moderate 3.2 eV electron band gap which increases the overall solar absorption in the UV band. The higher volume concentration of the acrylic binder also increases the solar absorption in the NIR region, thus further increasing the surface temperature. These factors lead to the reduction of the paint's solar reflectance, hindering its cooling properties. Based on theoretical studies and simulation, it was estimated that TiO2-based paints are unlikely to exceed a solar reflectance value of 92%, hence would not be a good candidate in achieving daytime subambient temperatures.12
3.7. Net radiative cooling power of BaSO4 and CaCO3 cooling paints
The net radiative cooling power of both cooling paints were calculated using eqn (1), taking into consideration the effects of both the radiative and non-radiative heat transfer processes. The average cooling power of the BaSO4 cooling paint was 71.0 W m−2 while the CaCO3 cooling paint was 69.9 W m−2. The average daytime cooling power of both paints were 47.5 W m−2 and 48.8 W m−2 respectively. As can be seen in Fig. 16, the net cooling power is typically higher during the night-time as there is no solar irradiation present which usually counteracts some of the radiative cooling performance during the day. A variety of factors can affect the net cooling power of the cooling paints including the humidity levels, wind speed, cloud opacity and the zenith angle.22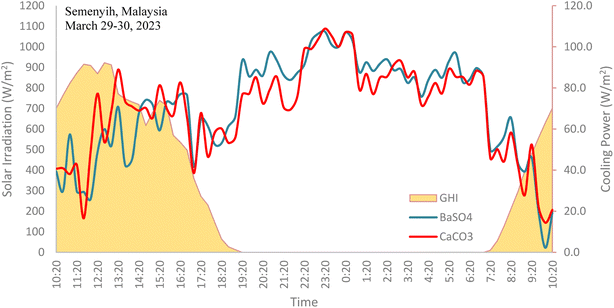 | ||
| Fig. 16 Field test results showing the net cooling powers of BaSO4 cooling paint and CaCO3 cooling paint. | ||
It is important to note that the net cooling power here is lower than those reported in recent research works regarding radiative cooling paints which are typically in the range of above 100 W m−2.10,11 There is a plethora of reasons why including the different climate conditions, ambient temperature, solar irradiation, etc. but by far the most significant factor is the non-radiative heat transfer effects. The experimental setup done in this work does not utilize any convection shield to cover the radiative cooling surface, like what is usually done in other research works. It was purposely done to see how radiative cooling paints would perform under real-life conditions, whereby any form of convection shield would not usually be present. This can cause a considerable portion of the cooling power to be lost through convection and conduction. Fig. 17 shows the net cooling power performance of the individual cooling paints and their temperature profile. Further research works are needed to develop a method to reduce the non-radiative transfer effects towards radiative cooling paints. Nevertheless, the results obtained by the cooling paints in Malaysia show great potential and can bring substantial benefits if adopted properly and at a large scale.
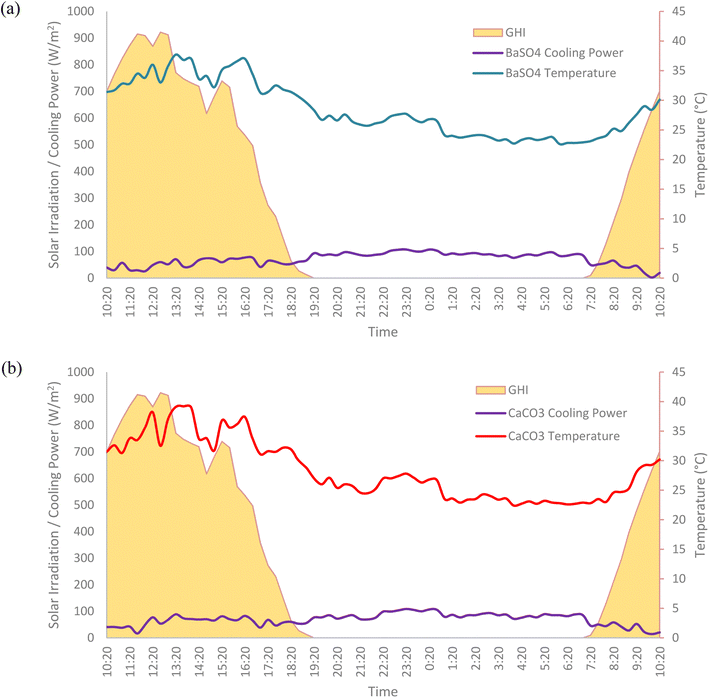 | ||
| Fig. 17 Net cooling power performance and the temperature profiles of (a) BaSO4 cooling paint. (b) CaCO3 cooling paint. | ||
3.8. Effect of various climate factors on net radiative cooling power
