Assessing the performance of various sorbents in micro-solid phase extraction cartridges for pesticide residue analysis in feed†
Ederina
Ninga
 *,
Elena
Hakme
and
Mette Erecius
Poulsen
*,
Elena
Hakme
and
Mette Erecius
Poulsen
National Food Institute, Technical University of Denmark, DTU-Food, Lyngby, Denmark. E-mail: edni@food.dut.dk
First published on 3rd June 2024
Abstract
Newly designed micro-solid phase extraction cartridges are now available, reflecting the increasing shift towards laboratory automation, especially in the clean-up step for the analysis of pesticide residues in food and feed. In the present study, the introduction of different sorbents on the newly designed PAL µSPE CTC cartridges was investigated for the removal of matrix interferents and the recovery of pesticides. Eight cartridges containing different sorbent combinations and different amounts were used including EMR-lipid (not activated), Z-sep, chitin, C18, PSA, and GCB. The evaluation of co-extractive removal for each cartridge showed that the optimal choice for removing fatty acids was the cartridges containing PSA and Z-sep as clean-up sorbents. However, the presence of C18 and EMR-lipid was still required for the removal of sterols and tocopherols. Two grams of sample, fish feed (FF) and rapeseed cake (RSC) were extracted using QuEChERS citrate buffer, followed by a freeze-out step. The recoveries and repeatability of QuEChERS using µ-SPE clean-up were evaluated for 216 pesticide residues (112 compounds analyzed by GC-MS/MS and 143 compounds by LC-MS/MS, from which 39 compounds were analyzed using both techniques). The best results, with recovery between 70 and 120% and RSD <20%, were achieved when FF samples were cleaned-up with 15 mg EMR-lipid and 20 mg MgSO4. This was achieved for 94% of GC-amenable compounds and 86% of LC-amenable compounds. In the case of RSC, the best results were seen when samples were cleaned-up with the cartridge containing only 20 mg Z-sep and 20 mg MgSO4. This was achieved for 88% of GC-amenable compounds and 90% of LC-amenable compounds. Although these cartridges yielded optimal results in terms of recovery, their use could require more instrument maintenance, especially for GC-MS/MS, due to the lower removal of co-extractives.
1. Introduction
Ensuring food and feed safety is a universal priority. The demand for international trade increases the necessity for high throughput multiresidue analysis of pesticides in monitoring laboratories all over the world. Many monitoring laboratories do not only face a shortage of resources for instruments and consumables but also often experience limited human resources. Besides, feed and feed ingredients cover a wide range of commodities with various origins and chemical compositions. Due to the considerably more diverse chemical composition of feed compared to food, analyzing pesticide residues and controlling Maximum Residue Levels (MRLs) to comply with EU Regulation 396/2005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Directive 2002/32
1 and Directive 2002/32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 present significant challenges.
2 present significant challenges.
Anastassiades et al. in 2003![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 introduced the “quick, easy, cheap, effective, rugged and safe” (QuEChERS) method for sample preparation and determination of pesticide residues. Different modified versions of QuEChERS are used worldwide for residue analysis including not only pesticides but also environmental contaminants,4–7 veterinary drugs,8–11 and natural toxin.12 The QuEChERS method is based on two main steps, sample extraction with acetonitrile and phase separation, followed by dispersive solid phase extraction (d-SPE) clean-up using PSA and/or C18 as a sorbent for pesticide residue analysis in fruits and vegetables. Although it produces reliable results for fruits and vegetables, when it is used in other more complex and difficult matrices, other sorbents (alone or in combination) give better results in the removal of co-extractive compounds. Various sorbents, including Z-sep and EMR-lipid, were used in different applications for the analysis of pesticides and veterinary drugs in complex food matrices.13–17
3 introduced the “quick, easy, cheap, effective, rugged and safe” (QuEChERS) method for sample preparation and determination of pesticide residues. Different modified versions of QuEChERS are used worldwide for residue analysis including not only pesticides but also environmental contaminants,4–7 veterinary drugs,8–11 and natural toxin.12 The QuEChERS method is based on two main steps, sample extraction with acetonitrile and phase separation, followed by dispersive solid phase extraction (d-SPE) clean-up using PSA and/or C18 as a sorbent for pesticide residue analysis in fruits and vegetables. Although it produces reliable results for fruits and vegetables, when it is used in other more complex and difficult matrices, other sorbents (alone or in combination) give better results in the removal of co-extractive compounds. Various sorbents, including Z-sep and EMR-lipid, were used in different applications for the analysis of pesticides and veterinary drugs in complex food matrices.13–17
Despite the advantages that the QuEChERS method offers, there are still some disadvantages such as insufficient clean-up and difficulty automating. To reduce labor and improve precision, laboratory automation has been implemented through the utilization of robotic sample preparation tools in sample clean-up.18 The micro-solid-phase-extraction (µSPE) clean-up method is a clean-up process, where the sample extract is delivered via a syringe at the desired volume and flow rate. The unwanted matrix components are retained on the cartridge, while the compounds of interest are eluted from the column.19 The method was introduced by Morris and Schriner in 2015.20 Later, Lehotay et al.21 applied this technique for the determination of residues in different food matrices. In the following years, the use of µSPE clean-up was demonstrated to be effective for the analysis of pesticide residues in different commodities such as cereals, fish, lamb, hemp, tea, and spice.6,18,22–29
A current drawback of µSPE cartridges is a lack of different sorbent packings.
There are two types of µSPE cartridges mainly used in pesticide analyses, known as ITSP (Instrument Top Sample Preparation) µSPE cartridges. A total sorbent amount of 35 mg containing 8 mg of Z-sep, 23 mg of C18, and 1 mg of CarbonX is suggested for LC analysis. Another cartridge, comprising a combination of PSA (12 mg)/C18 (12 mg)/CarbonX (1 mg) and anhydrous MgSO4 (20 mg) sorbents, is intended for GC analysis19 The main limitation of the ITSP µSPE cartridges is related to the amount of sorbent that can be packed (up to 45 mg) and their low flow rate (2 µL s−1). These limitations were overcome by a new product, PAL µSPE cartridges, introduced by CTC Analytics (Zwingen, Basel-Landschaft; Switzerland) in 2022, which can accommodate up to 150 mg of sorbent and up to a 10 µL s−1 flow rate. The PAL µSPE cartridges are septumless and composed of two pieces of polypropylene pressed very tightly together, which significantly lowers the risk of leakage that has been observed in the ITPS µSPE cartridges at flow rates greater than 2 µL s−1. The new µSPE cartridge design allows the application of a higher flow rate.30
The aim of the present study was to assess the effect of the introduction of different sorbents on the new PAL µSPE CTC cartridges in terms of sample clean-up efficiency and acceptable levels of pesticide recoveries and reproducibility at 0.01 mg kg−1. Various sorbent (EMR-lipid, Z-sep, PSA, C18, chitin and graphitized carbon black) combinations and amounts were selected, matching the typical combinations in conventional d-SPE methods used in feed analysis. Recoveries and repeatability were evaluated for the customized cartridges for 216 pesticide residues in two fatty feed matrices, fish feed (FF), and rapeseed cake (RSC). The results were further evaluated for matrix removal. FF contains 38% proteins, 34% crude fats, and additives such as astaxanthin.31 The RSC is rich in crude protein (∼30%), crude fiber (∼11%), and crude fat (approximately 17%).32
2. Materials and methods
2.1. Chemicals and reagents
Pure standards (purity > 96%) of pesticides were purchased from Sigma-Aldrich and LGC Standards. Stock solutions were prepared at a concentration of 1 mg mL−1 for each compound and stored at −20 °C. The solvent used for the solution preparation was either toluene or methanol depending on analyte solubility and stability. The stock solutions were combined in a mixture at a concentration of 10 mg L−1. A full list of the compounds investigated in this study and their chemical properties is given in Table S1.†Acetonitrile and methanol of HPLC-grade were purchased from Merck. Deionized water of 18.2 MΩ cm was obtained using an E-Pure system from Barnstead/Thermolyne Premade. A mixture of salts containing 6.5 grams of MgSO4, NaCl, C6H9Na3O9, and C6H9NaO8 (at a ratio of 4/1/1/0.5) in 15 mL polypropylene (PP) tubes was purchased from Merck (Sigma Aldrich, Germany).
Eight different customized µSPE cartridges were obtained from CTC Analytics (Zwingen, Basel-Landschaft; Switzerland). The cartridge sorbent and amounts used in this study are given in Table 1. The sorbents were: EMR-Lipid (EMR), Z-sep, Chitin, C18, PSA, and GCB. The EMR was used in two different amounts in the cartridges, referred to as EMR-low for the sorbent combination containing 15 mg EMR and 20 mg MgSO4, and EMR-high for 30 mg EMR and the same amount of MgSO4. The same approach was taken with chitin; two cartridges were utilized, namely chitin-low for the sorbent combination containing 15 mg chitin and 20 mg MgSO4, and chitin-high for 30 mg chitin with the same amount of MgSO4. The cartridge containing 20 mg Z-sep and 20 mg MgSO4 is referred to as Z-sep. The cartridges containing a mixture similar to ISTP µSPE (12 mg PSA/12 mg C18/1 mg GCB/20 mg MgSO4 and the one containing 8 mg of Z-sep, 23 mg of C18, and 1 mg of GCB) are referred to as µSPE-GC and µSPE-LC cartridges. The cartridge containing a combination of 15 mg C18 and 20 mg MgSO4 is referred to as C18 in the text. It is important to note that these names are used solely for the purposes of this study.
| Name | Sorbent composition | Sorbent amount in mg | Total amount in mg |
|---|---|---|---|
| EMR-low | EMR lipid/MgSO4 | 15/20 | 35 |
| EMR-high | EMR lipid/MgSO4 | 30/20 | 50 |
| Z-sep | Z-sep/MgSO4 | 20/20 | 40 |
| Chitin-low | Chitin/MgSO4 | 15/20 | 35 |
| Chitin-high | Chitin/MgSO4 | 30/20 | 50 |
| C18 | C18/MgSO4 | 15/20 | 35 |
| µSPE-GC | C18/PSA/GCB/MgSO4 | 12/12/1/20 | 45 |
| µSPE-LC | C18/Z-sep/GCB | 21/8/1 | 30 |
2.2. Sample preparation and extraction
The RSC blank sample was provided by the European reference laboratory for cereals and feeding stuff (EURL-CF), where it was grown in connection with the 15th European Commission's Proficiency Test on Cereals and Feed, EUPT-CF15.33,34 The FF samples were salmon feed (EFICO) produced by BioMar, Denmark.The samples were homogenized using an Ultra Centrifugal Mill ZM 200. Two grams of homogenized blank samples were spiked with 100 µL of 0.2 mg L−1 pesticide mix solution to yield a concentration of 0.01 mg kg−1. Additionally, 100 µL of 0.2 mg L−1 procedural standard consisting of azoxystrobin-d4, dichlorvos-d6 and etofenprox-d5 were added. Blank samples were prepared for quality control measures.
The samples were extracted using the QuEChERS citrate-buffered method. Initially 10 mL of water was added to the sample and mixed. Then, 10 mL of acetonitrile was added for the extraction. The samples were shaken for 1 minute at 750 rpm using a Geno Grinder 2010. For phase separation, a mixture of 6.5 gram of salts, containing MgSO4, NaCl, C6H9Na3O9, and C6H9NaO8 (at a ratio of 4/1/1/0.5), was added to the extracts and shaken for another minute, followed by 10 min centrifugation at 4500 with a Thermo Multifuge X3FR. Eight milliliters of supernatant were transferred to a 15 mL polypropylene tube and stored in a freezer at −80 °C for at least 1 hour. After freezing-out, the extract was thawed and centrifuged for another 10 min at 4500 rpm at 5 °C.
