Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
n-Type thermoelectric behavior in oxyethylene surfactant/carbon nanotubes†
Shinichi
Hata
 *a,
Huynh
Le Thu Thao
a,
Hiroki
Ihara
b,
Yukou
Du
*a,
Huynh
Le Thu Thao
a,
Hiroki
Ihara
b,
Yukou
Du
 c,
Yukihide
Shiraishi
c,
Yukihide
Shiraishi
 *a and
Naoki
Toshima
*a and
Naoki
Toshima
 d
d
aDepartment of Applied Chemistry, Faculty of Engineering, Sanyo-Onoda City University, Daigaku-dori, 1-1-1, Sanyo-Onoda, Yamaguchi 756-0884, Japan. E-mail: hata@rs.socu.ac.jp; shiraishi@rs.socu.ac.jp
bGraduate School of Engineering, Sanyo-Onoda City University, Daigakudo-ri, 1-1-1, Sanyo-Onoda, Yamaguchi 756-0884, Japan
cCollege of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
dSanyo-Onoda City University, Daigaku-dori, 1-1-1, Sanyo-Onoda, Yamaguchi 756-0884, Japan
First published on 8th December 2022
Abstract
Oxyethylene surfactants were investigated as electron donors for thermoelectric (TE) carbon nanotubes (CNTs). The oxygen-barrier properties of the surfactant wrapping the NT surface and electron-donating effect of oxyethylene oxygen atoms enabled the preparation of n-type CNTs. The TE output of these NTs was comparable with that of conventionally used dopants.
Flexible and lightweight carbon nanotube (CNT)-based thermoelectric (TE) modules are attracting attention in a wide range of applications (e.g., organic solar cells,1 field-effect transistors,2 wearable devices)3 because they do not contain toxic elements and can be operated at waste heat temperatures (below 150 °C).4–6 Research on various aspects of CNT thin films, including their thermal conductivity and thermoelectric conversion efficiency, has been conducted. However, in-plane thermal conductivity measurements of freestanding thin films made of CNTs or conductive polymers are especially difficult because of unfavorable heat flow in the films.7,8 The conversion efficiency of CNT films can be estimated from their thermal power factor (PF):
| PF = S2σ, | (1) |
CNT doping has been extensively studied since its discovery in 1993.10 However, the concept of n-type doping remains incompletely understood11 because the electronic properties (e.g., thermoelectromotive force and local density of states) of CNTs with large specific surface areas (200–1000 m2 g−1)12 are very sensitive to their chemical environment and, thus, easily switch to the p-type state. Radical anions in the graphene backbone created by electron doping (e.g., ammonia,13 alkali metals)14 can react with electron donors, such as atmospheric oxygen, CO2, and water.15 Polyethylenimine has been successfully used as a Lewis-base molecular dopant; here, the lone pair of electrons on the nitrogen atom is partially injected into the NT conduction band (Fig. S1, ESI†).16 This finding has led to investigations of other amine (e.g., hydrazine,17 diethylenetriamine,18 naphthalene diimide,19 reduced 1,1′-dibenzyl-4,4′-bipyridinium dichloride)20 and phosphine (e.g., 1,3-bis(diphenylphosphino)propane)21 compounds. Considerable progress has been made in synthesizing n-type CNTs, particularly when potassium/crown ether complexes are used as doping reagents.22 The dopant function design of cationic compounds (e.g., imidazole,23 gemini surfactant24) has steadily evolved; these compounds can impart long-term air stability and power generation properties. However, with a few exceptions, research remains focused on oil-soluble compounds and complex synthetic molecules that are difficult to handle owing to their toxicity and strong odor. Given disposal, reuse, and cost-effectiveness concerns and the expectation of the future widespread and long-term use of TE power sources in residential settings, the development of a new class of mild, naturally derived n-dopants characterized by high availability, biodegradability, and low toxicity is an important endeavor.25
Herein, we focused on candidate naturally derived electron donors containing negatively charged oxygen atoms with unshared electron pairs in their molecular structure to investigate the versatility of metal- and amine-free molecular dopants. Specifically, the dopant functions of tetraethylene glycol (EO4) and its surfactant molecular equivalent, tetraethylene glycol monododecyl ether (C12EO4), are investigated (Fig. 1). Research on oxygen-rich compounds to prepare n-type materials is limited to polyoxyethylene26 and polyvinyl alcohol,27 and the relationship between amphiphilic dopants and n-type CNTs remains unclear. In this study, preliminary findings on the preparation of n-type CNTs based on intermolecular electron transfer between C12EO4 and NTs using nonionic surfactants are presented.