The humidity levels also play a significant role affecting the net cooling power of the radiative cooling surface. Based on Fig. 18, when plotted against the relative humidity, there is an inverse relationship between the net cooling power and humidity. As the relative humidity increases, the water vapour content in the air also increases. This in turn reduces the amount of heat that can be radiated to the surroundings by the cooling paints as water vapour interferes by absorbing and re-emitting the longwave radiation emitted.23 With water vapour being the most prominent source of IR absorption in the atmosphere, the increase in water vapour causes the decrease of atmospheric transmittance and the increase of spectral irradiance in the atmospheric window. Thus, leading to the cooling paint absorbing more atmospheric radiation and limiting its cooling performance.24 It was also suggested that in warm humid environments like in Malaysia, the high humidity and precipitable water vapor (PWV) levels causes the secondary atmospheric window (16 to 25 μm) to close.25 This would inevitably hinder any potential additional cooling power obtained from radiative cooling surfaces.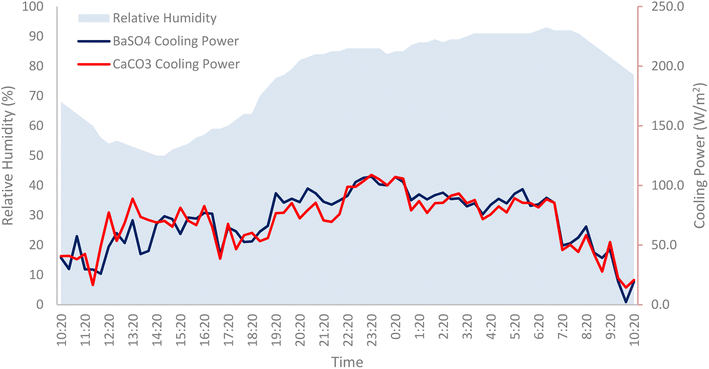 | ||
| Fig. 18 Relative humidity levels plotted against the cooling powers of BaSO4 cooling paint and CaCO3 cooling paint. | ||
The local wind speed, Vwind also plays a crucial role in the non-radiative heat transfer processes, as is evident from eqn (6) with the correlation for the local heat transfer coefficient being directly proportional to Vwind. Heat transfer through convection may either be beneficial or detrimental to the radiative cooling device, depending on the application. For subambient applications, such as this, it can be detrimental and needs to be suppressed as much as possible.26 A higher local wind speed will aggravate the free convection heat transfer from the cooling paint surface with the surrounding warmer air.27Fig. 19 shows a plot of the local wind speeds against the calculated net cooling power obtained from the field test.
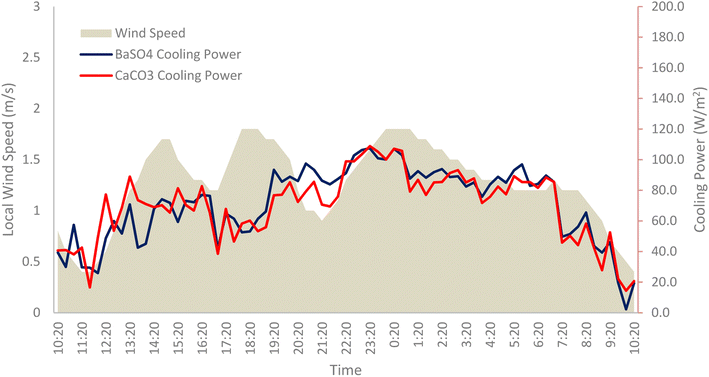 | ||
| Fig. 19 Local wind speeds plotted against the cooling powers of BaSO4 cooling paint and CaCO3 cooling paint. | ||
On average, the non-radiative heat transfer effects account for approximately 25–26% of the loss in the net cooling power of both cooling paints. Thus, there is a dire need to minimize these losses in real world applications to achieve maximum cooling performance and efficiency. Clearly, it is not possible to alter the weather conditions at a certain location, and the most practical solution would be to install some form of convection shield to insulate the top of the surface that faces the sky and the incoming solar irradiation. Liu et al., proposed employing a “tilt strategy and wind cover strategy” to minimize the effects of the non-radiative heat exchange caused by the winds.28
While it may not be a practical solution to apply for the painting of buildings, it could be utilized to improve the performance of a wide range of heat exchangers, including air fin coolers and external radiators of air-conditioning systems. A suitable insulating material would need to have a few key characteristics including a high transmittance across the entire infra-red (IR) band, high mechanical strength to persist through harsh weather conditions, high durability as well as a relatively low cost.29 Such a material that possesses all these characteristics is yet to be developed, but the closest solution currently is utilizing a polyethylene (PE) film.30 Further investigation and research are needed to realize the full potential of radiative cooling paints.