2.3. Automatic sample cleanup
A customized Thermo Scientific™ TriPlus™ RSH™ (based on a PAl3_RTC autosampler from CTC Analytics) controlled with Chromeleon software was used for clean-up. The system contains two independent tray holders dedicated to µSPE in parallel, one for ITSP µSPE and the other for PAL µSPE. A third tray intended to be used for sample dilution and calibration curve preparation is also included in the configuration. The system is shown in Fig. S1.†The µSPE clean-up workflow, previously described by Hakme & Poulsen,29 was extended with additional sample preparation, namely, sample dilution, and automatic addition of internal standards.
The clean-up procedure was performed with a 250 µL aliquot of extract. The extract was eluted from the cartridges at 2 µL s−1. The extract volume and flow rate used were recommended at the time of the experiment from the PAL µSPE producer. To align with the matrix amount in the calibration standard, the cleaned extract was diluted with acetonitrile (100 µL extract + 100 µL acetonitrile) and automatically transferred into a clean sample vial. After the dilution, a 20 µL quality standard mix was added and the extract was thoroughly mixed. In Table S2† are given the detailed steps of the updated workflow.
2.4. Assessment of co-extractive removal
Blank sample extracts (for both matrices) obtained with QuEChERS without clean-up, along with the cleaned extracts obtained using various µ-SPE cartridges as previously described, were injected into a GC-MS. A comparison between the total ion chromatograms (TICs) of the sample extract before and after µSPE clean-up was made using the following formula: [(arearaw extract − areacleaned extract)/arearaw extract] × 100.2.5. Assessment of pesticide recovery
For the evaluation of sorbent effects on the analyte loss, uncleaned spiked extracts were combined after the centrifugation step and were mixed thoroughly in order to make a uniformed extract. Finally, at least 1 mL was transferred to a 2 mL glass vial and placed on the sample tray on a µSPE sample tray holder for clean-up. Five portions for each type of µ-SPE cartridge were used. For quantification, matrix-matched calibration standards were prepared by using a blank sample extract cleaned through the same sorbent. The extracts were analyzed by GC-MS/MS and LC-MS/MS. Recovery in percentage and repeatability expressed as relative standard deviation (RSD) were calculated.2.6. Analytical instrument
For gas chromatographic separation, a Thermo Scientific™ Trace™ 1310 Gas Chromatograph coupled to a Thermo Scientific™ TriPlus™ RSH autosampler was used. The injection volume was 1 µL and a programmable temperature vaporizer (PTV) large volume mode was used with a PTV baffle liner 2 × 2.75 × 120 mm from Thermo Scientific™. The injection temperature was 70 °C and the split flow rate was set to 15 mL min−1 for 1 min at 70 kPa during the injection phase. Afterward, the split vent was closed, and the inlet was heated up to 210 °C at 5 °C s−1 and held for 2 min. To remove any high boiling residue inside the inlet, the inlet temperature was finally ramped to 330 °C and a split vent flow rate of 75 mL min−1 was set for 10 min. Ultrahigh purity helium was used as the carrier gas at a flow rate of 1.2 mL min−1. A capillary column TG-5SILMS W/5m Safeguard, 30 m length, 0.25 mm internal diameter and 0.25 µm film thickness, was used. The program oven temperature started at 60 °C for 1.5 min, and then ramped up to 25 °C min−1 at 90 °C for 1.5 min, up to 180 °C at 25 °C min−1, and then up to 280 °C at 5 °C min−1 and finally up to 300 °C at 10 °C min−1 for 12 min. For the mass spectrometric analysis, a Thermo Scientific™ TSQTM 8000 Evo was used. The MS has been upgraded with an advanced electron ionization source (AEI) operated with an electron energy of 50 eV. The transfer line was set at 280 °C and the ion source temperature was set at 300 °C. The analyses were performed in multiple reaction monitoring mode (MRM).For liquid chromatographic separation, an LC system Thermo Ultimate 3000 and a mass spectrometer Bruker EVOQ were used. The analytes were separated on a Waters Accuity UPLC BEH C18 1.7 µm × 2.1 × 100 mm reversed-phase column. The injection volume was 1 µL. The eluents consisted of Milli-Q water with 0.1% formic acid and 5 mM ammonia solution (A eluent) and methanol (B eluent). A flow rate of 0.4 mL min−1 was applied. The analytes were separated using a gradient elution program. Before every injection the column was equilibrated with 2% B eluent. After the injection, eluent B increases up to 35% within 0.1 min and then up to 98% in seven min. For three more minutes, the eluent remains still and 98% of B eluent is then maintained for 3 min. In the last step, the eluent goes back to 2% for only 0.1 min. The mass spectrometer was operated in MRM mode and using both positive and negative electrospray ionization (ESI).
The MS/MS conditions for the GC and LC analytes are given in Tables S3 and S4.†
3. Results and discussion
3.1. Co-extractive removal
A visual comparison of the transparency and color of the extracts obtained with QuEChERS without clean-up and the cleaned extracts obtained with the different µ-SPE cartridges are shown in Fig. 1. In both cases, the color intensity (the green in RSC and red in FF) decreased significantly when GCB was used (cartridges µSPE-GC and µSPE-LC). In the RSC, the extract became colorless when cleaned with the cartridges containing 30 mg of EMR. The removal of color for both sorbents has been previously shown in different studies using dispersive clean-up.16,35,36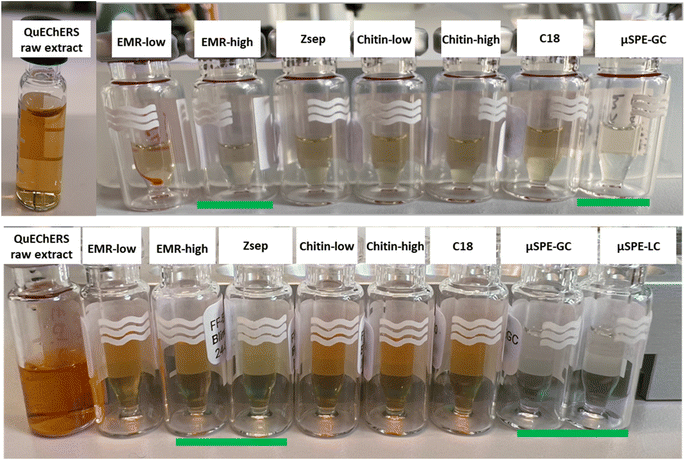 | ||
| Fig. 1 Vials containing cleaned extracts of RSC (above) and FF (below), including the raw extracts and the cleaned ones with different types of cartridges. | ||
To further explore the clean-up effect of different sorbent compositions, an evaluation of the co-extractive efficiency removal was made in terms of the chromatographic background by comparing the TICs of the sample extract before and after µSPE clean-up using the formula in paragraph 2.4. The TICs obtained from GC-MS scan are shown in Fig. S2–S10.†Fig. 2 shows the percentage of co-extractive removed from FF and RSC using each of the cartridges.
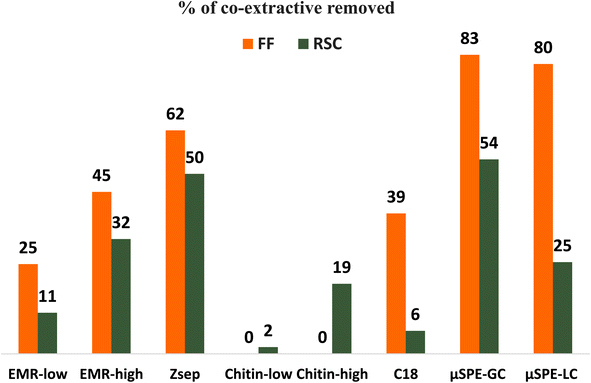 | ||
| Fig. 2 Percentage of co-extractive compounds removed from FF and RSC by comparing the TICs before and after the clean-up for each matrix and cartridge. | ||
For the RSC, the lowest removal efficiency was observed with the Chitin-low cartridge, where there was approximately a 2% difference between the TIC areas of the cleaned and uncleaned extracts, followed by ∼6% for the C18 cartridge, ∼11% for EMR-low and ∼20% for chitin-high. Improved clean-up was observed with µSPE-LC, EMR-high, Z-sep, and µSPE-GC cartridges, where the co-extractive removal was assessed to be ∼25, 32, 50, and 54% respectively.
For the FF, the clean-up removal efficiency was approximately ∼25% for the EMR-low cartridges, followed by ∼39% for C18, ∼45% for EMR-high, ∼62% for Z-sep, ∼80% for µSPE-LC and ∼83% for µSPE-GC. For both cartridges containing chitin, there was no significant difference between the TICs, before and after the clean-up, suggesting that chitin did not have any effect on matrix removal.
In RSC extracts, the main detected compounds were phytosterols stigmasterol (Rt. 30.3 min) and campesterol (Rt. 31.3 min). Again, EMR-high and C18 cartridges seem to play a major role in the removal of these compounds. The sample clean-up through these two cartridges completely removed stigmasterol and lowered the area of campesterol by approximately 94% and 97%, respectively. The removal efficiency of sterol is in line with other studies done on SPE or d-SPE clean-up.5,38,39
The total ion chromatogram of a FF raw extract and cleaned extract in different cartridges (C18, EMR-high, µSPE-LC, Z-sep and µSPE-GC) showing the effect of different cartridges on fatty acids and sterols region is given in Fig. 3.
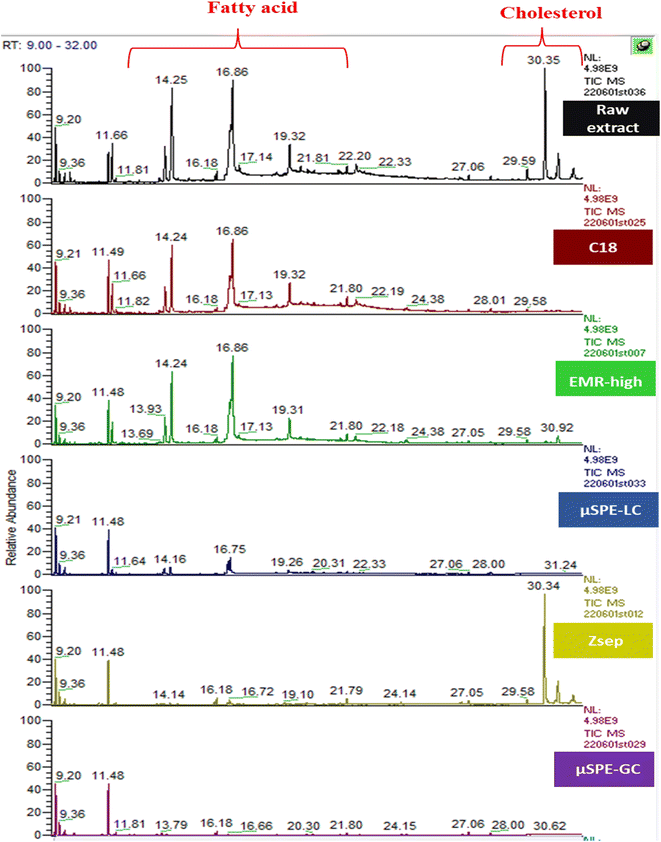 | ||
| Fig. 3 Total ion chromatogram of the FF extract before clean-up and after µSPE clean-up with C18, EMR-high, µSPE-LC, Z-sep and µSPE-GC. | ||
As a conclusion, in terms of the clean-up efficiency, the cartridges containing EMR (EMR-high) and C18 did not play any significant role in fatty acid removal, but they removed up to 100% of sterols in both matrices. Although these compounds are eluting at the end of the chromatogram, their removal is important to extend the life of the GC column.
The best removal efficiency for the fatty acid was achieved with the cartridges containing PSA (µSPE-GC) and Z-sep (Z-sep and µSPE-LC), but Z-sep did not have a similar effect on the sterol's region. The presence of GCB had a positive visual effect on the FF, most probably due to the removal of carotenoid. EMR and C18 have almost the same clean-up efficiency for sterols and phytosterols, but EMR seems to have a positive effect on color removal especially due to the removal of chlorophyll in the RSC extract compared to C18. Increasing the amount of the sorbent Z-sep improved the removal efficiency of fatty acids. A comparison between the two cartridges containing the same total amount of sorbents, 35 mg each, EMR-low and C18, showed differences in the total area removal of co-extractives.