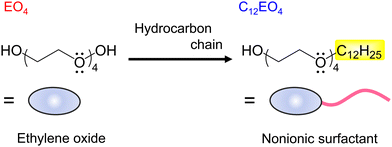 | ||
| Fig. 1 Molecular structures of tetraethylene glycol (EO4) and tetraethylene glycol monodododecyl ether (C12EO4). | ||
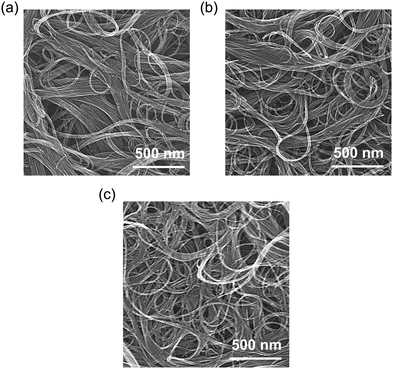
Detailed information on the materials, experimental techniques, and characterization methods is provided in the ESI.† An overview of the film preparation process is shown in Fig. S2 (ESI†). The dispersion behavior and microscopic orientation of the CNT bundles in the fabricated films were observed by SEM (Fig. 2). The average diameters of pure CNT, EO4/CNT, and C12EO4/CNT were estimated to be 41.8 ± 33.7, 38.0 ± 17.6, and 27.6 ± 16.1 nm, respectively. EO4 incorporation reduced van der Waals forces and π–π interactions between CNT bundles.28 The adsorption capacity of the films increased with the hydrocarbon chain length of the surfactant and promoted the divergence of NT bundles. C12EO4 weakened chemical interactions between CNTs, causing the CNT ropes to separate and form fiber bundle structures in the films. Thus, the induction of hydrophilic properties on the outer NT wall surface by adsorbed C12EO4 appears important for the dispersion of the CNTs in aqueous media.
The TE properties of the fabricated films (Fig. 3) were investigated in the temperature range of 330–390 K. The S values of the EO4/CNT film at each measurement temperature ranged from 53.2 to 58.3 μV K−1, and its semiconductor properties were p-type. This finding is consistent with the carrier properties of CNTs in air (S = 62.3 μV K−1).29 By contrast, the S values of the C12EO4/CNT film were consistently negative (range = −62.5 to −52.7 μV K−1), regardless of the measurement temperature. C12EO4, a carrier for NTs, acts as an n-type dopant that converts the charge carriers from holes to electrons, and its σ value is higher than that of EO4 at all measurement temperatures. This finding is supported by the SEM observations, where the increased dispersion ability of the surfactant, which features hydrocarbon chains attached to EO4, results in finer NT bundles. The S and σ measurements showed that the PF values of C12EO4 were consistently superior to those of EO4 with increasing temperature. At 363 K, the PF of C12EO4/CNT peaked at 214.1 μW m−1 K−2, corresponding to an improvement of ∼2.8 times compared with that of EO4/CNT.