Several other factors also play a key role affecting the net radiative cooling power of both the cooling paints. A high cloud coverage during the day would significantly reduce the amount of solar irradiation directed onto a cooling surface, thus hindering its overall emissivity and net cooling power.31 Rain is another crucial factor, which can have a beneficial or detrimental impact on the cooling performance. Moderate rain could actually help clean the radiative cooling surfaces that have been covered with dust, dirt or any debris and improve its overall reflectivity. On the other hand, continuous heavy rain would result in water accumulation on the surface, which can significantly hinder its cooling performance.32 The scope of this research work does not fully study these factors and further investigation is required.
3.9. Indoor house temperature results
Miniature houses were built as part of the field test setup to observe the indoor cooling effects of the cooling paints vs. commercial white paint (Dulux Aura High Gloss) as well as a house without any paint (No Paint). As expected, the house with no paint recorded the highest indoor temperature throughout the daytime when solar irradiation was present. The mean temperature reduction recorded during the daytime when compared to “No Paint” was −1.5 °C for BaSO4 cooling paint and −1.2 °C for CaCO3 cooling paint, reaching a peak temperature difference of −4.6 °C and −4.3 °C respectively. When compared against the “Commercial White Paint” house, the mean temperature reduction recorded during the daytime was −0.9 °C for BaSO4 cooling paint and −0.6 °C for CaCO3 cooling paint, reaching a peak temperature difference of −2.5 °C and −1.9 °C respectively. Fig. 20 shows the temperature difference between the cooling paint houses with the other house setups.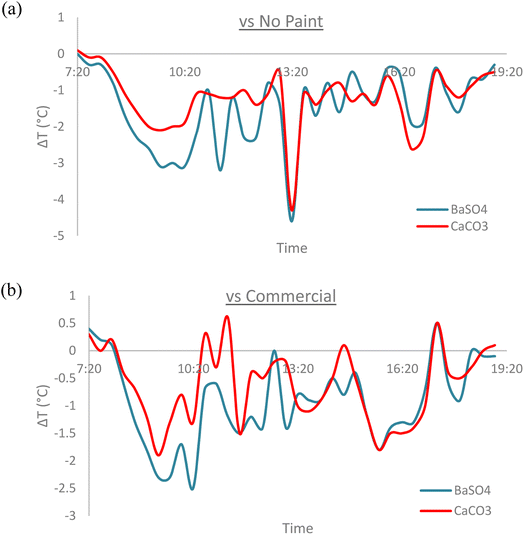 | ||
| Fig. 20 Indoor temperature difference during the daytime with (a) “No Paint” house for both cooling paints. (b) “Commercial” house for both cooling paints. | ||
If employed on a larger scale with an even bigger cooling surface area, it is expected that the cooling performance would remain similar. While this is clearly not enough to negate the need of air conditioning, especially in a warm and humid country like Malaysia, the cooling paints are able to act as a supplement to further decrease cooling energy demands.33 Interest in passive radiative cooling technologies, especially paint coatings, will only continue to increase in the near future due to its cost saving attributes.34
3.10. Performance comparison of BaSO4 and CaCO3 cooling paints
Based on the performance of both cooling paints, it is undeniable that the BaSO4 cooling paint shows consistently better cooling performance throughout all the tests conducted, compared to the CaCO3 cooling paint. Nevertheless, the differences between both the cooling paints are not too significant, as they have a very small percentage difference for a majority of the parameters. The reported higher solar reflectance and sky (atmospheric) window emissivity values of the BaSO4-acrylic paint helps it edge out the CaCO3-acrylic paint in terms of cooling performance. From a visual perspective, the BaSO4 cooling paint also has a more distinct whiter colour, with Li et al. dubbing it as “ultrawhite” whereas the CaCO3 cooling paint has a slight greyish tint in the overall white colour.11 BaSO4 cooling paint also outperformed the CaCO3 on a majority of the different surface types including cardboard, tin, wood and porcelain. Table 5 shows a summary of the results obtained from both paints. The figure of merit RC was calculated using eqn (7). The r value was taken to be 10 as a 300 K blackbody emission was assumed.| Parameter | BaSO4 cooling paint | CaCO3 cooling paint | Difference (%) |
|---|---|---|---|
| Reported solar reflectance (%) | 98.1 (ref. 11) | 95.5 (ref. 12) | 2.65 |
| Reported sky window emissivity | 0.95 (ref. 11) | 0.94 (ref. 12) | 1.05 |
| Minimum subambient temperature reduction (°C) | −0.2 | −0.2 | 0 |
| Maximum subambient temperature reduction (°C) | −6.1 | −6 | 1.64 |
| Mean subambient temperature reduction (°C) | −2.0 | −2.0 | 0 |
| Mean daytime net cooling power (W m−2) | 47.5 | 48.8 | 2.74 |