Considering the overall removal efficiency, the best cartridge was the one containing PSA (µSPE-GC) and Z-sep. It is also important to note that EMR requires the addition of water prior to clean-up in order for it to work well.16,35 During our study, this step was not performed due to the way in which the sorbent was combined in the cartridges containing EMR. Both cartridges, EMR-low and EMR-high, contained 20 mg MgSO4 in their sorbent combination mix.
3.2. Recovery study
To further investigate the effect of different sorbents used in the PAL µSPE cartridges, recovery experiments were performed. The five sorbent combinations that demonstrate the highest efficiency in removing matrix components, EMR-low, EMR-high, Z-sep, µSPE-GC and µSPE-LC, were selected for the recovery study.Recoveries were calculated for 216 pesticides in the two matrices. Of those, 112 compounds were analysed by GC-MS/MS and 143 compounds by LC-MS/MS. Thirty-nine compounds were analyzed by both GC-MS/MS and LC-MS/MS. The 255 average recoveries (%) and RSDs of spiked samples at 0.01 mg kg−1 (n = 5) of FF and RSC are given in Table 2.
| Nr | Analytes | Tool | EMR-low | EMR-high | Z-sep | µSPE-GC | µSPE-LC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FF | RSC | FF | RSC | FF | RSC | FF | RSC | FF | RSC | |||
| 1 | 2-Phenylphenol | GC | 96 (8) | 105 (8) | 128 (6) | 118 (8) | 130 (15) | 116 (5) | 104 (6) | 115 (13) | 127 (10) | 112 (11) |
| 2 | 3-Hydroxycarbofuran | LC | 83 (14) | 135 (7) | 94 (6) | 117 (4) | 94 (9) | 113 (12) | 87 (9) | 119 (9) | 93 (6) | — |
| 3 | Acephate | LC | 85 (8) | 104 (8) | 82 (5) | 96 (10) | 74 (10) | 89 (15) | 69 (16) | 93 (13) | 84 (8) | 94 (12) |
| 4 | Acetamiprid | LC | 89 (5) | 47 (24) | 92 (4) | 47 (26) | 99 (8) | 105 (5) | 91 (5) | 107 (1) | 87 (4) | 93 (6) |
| 5 | Acrinathrin | GC | 97 (4) | 78 (28) | 113 (3) | 96 (54) | 112 (9) | 100 (26) | 100 (16) | — | 101 (8) | — |
| 6 | Aldicarb | LC | 82 (11) | 104 (34) | 91 (24) | 86 (20) | 92 (20) | 68 (28) | 99 (13) | 145 (13) | 91 (10) | 99 (14) |
| 7 | Aldicarb-sulfone | LC | 94 (17) | — | 105 (12) | — | 99 (11) | - | 95 (9) | — | 87 (8) | — |
| 8 | Aldicarb-sulfoxide | LC | 68 (7) | 89 (15) | 75 (8) | 82 (4) | 64 (11) | 86 (18) | 78 (11) | 93 (14) | — | 82 (26) |
| 9 | Aldrin | GC | 65 (14) | 62 (9) | 66 (8) | 51 (17) | 69 (16) | 60 (9) | 61 (12) | 61 (71) | 51 (9) | 128 (86) |
| 10 | Atrazine | LC | 101 (8) | 105 (5) | 123 (3) | 96 (3) | 90 (2) | 110 (3) | 94 (2) | 109 (5) | 82 (6) | — |
| 11 | Azinphos-ethyl | LC | 79 (13) | 118 (13) | 98 (6) | 107 (14) | 108 (8) | 112 (10) | 101 (20) | 101 (17) | 83 (7) | 91 (10) |
| 12 | Azinphos-methyl | LC | 85 (6) | 105 (8) | 98 (5) | 112 (6) | 98 (10) | 105 (4) | 90 (8) | 102 (9) | 76 (7) | 94 (4) |
| 13 | Azoxystrobin | GC | 95 (3) | 114 (4) | 111 (5) | 102 (8) | 128 (10) | 107 (3) | 104 (6) | 111 (18) | 111 (8) | 88 (17) |
| Azoxystrobin | LC | 89 (4) | 112 (4) | 104 (4) | 103 (3) | 97 (9) | 111 (7) | 94 (5) | 108 (2) | 88 (4) | 104 (5) | |
| 14 | Bifenthrin | GC | 91 (11) | 60 (17) | 88 (5) | 47 (9) | 99 (9) | 71 (18) | 115 (8) | 78 (8) | 104 (10) | 51 (14) |
| Bifenthrin | LC | 75 (4) | 59 (9) | 82 (12) | 64 (12) | 84 (20) | 64 (7) | 72 (17) | 43 (14) | 61 (15) | 39 (6) | |
| 15 | Bitertanol | GC | 91 (5) | 92 (12) | 108 (2) | 42 (22) | 109 (8) | 121 (15) | 88 (3) | — | — | — |
| Bitertanol | LC | 84 (8) | 115 (7) | 98 (12) | 95 (17) | 92 (10) | 103 (6) | 94 (3) | 102 (4) | 82 (13) | 94 (7) | |
| 16 | Boscalid | GC | 93 (3) | 105 (4) | 108 (2) | 101 (5) | 117 (9) | 113 (6) | 94 (6) | 144 (34) | 99 (10) | — |
| Boscalid | LC | 92 (10) | 109 (10) | 90 (5) | 110 (6) | 92 (3) | 101 (6) | 84 (7) | — | 65 (5) | — | |
| 17 | Bromophos-ethyl | GC | 79 (5) | 71 (10) | 89 (5) | 63 (7) | 106 (13) | 77 (3) | 77 (13) | 66 (8) | 64 (7) | 56 (10) |
| 18 | Bromopropylate | GC | 87 (7) | 77 (4) | 103 (5) | 78 (4) | 109 (8) | 95 (3) | 98 (8) | 84 (3) | 94 (10) | 74 (7) |
| 19 | Bromoxynil | LC | 100 (10) | 121 (11) | 92 (25) | 104 (3) | — | 87 (5) | 69 (25) | 94 (16) | 45 (17) | 83 (26) |
| 20 | Bromuconazole | GC | 93 (9) | 103 (14) | 92 (7) | 101 (11) | 116 (10) | 106 (10) | 99 (4) | 113 (18) | 84 (14) | 89 (5) |
| Bromuconazole | LC | — | 121 (9) | — | 103 (15) | — | 103 (12) | - | 108 (19) | — | 91 (18) | |
| 21 | Bupirimate | GC | 99 (4) | 104 (10) | 103 (5) | 105 (8) | 118 (10) | 118 (4) | 105 (9) | 116 (11) | 103 (6) | 99 (7) |
| 22 | Buprofezin | LC | 83 (5) | 85 (3) | 88 (5) | 78 (5) | 92 (4) | 91 (3) | 82 (5) | 84 (5) | 72 (2) | 67 (6) |
| 23 | Cadusafos | GC | 97 (4) | 104 (6) | 106 (3) | 98 (4) | 118 (11) | 115 (5) | 99 (7) | 113 (12) | 95 (9) | 93 (9) |
| Cadusafos | LC | 86 (4) | 104 (2) | 96 (3) | 98 (3) | 96 (4) | 103 (2) | 90 (4) | 100 (4) | 75 (4) | 84 (4) | |
| 24 | Carbaryl | LC | 86 (4) | 107 (1) | 100 (5) | 101 (10) | 98 (8) | 106 (6) | 93 (2) | 123 (4) | 90 (2) | 107 (6) |
| 25 | Carbendazim | LC | 84 (5) | 62 (11) | 85 (7) | 51 (12) | 89 (3) | 81 (5) | 77 (3) | 82 (5) | 44 (3) | — |
| 26 | Carbofuran | LC | 82 (4) | 140 (4) | 95 (3) | 133 (5) | 91 (4) | 124 (6) | 87 (2) | 150 (4) | 79 (2) | 107 (5) |
| 27 | Carboxin | GC | 101 (6) | 90 (4) | 116 (5) | 92 (7) | 117 (10) | 107 (6) | 105 (6) | 116 (10) | 118 (12) | 100 (3) |
| Carboxin | LC | 87 (5) | 91 (6) | 102 (5) | 93 (8) | 99 (5) | 100 (5) | 93 (6) | 104 (5) | 88 (5) | 93 (7) | |
| 28 | Chlorfenapyr | GC | 111 (16) | 79 (39) | 113 (5) | 109 (16) | 96 (19) | 93 (16) | 113 (18) | 117 (36) | 114 (10) | 111 (7) |
| 29 | Chlorfenson | GC | 91 (4) | 102 (5) | 106 (3) | 86 (8) | 111 (8) | 104 (4) | 95 (4) | 82 (14) | 95 (6) | 94 (12) |
| 30 | Chlorfenvinphos | GC | 100 (2) | 110 (12) | 109 (4) | 106 (7) | 118 (7) | 120 (6) | 105 (5) | 123 (8) | 100 (8) | 114 (16) |
| 31 | Chlormephos | GC | 118 (28) | 97 (12) | 138 (25) | 101 (12) | 81 (10) | 98 (5) | 91 (5) | 103 (11) | 88 (11) | 96 (5) |
| 32 | Chlorobenzilate | GC | 90 (4) | 97 (3) | 105 (2) | 94 (5) | 113 (11) | 103 (3) | 99 (3) | 102 (4) | 96 (8) | 88 (2) |
| 33 | Chlorpropham | GC | 105 (10) | 94 (13) | 123 (7) | 88 (14) | 122 (23) | 115 (3) | 89 (14) | 113 (6) | 96 (13) | 98 (2) |
| 34 | Chlorpyrifos | GC | 85 (6) | 93 (11) | 102 (6) | 82 (10) | 109 (14) | 97 (7) | 94 (8) | 86 (18) | 81 (15) | 76 (26) |
| Chlorpyrifos | LC | 82 (7) | 86 (4) | 102 (4) | 85 (9) | 90 (6) | 82 (2) | 78 (3) | 75 (6) | 66 (2) | 62 (15) | |
| 35 | Chlorpyrifos-methyl | GC | 99 (13) | 97 (10) | 120 (6) | 104 (8) | 141 (18) | 104 (7) | 99 (19) | 106 (6) | 113 (15) | 104 (18) |
| 36 | Clethodim | LC | — | 100 (8) | — | 112 (8) | — | 99 (7) | — | — | — | 99 (16) |
| 37 | Clofentezine | GC | 94 (6) | 82 (16) | 112 (14) | 100 (15) | 103 (22) | 68 (10) | — | — | 80 (12) | 67 (19) |
| 38 | Clomazone | GC | 101 (5) | 104 (5) | 113 (1) | 102 (4) | 120 (10) | 121 (5) | 103 (9) | 118 (4) | 104 (8) | 108 (6) |
| 39 | Clothianidin | LC | 90 (5) | 40 (36) | 94 (6) | 107 (11) | 96 (4) | 115 (11) | — | 107 (14) | 83 (7) | 103 (7) |
| 40 | Cyazofamid | LC | 88 (6) | 114 (2) | 98 (5) | 101 (2) | 98 (10) | 112 (6) | 94 (5) | 111 (4) | 89 (8) | 99 (5) |
| 41 | Cyfluthrin | GC | 93 (3) | 84 (6) | 109 (4) | 80 (7) | 68 (10) | 94 (8) | 114 (27) | 88 (9) | 99 (9) | 95 (24) |
| 42 | Cyhalothrin-lambda | GC | 95 (8) | 96 (9) | 114 (3) | 87 (10) | 113 (10) | 28 (49) | 115 (20) | 75 (8) | 103 (10) | 55 (20) |
| 43 | Cypermethrin | LC | 77 (6) | 59 (13) | 103 (18) | 90 (9) | 85 (10) | 72 (9) | 70 (26) | 75 (8) | 64 (11) | 83 (38) |