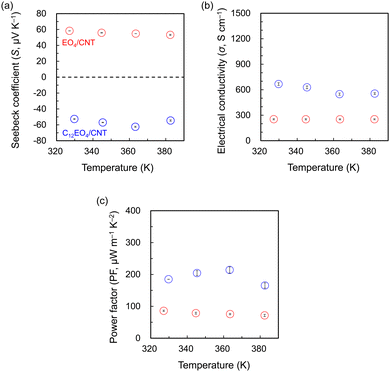 | ||
| Fig. 3 Temperature dependence of the (a) Seebeck coefficient, (b) electrical conductivity, and (c) thermoelectric (TE) power factor of the TE films. Red circles, EO4/CNT; blue circles, C12EO4/CNT. | ||
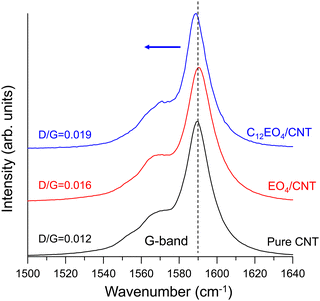
Fig. 4 shows the normalized spectrum of the G band associated with the vibration of the carbon atoms along the NT plane. After doping with C12EO4, the G band of the CNTs shifted slightly from 1589 cm−1 to the lower frequency region. This behavior is well observed during electron transfer from electron donors to NTs,19 indicating that electron doping from C12EO4 to CNTs has occurred. The Raman D/G ratios of the pure, EO4-doped, and C12EO4-doped CNTs, which indicate their crystallinity, were 0.012, 0.016, and 0.019, respectively. No significant changes in D-band intensity were observed during the doping process. In particular, no significant structural defects occurred after doping. These findings indicate that the noncovalent binding of C12EO4 to CNTs triggers electron transfer from the dopant to the CNTs. The results thus far confirm that EO4 does not serve as an n-dopant for NTs, in contrast to C12EO4. In the case of EO4, the ether oxygen in its oxyethylene group is a highly hydrophilic functional group that hydrogen-bonds with water molecules.30 Thus, EO4 has poor adsorption and dispersion capacities for NTs and does not effectively deband CNTs in water.
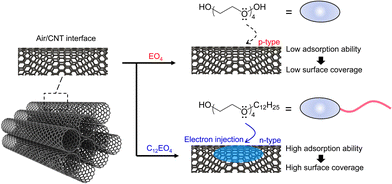
The specific surface area of bare NTs is ∼56% smaller than that of pure CNTs, and most of the CNT surface is an interface accessible to molecular O2 (Fig. S3, ESI†). The low coverage of adsorbed EO4 could promote electrophilic reactions between molecular O2 and the electron-activated sites of the NTs. This factor is responsible for the dominant doping of atmospheric oxygen by electron injection from the dopant into the CNTs, resulting in p-type properties, as observed in the pure CNTs. In the case of C12EO4, the molecule has hydrophilic and hydrophobic groups in its structure and is adsorbed on the NT wall. Its critical micelle concentration is very low at 0.06 mM,31 which may indicate an intrinsically favorable interfacial adsorption capacity. Using TGA, we determined that the apparent amount of surfactant in the film was 23.1 wt% (Fig. S4, ESI†). The C12EO4 molecules weaken chemical interactions between the CNTs, causing the CNT ropes to separate and form a fiber bundle structure in the film. This highly interconnected network may play an important role in increasing the number of conduction pathways available for charge transport.32,33 The hydrophilic groups of C12EO4 appear to be in contact with the NT wall; the results of systematic investigations with anionic and cationic surfactants indicate the ionic nature of the surfactant and confirm that the headgroup determines the type of charge carriers in the doped CNT.34 In this work, the ether oxygen of the C12EO4 headgroup contains an isolated electron pair, which injects electrons into the CNTs during adsorption. Preliminary results obtained with the dopant polymer polyethylene glycol indicate that electrons isolated from the oxygen atom can shift the Fermi level upward and the work function downward, changing the positive S of CNTs to negative.26 In addition, the specific surface area occupied by bare NTs is approximately 85% smaller than that of pure CNTs, and most of the CNT surface is wrapped by surfactant molecules (Fig. S3, ESI†). The adsorbed surfactant layer can conform to the curvature of the NT bundle, resulting in an irregular structure that is soft, flexible, and fluid. Such a structure provides multipoint protection around negatively charged doping sites. The number of oxygen doping sites must be considerably smaller than the number of negatively charged doping sites because the specific surface area of the bare NTs decreases as the coverage of the adsorbed surfactant increases. In such samples, molecular oxygen adsorption is inhibited, and doping with C12EO4 oxyethylene units render the CNT surface electron-rich, stabilizing the delocalized negative charge of n-type CNTs (Fig. 5). Recent theoretical calculations and experimental results have shown that cationic compounds (e.g., imidazole,23 gemini surfactants24) can tightly cover the surface of CNTs, inhibiting interaction with O2 molecules. To test whether oxyethylene surfactants can indeed act as dispersants and dopants, nonionic surfactants with 6 or 8 mol of ethylene oxide added to dodecyl ether were studied (Fig. S5, ESI†). The doped CNTs obtained were n-type, regardless of the surfactant concentration in the aqueous solution used to disperse them in their original state. A comparison of a series of basic molecular structures indicates that oxyethylene surfactants are useful reagents capable of simultaneous carrier conversion and NT dispersion. Preliminary studies confirm that EO4/CNTs are p-type and C12EO4/CNTs are n-type in the dopant concentration range of 4.3–17.0 mM in CNT dispersions (Fig. S6, ESI†). These dopant features are achieved by introducing hydrocarbons into oxyethylene, which is an important structural feature for n-dopants with a simple structure.
