Open Access Article
Open Access Article(Chlorosulfonyl)(trifluoromethanesulfonyl)imide—a versatile building block for battery electrolytes†
Letao
Jin
,
Ziyu
Song
,
Heng
Zhang
 *,
Zhibin
Zhou
*,
Zhibin
Zhou
 and
Wenfang
Feng
and
Wenfang
Feng
 *
*
Key Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry of Education), School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan 430074, China. E-mail: fengwenfang@hust.edu.cn
First published on 1st June 2023
Abstract
Alkali metal salts with trifluoromethanesulfonimide anions ([N(SO2CF3)(SO2R)]−) are intriguing electrolyte components for high-performance rechargeable batteries, owing to their good thermal, chemical, and electrochemical stability. (Chlorosulfonyl)(trifluoromethanesulfonyl)imide (HN[(SO2Cl)(SO2CF3)], H[ClTFSI]) is a crucial precursor to access these important sulfonimide salts. In this work, the chemical properties of H[ClTFSI] in the presence of various kinds of tertiary amines are systematically investigated. It has been demonstrated that H[ClTFSI] possesses versatile reactive pathways under different reaction conditions, mainly including neutralization, nucleophilic substitution, and intra-/inter-molecular eliminative reactions. These reactions give a wide array of new compounds that are of great interest for battery use, particularly the sulfonimide-based zwitterion compounds with sufficient chemical and thermal stability (>190 °C). The present work provides a useful guideline for utilizing H[ClTFSI] as an efficient and versatile building block for battery electrolytes.
Introduction
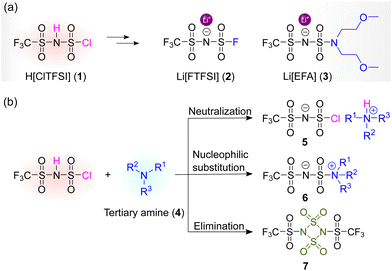
Alkali metal salts and ionic liquids (ILs) with the trifluoromethanesulfonimide anion ([N(SO2CF3)(SO2R)]−) have attracted increasing attention in a wide range of research domains (e.g., battery electrolytes, catalysts), due to their advantages of sufficient thermal, chemical, and electrochemical stability, etc.1–6 At present, trifluoromethanesulfonimide-based compounds are primarily synthesized through the nucleophilic substitution reactions between (chlorosulfonyl)(trifluoromethanesulfonyl)imide (HN[(SO2Cl)(SO2CF3)], H[ClTFSI] (1); Fig. 1a) and a variety of nucleophiles (e.g., F− ion, RSO2NHK).7–10 This is mainly owing to the fact that H[ClTFSI] is readily accessible from low-cost and commercially available materials (i.e., trifluoromethanesulfonamide, thionyl chloride, and chlorosulfonic acid; 95% yield).8Starting from H[ClTFSI], several trifluoromethanesulfonimide-based compounds of interest for battery use have been obtained. For example, the fluorination of H[ClTFSI] with certain metal fluorides (e.g., antimony trifluoride (SbF3), potassium fluoride (KF)) affords (fluorosulfonyl)(trifluoromethanesulfonyl)imide (H[N(SO2F)(SO2CF3)], H[FTFSI]) in high yield (>95%) under mild conditions. Subsequently, the acid H[FTFSI] could be easily turned into battery-grade (>99.99% purity) alkali metal salts and ILs with the [FTFSI]− anion via simple neutralization and/or metathesis reactions.8 The as-obtained lithium (fluorosulfonyl)(trifluoromethanesulfonyl)imide (Li[N(SO2F)(SO2CF3)], Li[FTFSI] (2); Fig. 1a) has been deemed as a promising alternative to the state-of-the-art electrolyte salts (i.e., LiPF6) for next-generation rechargeable lithium batteries.11–15 Alternatively, the direct treatments of H[ClTFSI] with secondary amines give several kinds of partially fluorinated sulfonimide salts (e.g., Li[N(SO2CF3)(SO2R)], R = N(CH2CH2OCH3)2 (3); Fig. 1a), which are capable of improving the selectivity of cation transport in polymer electrolytes due to the enhanced interactions between anionic species and polymer backbones.9,10
The preparative processes of the aforementioned trifluoromethanesulfonimide-based compounds generally involve the utilization of certain tertiary amines as deprotonation reagents to suppress the acid–base reaction of selected nucleophiles (e.g., RSO2NHK) considering the strong acidity of H[ClTFSI] (pKa < −11 in 1,2-dichloroethane16). Yet, the reactions between H[ClTFSI] and tertiary amines tend to be sophisticated (e.g., neutralization, nucleophilic substitution, and intra-/inter-molecular eliminative reactions; Fig. 1b), and are highly dependent on the nature of the tertiary amines and the adopted reaction conditions.5,17 Hitherto, explicit reaction pathways of H[ClTFSI] in the presence of tertiary amines remain to be elucidated, particularly with the ever-increasing interest in trifluoromethanesulfonimide-based compounds.