| 44 | Cyproconazole | GC | — | — | — | — | 102 (10) | 112 (10) | 101 (6) | 121 (6) | 98 (5) | 102 (7) |
| 45 | Cyprodinil | GC | 84 (5) | 83 (6) | 96 (9) | 86 (9) | 108 (9) | 102 (10) | 86 (14) | 92 (7) | 38 (17) | 86 (42) |
| 46 | Deltamethrin_cis | GC | 102 (7) | 90 (11) | 115 (2) | 83 (13) | 89 (21) | 82 (7) | 94 (18) | 72 (40) | 105 (12) | 69 (36) |
| Deltamethrin_cis | LC | 69 (8) | — | 93 (7) | — | 99 (4) | 72 (7) | 85 (18) | 76 (9) | 81 (8) | 81 (39) | |
| 47 | Demeton-S-methyl | GC | 107 (7) | 112 (8) | 111 (7) | 114 (15) | 119 (5) | 104 (5) | 112 (14) | 125 (8) | 119 (14) | 219 (48) |
| Demeton-S-methyl | LC | — | 113 (5) | — | 112 (5) | 98 (6) | 105 (6) | 88 (7) | 107 (8) | 92 (8) | 92 (5) | |
| 48 | Demeton-S-methylsulfone | LC | 85 (13) | 103 (14) | 132 (24) | 93 (15) | 136 (35) | 114 (6) | 76 (54) | 115 (9) | 86 (44) | 123 (6) |
| 49 | Diazinon | GC | 101 (6) | 109 (6) | 112 (7) | 101 (8) | 114 (12) | 118 (4) | 101 (4) | 102 (5) | 92 (10) | 97 (11) |
| Diazinon | LC | 84 (4) | 99 (8) | 94 (8) | 97 (7) | 97 (6) | 102 (4) | 83 (8) | 97 (5) | 78 (3) | 90 (8) | |
| 50 | Dichlorprop | LC | 95 (27) | 64 (44) | — | — | — | — | — | — | — | — |
| 51 | Dichlorvos | GC | 85 (10) | 66 (11) | 81 (2) | 67 (17) | 67 (8) | 73 (7) | 87 (7) | 98 (13) | 86 (8) | 97 (14) |
| Dichlorvos | LC | 69 (10) | 67 (4) | 73 (4) | 42 (14) | 51 (10) | 71 (5) | 74 (5) | 99 (6) | 70 (7) | 60 (10) | |
| 52 | Dicloran | GC | 86 (11) | 90 (5) | 103 (9) | 91 (10) | 111 (19) | 98 (2) | 83 (5) | 108 (12) | 89 (16) | 82 (7) |
| 53 | Dicofol-pp | GC | 104 (12) | 107 (7) | 122 (3) | 109 (4) | 113 (9) | 112 (4) | 100 (6) | 116 (7) | 105 (6) | 104 (13) |
| 54 | Dieldrin | GC | 92 (10) | 80 (16) | — | — | 100 (7) | 87 (19) | 66 (18) | 45 (85) | 82 (37) | — |
| 55 | Difenoconazole | GC | 93 (4) | 109 (4) | 103 (4) | 95 (3) | 110 (11) | 107 (3) | 96 (7) | 114 (3) | 84 (12) | 93 (4) |
| Difenoconazole | LC | 85 (3) | 96 (2) | 88 (4) | 99 (4) | 90 (8) | 104 (2) | 86 (3) | 99 (7) | 67 (7) | 85 (8) | |
| 56 | Diflubenzuron | LC | 95 (10) | 108 (3) | 86 (2) | 113 (6) | 99 (11) | 101 (7) | 86 (5) | 95 (5) | 75 (4) | 83 (5) |
| 57 | Dimethoate | LC | 88 (3) | 112 (5) | 99 (2) | — | 97 (8) | 117 (4) | 98 (3) | 124 (3) | 88 (3) | 111 (3) |
| 58 | Dimethomorph | GC | 102 (4) | 110 (5) | 111 (11) | 100 (6) | 114 (9) | 112 (10) | 109 (4) | 116 (3) | 112 (10) | 107 (3) |
| 59 | Dinoterb | LC | 98 (15) | 102 (28) | — | 97 (16) | 89 (16) | 91 (9) | 70 (40) | 95 (19) | 81 (22) | 64 (14) |
| 60 | Diphenylamine | GC | 93 (6) | 87 (6) | 109 (7) | 97 (5) | 117 (9) | 101 (4) | 96 (10) | 99 (3) | 123 (12) | 94 (10) |
| 61 | Disulfoton | GC | 84 (14) | 93 (12) | 109 (5) | 87 (66) | 109 (14) | 118 (11) | 98 (8) | 110 (12) | 112 (11) | 66 (26) |
| Disulfoton | LC | 81 (12) | 107 (9) | 96 (16) | 90 (5) | 87 (4) | 91 (7) | 77 (13) | 82 (7) | 88 (10) | 81 (12) | |
| 62 | Ditalimphos | LC | 91 (4) | 111 (4) | 101 (3) | 96 (5) | 95 (4) | 111 (4) | 54 (8) | 93 (4) | 88 (6) | 96 (4) |
| 63 | DMF | LC | 85 (5) | 108 (4) | 97 (3) | 100 (6) | 91 (6) | 109 (5) | 87 (5) | 108 (3) | 90 (2) | 95 (3) |
| 64 | DMST | LC | 75 (5) | 118 (8) | 89 (5) | 112 (6) | 87 (7) | 115 (7) | 91 (5) | 109 (6) | 82 (4) | 101 (6) |
| 65 | Endosulfan-alpha | GC | 89 (13) | 95 (14) | 93 (10) | 53 (12) | 100 (5) | 91 (17) | 86 (17) | 92 (13) | 72 (10) | 68 (14) |
| 66 | Endosulfan-beta | GC | 86 (18) | 93 (10) | 100 (20) | 58 (30) | 119 (21) | 99 (8) | 89 (13) | 85 (7) | 90 (17) | 79 (11) |
| 67 | Endosulfan-sulfate | GC | 96 (8) | 100 (5) | 107 (12) | 90 (12) | 125 (8) | 99 (12) | 99 (7) | — | 99 (6) | 80 (18) |
| 68 | Endrin | GC | 89 (35) | 76 (26) | 96 (26) | 75 (18) | 115 (17) | — | 82 (11) | — | 64 (18) | — |
| 69 | EPN | GC | 93 (7) | 99 (2) | 108 (5) | 87 (11) | 120 (8) | 104 (5) | 98 (9) | 101 (7) | 97 (14) | 88 (8) |
| 70 | Epoxiconazole | GC | 95 (4) | 116 (6) | 109 (2) | 99 (7) | 113 (10) | 116 (4) | 103 (3) | 226 (92) | 96 (7) | 141 (72) |
| Epoxiconazole | LC | 80 (6) | 114 (8) | 90 (9) | 108 (6) | 98 (10) | 114 (5) | 86 (7) | 247 (77) | 87 (8) | 139 (73) | |
| 71 | Ethiofencarb | LC | 89 (5) | 86 (14) | 101 (6) | 93 (7) | 99 (7) | 108 (6) | 90 (7) | 104 (7) | 91 (9) | 96 (5) |
| 72 | Ethion | GC | 109 (6) | 94 (3) | 109 (5) | 89 (8) | 117 (7) | 100 (3) | 112 (5) | 100 (7) | 109 (10) | 84 (11) |
| Ethion | LC | 99 (6) | 95 (4) | 97 (4) | 90 (3) | 95 (4) | 91 (2) | 96 (4) | 83 (7) | 87 (2) | 79 (6) | |
| 73 | Ethoprophos | GC | 98 (5) | 108 (6) | 109 (4) | 113 (7) | 121 (8) | 116 (2) | 102 (8) | 110 (5) | 101 (7) | 103 (13) |
| Ethoprophos | LC | 84 (8) | 120 (6) | 93 (11) | 93 (5) | 97 (10) | 109 (11) | 79 (3) | 103 (7) | 89 (7) | 92 (8) | |
| 74 | Ethoxyquin | LC | — | 75 (8) | — | 83 (13) | — | 88 (8) | — | 90 (20) | — | 74 (17) |
| 75 | Etofenprox | GC | 82 (5) | 51 (5) | 92 (3) | 64 (6) | 102 (7) | 76 (19) | 78 (8) | 63 (2) | 69 (8) | 54 (5) |
| Etofenprox | LC | 72 (9) | 50 (8) | 76 (12) | 42 (29) | 83 (5) | 66 (3) | 72 (6) | 53 (10) | 54 (10) | 43 (4) | |
| 76 | Fenamiphos | LC | 90 (3) | 112 (5) | 89 (4) | 100 (4) | 97 (6) | 115 (5) | 90 (6) | 104 (5) | 87 (4) | 95 (6) |
| 77 | Fenamiphos-sulfone | LC | 92 (6) | 111 (4) | 96 (11) | 104 (7) | 97 (10) | 102 (9) | 93 (6) | 107 (9) | 86 (7) | 100 (5) |
| 78 | Fenarimol | GC | 92 (6) | 106 (3) | 110 (4) | 92 (6) | 108 (8) | 106 (3) | 97 (2) | 111 (2) | 94 (8) | 97 (7) |
| 79 | Fenazaquin | LC | 68 (4) | 71 (4) | 65 (2) | 60 (4) | 78 (5) | 77 (4) | 53 (8) | 54 (5) | 28 (5) | 31 (5) |
| 80 | Fenbuconazole | GC | 100 (3) | 110 (2) | 110 (3) | 110 (3) | 117 (9) | 113 (7) | 101 (6) | 118 (2) | 101 (8) | 106 (4) |
| Fenbuconazole | LC | 89 (21) | 131 (9) | 90 (23) | 92 (10) | 97 (19) | 108 (11) | 94 (18) | 102 (11) | 86 (14) | 101 (10) | |
| 81 | Fenitrothion | GC | 100 (5) | 97 (8) | 122 (5) | 109 (11) | 123 (9) | 113 (8) | 105 (7) | 109 (12) | 104 (11) | 116 (17) |
| 82 | Fenoxycarb | GC | 97 (5) | 102 (16) | 114 (5) | 108 (8) | 110 (16) | 99 (13) | 89 (12) | — | 108 (13) | 96 (15) |
| Fenoxycarb | LC | 87 (6) | 112 (5) | 101 (3) | 105 (3) | 95 (5) | 106 (3) | 89 (4) | 106 (3) | 87 (4) | 99 (6) | |
| 83 | Fenpropathrin | GC | 99 (18) | 101 (6) | 106 (23) | 122 (13) | 102 (13) | — | 94 (18) | — | 100 (69) | — |
| 84 | Fenpropidin | LC | 61 (7) | 93 (8) | — | 71 (11) | 65 (14) | 104 (5) | 26 (10) | — | 79 (6) | 90 (8) |
| 85 | Fenpropimorph | GC | 71 (2) | 99 (4) | 50 (3) | 83 (5) | 75 (7) | 101 (6) | 88 (5) | 83 (3) | 98 (7) | 104 (13) |
| 86 | Fenson | GC | 95 (5) | 105 (5) | 111 (4) | 106 (10) | 115 (8) | 110 (6) | 149 (78) | 109 (5) | 97 (9) | 100 (4) |
| 87 | Fenthion | GC | 99 (2) | 109 (10) | 107 (5) | 101 (2) | 116 (9) | 106 (7) | 100 (11) | 110 (8) | 104 (7) | 99 (15) |
| Fenthion | LC | 85 (10) | 83 (17) | 94 (7) | 92 (14) | 100 (10) | 94 (12) | 102 (9) | 97 (11) | 87 (16) | 82 (14) | |
| 88 | Fenthion-oxon | LC | 20 (78) | 99 (9) | 91 (25) | 107 (6) | 86 (21) | 109 (3) | 95 (16) | 106 (1) | 97 (19) | 100 (2) |
| 89 | Fenthion-oxon-sulfone | LC | 85 (7) | 121 (12) | 96 (5) | 115 (10) | 92 (8) | 118 (7) | 87 (6) | 119 (9) | 83 (6) | 111 (5) |
| 90 | Fenthion-oxon-sulfoxide | LC | 82 (4) | 102 (5) | 82 (2) | 92 (5) | 67 (6) | 97 (6) | 87 (3) | 100 (2) | 81 (3) | 99 (3) |
| 91 | Fenthion-sulfone | LC | 85 (13) | 93 (15) | 107 (8) | 133 (7) | 104 (6) | 113 (10) | 91 (8) | 117 (19) | 96 (3) | 94 (19) |
| 92 | Fenthion-sulfoxide | LC | 93 (6) | 108 (9) | 98 (7) | 101 (10) | 94 (3) | 110 (12) | 91 (3) | 114 (3) | 85 (7) | 102 (5) |
| 93 | Fenvalerate | GC | 92 (3) | 75 (4) | 105 (4) | 72 (10) | 112 (9) | 89 (8) | 88 (11) | 83 (14) | 92 (8) | 83 (24) |
| 94 | Fipronil | LC | 90 (17) | 123 (13) | 86 (18) | 100 (21) | 100 (15) | 109 (22) | 116 (19) | 143 (11) | 55 (4) | 104 (16) |
| 95 | Fluazifop-p-butyl | GC | 98 (6) | 98 (9) | 109 (6) | 93 (7) | 118 (14) | 96 (6) | 100 (5) | 107 (3) | 99 (8) | 89 (5) |
| Fluazifop-p-butyl | LC | 92 (5) | 98 (3) | 103 (5) | 93 (6) | 98 (6) | 102 (2) | 91 (7) | 96 (7) | 80 (4) | 84 (8) | |
| 96 | Fludioxonil | GC | 96 (6) | 105 (6) | 110 (6) | 99 (13) | 118 (10) | 113 (12) | 111 (3) | 123 (3) | 100 (12) | 109 (7) |
| 97 | Flufenoxuron | GC | 93 (10) | 123 (23) | 126 (13) | 100 (13) | 122 (8) | 119 (37) | 48 (17) | — | — | 80 (52) |
| 98 | Fluoxastrobin | LC | 91 (1) | 115 (4) | 102 (4) | 112 (3) | 101 (4) | 109 (4) | 94 (5) | 110 (4) | 88 (2) | 108 (8) |
| 99 | Fluquinconazole | GC | 95 (4) | 110 (9) | 109 (4) | 101 (7) | 118 (9) | 109 (6) | 103 (3) | 124 (4) | 100 (8) | 102 (5) |
| 100 | Flusilazole | LC | 88 (3) | 114 (6) | 92 (2) | 121 (17) | 109 (7) | 112 (2) | 97 (5) | 107 (6) | 86 (4) | 99 (3) |
| 101 | Flutriafol | GC | 101 (9) | 113 (4) | 115 (2) | 111 (5) | 92 (10) | 108 (6) | 104 (4) | 127 (3) | 103 (9) | 108 (7) |
| 102 | Fluvalinate-tau | GC | 99 (24) | 26 (134) | 124 (7) | 96 (14) | 134 (9) | 88 (12) | 99 (15) | — | 96 (7) | — |
| 103 | Fosthiazate | LC | 87 (4) | 110 (6) | 101 (3) | 103 (4) | 99 (4) | 112 (4) | 94 (2) | 114 (4) | 90 (7) | 100 (6) |
| 104 | HCH-alpha | GC | 85 (6) | 78 (13) | 108 (4) | 100 (8) | 105 (6) | 101 (7) | 95 (6) | 89 (14) | 95 (11) | 95 (14) |
| 105 | HCH-beta | GC | 95 (6) | 80 (24) | 88 (56) | 85 (14) | 124 (8) | 117 (19) | 102 (4) | 89 (21) | 106 (6) | 96 (22) |
| 106 | Heptenophos | GC | 99 (4) | 112 (5) | 115 (3) | 112 (8) | 119 (8) | 114 (6) | 105 (6) | 113 (4) | 110 (7) | 114 (20) |
| Heptenophos | LC | 87 (7) | 109 (4) | 98 (3) | 106 (3) | 95 (7) | 110 (2) | 94 (6) | 112 (2) | 84 (2) | 103 (4) | |
| 107 | Hexaconazole | LC | 93 (6) | 100 (7) | 83 (6) | 87 (15) | 76 (9) | 103 (11) | 93 (7) | 93 (10) | 75 (11) | 73 (6) |
| 108 | Hexythiazox | GC | 81 (11) | 85 (14) | 100 (3) | 60 (37) | 100 (2) | 92 (12) | 95 (5) | 87 (12) | 87 (12) | 53 (11) |
| Hexythiazox | LC | 81 (3) | 79 (6) | 88 (4) | 83 (3) | 86 (5) | 82 (3) | 78 (4) | 82 (3) | 69 (3) | 64 (2) | |
| 109 | Imazalil | LC | 75 (8) | 67 (15) | 66 (21) | 65 (14) | 57 (7) | 90 (8) | 77 (6) | 88 (7) | 75 (7) | 78 (13) |
| 110 | Imidacloprid | LC | 92 (4) | 107 (12) | 90 (2) | 114 (3) | 94 (5) | 99 (13) | 93 (6) | 96 (12) | 86 (5) | 98 (7) |
| 111 | Indoxacarb | LC | 96 (9) | 96 (16) | 108 (9) | 113 (9) | 112 (7) | 108 (6) | 91 (24) | 116 (6) | 73 (12) | 96 (23) |
| 112 | Iodosulfuron-methyl-sodium | LC | 80 (17) | 97 (18) | 94 (12) | 100 (8) | 89 (15) | 101 (6) | — | 85 (12) | 58 (18) | 102 (13) |
| 113 | Iprodione | GC | 93 (12) | 78 (65) | 108 (6) | 120 (14) | 97 (15) | 130 (18) | 105 (34) | — | 101 (13) | 154 (30) |
| 114 | Iprovalicarb | LC | 88 (3) | 112 (4) | 99 (2) | 108 (3) | 96 (4) | 110 (3) | 92 (3) | 106 (3) | 88 (3) | 105 (3) |
| 115 | Isofenphos-methyl | GC | 100 (5) | 111 (4) | 114 (1) | 107 (6) | 122 (9) | 111 (6) | 107 (4) | 120 (3) | 107 (10) | 107 (5) |
| 116 | Isoprothiolane | GC | 95 (7) | 120 (2) | 105 (4) | 114 (10) | 119 (8) | 112 (9) | 93 (7) | 119 (3) | 103 (9) | 110 (6) |
| Isoprothiolane | LC | 87 (3) | 110 (4) | 98 (2) | 105 (3) | 100 (6) | 108 (3) | 91 (5) | 105 (3) | 85 (2) | 101 (5) | |
| 117 | Isoproturon | LC | 86 (5) | 105 (5) | 89 (3) | 103 (3) | 93 (7) | 109 (3) | 94 (2) | 107 (5) | 80 (7) | 101 (9) |
| 118 | Jodofenfos | GC | 82 (3) | 80 (7) | 97 (3) | 75 (17) | 108 (11) | 91 (10) | 80 (4) | 75 (9) | 70 (8) | 62 (18) |
| 119 | Kresoxim-methyl | GC | 101 (9) | 116 (7) | 115 (5) | 110 (4) | 131 (7) | 116 (8) | 112 (8) | 114 (5) | 110 (10) | 108 (5) |
| 120 | Lindane | GC | 96 (6) | 46 (90) | 107 (5) | 77 (25) | 104 (6) | 40 (96) | 94 (4) | — | 108 (3) | 105 (22) |
| 121 | Linuron | LC | 101 (17) | 109 (28) | 93 (15) | 105 (14) | 99 (8) | 103 (30) | — | 96 (12) | — | 88 (13) |
| 122 | Malaoxon | LC | 92 (3) | 115 (5) | 103 (6) | 108 (5) | 99 (7) | 108 (8) | 92 (3) | 119 (4) | 95 (3) | 103 (5) |
| 123 | Malathion | LC | 94 (4) | 116 (2) | 101 (8) | 104 (5) | 99 (6) | 107 (6) | 92 (5) | 115 (7) | 93 (7) | 102 (4) |
| 124 | Mecarbam | LC | 94 (2) | 111 (3) | 102 (3) | 102 (9) | 97 (5) | 108 (4) | 93 (4) | 107 (5) | 87 (4) | 101 (8) |
| 125 | Mepanipyrim | LC | 93 (9) | 109 (16) | 78 (16) | 54 (12) | 102 (15) | 96 (11) | 75 (5) | 72 (19) | 45 (15) | 78 (18) |
| 126 | Metaflumizone | LC | 88 (4) | 102 (5) | 103 (2) | 75 (38) | 94 (7) | 95 (5) | 71 (11) | 81 (6) | 57 (9) | 78 (9) |
| 127 | Metalaxyl | LC | 84 (4) | 107 (5) | 95 (5) | 98 (3) | 100 (6) | 108 (3) | 95 (3) | 108 (4) | 85 (6) | 103 (8) |
| 128 | Metconazole | LC | 81 (14) | 97 (8) | 81 (10) | 95 (9) | 84 (12) | 101 (8) | 83 (13) | 94 (10) | 74 (7) | 74 (10) |
| 129 | Methamidophos | LC | 71 (4) | 81 (4) | 77 (3) | 74 (4) | 46 (4) | 72 (3) | 75 (8) | 87 (2) | 67 (3) | 83 (5) |
| 130 | Methidathion | GC | 98 (2) | 119 (6) | 113 (2) | 121 (8) | 119 (9) | 116 (8) | 99 (6) | 118 (6) | 108 (8) | 120 (14) |
| 131 | Methiocarb | LC | 87 (7) | 111 (9) | 102 (6) | 105 (9) | 100 (10) | 114 (6) | 92 (16) | 121 (13) | 84 (11) | 109 (10) |
| 132 | Methiocarb-sulfone | LC | 88 (5) | 122 (3) | 104 (3) | 118 (7) | 97 (6) | 119 (4) | — | — | 95 (9) | 118 (4) |
| 133 | Methiocarb-sulfoxide | LC | 86 (5) | 103 (4) | 90 (4) | 94 (3) | 74 (9) | 101 (6) | 103 (11) | — | 81 (6) | 96 (5) |
| 134 | Methomyl | LC | 123 (14) | 171 (17) | 139 (13) | 195 (10) | 102 (13) | 204 (19) | 116 (11) | 116 (14) | 99 (18) | 148 (17) |
| 135 | Methoxychlor | GC | 305 (24) | 88 (8) | 201 (15) | 92 (7) | 111 (10) | 100 (8) | 97 (7) | 95 (3) | 102 (13) | 85 (12) |
| 136 | Methoxyfenozide | LC | 93 (6) | 113 (5) | 102 (6) | 110 (14) | 98 (1) | 110 (9) | 88 (7) | 116 (17) | 84 (4) | 102 (6) |
| 137 | Metribuzin | GC | 101 (9) | 160 (7) | 112 (2) | 132 (9) | 121 (7) | 135 (18) | 108 (3) | 122 (8) | 111 (6) | 110 (4) |
| 138 | Metsulfuron-methyl | LC | 92 (5) | 99 (4) | 96 (3) | 105 (4) | 99 (6) | 102 (6) | 23 (40) | 81 (3) | 86 (3) | 92 (8) |
| 139 | Mevinphos | LC | 87 (5) | 101 (4) | 98 (5) | 99 (3) | 95 (5) | 112 (5) | 93 (6) | 109 (2) | 88 (3) | 98 (8) |
| 140 | Monocrotophos | LC | 84 (3) | 101 (9) | 87 (6) | 97 (4) | 86 (3) | 101 (6) | 91 (5) | 106 (6) | 84 (6) | 105 (6) |
| 141 | Monolinuron | LC | 88 (4) | 102 (7) | 98 (8) | 99 (10) | 100 (5) | 114 (6) | 96 (6) | 115 (6) | 88 (4) | 98 (2) |
| 142 | Myclobutanil | GC | 94 (3) | 123 (9) | 105 (2) | 100 (14) | 117 (13) | 109 (15) | 107 (4) | 123 (17) | 102 (5) | 117 (10) |
| 143 | Nuarimol | GC | 92 (4) | 113 (4) | 102 (3) | 105 (3) | 112 (8) | 113 (8) | 106 (5) | 120 (6) | 103 (7) | 101 (5) |
| 144 | Ofurace | LC | 88 (7) | 102 (7) | 104 (5) | 105 (11) | 103 (7) | 115 (9) | 92 (9) | 115 (5) | 87 (5) | 105 (7) |
| 145 | Omethoate | LC | 78 (3) | 98 (16) | 81 (6) | 95 (8) | 67 (6) | 112 (9) | 77 (6) | 85 (5) | 84 (3) | 86 (22) |
| 146 | Oxadixyl | GC | 100 (3) | 129 (18) | 114 (4) | 101 (10) | 121 (12) | 119 (11) | 103 (3) | 104 (3) | 110 (8) | 101 (4) |
| 147 | Oxamyl | LC | 87 (4) | 113 (4) | 97 (4) | 104 (5) | 94 (3) | 108 (3) | 85 (5) | 108 (5) | 81 (7) | 103 (3) |
| 148 | Oxycarboxin | LC | 91 (1) | 110 (4) | 99 (3) | 109 (8) | 98 (7) | 110 (4) | 89 (4) | 108 (6) | 91 (2) | 107 (6) |
| 149 | Oxydemeton-methyl | LC | 74 (3) | 95 (4) | 60 (5) | 80 (3) | 15 (14) | 94 (5) | 85 (5) | 97 (7) | 66 (2) | 105 (7) |
| 150 | Paclobutrazol | GC | 104 (77) | 112 (6) | 111 (4) | 109 (3) | 108 (12) | 116 (8) | 104 (5) | 122 (1) | 102 (12) | 107 (5) |
| 151 | Paraoxon-methyl | LC | 95 (20) | 50 (78) | 119 (78) | 86 (5) | 79 (50) | 103 (22) | 69 (61) | 81 (15) | 63 (33) | 70 (55) |
| 152 | Parathion | GC | 84 (10) | 118 (9) | 117 (5) | 101 (6) | 121 (12) | 107 (11) | 100 (6) | 112 (16) | 107 (16) | 93 (18) |
| 153 | Parathion-methyl | GC | 105 (5) | 115 (12) | 118 (9) | 122 (8) | 127 (7) | 115 (7) | 112 (3) | 104 (11) | 107 (13) | 110 (13) |
| 154 | Penconazole | GC | 98 (5) | 106 (3) | 92 (4) | 99 (6) | 110 (10) | 108 (5) | 99 (8) | 118 (4) | 93 (7) | 85 (2) |
| Penconazole | LC | 84 (12) | 109 (8) | 80 (5) | 92 (9) | 95 (6) | 106 (7) | 89 (6) | 101 (6) | 72 (4) | 89 (8) | |
| 155 | Pencycuron | GC | 93 (10) | 116 (7) | 75 (11) | 108 (7) | 101 (7) | 99 (10) | 96 (9) | 106 (11) | 103 (7) | 104 (20) |
| Pencycuron | LC | 81 (6) | 91 (3) | 94 (2) | 84 (4) | 94 (7) | 91 (5) | 85 (4) | 86 (4) | 76 (3) | 77 (6) | |
| 156 | Pendimethalin | GC | 86 (6) | 84 (2) | 106 (13) | 77 (8) | 112 (12) | 87 (13) | 90 (10) | 86 (3) | 83 (8) | 71 (8) |
| Pendimethalin | LC | 81 (6) | 77 (7) | 91 (5) | 71 (5) | 91 (4) | 82 (2) | 77 (4) | 75 (4) | 68 (4) | 62 (2) | |
| 157 | Phenthoate | GC | 107 (3) | 104 (11) | 119 (5) | 101 (4) | 157 (10) | 109 (4) | 105 (8) | 111 (7) | 117 (11) | 122 (20) |
| 158 | Phosalone | GC | 85 (6) | — | 118 (2) | 51 (28) | 103 (23) | 73 (23) | 86 (14) | — | 97 (9) | — |
| 159 | Phosmet | LC | 92 (5) | 114 (10) | 96 (8) | 113 (7) | 103 (10) | 104 (9) | 93 (11) | 97 (10) | 79 (11) | 99 (10) |
| 160 | Phosmet-oxon | LC | 92 (3) | 111 (5) | 102 (4) | 102 (4) | 95 (6) | 108 (2) | 91 (2) | 99 (2) | 87 (3) | 102 (4) |
| 161 | Phosphamidon | LC | 90 (7) | 128 (10) | 107 (5) | 109 (8) | 88 (5) | 109 (4) | 90 (3) | 109 (9) | 91 (8) | 98 (12) |
| 162 | Phoxim | LC | 87 (6) | 98 (11) | 101 (9) | 97 (4) | 101 (7) | 103 (6) | 88 (3) | 99 (6) | 79 (6) | 88 (5) |
| 163 | Pirimicarb | GC | 94 (5) | 106 (6) | 108 (6) | 98 (11) | 120 (7) | 105 (6) | 101 (2) | 110 (4) | 97 (8) | 109 (9) |
| Pirimicarb | LC | 85 (4) | 105 (2) | 95 (3) | 96 (3) | 91 (5) | 100 (5) | 86 (5) | 102 (4) | 83 (3) | 91 (5) | |
| 164 | Pirimiphos-methyl | GC | 109 (9) | 93 (7) | 125 (2) | 95 (9) | 143 (16) | 108 (4) | 103 (12) | 104 (7) | 118 (12) | 100 (15) |
| Pirimiphos-methyl | LC | 89 (12) | 101 (7) | 101 (4) | 80 (5) | 104 (11) | 96 (4) | 75 (11) | 95 (2) | 79 (6) | 82 (8) | |
| 165 | Prochloraz | LC | 82 (5) | 105 (2) | 82 (5) | 93 (5) | 87 (8) | 100 (5) | 85 (2) | 97 (4) | 62 (3) | 80 (5) |
| 166 | Procymidone | GC | 101 (4) | 120 (2) | 116 (5) | 102 (7) | 121 (3) | 113 (7) | 99 (8) | 108 (8) | 101 (5) | 108 (4) |
| 167 | Profenofos | GC | 90 (8) | 97 (8) | 101 (4) | 105 (17) | 115 (12) | 113 (7) | 97 (17) | 99 (12) | 83 (6) | 110 (21) |
| 168 | Propamocarb | LC | 36 (6) | 54 (13) | — | 14 (19) | 25 (10) | 47 (6) | 50 (46) | 35 (6) | 116 (4) | 69 (7) |
| 169 | Propargite | LC | 78 (10) | 89 (3) | 86 (5) | 87 (3) | 86 (20) | 107 (21) | 65 (29) | 84 (4) | 78 (19) | 80 (6) |
| 170 | Propiconazole | GC | 95 (5) | 91 (13) | 97 (3) | 92 (7) | 115 (7) | 107 (5) | 100 (3) | 112 (5) | 93 (8) | 94 (9) |
| Propiconazole | LC | — | 96 (6) | — | 92 (9) | 97 (7) | 106 (12) | 71 (4) | 98 (9) | 62 (10) | 73 (7) | |
| 171 | Propoxur | LC | 85 (6) | 112 (4) | 101 (4) | 97 (8) | 102 (5) | 113 (7) | 92 (8) | 111 (6) | 92 (3) | 100 (5) |
| 172 | Propyzamide | GC | 74 (16) | 97 (9) | 131 (6) | 117 (9) | 135 (12) | 111 (16) | 119 (15) | 108 (18) | 86 (33) | 109 (15) |
| Propyzamide | LC | 88 (5) | 94 (9) | 99 (5) | 93 (9) | 97 (9) | 106 (3) | 90 (8) | 103 (6) | 85 (3) | 92 (4) | |
| 173 | Prosulfocarb | LC | 84 (3) | 78 (4) | 92 (3) | 79 (4) | 92 (6) | 85 (6) | 84 (4) | 82 (5) | 75 (3) | 72 (9) |
| 174 | Prosulfuron | LC | 90 (9) | 113 (3) | 100 (3) | 117 (6) | 101 (3) | 99 (12) | — | 95 (9) | 85 (12) | 97 (9) |
| 175 | Prothioconazole-desthio | LC | 95 (11) | 108 (5) | 72 (27) | 97 (15) | 87 (12) | 93 (7) | 58 (93) | 103 (7) | 67 (12) | 92 (17) |
| 176 | Prothiofos | GC | 78 (9) | 53 (11) | 89 (7) | 62 (7) | 106 (15) | 78 (18) | 77 (4) | 66 (21) | 69 (9) | 51 (16) |
| 177 | Pymetrozine | LC | 40 (14) | 29 (6) | 20 (11) | 19 (19) | 22 (7) | 35 (7) | 58 (10) | 40 (9) | 30 (7) | 34 (9) |
| 178 | Pyraclostrobin | LC | 92 (3) | 106 (6) | 97 (2) | 100 (4) | 95 (7) | 101 (5) | 78 (6) | 242 (84) | 61 (5) | 134 (83) |
| 179 | Pyrazophos | GC | 103 (5) | — | 113 (5) | 99 (16) | 123 (8) | 91 (10) | 89 (7) | — | 81 (9) | 137 (143) |
| 180 | Pyridaben | GC | 92 (4) | 72 (6) | 100 (3) | 71 (4) | 107 (11) | 71 (25) | 84 (7) | 76 (3) | 83 (10) | 60 (5) |
| 181 | Pyridaphenthion | GC | 98 (3) | 123 (13) | 109 (2) | 118 (9) | 119 (7) | 128 (14) | 104 (6) | 107 (21) | 105 (12) | 134 (18) |
| 182 | Pyridate | LC | — | 46 (25) | — | 38 (5) | — | 76 (7) | — | — | — | 45 (2) |
| 183 | Pyrimethanil | GC | 87 (6) | 97 (9) | 99 (2) | 93 (12) | 104 (17) | 112 (9) | 83 (8) | 108 (6) | 49 (10) | 86 (7) |
| Pyrimethanil | LC | 98 (11) | 90 (8) | 83 (7) | 85 (13) | 95 (9) | 101 (7) | 85 (8) | 88 (10) | 44 (17) | 82 (15) | |
| 184 | Pyriproxyfen | GC | 88 (4) | 76 (4) | 100 (2) | 83 (6) | 109 (10) | 87 (10) | 86 (8) | 79 (4) | 82 (7) | 70 (6) |
| Pyriproxyfen | LC | 83 (3) | 79 (4) | 86 (5) | 78 (4) | 88 (8) | 82 (4) | 79 (2) | 73 (5) | 69 (4) | 65 (6) | |
| 185 | Quinoxyfen | GC | 76 (4) | 72 (5) | 82 (4) | 70 (2) | 96 (9) | 84 (12) | 66 (4) | 63 (4) | 46 (10) | 48 (5) |
| Quinoxyfen | LC | 66 (5) | 71 (6) | 76 (5) | 69 (9) | 73 (6) | 75 (5) | 58 (8) | 57 (7) | 37 (5) | 45 (12) | |
| 186 | Simazine | LC | 82 (8) | — | 94 (9) | — | 93 (16) | — | 84 (10) | — | 78 (10) | — |
| 187 | Spinosad | LC | 26 (12) | 77 (17) | — | — | 29 (13) | 85 (18) | 71 (11) | 76 (14) | 69 (14) | 75 (11) |
| 188 | Spirodiclofen | LC | 85 (7) | 82 (7) | 92 (7) | 70 (4) | 94 (10) | 84 (13) | 80 (12) | 67 (12) | 74 (4) | 66 (9) |
| 189 | Spiroxamine | LC | 48 (6) | 84 (4) | — | 52 (14) | 56 (9) | 96 (4) | 27 (5) | 8 (18) | 78 (7) | 89 (5) |
| 190 | Tebuconazole | GC | 92 (8) | 99 (4) | 105 (6) | 100 (7) | 104 (6) | 110 (11) | 100 (7) | 112 (6) | 96 (8) | 98 (4) |
| Tebuconazole | LC | 85 (11) | 96 (8) | 82 (10) | 93 (13) | 90 (10) | 92 (9) | 37 (137) | 93 (14) | 79 (12) | 82 (5) | |
| 191 | Tebufenozide | LC | 90 (11) | 133 (10) | 103 (13) | 117 (5) | 122 (11) | 113 (15) | 104 (6) | 106 (6) | 95 (12) | 101 (14) |
| 192 | Tebufenpyrad | GC | 91 (4) | 98 (10) | 102 (2) | 90 (3) | 106 (8) | 95 (6) | 93 (5) | 100 (4) | 87 (10) | 79 (7) |
| Tebufenpyrad | LC | 90 (5) | 90 (4) | 70 (5) | 84 (5) | 92 (6) | 91 (5) | 81 (8) | 88 (5) | 75 (5) | 71 (8) | |
| 193 | Tecnazene | GC | 87 (7) | 89 (10) | 94 (9) | 74 (5) | 95 (10) | 96 (6) | 86 (14) | 80 (6) | 80 (11) | 68 (12) |
| 194 | Teflubenzuron | LC | 90 (22) | 97 (21) | 108 (16) | 66 (25) | 87 (19) | 110 (4) | 79 (17) | 88 (12) | 47 (14) | 85 (29) |
| 195 | Tefluthrin | GC | 94 (3) | 78 (11) | 104 (1) | 70 (12) | 115 (10) | 89 (10) | 97 (4) | 78 (14) | 88 (7) | 64 (8) |
| 196 | Tetraconazole | GC | 95 (4) | 121 (2) | 111 (5) | 103 (11) | 122 (8) | 112 (6) | 95 (46) | 114 (7) | 90 (54) | 112 (9) |
| 197 | Tetradifon | GC | 78 (8) | 70 (9) | 94 (10) | 81 (7) | 112 (14) | 91 (8) | 85 (7) | 81 (6) | 75 (6) | 74 (6) |
| 198 | Thiabendazole | LC | 72 (5) | 108 (11) | 68 (6) | 101 (7) | 49 (9) | 79 (8) | 72 (7) | 70 (12) | 37 (5) | 66 (9) |
| 199 | Thiacloprid | LC | 89 (4) | 115 (4) | 96 (4) | 102 (3) | 101 (8) | 103 (3) | 89 (5) | 113 (6) | 90 (6) | 104 (6) |
| 200 | Thiamethoxam | LC | 88 (6) | 155 (11) | 91 (13) | 100 (13) | 83 (5) | 112 (11) | 79 (10) | 108 (9) | 79 (5) | 114 (10) |
| 201 | Thiodicarb | LC | 74 (5) | — | 83 (4) | — | 81 (8) | — | 77 (5) | — | 71 (4) | — |
| 202 | Thiometon | LC | — | 82 (29) | 52 (83) | 89 (23) | 99 (9) | 105 (4) | — | 108 (23) | 82 (25) | 118 (14) |
| 203 | Tolclofos-methyl | GC | 95 (4) | 97 (6) | 109 (2) | 101 (12) | 121 (9) | 104 (3) | 95 (4) | 95 (5) | 94 (9) | 94 (14) |
| 204 | Triadimefon | GC | 94 (4) | 124 (7) | 108 (9) | 111 (4) | 118 (13) | 121 (11) | 102 (6) | 116 (8) | 107 (9) | 106 (12) |
| 205 | Triadimenol | LC | 89 (4) | 108 (5) | 92 (7) | 104 (5) | 94 (5) | 106 (5) | 88 (6) | 103 (5) | 84 (6) | 93 (7) |
| 206 | Triallate | LC | 72 (17) | 70 (3) | 81 (10) | 76 (9) | 91 (9) | 76 (8) | 75 (10) | 67 (3) | 60 (19) | 53 (5) |
| 207 | Triazophos | GC | 103 (4) | 91 (16) | 117 (5) | 111 (5) | 118 (13) | 117 (11) | 103 (6) | 104 (6) | 103 (9) | 112 (16) |
| Triazophos | LC | 91 (2) | 110 (3) | 100 (3) | 103 (3) | 100 (4) | 109 (3) | 94 (5) | 108 (3) | 84 (4) | 99 (4) | |
| 208 | Trichlorfon | GC | 77 (8) | 67 (11) | 81 (3) | 67 (18) | 67 (7) | 79 (17) | 87 (6) | 98 (13) | 88 (8) | 97 (13) |
| 209 | Tricyclazole | GC | 98 (11) | 120 (8) | 115 (8) | 110 (9) | 121 (8) | 118 (9) | 105 (9) | 115 (6) | 101 (2) | 101 (9) |
| Tricyclazole | LC | 76 (4) | 98 (5) | 77 (3) | 90 (3) | 18 (16) | 51 (7) | 78 (5) | 94 (4) | 47 (5) | 56 (6) | |
| 210 | Trifloxystrobin | LC | 94 (5) | 104 (5) | 102 (3) | 102 (4) | 101 (6) | 111 (2) | 92 (4) | 106 (3) | 86 (3) | 94 (5) |
| 211 | Triflumuron | LC | 88 (4) | 105 (4) | 93 (5) | 104 (2) | 100 (5) | 107 (3) | 89 (5) | 101 (4) | 74 (6) | 93 (7) |
| 212 | Trifluralin | GC | 96 (4) | 82 (7) | 106 (3) | 86 (12) | 116 (13) | 93 (7) | 97 (5) | 86 (6) | 100 (11) | 76 (13) |
| 213 | Triticonazole | LC | 85 (6) | 111 (7) | 91 (9) | 102 (6) | 73 (12) | 108 (6) | 81 (4) | 109 (5) | 82 (3) | 91 (6) |
| 214 | Vamidothion | LC | 87 (2) | 100 (11) | 90 (5) | 95 (5) | 83 (7) | 101 (4) | 92 (4) | 112 (9) | 84 (9) | 104 (14) |
| 215 | Vinclozolin | GC | 96 (8) | 108 (5) | 112 (5) | 108 (9) | 125 (11) | 109 (9) | 103 (8) | 105 (3) | 113 (10) | 103 (8) |
| 216 | Zoxamide | LC | 88 (10) | 99 (9) | 96 (4) | 92 (7) | 96 (4) | 104 (3) | 94 (3) | 101 (7) | 85 (8) | 85 (6) |
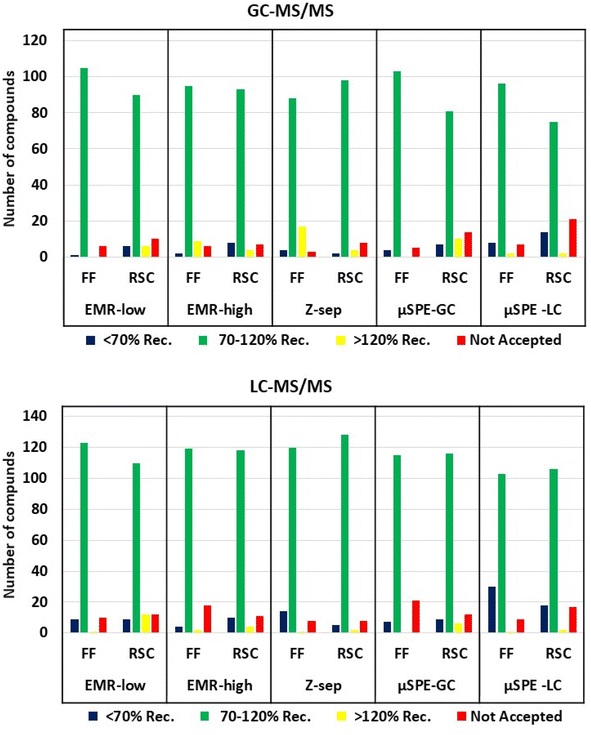
For the FF, the cartridges that resulted in the highest percentage of compounds with recoveries between 70 and 120% were EMR-low with 105 (94%) compounds and µSPE-GC with 103 (92%), followed by µSPE-LC 96 (86%), EMR-high 95 (85%) and Z-sep 88 (79%).
For RSC, the best performance was obtained with the cartridges containing Z-sep for 98 compounds (88%), EMR-high for 93 (83%), and EMR-low for 90 (80%). When both cartridges containing GCB (µSPE-GC and µSPE-LC) were used, recoveries between 70 and 120% could only be achieved for 81 (72%) and 75 (67%) compounds, respectively.
Poor results were obtained when the extract was cleaned with the µSPE-LC cartridge. This cartridge does not contain MgSO4; therefore the water content in the extract was not removed and this could have affected the column, and thus this cartridge is not recommended for GC-MS/MS analysis.
In the LC-MS/MS, FF sample extracts were analyzed for 143 pesticides and metabolites. The results showed that 123 (86%) compounds for the EMR-low cartridge had recoveries between 70 and 120%, followed by EMR-high, Z-sep, µSPE-GC and µSPE-LC with 119 (83%), 120 (84%), 115 (80%) and 103 (72%), respectively.
A comparison of recoveries in the RSC spiked samples showed the largest number of compounds with recoveries between 70 and 120% when the extract was cleaned throughout Z-sep with 128 (90%), followed by EMR-low, EMR-high, µSPE-GC and µSPE-LC with 110 (77%), 118 (83%), 116 (81%) and 106 (74%).
The number of compounds within different ranges of recoveries analyzed by GC-MS/MS and LC-MS/MS, in FF and RSC, is shown in Fig. 4.
Compounds with a planar structure have been previously shown to be affected by GCB. The same was observed in our study. Planar compounds such as quinoxyfen gave poor recovery in both matrices and both instruments, between 37 and 45% with µSPE-GC and µSPE-LC cartridges. To overcome this issue, an isotopically labeled standard can be used to normalize potential losses30 when still using GCB or removal or replacement with other sorbents may be considered. For instance, good recoveries were obtained for quinoxyfen with cartridges containing EMR and Z-sep sorbents (76% and 73%, respectively).
Acid compounds, fenpropidin, iodosulfuron-methyl-sodium, and metsulfuron-methyl resulted in low recoveries or unacceptable RSD (>20%) when the extract was cleaned-up using the µSPE-GC cartridge, due to their interaction with PSA.
Increasing the amount of sorbent in the case of EMR did not significantly affect compound recoveries but it improved the sample clean-up, as reflected by the reduced TIC background. The lack of EMR “activation” during the clean-up may have affected the recoveries for some compounds.
4. Conclusions
The study investigated the performance of different sorbents and amounts in µSPE cartridges using an automatic clean-up and sample preparation workflow. Eight different sorbent combinations, including EMR, Z-sep, chitin, C18, PSA, and GCB, were tested in terms of clean-up efficiency. The cartridges containing only EMR and C18 did not play any significant role in fatty acid removal, but they removed up to 100% of sterols in both FF and RSC matrices. The best removal efficiency for fatty acids was achieved with the cartridges containing PSA (µSPE-GC) and Z-sep (Z-sep and µSPE-LC). However, the Z-sep cartridge, without C18, did not have a similar effect on the removal of sterols. The presence of GCB had a positive visual effect on the FF extract, most probably due to the removal of carotenoid. For RSC, GCB and EMR had the same effect on color removal proving once again that the role of GCB could be reconsidered in the cartridge's composition, by replacing or lowering its amount. Increasing the amount of Z-sep improved the removal efficiency of fatty acids. Overall, the clean-up with the different sorbents introduced into the new µSPE cartridges showed similar clean-up efficiency to conventional d-SPE proving that the automatic clean-up has equal performance.In terms of recovery and precision, five cartridges were investigated. The best results with recovery between 70 and 120% and RSD<20% were achieved when FF samples were cleaned-up with EMR-low (94% for the compound analysis by GC-MS/MS and 86% for the ones analyzed by LC-MS/MS). In the case of RSC, the optimal results were obtained when samples were cleaned-up with the cartridge containing only Z-sep (98% by GC-MS/MS and 88% by LC-MS/MS). Although these cartridges give the best results in terms of recovery, their use could require more instrument maintenance, especially for GC-MS/MS, due to the lower removal of co-extractives. To avoid this potential challenge, a novel sorbent combination, which includes Z-sep and EMR, can be introduced into the new type of cartridge and further investigated for pesticide residue analyses.
Conflicts of interest
The authors report no conflicts of interest.Acknowledgements
The current study was performed within the framework of the European Union Reference Laboratory (EURL) for pesticide residues in cereals and feeding stuff financed by the European Commission. The authors also thank Thomi Preiswerk, Mario Mirabelli, Gwen Lim Sin Yee and Say Kotchanoot Srikham from CTC Analytics for providing, programming, and installing the PAL3-RTC robotic liquid handler, and CTC Analytics (Zwingen, Basel-Landschaft; Switzerland) for supplying customized PAL µSPE mini-cartridges with different sorbent combinations for use in the study. We greatly appreciate the technical assistance of Ban M. Kadhum and Susanne Pless.References
- European Parliament, Regulation (Ec) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/eec, Off. J. Eur. Communities: Inf. Not., 2005, 70, 1–16 Search PubMed
.
- European Parliament, Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed, Official Journal of the European Communities, 2013, L 269, 1–15 Search PubMed
.
- M. Anastassiades, S. J. Lehotay, D. Štajnbaher and F. J. Schenck, Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction”, J. AOAC Int., 2003, 86, 412–431 CrossRef CAS PubMed
.
- Y. Sapozhnikova and S. J. Lehotay, Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography-tandem mass spectrometry, Anal. Chim. Acta, 2013, 758, 80–92 CrossRef CAS PubMed
.
- Y. Sapozhnikova and S. J. Lehotay, Evaluation of different parameters in the extraction of incurred pesticides and environmental contaminants in fish, J. Agric. Food Chem., 2015, 63, 5163–5168 CrossRef CAS PubMed
.
- Y. Sapozhnikova, High-throughput analytical method for 265 pesticides and environmental contaminants in meats and poultry by fast low pressure gas chromatography and ultrahigh-performance liquid chromatography tandem mass spectrometry, J. Chromatogr. A, 2018, 1572, 203–211 CrossRef CAS PubMed
.
- Y. Sapozhnikova, Evaluation of low-pressure gas chromatography-tandem mass spectrometry method for the analysis of >140 pesticides in fish, J. Agric. Food Chem., 2014, 62, 3684–3689 CrossRef CAS PubMed
.
- T. Yamaguchi, M. Okihashi, K. Harada, K. Uchida, Y. Konishi, K. Kajimura, K. Hirata and Y. Yamamoto, Rapid and easy multiresidue method for the analysis of antibiotics in meats by ultrahigh-performance liquid chromatography-tandem mass spectrometry, J. Agric. Food Chem., 2015, 63, 5133–5140 CrossRef CAS PubMed
.
- M. M. Aguilera-Luiz, J. L. M. Vidal, R. Romero-González and A. G. Frenich, Multi-residue determination of veterinary drugs in milk by ultra-high-pressure liquid chromatography-tandem mass spectrometry, J. Chromatogr. A, 2008, 1205, 10–16 CrossRef CAS PubMed
.
- D. Hurtaud-Pessel, T. Jagadeshwar-Reddy and E. Verdon, Development of a new screening method for the detection of antibiotic residues in muscle tissues using liquid chromatography and high resolution mass spectrometry with a LC-LTQ-Orbitrap instrument, Food Addit. Contam.: Part A, 2011, 28, 1340–1351 CrossRef CAS PubMed
.
- P. Gago-Ferrero, A. A. Bletsou, D. E. Damalas, R. Aalizadeh, N. A. Alygizakis, H. P. Singer, J. Hollender and N. S. Thomaidis, Wide-scope target screening of >2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes, J. Hazard. Mater., 2020, 387, 121712 CrossRef CAS PubMed
.
- E. Miró-Abella, P. Herrero, N. Canela, L. Arola, F. Borrull, R. Ras and N. Fontanals, Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography-tandem mass spectrometry, Food Chem., 2017, 229, 366–372 CrossRef PubMed
.
- A. B. Martínez-Piernas, P. Plaza-Bolaños, A. Gilabert and A. Agüera, Application of a fast and sensitive method for the determination of contaminants of emerging concern in wastewater using a quick, easy, cheap, effective, rugged and safe-based extraction and liquid chromatography coupled to mass spectrometry, J. Chromatogr. A, 2021, 1653, 462396 CrossRef PubMed
.
- X. Yu, X. Wu, Y. Xie, K. Tong, M. Wang, J. Li, C. Fan and H. Chen, Development and Validation of a Method for Determination of 43 Antimicrobial Drugs in Western-Style Pork Products by UPLC-MS/MS with the Aid of Experimental Design, Molecules, 2022, 27, 8283 CrossRef CAS PubMed
.
- K. Sugitate and M. Saka, Decrease in the matrix enhancement effect on pesticides analysis with GC-MS using new types of solid-phase extraction column, J. Pestic. Sci., 2015, 40, 87–91 CrossRef CAS
.
- L. Han, J. Matarrita, Y. Sapozhnikova and S. J. Lehotay, Evaluation of a recent product to remove lipids and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods, J. Chromatogr. A, 2016, 1449, 17–29 CrossRef CAS PubMed
.
- M. Imran, A. Raza, H. Faisal Usman, M. Mubasher, N. U. Din, M. Amir Nadeem, M. Irfan Ashiq, M. Amjad and M. A. Tahir, Enhanced Matrix Removal-Lipid (EMR-L): a novel approach for efficient clean-up in systemic toxicological analysis of drugs and pesticides, Aust. J. Forensic Sci., 2023, 1–15 Search PubMed
.
- A. Goon, R. Shinde, B. Ghosh and K. Banerjee, Application of automated mini–Solid-phase extraction cleanup for the analysis of pesticides in complex spice matrixes by GC-MS/MS, J. AOAC Int., 2021, 103, 40–45 CrossRef PubMed
.
-
H. J. Hübschmann, Automated Sample Preparation: Methods for GC-MS and LC-MS, John Wiley & Sons, 2022 Search PubMed
.
- B. D. Morris and R. B. Schriner, Development of an automated column solid-phase extraction cleanup of QuEChERS extracts, using a zirconia-based sorbent, for pesticide residue analyses by LC-MS/MS, J. Agric. Food Chem., 2015, 63, 5107–5119 CrossRef CAS PubMed
.
- S. J. Lehotay, L. Han and Y. Sapozhnikova, Automated Mini-Column Solid-Phase Extraction Cleanup for High-Throughput Analysis of Chemical Contaminants in Foods by Low-Pressure Gas Chromatography-Tandem Mass Spectrometry, Chromatographia, 2016, 79, 1113–1130 CrossRef CAS PubMed
.
- L. Han and Y. Sapozhnikova, Semi-automated high-throughput method for residual analysis of 302 pesticides and environmental contaminants in catfish by fast low-pressure GC-MS/MS and UHPLC-MS/MS, Food Chem., 2020, 319, 126592 CrossRef CAS PubMed
.
- N. Michlig, S. J. Lehotay, A. R. Lightfield, H. Beldoménico and M. R. Repetti, Validation of a high-throughput method for analysis of pesticide residues in hemp and hemp products, J. Chromatogr. A, 2021, 1645, 462097 CrossRef CAS PubMed
.
- S. H. Monteiro, S. J. Lehotay, Y. Sapozhnikova, E. Ninga and A. R. Lightfield, High-Throughput Mega-Method for the Analysis of Pesticides, Veterinary Drugs, and Environmental Contaminants by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry and Robotic Mini-Solid-Phase Extraction Cleanup + Low-Pressure Gas Chroma, J. Agric. Food Chem., 2021, 69, 1159–1168 CrossRef CAS PubMed
.
- S. H. Monteiro, S. J. Lehotay, Y. Sapozhnikova, E. Ninga, G. C. Moura Andrade and A. R. Lightfield, Validation of the QuEChERSER mega-method for the analysis of pesticides, veterinary drugs, and environmental contaminants in tilapia (Oreochromis niloticus), Food Addit. Contam.: Part A, 2022, 39, 699–709 CrossRef CAS PubMed
.
- E. Ninga, S. J. Lehotay, Y. Sapozhnikova, A. R. Lightfield, G. D. Strahan and S. H. Monteiro, Analysis of Pesticides, Veterinary Drugs, and Environmental Contaminants in Goat and Lamb by the QuEChERSER Mega-Method, Anal. Methods, 2022, 14, 2761–2770 RSC
.
- E. Ninga, Y. Sapozhnikova, S. J. Lehotay, A. R. Lightfield and S. H. Monteiro, High-Throughput Mega-Method for the Analysis of Pesticides, Veterinary Drugs, and Environmental Contaminants by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry and Robotic Mini-Solid-Phase Extraction Cleanup + Low-Pressure Gas Chroma, J. Agric. Food Chem., 2021, 69, 1169–1174 CrossRef CAS PubMed
.
- P. Eyring, T. Preiswerk, H. L. Frandsen and L. Duedahl-Olesen, Automated micro-solid-phase extraction clean-up of polycyclic aromatic hydrocarbons in food oils for analysis by gas chromatography–orbital ion trap mass spectrometry, J. Sep. Sci., 2021, 44, 600–608 CrossRef CAS PubMed
.
- E. Hakme and M. E. Poulsen, Evaluation of the automated micro-solid phase extraction clean-up system for the analysis of pesticide residues in cereals by gas chromatography-Orbitrap mass spectrometry, J. Chromatogr. A, 2021, 1652, 462384 CrossRef CAS PubMed
.
- N. Michlig and S. J. Lehotay, Evaluation of a septumless mini-cartridge for automated solid-phase extraction cleanup in gas chromatographic analysis of >250 pesticides and environmental contaminants in fatty and nonfatty foods, J. Chromatogr. A., 2022, 1685, 463596 CrossRef CAS PubMed
.
- EFICO Enviro 940 8 mm, https://www.biomar.com/da-dk.
- R. Leming and A. Lember, Chemical composition of expeller-extracted and cold-pressed rapeseed cake, Agraarteadus, 2005, 16, 103–109 Search PubMed
.
-
M. E. Poulsen, E. Ninga and E. Hakme, Proficiency Test on Pesticide Residues in Rapeseed Cake, p. 2021 Search PubMed
.
- E. Hakme, S. S. Herrmann and M. E. Poulsen, European Union Proficiency Tests for pesticide residues in cereals and feedstuff, from 2007 to 2022- Data collection experience, Food Control, 2023, 152, 109867 CrossRef
.
- E. Hakme, A. Lozano, S. Uclés, M. M. Gómez-Ramos and A. R. Fernández-Alba, High-throughput gas chromatography-mass spectrometry analysis of pesticide residues in spices by using the enhanced matrix removal-lipid and the sample dilution approach, J. Chromatogr. A, 2018, 1573, 28–41 CrossRef CAS PubMed
.
- S. Mandal, R. Poi, I. Ansary, D. K. Hazra, S. Bhattacharyya and R. Karmakar, Validation of a modified QuEChERS method to determine multiclass multipesticide residues in apple, banana and guava using GC-MS and LC-MS/MS and its application in real sample analysis, SN Appl. Sci., 2020, 2, 188 CrossRef CAS
.
- S. S. Herrmann and M. E. Poulsen, Clean-up of cereal extracts for gas chromatography-tandem quadrupole mass spectrometry pesticide residues analysis using primary secondary amine and C18, J. Chromatogr. A, 2015, 1423, 47–53 CrossRef CAS PubMed
.
- K. Sugitate, S. Nakamura, N. Orikata, K. Mizukoshi, M. Nakamura, A. Toriba and K. Hayakawa, Search of components causing matrix effects on GC/MS for pesticide analysis in food, J. Pestic. Sci., 2012, 37, 156–163 CrossRef CAS
.
- S. J. Lehotay, K. Maštovská and S. J. Yun, Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes, J. AOAC Int., 2005, 88, 630–638 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay00226a |
| This journal is © The Royal Society of Chemistry 2024 |