| Mean night-time net cooling power (W m−2) | 91.3 | 86.5 | 5.26 |
| Mean net cooling power (W m−2) | 71.0 | 69.9 | 1.55 |
| RC figure of merit | 0.68 | 0.42 | 38.38 |
Overall, it is safe to conclude that BaSO4 cooling paint is the preferred choice rather than the CaCO3 cooling paint. The only benefit that would arise from adopting CaCO3 as the preferred cooling paint pigment is its lower cost when compared to pure BaSO4. That may also be a key factor of consideration if widespread and large-scale implementation of radiative cooling paints were to occur. Further investigation should be conducted to find ways to improve the efficiency of cooling paints in tropical climates like in Malaysia.
4. Conclusion
To sum up, two types of cooling paints were fabricated utilizing BaSO4 and CaCO3 as the respective pigments. After testing, the most optimum cooling paint configuration consisted of acrylic resin acting as the binder and dimethylformamide (DMF) as the solvent. For the BaSO4 cooling paint, the volume concentration of the pigment was set at 60% while the volume concentration for the CaCO3 cooling paint was set at 70%, both of which are significantly higher than the pigment concentration of commercial paints. Both cooling paints possess a remarkably high solar reflectance and emissivity in the atmospheric window (8–13 μm), allowing it to retain the temperature of painted surfaces to even below ambient temperature levels. The paints were also tested against commercial white paint (Dulux Aura High Gloss) on various types of surfaces (i.e., cardboard, wood, zinc, tin, and porcelain) and showed considerably lower surface temperatures for each material.The field test results showed that both cooling paints were able to achieve remarkable subambient temperatures throughout the entire day, even when placed under direct solar irradiation during daytime. The BaSO4 cooling paint achieved a mean subambient temperature reduction of −2 °C, a peak of −6.1 °C and a mean cooling power of 71.0 W m−2 while the CaCO3 cooling paint yielded a mean temperature reduction of −2 °C, a peak of −6 °C and a mean cooling power of 69.9 W m−2. When compared against various climate factors, it was concluded that the net cooling power of the paints had an inverse relationship with both the relative humidity levels and the local wind speed. The heat transfer through non-radiative processes such as conduction and convection accounted for a considerable percentage of the net cooling power reduction. The indoor temperatures of the miniature houses were also consistently lower for both cooling paints compared to commercial white paint as well as no paint during the daytime. Between the two, the BaSO4 cooling paint yielded consistently better results compared to the CaCO3 cooling paint and is deemed more favourable due to its higher cooling performance as well as its whiter appearance.
There are many challenges posed by the climate conditions in Malaysia when attempting to optimize the cooling performance of both paints. The high humidity levels, local wind speed and frequent tropical rains can be detrimental to their performance. Nevertheless, this experimental work yielded very promising results. A maximum subambient temperature reduction of −6.1 °C and −6 °C under peak solar irradiation and an average daytime cooling power of 47.5 W m−2 and 48.8 W m−2 for the BaSO4 cooling paint and CaCO3 cooling paint respectively, is already a remarkable feat. While the radiative cooling paints would be most efficient under dry and warm weather conditions such as in desert climates, its effects in Malaysia's tropical climate may also prove to be significant in reducing the active cooling requirements and energy consumption within the region.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the Department of Chemical and Environmental Engineering, University of Nottingham Malaysia. A. K. K. acknowledges financial support for a postdoc fellowship through the NVEC0022 grant from Cquestr8 Sdn. Bhd. The authors also thank Qisya Izanti for her help in the sample fabrication process and field test.References
- A. Mikhaylov, N. Moiseev, K. Aleshin and T. Burkhardt, Global Climate Change And Greenhouse Effect, Entrep. Sustain. Issues, 2020, 7, 2897–2913 Search PubMed
.