In this work, the chemical properties of H[ClTFSI] are systemically investigated, including their chemical reactivities in the presence of various kinds of tertiary amines at different temperatures and hydrolytic decompositions. Surprisingly, a new family of chemically stable zwitterion compounds is obtained by treating H[ClTFSI] with certain tertiary amines. Additionally, the structural identifications and thermal properties of these interesting zwitterion compounds are also provided.
Results and discussion
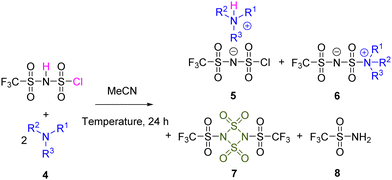
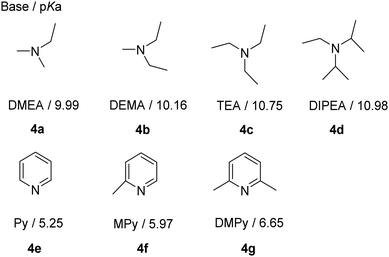
To reveal the chemical reactivities of H[ClTFSI] vs. tertiary amines, several aliphatic and aromatic tertiary amines were selected, including dimethylethylamine (DMEA, 4a), diethylmethylamine (DEMA, 4b), triethylamine (TEA, 4c), N,N-diisopropylethylamine (DIPEA, 4d), pyridine (Py, 4e), 2-methylpyridine (MPy, 4f), and 2,6-dimethylpyridine (DMPy, 4g). The impact of the reaction temperature and feeding ratios between H[ClTFSI]/tertiary amines was studied, and the results are shown in Table 1 and Table S1 (ESI†). The assignments of chemical shift values (δ) and spin–spin coupling constants (J) are summarized in Table S2 (ESI†).| Entry | Base | Temperature/°C | NMR yield/% | |||
|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | |||
| a The 5, 6, 7, and 8 represent neutralization products, nucleophilic substitution products, elimination products, and trifluoromethanesulfonamide (CF3SO2NH2), respectively. | ||||||
| 1 | 4a | 20 | 15 | 78 | 0 | 7 |
| 2 | 4b | 20 | 27 | 66 | 1 | 6 |
| 3 | 4c | 20 | 30 | 60 | 5 | 5 |
| 4 | 4d | 20 | 94 | 0 | 0 | 6 |
| 5 | 4e | 20 | 45 | 42 | 3 | 10 |
| 6 | 4f | 20 | 74 | 10 | 9 | 7 |
| 7 | 4g | 20 | 95 | 0 | 0 | 5 |
| 8 | 4a | 80 | 67 | 7 | 18 | 8 |
| 9 | 4b | 80 | 48 | 24 | 23 | 5 |
| 10 | 4c | 80 | 43 | 33 | 15 | 9 |
| 11 | 4d | 80 | 4 | 0 | 10 | 86 |
| 12 | 4e | 80 | 43 | 46 | 2 | 9 |
| 13 | 4f | 80 | 74 | 10 | 6 | 10 |
| 14 | 4g | 80 | 89 | 0 | 0 | 11 |
|
||||||
Under room temperature (20 °C), the addition of equimolar tertiary amines into the solution of H[ClTFSI] in acetonitrile (MeCN) rapidly affords the corresponding neutralization compounds 5 as the main products (79–95%; Table S1, ESI†), irrespective of the chemical structures of tertiary amines. This result implies that the selected tertiary amines mainly function as deprotonation reagents under mild conditions, which is well rationalized by the significant difference (>16 orders of magnitudes) in Brønsted acidity (e.g., pKa < −11 in 1,2-dichloroethane for H[ClTFSI]16vs. > 5 for tertiary amines18,19).