An atmospheric stability test of C12EO4/CNT was conducted to confirm carrier persistence in n-type materials (Fig. S7, ESI†). In the initial 4–5 d of the test, the S value was almost zero and the electron carriers in the CNTs were nearly deactivated. Thus, the PF value was also close to 0 even after 7 d. By contrast, when the dopant concentration was changed from 4.3 to 17.0 mM (an increase of ∼4 times), the S of C12EO4/CNT showed a negative value even after 7 d. Increasing the oxyethylene surfactant concentration further extended the adsorbed surfactant wrapping area on the CNT surface and appeared to inhibit n-type material degradation owing to atmospheric oxygen. This result indicates that n-type lifetimes can be improved by optimizing the dopant concentration in the film sample.
Table S1 (ESI†) summarizes the preparation and properties of the n-type CNT materials developed in this study and previous work.7,19,21,22,26,27,35–44 With a few exceptions, the TE performance of C12EO4/CNTs is comparable with that of n-type films impregnated with solutions containing high concentrations of metal ions, amine compounds, and ammonium salts in organic solvents. The proposed approach is an environmentally friendly, economically feasible solution because n-type CNTs are prepared at a very low oxygen-based surfactant concentration of 4.3 mM without the need for metal ions or organic solvents. Research on converting CO2 and biomass into organic resources of oxygen atoms through catalytic reactions is gaining momentum.45,46 The results of this study could be adapted to contribute to a carbon-neutral society.
In summary, C12EO4, a surfactant with high adsorption capacity, can cover ∼85% of the CNT surface, inhibiting oxygen adsorption. Electron donation from oxyethylene units to the NTs occurs simultaneously, resulting in functionalized n-type CNTs. The TE performance of the CNTs (214.1 μW m−1 K−2 at 363 K) is comparable with that of conventional CNTs incorporating nitrogen-based dopants. Further studies are needed to identify the optimal semiconductor/metal ratio of surfactant-wrapped CNT films and investigate the detailed electronic structure and carrier transport properties of the resultant materials. Such work would contribute to the future functionalization of flexible electronics.
Author contributions
S. H. and Y. S. developed the original concept and designed the experiments. S. H. wrote the manuscript. S. H. and H. L. T. T. performed the material synthesis and characterization. H. I. supported the analysis of the results. Y. D. and N. T. provided advice regarding the interpretation of the results. S. H. and Y. S. supervised the project. All authors contributed to the discussion of the results.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.Acknowledgements
This study was supported in part by KAKENHI projects (No. 21K14428 and 19K05633 awarded to S. H. and Y. S., respectively) from the JSPS and a Sasakawa Scientific Research Grant from The Japan Science Society, Japan.Notes and references
- Z. Li, V. Saini, E. Dervishi, V. P. Kunets, J. Zhang, Y. Xu, A. R. Biris, G. J. Salamo and A. S. Biris, Appl. Phys. Lett., 2010, 96, 033110 CrossRef.
- A. Javey, R. Tu, D. B. Farmer, J. Guo, R. G. Gordon and H. Dai, Nano Lett., 2005, 5, 345–348 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhang and G. Chen, Carbon Energy, 2020, 2, 408–436 CrossRef CAS.
- J. L. Blackburn, A. J. Ferguson, C. Cho and J. C. Grunlan, Adv. Mater., 2018, 30, 1704386 CrossRef PubMed.
- N. Toshima, Synth. Met., 2017, 225, 3–21 CrossRef CAS.
- C. Yu, Y. S. Kim, D. Kim and J. C. Grunlan, Nano Lett., 2008, 8, 4428–4432 CrossRef CAS PubMed.
- K. Chatterjee, A. Negi, K. Kim, J. Liu and T. K. Ghosh, ACS Appl. Energy Mater., 2020, 3, 6929–6936 CrossRef CAS.
- Z. Liang, L. Chen and G. C. Bazan, Adv. Electron. Mater., 2019, 5, 1900650 CrossRef CAS.
- Y. Sun, C. A. Di, W. Xu and D. Zhu, Adv. Electron. Mater., 2019, 5, 1800825 CrossRef CAS.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603–605 CrossRef CAS.
- V. Derycke, R. Martel, J. Appenzeller and P. Avouris, Appl. Phys. Lett., 2002, 80, 2773–2775 CrossRef CAS.
- K. Kobashi, S. Ata, T. Yamada, D. N. Futaba, T. Okazaki and K. Hata, ACS Appl. Nano Mater., 2019, 2, 4043–4047 CrossRef CAS.
- J. Kong, N. R. Franklin, C. Zhou, M. G. Chapline, S. Peng, K. Cho and H. Dai, Science, 2000, 287, 622–625 CrossRef CAS PubMed.
- C. Zhou, J. Kong, E. Yenilmez and H. Dai, Science, 2000, 290, 1552–1555 CrossRef CAS PubMed.
- J. T. Quinn, J. Zhu, X. Li, J. Wang and Y. Li, J. Mater. Chem. C, 2017, 5, 8654–8681 RSC.
- M. Shim, A. Javey, N. W. Shi Kam and H. Dai, J. Am. Chem. Soc., 2001, 123, 11512–11513 CrossRef CAS PubMed.
- K. S. Mistry, B. A. Larsen, J. D. Bergeson, T. M. Barnes, G. Teeter, C. Engtrakul and J. L. Blackburn, ACS Nano, 2011, 5, 3714–3723 CrossRef CAS PubMed.
- G. Wu, C. Gao, G. Chen, X. Wang and H. Wang, J. Mater. Chem. A, 2016, 4, 14187–14193 RSC.
- G. Wu, Z.-G. Zhang, Y. Li, C. Gao, X. Wang and G. Chen, ACS Nano, 2017, 11, 5746–5752 CrossRef CAS PubMed.
- S. M. Kim, J. H. Jang, K. K. Kim, H. K. Park, J. J. Bae, W. J. Yu, I. H. Lee, G. Kim, D. D. Loc, U. J. Kim, E.-H. Lee, H.-J. Shin, J.-Y. Choi and Y. H. Lee, J. Am. Chem. Soc., 2009, 131, 327–331 CrossRef CAS PubMed.
- Y. Nonoguchi, K. Ohashi, R. Kanazawa, K. Ashiba, K. Hata, T. Nakagawa, C. Adachi, T. Tanase and T. Kawai, Sci. Rep., 2013, 3, 3344 CrossRef PubMed.
- Y. Nonoguchi, M. Nakano, T. Murayama, H. Hagino, S. Hama, K. Miyazaki, R. Matsubara, M. Nakamura and T. Kawai, Adv. Funct. Mater., 2016, 26, 3021–3028 CrossRef CAS.
- Y. Nakashima, R. Yamaguchi, F. Toshimitsu, M. Matsumoto, A. Borah, A. Staykov, M. S. Islam, S. Hayami and T. Fujigaya, ACS Appl. Nano Mater., 2019, 2, 4703–4710 CrossRef CAS.
- S. Hata, K. Maeshiro, M. Shiraishi, Y. Du, Y. Shiraishi and N. Toshima, ACS Appl. Electron. Mater., 2022, 4, 1153–1162 CrossRef CAS.
- M. Massetti, F. Jiao, A. J. Ferguson, D. Zhao, K. Wijeratne, A. Würger, J. L. Blackburn, X. Crispin and S. Fabiano, Chem. Rev., 2021, 121, 12465–12547 CrossRef CAS PubMed.
- S. Wang, J. Wu, F. Yang, H. Xin, L. Wang and C. Gao, ACS Appl. Mater. Interfaces, 2021, 13, 26482–26489 CrossRef CAS PubMed.
- S. Horike, T. Fukushima, T. Saito, T. Kuchimura, Y. Koshiba, M. Morimoto and K. Ishida, Mol. Syst. Des. Eng., 2017, 2, 616–623 RSC.
- M. Wong, M. Paramsothy, X. Xu, Y. Ren, S. Li and K. Liao, Polymer, 2003, 44, 7757–7764 CrossRef CAS.
- S. Hata, M. Kusada, S. Yasuda, Y. Du, Y. Shiraishi and N. Toshima, Appl. Phys. Lett., 2021, 118, 243904 CrossRef CAS.
- R. Dong and J. Hao, Chem. Rev., 2010, 110, 4978–5022 CrossRef CAS PubMed.
- L.-J. Chen, S.-Y. Lin, C.-C. Huang and E.-M. Chen, Colloids Surf., A, 1998, 135, 175–181 CrossRef CAS.
- E. Bekyarova, M. E. Itkis, N. Cabrera, B. Zhao, A. Yu, J. Gao and R. C. Haddon, J. Am. Chem. Soc., 2005, 127, 5990–5995 CrossRef CAS PubMed.
- J. W. Jo, J. W. Jung, J. U. Lee and W. H. Jo, ACS Nano, 2010, 4, 5382–5388 CrossRef CAS PubMed.
- S. Hata, M. Shiraishi, S. Yasuda, G. Juhasz, Y. Du, Y. Shiraishi and N. Toshima, Energy Mater. Adv., 2022, 9854657 CAS.
- T. Fukumaru, T. Fujigaya and N. Nakashima, Sci. Rep., 2015, 5, 7951 CrossRef CAS PubMed.
- S. L. Kim, K. Choi, A. Tazebay and C. Yu, ACS Nano, 2014, 8, 2377–2386 CrossRef CAS PubMed.
- C. Yu, A. Murali, K. Choi and Y. Ryu, Energy Environ. Sci., 2012, 5, 9481 RSC.
- W. Zhou, Q. Fan, Q. Zhang, L. Cai, K. Li, X. Gu, F. Yang, N. Zhang, Y. Wang, H. Liu, Z. Weiya and X. Sishen, Nat. Commun., 2017, 8, 14886 CrossRef CAS PubMed.
- X. Cheng, X. Wang and G. Chen, J. Mater. Chem. A, 2018, 6, 19030–19037 RSC.
- Y. Liu, Q. Dai, Y. Zhou, B. Li, X. Mao, C. Gao, Y. Gao, C. Pan, Q. Jiang and Y. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 29320–29329 CrossRef CAS PubMed.
- S. Horike, Q. Wei, K. Kirihara and M. Mukaida, Chem. Phys. Lett., 2020, 755, 137801 CrossRef CAS.
- X. Nie, X. Mao, X. Li, J. Wu, Y. Liu, B. Li, L. Xiang, C. Gao, Y. Xie and L. Wang, Chem. Eng. J., 2021, 421, 129718 CrossRef CAS.
- S. Horike, Q. Wei, K. Akaike, K. Kirihara, M. Mukaida, Y. Koshiba and K. Ishida, Nat. Commun., 2022, 13, 3517 CrossRef CAS PubMed.
- Y. Wang, Q. Li, J. Wang, Z. Li, K. Li, X. Dai, J. Pan and H. Wang, Nano Energy, 2022, 93, 106804 CrossRef CAS.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- R. A. Pramudita, K. Nakao, C. Nakagawa, R. Wang, T. Mochizuki, H. Takato, Y. Manaka and K. Motokura, Energy Adv., 2022, 1, 385–390 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ya00226d |
| This journal is © The Royal Society of Chemistry 2023 |