-
H. Ritchie, M. Roser and P. Rosado, CO2 and Greenhouse Gas Emissions, Our World in Data [Internet]. 2020 May 11 [cited 2023 Mar 31], available from: https://ourworldindata.org/co2-and-greenhouse-gas-emissions Search PubMed
.
- Climate Change 2022: Impacts, Adaptation and Vulnerability, [Internet]. [cited 2023 Mar 31], available from: https://www.ipcc.ch/report/ar6/wg2/ Search PubMed.
-
J. Conti, P. Holtberg, J. Diefenderfer, A. LaRose, J. T. Turnure and L. Westfall, International Energy Outlook 2016 with Projections to 2040, 2016 May 1 [cited 2023 Mar 31], available from: http://www.osti.gov/servlets/purl/1296780/ Search PubMed
.
- Y. Dong, M. Coleman and S. A. Miller, Greenhouse Gas Emissions from Air Conditioning and Refrigeration Service Expansion in Developing Countries, Annu. Rev. Environ. Resour., 2021, 46, 59–83, DOI:10.1146/annurev-environ-012220-034103
.
- D. Zhao, A. Aili, Y. Zhai, S. Xu, G. Tan and X. Yin,
et al., Radiative sky cooling: Fundamental principles, materials, and applications, Appl. Phys. Rev., 2019, 6(2), 021306 Search PubMed
.
- M. N. Bahadori, Passive Cooling Systems in Iranian Architecture, Sci. Am., 1978, 238(2), 144–154 CrossRef
, available from: https://www.scientificamerican.com/article/passive-cooling-systems-in-iranian.
- U. Eicker and A. Dalibard, Photovoltaic–thermal collectors for night radiative cooling of buildings, Sol. Energy, 2011, 85(7), 1322–1335 CrossRef
.
- J. Mandal, Y. Fu, A. C. Overvig, M. Jia, K. Sun and N. N. Shi,
et al., Hierarchically porous polymer coatings for highly efficient passive daytime radiative cooling, Science, 2018, 362(6412), 315–319, DOI:10.1126/science.aat9513
.
- J. Lv, Z. Chen and X. Li, Calcium Phosphate Paints for Full-Daytime Subambient Radiative Cooling, ACS Appl. Energy Mater., 2022, 5(4), 4117–4124, DOI:10.1021/acsaem.1c03457
.
- X. Li, J. Peoples, P. Yao and X. Ruan, Ultrawhite BaSO4Paints and Films for Remarkable Daytime Subambient Radiative Cooling, ACS Appl. Mater. Interfaces, 2021, 13(18), 21733–21739, DOI:10.1021/acsami.1c02368
.
- X. Li, J. Peoples, Z. Huang, Z. Zhao, J. Qiu and X. Ruan, Full Daytime Sub-ambient Radiative Cooling in Commercial-like Paints with High Figure of Merit, Cell Rep. Phys. Sci., 2020, 1(10), 100221 CrossRef CAS
.
- K. M. Al-Obaidi, M. Ismail and A. M. Abdul Rahman, Passive cooling techniques through reflective and radiative roofs in tropical houses in Southeast Asia: a literature review, Front. Archit. Res., 2014, 3(3), 283–297 CrossRef
.
- M. M. S. Altamimi, U. Saeed and H. Al-Turaif, BaSO4/TiO2 Microparticle Embedded in Polyvinylidene Fluoride-Co-Hexafluoropropylene/Polytetrafluoroethylene Polymer Film for Daytime Radiative Cooling, Polymers, 2023, 15, 3876 CrossRef CAS PubMed
.
- S. Atiganyanun and P. Kumnorkaew, Effects of pigment volume concentration on radiative cooling properties of acrylic-based paints with calcium carbonate and hollow silicon dioxide microparticles, Int. J. Sustain. Energy, 2023, 42(1), 612–626, DOI:10.1080/14786451.2023.2221082
.