With the addition of an extra molar of tertiary amines, the reaction paths of H[ClTFSI] are largely diversified. As shown in Table 1, at room temperature, product 6 originating from the nucleophilic substitution reaction increases as follows: 0% (Entry 7, DMPy, 4g; Entry 4, DIPEA, 4d) < 10% (Entry 6, MPy, 4f) < 42% (Entry 5, Py, 4e) < 60% (Entry 3, TEA, 4c) < 66% (Entry 2, DEMA, 4b) < 78% (Entry 1, DMEA, 4a). This trend suggests that: (1) the utilization of aliphatic tertiary amines is likely to promote the nucleophilic substitution reactions due to their relatively stronger nucleophilicities vs. aromatic tertiary amines (e.g., Mayr's nucleophilicity parameter obtained from the reactions with benzhydrylium ions: 17.30 (TEA)20vs. 12.90 (Py)21 in dichloromethane); and (2) the presence of sterically hindering substituents also decreases the nucleophilicities of tertiary amines toward sulfonyl chloride groups (e.g., DMPy, 4gvs. Py, 4e; DIPEA, 4dvs. DMEA, 4a). It is interesting to note that DMPy and DIPEA with relatively lower nucleophilicity among the studied bases could be better replacements for the prevailing tertiary bases (i.e., TEA and Py)17,22 as deprotonation reagents for efficiently preparing alkali metal salts and ILs with trifluoromethanesulfonimide anions.
Under elevated temperatures (e.g., 80 °C), the eliminative reaction product (i.e., 2,4-bis((trifluoromethyl)sulfonyl)-1,3,2,4-dithiadiazetidine-1,1,3,3-tetraoxide, 7; Table 1) formed by dehydrochlorination of H[ClTFSI] is observed in all the cases. Effectively, the intra-/inter-molecular eliminative reaction product (7; Table 1), could be treated as a dimer of N-sulfonyltrifluoromethanesulfonylamide (CF3SO2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SO2) and accessed through the reaction of N-sulfinyltrifluoromethanesulfonamide (CF3SO2N
SO2) and accessed through the reaction of N-sulfinyltrifluoromethanesulfonamide (CF3SO2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) with sulfur trioxide (SO3) at low temperatures (ca. −80 °C; Reaction S1; Scheme S1, ESI†).23 The increased amounts of eliminative reaction product at higher temperatures (e.g., 23% (Entry 9, 80 °C) vs. 1% (Entry 2, 20 °C) for DEMA, 4b) would be ascribed to the higher activation energies of forming the S
O) with sulfur trioxide (SO3) at low temperatures (ca. −80 °C; Reaction S1; Scheme S1, ESI†).23 The increased amounts of eliminative reaction product at higher temperatures (e.g., 23% (Entry 9, 80 °C) vs. 1% (Entry 2, 20 °C) for DEMA, 4b) would be ascribed to the higher activation energies of forming the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond with the breakdown of the N–H and S–Cl bonds.24 Moreover, the contents of the elimination product are higher for those amines with higher pKa values (e.g., Entries 8–10 vs. Entries 12 and 13), which implies the impact of Brønsted basicity of tertiary amines on the reaction paths of H[ClTFSI].
N double bond with the breakdown of the N–H and S–Cl bonds.24 Moreover, the contents of the elimination product are higher for those amines with higher pKa values (e.g., Entries 8–10 vs. Entries 12 and 13), which implies the impact of Brønsted basicity of tertiary amines on the reaction paths of H[ClTFSI].
Besides, the product trifluoromethylsulfonamide (CF3SO2NH2, 8) is observed in most of the experiments, which is mainly due to the hydrolytic decomposition (i.e., cleavage of the S–N bond adjacent to the Cl atom) of H[ClTFSI] in the presence of trace amounts of water (Reaction S2; Scheme S1, ESI†). Effectively, as reported in the literature, a typical N-sulfonyl-containing compound, N-neopentyl sulfamate ((CH3)3CCH2NHSO3H), is also likely to undergo acid-catalyzed hydrolysis in aqueous solution, forming (CH3)3CCH2NH3+ and SO42− ions with the heterolytic scission of the S–N bond (Reaction S3; Scheme S1, ESI†).25 Our experiments show that nearly 20% (by mol) of H[ClTFSI] decomposes into CF3SO2NH2 and SO42− ions after being left in the mixture of H2O/MeCN (H[ClTFSI]/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by mol) at 20 °C for 24 h (Fig. S1, ESI†).
1, by mol) at 20 °C for 24 h (Fig. S1, ESI†).
According to the aforesaid experimental results, the chemical properties of H[ClTFSI] are summarized in Scheme 1. In the presence of tertiary amines (e.g., TEA, 4e), H[ClTFSI] mainly shows three types of chemical reaction pathways in non-aqueous media (e.g., MeCN), including (1) the neutralization reaction between the acidic proton in H[ClTFSI] and tertiary amines, yielding the ionic compounds with protonated amines (e.g., HNEt3+) as the cation and the structurally retained [ClTFSI]− as the anion (Reaction 1; Scheme 1); (2) the intra-/inter-molecular eliminative reaction of H[ClTFSI], liberating hydrogen chloride (HCl) and yielding CF3SO2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SO2 or its dimer ((CF3SO2N
SO2 or its dimer ((CF3SO2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SO2)2, Reaction 2; Scheme 1); and (3) the nucleophilic substitution reaction between the sulfonyl chloride group in H[ClTFSI] and tertiary amines, yielding a new family of ionic compounds which are generally known as zwitterions (Reaction 3; Scheme 1). In addition, H[ClTFSI] has a strong tendency to react with trace amounts of water as residual impurities in the aprotic media, yielding CF3SO2NH2 and HSO4− (Reaction 4; Scheme 1). These interesting features of H[ClTFSI] allow facile preparation of various kinds of electrolyte components, as detailed below.
SO2)2, Reaction 2; Scheme 1); and (3) the nucleophilic substitution reaction between the sulfonyl chloride group in H[ClTFSI] and tertiary amines, yielding a new family of ionic compounds which are generally known as zwitterions (Reaction 3; Scheme 1). In addition, H[ClTFSI] has a strong tendency to react with trace amounts of water as residual impurities in the aprotic media, yielding CF3SO2NH2 and HSO4− (Reaction 4; Scheme 1). These interesting features of H[ClTFSI] allow facile preparation of various kinds of electrolyte components, as detailed below.
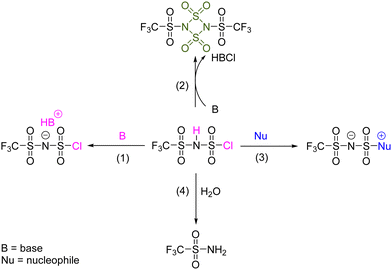 | ||
| Scheme 1 Reactive pathways between H[ClTFSI] and tertiary amines. The numbers on the arrow represent the sequence of the reactions in the main text. | ||
As well documented in the textbooks of organic chemistry, the Hinsberg reagent, benzene sulfonyl chloride, could be adopted to screen out tertiary amines from primary and secondary amines since the products formed with tertiary amines are generally unstable and sensitive toward water.26,27 Differing from previous understanding, the reactions between H[ClTFSI] and several tertiary amines give chemically stable compounds which could be easily isolated and characterized (Fig. S3–S14, ESI†). Fig. 2a and b show the single crystal structures of two zwitterion compounds obtained from the reactions with TEA and Py, respectively. The structural details of the crystals are provided in the ESI† (Tables S3–S8).
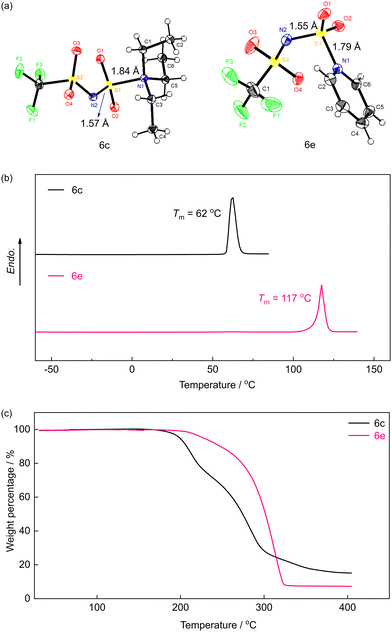 | ||
| Fig. 2 (a) Oak ridge thermal ellipsoid plot diagram of single crystal structures of 6c and 6e and their thermogravimetric analysis (b) and differential scanning calorimetry (c) traces. | ||
Very interestingly, these two zwitterion compounds (6c and 6e) remain chemically stable after stirring in the mixture of MeCN/H2O (H[ClTFSI]/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by mol) for 24 h at room temperature (Fig. S2, ESI†). As shown in Fig. 2a and b, the distances (i.e., S–N) between tertiary amines and sulfonyl groups are 1.87 and 1.79 Å for compounds 6c and 6e, respectively, which are slightly shorter than that of the classic Burgess reagent (i.e., CH3OC(
1, by mol) for 24 h at room temperature (Fig. S2, ESI†). As shown in Fig. 2a and b, the distances (i.e., S–N) between tertiary amines and sulfonyl groups are 1.87 and 1.79 Å for compounds 6c and 6e, respectively, which are slightly shorter than that of the classic Burgess reagent (i.e., CH3OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)N(−)SO2–N(+)Et3, 1.90 Å).28 This could be attributed to the presence of a conjugated sulfonimide structure, which allows better delocalization of negative charges in the as-obtained zwitterion compounds (Scheme S2, ESI†).29 Besides, the S–N bond between the negatively charged N atom and sulfonyl (1.57 Å for 6c, 1.55 Å for 6e) is shorter than the positively charged N atom (1.87 Å for 6c, 1.79 Å or 6e), which is also caused by the stronger conjugated delocalization effect of fluorinated sulfonimide.30
O)N(−)SO2–N(+)Et3, 1.90 Å).28 This could be attributed to the presence of a conjugated sulfonimide structure, which allows better delocalization of negative charges in the as-obtained zwitterion compounds (Scheme S2, ESI†).29 Besides, the S–N bond between the negatively charged N atom and sulfonyl (1.57 Å for 6c, 1.55 Å for 6e) is shorter than the positively charged N atom (1.87 Å for 6c, 1.79 Å or 6e), which is also caused by the stronger conjugated delocalization effect of fluorinated sulfonimide.30
Fig. 2c shows the thermogravimetric analysis (TGA) traces of two sulfonimide-type zwitterion compounds. The decomposition temperatures (Td) of the two zwitterion compounds are 198 and 227 °C, respectively, while the Burgess reagent generally decomposes at a much lower temperature of ca. 70 °C.31 This clearly suggests the superior chemical stability of the newly prepared zwitterion compounds, owing to better delocalization of charges via the sulfonimide center. It is important to note that, for battery applications, the electrolyte components should remain chemically stable up to a working temperature of ca. 100 °C; hence, the enhanced thermal stability of sulfonimide-type zwitterion compounds is highly desired for practical applications in these emerging domains.
Furthermore, Fig. 2d displays the differential scanning calorimetry (DSC) traces of the two zwitterion compounds (6c and 6e). Clearly, both compounds show relatively low melting points below 120 °C. In particular, the zwitterion with a triethylamine moiety (i.e., 6c) completely melts at a relatively low temperature of ca. 60 °C, behaving as typical IL materials (<100 °C melting point).6 These unique properties enable their potential applications as solvents for building highly conductive and thermally stable electrolyte materials. For example, utilizing these zwitterionic compounds as electrolyte additives to enhance the dissociation of metal salts in non-aqueous electrolytes.32–36
In conclusion, the chemical properties of H[ClTFSI] have been carefully examined, in view of its potential use as a building block for battery electrolytes. The sophisticated chemical reactivities of H[ClTFSI] originate from diversified reaction pathways of the sulfamoyl chloride group (–NHSO2Cl) in the presence of tertiary amines. Generally, for the reaction between H[ClTFSI] and tertiary amines, three kinds of reaction pathways are likely to occur, including neutralization, nucleophilic substitution, and intra-/inter-molecular elimination reactions. The above reactive pathways are mainly influenced by the chemical structure of tertiary amines and reacting temperatures. Hindered tertiary amines (e.g., DIPEA, DMPy) favour the formation of neutralization products and could be utilized as deprotonation reagents for preparing other interesting electrolyte materials. Most importantly, the reaction of H[ClTFSI] with non-hindered tertiary amines (e.g., TEA, Py) readily affords a new family of zwitterion compounds with good thermal and chemical stability (>190 °C). These intriguing results are anticipated to provide a solid reference for optimizing the synthetic methods of sulfonimide-based compounds and also inspire the design of robust electrolyte materials for battery applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Fundamental Research Funds for the Central Universities, HUST (2020kfyXJJS095), and the National Natural Science Foundation of China (No. 52203223 and 22279037).References
- B. A. Shainyan and L. L. Tolstikova, Chem. Rev., 2013, 113, 699–733 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- H. Zhang, G. G. Eshetu, X. Judez, C. Li, L. M. Rodriguez-Martinez and M. Armand, Angew. Chem., Int. Ed., 2018, 57, 15002–15027 CrossRef CAS PubMed.
- B. Tong, J. Wang, Z. Liu, L. Ma, Z. Zhou and Z. Peng, J. Power Sources, 2018, 384, 80–85 CrossRef CAS.
- Y. Cheng, X. Ou, J. Ma, L. Sun and Z. Ma, Eur. J. Org. Chem., 2019, 2019, 66–72 CrossRef CAS.
- C. Liu, X. Ma, F. Xu, L. Zheng, H. Zhang, W. Feng, X. Huang, M. Armand, J. Nie, H. Chen and Z. Zhou, Electrochim. Acta, 2014, 149, 370–385 CrossRef CAS.
- H. Han, Y. Zhou, K. Liu, J. Nie, X. Huang, M. Armand and Z. Zhou, Chem. Lett., 2010, 39, 472–474 CrossRef CAS.
- M. Beran, J. Příhoda and J. Taraba, Polyhedron, 2010, 29, 991–994 CrossRef CAS.
- H. Zhang, F. Chen, O. Lakuntza, U. Oteo, L. Qiao, M. Martinez-Ibanez, H. Zhu, J. Carrasco, M. Forsyth and M. Armand, Angew. Chem., Int. Ed., 2019, 58, 12070–12075 CrossRef CAS PubMed.
- M. Martinez-Ibañez, E. Sanchez-Diez, U. Oteo, I. Gracia, I. Aldalur, H. B. Eitouni, M. Joost, M. Armand and H. Zhang, Chem. Mater., 2022, 34, 3451–3460 CrossRef.
- H. Zhang, W. Feng, J. Nie and Z. Zhou, J. Fluorine Chem., 2015, 174, 49–61 CrossRef.
- W. Gorecki, C. Roux, M. Clémancey, M. Armand and E. Belorizky, Chem. Phys. Chem., 2002, 3, 620–625 CrossRef CAS.
- L. Qiao, U. Oteo, M. Martinez-Ibañez, A. Santiago, R. Cid, E. Sanchez-Diez, E. Lobato, L. Meabe, M. Armand and H. Zhang, Nat. Mater., 2022, 21, 455–462 CrossRef CAS.
- G. G. Eshetu, X. Judez, C. Li, M. Martinez-Ibanez, I. Gracia, O. Bondarchuk, J. Carrasco, L. M. Rodriguez-Martinez, H. Zhang and M. Armand, J. Am. Chem. Soc., 2018, 140, 9921–9933 CrossRef CAS PubMed.
- K. Beltrop, P. Meister, S. Klein, A. Heckmann, M. Grünebaum, H.-D. Wiemhöfer, M. Winter and T. Placke, Electrochim. Acta, 2016, 209, 44–55 CrossRef CAS.
- A. Kütt, T. Rodima, J. Saame, E. Raamat, V. Maemets, I. Kaljurand, I. A. Koppel, R. Y. Garlyauskayte, Y. L. Yagupolskii, L. M. Yagupolskii, E. Bernhardt, H. Willner and I. Leito, J. Org. Chem., 2011, 76, 391–395 CrossRef PubMed.
- H. Zhang, U. Oteo, H. Zhu, X. Judez, M. Martinez-Ibanez, I. Aldalur, E. Sanchez-Diez, C. Li, J. Carrasco, M. Forsyth and M. Armand, Angew. Chem., Int. Ed., 2019, 58, 7829–7834 CrossRef CAS PubMed.
- H. K. Hall, J. Am. Chem. Soc., 1957, 79, 5439–5441 CrossRef CAS.
- A. D. Gift, S. M. Stewart and P. Kwete Bokashanga, J. Chem. Educ., 2012, 89, 1458–1460 CrossRef CAS.
- J. Ammer, M. Baidya, S. Kobayashi and H. Mayr, J. Phys. Org. Chem., 2010, 23, 1029–1035 CrossRef CAS.
- F. Brotzel, B. Kempf, T. Singer, H. Zipse and H. Mayr, Chem. – Eur. J., 2007, 13, 336–345 CrossRef CAS.
- S. Feng, D. Shi, F. Liu, L. Zheng, J. Nie, W. Feng, X. Huang, M. Armand and Z. Zhou, Electrochim. Acta, 2013, 93, 254–263 CrossRef CAS.
- H. Roesky, M. Aramaki and L. Schönfelder, Z. Naturforsch., 1978, 33, 1072–1076 CrossRef.
- J. Meyer, V. Tajti, E. Carrascosa, T. Győri, M. Stei, T. Michaelsen, B. Bastian, G. Czakó and R. Wester, Nat. Chem., 2021, 13, 977–981 CrossRef CAS PubMed.
- D. C. Lohman, R. Wolfenden and D. R. Edwards, J. Org. Chem., 2012, 77, 2907–2910 CrossRef CAS PubMed.
- O. Hinsberg, Ber. Dtsch. Chem. Ges., 1890, 23, 2962–2965 CrossRef.
- G. Solomons and C. Fryhle, Organic Chemistry, John Wiley & Sons Inc., New York, 7th edn, 2002, pp. 975–976 Search PubMed.
- A. J. Arduengo, Y. Uchiyama, D. A. Dixon and M. Vasiliu, Aust. J. Chem., 2019, 72, 867–873 CrossRef CAS.
- U. Jäger, W. Sundermeyer and H. Pritzkow, Chem. Ber., 1987, 120, 1191–1195 CrossRef.
- L. Xue, C. W. Padgett, D. D. DesMarteau and W. T. Pennington, Solid State Sci., 2002, 4, 1535–1545 CrossRef CAS.
- T. A. Metcalf, R. Simionescu and T. Hudlicky, J. Org. Chem., 2010, 75, 3447–3450 CrossRef CAS.
- H. Ohno, M. Yoshizawa-Fujita and Y. Kohno, Phys. Chem. Chem. Phys., 2018, 20, 10978–10991 RSC.
- A. Islam, J. Li, M. Pervaiz, Z. Lu, M. Sain, L. Chen and X. Ouyang, Adv. Energy Mater., 2019, 9, 1803354 CrossRef.
- F. Makhlooghiazad, L. A. O’Dell, L. Porcarelli, C. Forsyth, N. Quazi, M. Asadi, O. Hutt, D. Mecerreyes, M. Forsyth and J. M. Pringle, Nat. Mater., 2022, 21, 228–236 CrossRef CAS PubMed.
- B. Tong, Z. Song, H. Wu, X. Wang, W. Feng, Z. Zhou and H. Zhang, Mater. Futures, 2022, 1, 042103 CrossRef.
- H. Zhang, L. Qiao, H. Kühnle, E. Figgemeier, M. Armand and G. G. Eshetu, Energy Environ. Sci., 2023, 16, 11–52 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2218540 and 2218543. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ya00110e |
| This journal is © The Royal Society of Chemistry 2023 |