- N. Makaremi, E. Salleh, M. Z. Jaafar and A. H. GhaffarianHoseini, Thermal comfort conditions of shaded outdoor spaces in hot and humid climate of Malaysia, Build. Environ., 2012, 48(1), 7–14 CrossRef
.
-
Solcast API Toolkit [Internet]. [cited 2023 Apr 3], available from: https://toolkit.solcast.com.au/historical/timeseries/request Search PubMed
.
- K. D. Weiss, Paint and coatings: A mature industry in transition, Prog. Polym. Sci., 1997, 22(2), 203–245 CrossRef CAS
.
- What is the difference between water-based paint and solvent-based paint?, Normans Media Ltd, Coventry, 2022 [Internet], [cited 2023 Mar 31] available from: https://www.quora.com/What-is-the-difference-between-a-solvent-based-and-a-water-based-paint-system#:˜:text=Solvent%2Dbased%20paint%20uses%20organic,resistant%20to%20wear%20and%20tear Search PubMed.
- A. W. Harrison and M. R. Walton, Radiative cooling of TiO2 white paint, Sol. Energy, 1978, 20(2), 185–188 CrossRef
.
- O. Berger, D. Inns and A. G. Aberle, Commercial white paint as back surface reflector for thin-film solar cells, Sol. Energy Mater. Sol. Cells, 2007, 91(13), 1215–1221 CrossRef CAS
.
- X. Berger and J. Bathiebo, Directional spectral emissivities of clear skies, Renew. Energy, 2003, 28(12), 1925–1933 CrossRef
.
- C. Y. Tso, K. C. Chan and C. Y. H. Chao, A field investigation of passive radiative cooling under Hong Kong's climate, Renew. Energy, 2017, 106, 52–61 CrossRef CAS
.
- C. Liu, Y. Wu, B. Wang, C. Y. Zhao and H. Bao, Effect of atmospheric water vapor on radiative cooling performance of different surfaces, Sol. Energy, 2019, 183, 218–225 CrossRef
.
- T. Suichi, A. Ishikawa, Y. Hayashi and K. Tsuruta, Performance limit of daytime radiative cooling in warm humid environment, AIP Adv., 2018, 8(5), 055124 CrossRef
.
- E. A. Goldstein, A. P. Raman and S. Fan, Sub-ambient non-evaporative fluid cooling with the sky, Nat. Energy, 2017, 2(9), 1–7 Search PubMed
. Available from: https://www.nature.com/articles/nenergy2017143.
- D. Zhao, A. Aili, Y. Zhai, J. Lu, D. Kidd and G. Tan,
et al., Subambient Cooling of Water: Toward Real-World Applications of Daytime Radiative Cooling, Joule, 2019, 3(1), 111–123 CrossRef CAS
.
- J. Liu, J. Zhang, D. Zhang, S. Jiao, J. Xing and H. Tang,
et al., Sub-ambient radiative cooling with wind cover, Renew. Sustain. Energy Rev., 2020, 130, 109935 CrossRef
.
- K. D. Dobson, G. Hodes and Y. Mastai, Thin semiconductor films for radiative cooling applications, Sol. Energy Mater. Sol. Cells, 2003, 80(3), 283–296 CrossRef CAS
.
- Z. Chen, L. Zhu, A. Raman and S. Fan, Radiative cooling to deep sub-freezing temperatures through a 24-h day–night cycle, Nat. Commun., 2016, 7(1), 1–5 CrossRef
. Available from: https://www.nature.com/articles/ncomms13729.
- M. M. Hossain and M. Gu, Radiative Cooling: Principles, Progress, and Potentials, Adv. Sci., 2016, 3(7), 1500360, DOI:10.1002/advs.201500360
.
- Y. Weng, W. Zhang, Y. Jiang, W. Zhao and Y. Deng, Effective daytime radiative cooling via a template method based PDMS sponge emitter with synergistic thermo-optical activity, Sol. Energy Mater. Sol. Cells, 2021, 230, 111205 CrossRef CAS
.
- J. P. Bijarniya, J. Sarkar and P. Maiti, Review on passive daytime radiative cooling: Fundamentals, recent researches, challenges and opportunities, Renew. Sustain. Energy Rev., 2020, 133, 110263 CrossRef
.
- H. Tang, S. Li, Y. Zhang, Y. Na, C. Sun and D. Zhao,
et al., Radiative cooling performance and life-cycle assessment of a scalable MgO paint for building applications, J. Clean. Prod., 2022, 380, 135035 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |