Comprehensive assessment of clean-up strategies for optimizing an analytical multi-method to determine pesticides and mycotoxins in Brazilian medicinal herbs using QuEChERS-LC-TQ-MS/MS
Marlos Eduardo
Zorzella Fontana
a,
Rosselei
Caiel da Silva
a,
Ingrid
Duarte dos Santos
ab,
Júlia Paula
Neu
 a,
Robson Dias
Wouters
a,
Robson Dias
Wouters
 a,
Paola Jennifer
Babinski
a,
Paola Jennifer
Babinski
 a,
Jessica Fernanda
Hoffmann
c,
Rochele Cassanta
Rossi
c,
Liliana
Essi
d and
Ionara Regina
Pizzutti
a,
Jessica Fernanda
Hoffmann
c,
Rochele Cassanta
Rossi
c,
Liliana
Essi
d and
Ionara Regina
Pizzutti
 *a
*a
aUFSM – Federal University of Santa Maria, Chemistry Department, Center of Research and Analysis of Residues and Contaminants (CEPARC), 97105-900, Santa Maria/RS, Brazil. E-mail: ionara.pizzutti@ceparc.com.br; Tel: +55 55 3220 9458
bUFSM – Federal University of Santa Maria, Food Science and Technology Department, 97105-900, Santa Maria/RS, Brazil
cUNISINOS – University of Vale do Rio dos Sinos, Health School – Professional Master's in Food, Nutrition and Health, 93022-000, São Leopoldo/RS, Brazil
dUFSM – Federal University of Santa Maria, Biology Department, 97105-900, Santa Maria/RS, Brazil
First published on 27th June 2024
Abstract
The use of medicinal herbs has increased significantly. However, the presence of pesticide residues and mycotoxins in medicinal herbs has generated constant discussion and concern among regulatory agencies. Developing and validating an analytical method for determining pesticides and mycotoxins in medicinal plants is challenging due to the naturally occurring substances in these plants. The purpose of this work was to develop and to optimize a sensitive, accurate, precise, effective QuEChERS method for simultaneous determination of over 160 pesticide and mycotoxin residues in complex medicinal plant matrices using LC-TQ-MS/MS. A comprehensive comparison of clean-up procedures and other parameters was conducted to achieve this goal. The validation procedure was performed according to SANTE 11312/2021. More polar analytes, such as acephate, methamidophos and omethoate, presented a higher negative matrix effect in both Melissa officinalis L. and Malva sylvestris L. However, other molecules, such as spirodiclofen, showed a 24% signal enhancement in M. officinalis and a 46% signal suppression in M. sylvestris, indicating that a representative matrix-matched calibration would lead to inaccurate quantification of the analyte. Accuracy and precision were satisfactory according to SANTE 11312/2021 for 157 pesticide residues and mycotoxins in M. officinalis and for 152 molecules in M. sylvestris. LOQs at 10 µg kg−1 were achieved for 117 pesticides in M. officinalis and 99 pesticides in M. sylvestris. Among the mycotoxins, all four aflatoxins (B1, B2, G1 and G2) presented LOQs of 5 µg kg−1, and ochratoxin A had an LOQ of 10 µg kg−1 in M. officinalis. The same LOQ values were shown for these mycotoxins in M. sylvestris, except for aflatoxin B2 and ochratoxin A, which had LOQs of 20 µg kg−1. Moreover, in Southern Brazil, there has been no previous study on mycotoxin and pesticide contamination in medicinal herbs. Therefore, the application of this method was assessed through the analysis of forty-two real samples. Imidacloprid was found in M. officinalis, and methyl pirimiphos was found in M. sylvestris. The proposed method not only serves as a helpful tool for routine monitoring but also offers a basis for further research on risk assessment and control in food safety.
Introduction
Brazil is one of the most biodiverse countries in the world, with plant species which have been widely used by the population for medicinal purposes as well as providing material for research into the search for new drugs against different diseases.1 During recent years, the consumption of natural resources has gained notoriety for its increase along with national policies related to traditional and complementary medicine. Medicinal plants have been an essential part of ancient healthcare practices and have become a valuable resource in the treatment of illnesses and pathologies.2Melissa officinalis L., popularly known as lemon balm, is an edible and medicinal plant belonging to the Lamiaceae. It has been traditionally used as a sedative, analgesic, and hypnotic,3 and with its antioxidant effects being beneficial to the brain, as a treatment for memory disorders and Alzheimers.4,5
Another plant that presents therapeutic properties is Malva sylvestris L., known as high mallow, which is another important medicinal plant and has been considered a good candidate for drug discovery.6 Currently distributed worldwide, M. sylvestris presents anti-inflammatory properties mainly due to the presence of some flavonoids and mucilage. M. sylvestris has been used to treat many diseases, such as gingivitis, toothache, abdominal pain, gastrointestinal disorders, and diarrhea. In addition, its flowers are recommended for acne, the treatment of eczema, and inflammatory diseases.7
The growing demand for medicinal plants requires an increase in production and thus, it is necessary to protect them from pests, increase their production and shelf life whilst reducing post-harvest and storage losses. Therefore, like other plants, medicinal herbs can not only be exposed to pesticides during agricultural practices but also contaminated by mycotoxins during processing and storage.8,9
According to Sedova,10 mycotoxins, pesticide residues, and toxic heavy metals are the most common chemical pollutants found in tea and medicinal herbs during production, storage, and consumption. Through eating polluted foods, chemical pollutants may cause significant health issues, such as carcinogenesis, immunosuppression, teratogenicity, as well as hepatotoxic, genotoxic, and nephrotoxic effects11,12 and result in huge commercial losses. For these reasons, the quality and safety of medicinal plants are of big concern13 and specific legislation for these matrices need to be created in order to control contamination by pesticide residues and mycotoxins. Since 2018, Brazilian legislation recommends the determination of pesticides according to RDC n° 105/2016 (ref. 14) and mycotoxins on herbal products, in all registration requests and post-registration petitions.
Several analytical methods can be used to identify and quantify this large variety of chemical compounds.15 In an effort to reduce the number of methods needed to perform a complete chemical analysis, recent trends have focused on the development of multi-residue16,17 and multi-class methods.18–20 Development of improved methods for multi-mycotoxin and multi-pesticide analysis, including sample preparation and extraction and detection parameters, has become an increasingly large research field due to co-occurrence processes while still responding to the wide range of physicochemical properties and low residue levels found in different matrices.21 These analyses are difficult since the analytes have varied properties and polarity. As a result, selecting the best extraction process can be difficult.13,22
Different methods for multi-compound analysis have been proposed for the analysis of mycotoxins and pesticides, in which ultra-performance liquid chromatography (UHPLC) coupled with tandem mass spectrometry (MS/MS) has become the technique of choice for the analysis of a wide range of contaminants in food. It allows the simultaneous determination and accurate quantification of several analytes at very low concentrations in complex matrices in a short chromatographic run time.23,24 It is important to have effective and reliable analytical methods for the determination of mycotoxins and pesticides at the legislated levels in representative samples, not only to perform accurate risk assessments, but also to enforce the regulatory limits established worldwide.21
A QuEChERS (quick, easy, cheap, effective, rugged and safe) method originally used just for pesticide residue analysis in vegetables and fruits16,17,25 has been further modified for pesticide determination in several matrices. Currently, this method is quickly becoming one of the most popular dispersive solid-phase extraction (d-SPE) methods in food safety.26 Parameters such as time, solvent consumption, simplicity, selectivity, and sensitivity are crucial when considering an appropriate extraction/clean-up strategy.21 According to recent investigations, different types of adsorbents, such as primary secondary amines (PSA), octadecyl (C18), and graphitized carbon black (GCB) have been used based on their physical and chemical properties.27,28
However, while many analytical methods have been reported for the determination of pesticides and mycotoxins in different foodstuffs,29,30 there is a lack of a simple and generic method for the simultaneous determination of such residues in medicinal plants due to matrices complexity as well as the diversity of species. Due to low water content, natural pigments, essential oils, and a high number of undesired components such as sugars, phenolics, and flavonoids, medicinal plants present more complicated interference when compared with other matrices, like fruits and vegetables.31 In addition, different species and parts of plants can affect analyte responses, making the development of analytical procedures a challenging task. Thus, it is necessary to develop a general multiclass-residue method to monitor different kinds of residues in medicinal plants, such as M. officinalis and M. sylvestris.
So far, there are no representative matrices for different medicinal parts and families, indicating that it is necessary to validate each medicinal plant separately. Additionally, even employing LC-MS/MS techniques for quantification, the present work is very significant considering that it is necessary to apply sample preparation for two distinct complex matrices whilst being able to minimize interference effects in addition to extracting with acceptable accuracy and precision the distinct classes of compounds (pesticides and mycotoxins).
The purpose of this work was to develop and optimize a sensitive, precise, effective QuEChERS method for the analysis of over 160 compounds in medicinal plant matrices by LC-MS/MS. As far as we know, the present study is the first method for simultaneous analysis of pesticides and mycotoxins in complex matrices such as M. sylvestris (flowers) and M. officinalis (leaves). In this matter, a comprehensive comparison of clean-up procedure efficiencies and other parameters were evaluated to achieve this goal. To ensure the adequate analysis of the selected mycotoxins and pesticides in medicinal plant samples, a validation process was ultimately performed for the most efficient extraction procedure. Moreover, in South Brazil, there has been no study on mycotoxin and pesticide contamination in medicinal herbs and an application of the method was assessed through the analysis of forty-two real samples. The proposed method not only works as a helpful tool for routine and surveillance monitoring but also offers a basis for further research on risk assessment and control in food safety.
Experimental
Chemicals and reagents
All reagents used were of at least analytical grade purity. Acetonitrile and acetone were obtained from Merck (Darmstadt, Germany), while methanol and toluene were purchased from Honeywell Chromasolv (Seelze, Germany). Anhydrous magnesium sulfate and sodium chloride were obtained from Êxodo Científica (São Paulo, Brazil), and formic acid from JT Baker (Deventer, Netherlands). Ultrapure water (resistivity of 18.2 MΩ cm) was obtained using a Milli-Q purification system (Millipore, Bedford, MA, USA).Two dispersive SPE (d-SPE) kits (Agilent Technologies, Santa Clara, CA, USA) were used for clean-up purposes. These kits contained 25 mg of primary-secondary amine (PSA), 2.5 mg of graphitized carbon (GCB) and 150 mg of MgSO4 (pigmented fruits and vegetables) (tests B and C – Table 1), or 25 mg of PSA, 7.5 mg of GCB and 150 mg of MgSO4 (highly pigmented fruits and vegetables) (tests D and E – Table 1).
| Extraction protocol | A | B | C | D | E |
|---|---|---|---|---|---|
| Slurry portion | 10 g of M. officinalis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) or 14 g of M. sylvestris (1 4 ratio) or 14 g of M. sylvestris (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) 6 ratio) |
||||
| Extraction solvent | 10 mL of acetonitrile | 10 mL of acetonitrile + 1% formic acid | |||
| Partitioning salts | 4 g MgSO4 + 1 g NaCl | ||||
| Clean-up | — | 1 mL upper layer to 25 mg of PSA, 2.5 mg of GCB and 150 mg of MgSO4 | 1 mL upper layer to 50 mg of PSA, 5 mg of GCB and 300 mg of MgSO4 | 1 mL upper layer to 25 mg of PSA, 7.5 mg of GCB and 150 mg of MgSO4 | |
| Dilution | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Analysis | LC-MS/MS | ||||
Reference standards
Reference standards of pesticides (purity > 97%) were obtained from Dr Ehrenstorfer (Augsburg, Germany), while the mycotoxin standards (purity > 98%) were obtained from Fermentek Biotechnology (Jerusalem, Israel) and Sigma-Aldrich (St. Louis, USA).Individual stock solutions of pesticides (1000 mg L−1) were prepared by dissolving the reference standards in toluene, methanol, or acetone, depending on their solubility. Similarly, individual stock solutions of mycotoxins (500 or 1000 mg L−1) were prepared in acetonitrile or methanol. A standard mixture solution of 150 pesticides (1 mg L−1) was prepared by diluting 100 µL of each stock solution in 100 mL of 0.1% formic acid in methanol (v/v). The 11 mycotoxins were divided into two groups based on their sensitivity in the liquid chromatographer-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) system. Group M1 included aflatoxins B1, B2, G1, G2, and ochratoxin A, while Group M2 included diacetoxyscirpenol (DAS), deoxynivalenol (DON), fumonisins B1 and B2, T2-toxin, zearalenone (ZEN). A solution containing 0.5 mg L−1 of standard mixture M1 and 25 mg L−1 of M2 was prepared by appropriately diluting the stock solutions with acetonitrile.
Analytical work solutions of pesticides and mycotoxins were prepared by suitably diluting the mixture solutions with acetonitrile. All solutions were stored at −18 °C in amber glass.
LC-MS/MS
Chromatographic analysis was performed using an Agilent 1260 prime II Liquid chromatography system coupled to a triple quadrupole mass spectrometer (LC-TQ-MS/MS) (ULTIVO, Agilent technologies, USA) with an Agilent Jet Stream Technology ion source (AJS), operating in dynamic multiple reaction monitoring (dMRM) mode. Chromatographic separations were carried out on an Infinity Lab Poroshell 120 EC1-C18 (2.1 mm i.d. O 100 mm O2.7 µm) reverse phase analytical column coupled to a pre-column (UHPLC GUARD Infinity Lab Poroshell) of the same stationary phase. Water (A) and acetonitrile (B), both acidified with 0.1% (v/v) formic acid, were used as the mobile phase at a constant flow rate of 0.3 mL min−1. The gradient elution program ranged from 20 to 90% B from 0 to 5 min. This condition was maintained for 4 min, then changed to 95% B from 9 to 9.25 min and maintained for 2 min. Finally, the mobile phase was changed to the initial composition from 11.25 to 14 min. The chromatographic column was maintained at 45 °C (±0.5 °C) and the injection volume was 2 µL.All mass spectrometer parameters were optimized using the Optimizer software version 1.1 (Agilent, USA).
Sample preparation
According to the Brazilian pharmacopeia,32 the pharmacologically active parts of M. officinalis are the dried leaves, while for M. sylvestris, they are the entire or fragmented dried flowers. Therefore, all samples used as blank samples (free of pesticides and mycotoxins) were in accordance with Brazilian pharmacopeia criteria.Commercially available organic samples identified by sellers as M. officinalis and M. sylvestris were purchased from local pharmacies in Santa Maria city, Brazil. These samples were checked for the absence of pesticides and mycotoxins before being used as blank samples for method optimization and validation.
Samples were obtained individually or in groups of packages with the same lot number, containing a minimum amount of 200 g, as recommended by sampling methods.33 The dried leaves and flowers were ground separately in a multiprocessor and sieved (granulometry 1 µm). Before the extraction procedure, the samples were hydrated for 30 min with ultrapure water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (w/w) for M. officinalis and M. sylvestris, respectively, at 8 °C, forming a slurry.
6 (w/w) for M. officinalis and M. sylvestris, respectively, at 8 °C, forming a slurry.
Extraction procedure
The extraction procedure employed was a modification of the QuEChERS method using the highly pigmented fruits and vegetables clean-up kit from Agilent Technologies. A slurry of 10 g (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) of M. officinalis leaves or 14 g (1
4 ratio) of M. officinalis leaves or 14 g (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) of M. sylvestris flowers was weighed in a 50 mL PTFE centrifuge tube. Subsequently, 10 mL of acetonitrile acidified with 1% formic acid was added, along with 40 µL of propoxur, which served as the internal standard solution. It should be noted that the concentration of propoxur in this study was 20 ng mL−1. The tubes were shaken using an automatic mechanical shaker (Orbital Shaker 3016, Gesellschaft für Labortechnik mbH, Germany) for 1 minute. Following this, 4 g of magnesium sulfate and 1 g of sodium chloride were added, and the samples were vortexed for an additional 1 minute. The extracts were then centrifuged at 4000 rpm for 4 minutes, and 1 mL of the supernatant was transferred to a dispersive clean-up kit. After homogenizing the tubes in a vortex for 1 minute, they were centrifuged again (4000 rpm, 4 minutes), and 0.5 mL of the extract was transferred to a vial and diluted with 0.5 mL of acetonitrile/water (1
6 ratio) of M. sylvestris flowers was weighed in a 50 mL PTFE centrifuge tube. Subsequently, 10 mL of acetonitrile acidified with 1% formic acid was added, along with 40 µL of propoxur, which served as the internal standard solution. It should be noted that the concentration of propoxur in this study was 20 ng mL−1. The tubes were shaken using an automatic mechanical shaker (Orbital Shaker 3016, Gesellschaft für Labortechnik mbH, Germany) for 1 minute. Following this, 4 g of magnesium sulfate and 1 g of sodium chloride were added, and the samples were vortexed for an additional 1 minute. The extracts were then centrifuged at 4000 rpm for 4 minutes, and 1 mL of the supernatant was transferred to a dispersive clean-up kit. After homogenizing the tubes in a vortex for 1 minute, they were centrifuged again (4000 rpm, 4 minutes), and 0.5 mL of the extract was transferred to a vial and diluted with 0.5 mL of acetonitrile/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) containing the injection internal standard solution of PCB-153 at a concentration of 100 ng mL−1.
1, v/v) containing the injection internal standard solution of PCB-153 at a concentration of 100 ng mL−1.
To develop a fast extraction protocol that causes less damage to the chromatographic system, which is robust and reliable, and still presents acceptable recovery rates, five preliminary studies were conducted to evaluate the accuracy, precision and matrix effects. For all tests, the slurries of M. officinalis and M. sylvestris samples were spiked (n = 3) at two different levels with pesticides (10 and 70 µg kg−1) and mycotoxins (group 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 20 µg kg−1; group 2
2 and 20 µg kg−1; group 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 1000 µg kg−1), simultaneously.
100 and 1000 µg kg−1), simultaneously.
Solvent extraction evaluation
Since the proposed method aims to extract a variety of target analytes with different polarities, pKa, and other chemical properties, two approaches were tested to evaluate the recovery rate of analytes. The first approach used pure acetonitrile according to the original QuEChERS method. The second approach employed acetonitrile acidified with 1% (v/v) formic acid to improve recovery, especially for mycotoxins.Sorbent evaluation for clean-up
The absence of and different proportions of dispersive solid-phase extraction (d-SPE) sorbents (Table 1) were tested for selectivity, sensitivity, reliability, acceptable accuracy and precision, and to achieve less damage to the chromatographic system. Mixtures of primary secondary amine (PSA) and graphitized carbon black (GCB) were tested to remove pigments (mostly chlorophyll), sugars, lipids, flavonoids, acids, and carotenoids.34Method performance
Analyte identification and confirmation were conducted according to SANTE document 11312/2021,35 including retention time standard (±0.1 min), and at least two product ions with fully overlapping peaks and ion ratio within ± 30%.Method validation
A validation protocol in accordance with SANTE document 11312/2021 (ref. 35) was conducted for the simultaneous determination of pesticides and mycotoxins in M. officinalis and M. sylvestris. The analytical method validation assessed the following parameters: sensitivity, selectivity, linearity of the analytical curves, matrix effects, trueness (expressed as recovery percentage), precision as repeatability RSDr and reproducibility (RSDWR), limit of detection (LOD), and limit of quantification (LOQ).For linearity, sensitivity, and matrix effect evaluation, seven different solutions for each concentration were prepared. For pesticides and mycotoxins of group 1, the concentrations of the solutions were 0.1, 0.5, 1, 5, 10, 25, 50 and 100 ng mL−1. For mycotoxins of group 2 the concentrations of analytical solutions prepared in neat organic solvent (acetonitrile) and in blank M. officinalis and M. sylvestris extracts were 5, 25, 50, 250, 500, 1250, 2500 and 5000 ng mL−1. Each solution was injected seven times.
The LOD was considered the lowest concentration level, injected repeatedly, obtained from 7 injections of an analytical solution prepared in blank matrix extract with a signal-to-noise ratio (S/N) ≥ 3. The LOQ was considered the lowest concentration level spiked with acceptable accuracy (70–120%) and precision (RSD ≤ 20%) obtained by the proposed analytical method.
Spiking/recovery experiments were performed by two different analysts on two different days to evaluate method reproducibility (RSDWR). Matrix effects were calculated as described by Dias et al.36 For accuracy (trueness and precision), recovery experiments were conducted by spiking blank M. officinalis and M. sylvestris at concentration levels of 10, 20, 50, and 70 µg kg−1 for pesticides; 2, 5, 10, and 20 µg kg−1 for mycotoxins of group 1; and 100, 250, 500, and 1000 µg kg−1 for mycotoxins of group 2. Seven replicates for each spiked level (n = 7) were performed by each analyst on two different days, totaling fourteen replicates (n = 14). All samples were extracted as mentioned in the section ‘Extraction Procedure’.
Repeatability (RSDr) was calculated for each analyst from recovery experiments performed using the same extraction protocol, quantification method, system, and blank sample on the same day. Reproducibility (RSDWR) was obtained via intermediate precision assessment by executing the same recovery experiments with different analysts, with a one-week interval between recovery experiments.
Sampling
The medicinal herb samples were obtained from the Public Market in Porto Alegre city, Rio Grande do Sul State, Brazil, due to the commercialization, consumer turnover, and location. The samples were collected from 10 commercial stores between May 2021 and July 2022. Each sample consisted of at least 200 g of medicinal herbs, comprising 23 samples of M. officinalis leaves and 19 samples of M. sylvestris flowers, totaling 42 samples over the course of the study.Results and discussion
Over the years, with the rise in food inspection and the escalating demand for quality control analyses, coupled with the need for promptly delivering results, multianalyte methods have garnered attention for their ability to analyze a diverse range of substances in a single operation. Methods enabling the simultaneous detection of pesticides and mycotoxins are available for various matrices, including fruits,37 cereals,38–40 wine,41 eggs,42 feed,15,43,44 raw coffee,45 and even some teas,18,46 spices, medicinal herbs,47 and infant milk formulae.48While there are methods available for analyzing teas and spices, these primarily focus on green and black teas. Additionally, there is currently no validated method for the simultaneous analysis of mycotoxins and pesticides in medicinal herbs, specifically comparing the dried flowers of M. sylvestris and the dried leaves of M. officinalis.
Clean-up optimization
Each matrix submitted to an extraction protocol for the analysis of residues and contaminants must undergo an optimization process. This optimization improves the selectivity of the analytes, reduces the matrix effect, and achieves quantification limits at low concentration levels while maintaining the accuracy and precision required in an analytical method.Medicinal herbs are particularly challenging matrices containing various extractable compounds such as pigments, essential oils, and flavonoids that may cause notable matrix effects in chromatographic analysis. The concern is not only about signal suppression caused by the co-extracts but also the potential damage caused to the systems, reducing the overall lifespan of the consumables. In addition, in long injection sequences, dirt can accumulate in the ionization source, decreasing the detectability along the sequence. Thus, a sample injected at the beginning and at the end of the sequence can present significant deviations in results, decreasing the accuracy and precision of the method.34
To improve method performance, different sorbent quantities were compared via recovery experiments, applying the following spike levels for mycotoxins groups: group 1: 2 and 20 µg kg−1; group 2: 100 and 1000 µg kg−1; and for pesticides: 10 and 70 µg kg−1, n = 3.
No clean-up step and two d-SPE kits (25 mg PSA + 2.5 mg GCB + 150 mg MgSO4 (tests B and C), and 25 mg PSA, 7.5 mg GCB + 150 mg of MgSO4 (tests D and E)) were tested (Table 1). The results are shown in Fig. 1 and 2, respectively, for the mycotoxins and pesticides. The concentration levels 1 and 2 were, respectively, 10 and 50 µg kg−1 for the pesticides; 2 and 10 µg kg−1 for the mycotoxins of group 1; and 100 and 500 µg kg−1 for the mycotoxins of group 2.
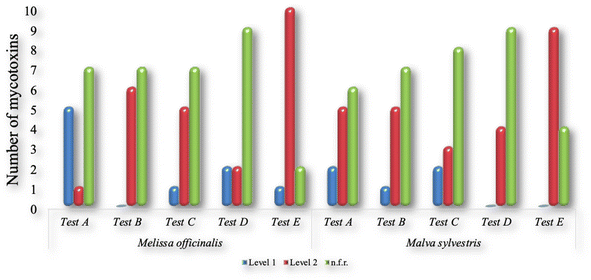 | ||
| Fig. 1 Number of mycotoxins presenting recoveries within the range of 70–120% in assays A, B, C, D and E, for M. officinalis and M. sylvestris. | ||
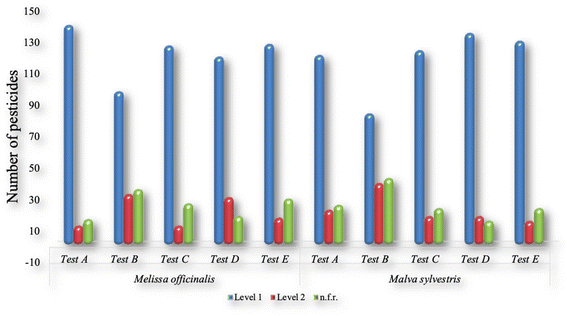 | ||
| Fig. 2 Number of pesticides presenting recoveries within the range of 70–120% in assays A, B, C, D and E, for M. officinalis and M. sylvestris. | ||
When no clean-up step was conducted, a highly pigmented extract was obtained for both matrices, causing the extensive deposition of co-extractives in the ion source, decreasing precision and causing a significant loss in detectability within the same injection sequence.
To efficiently remove pigment interferences from the extracts, graphitized carbon black (GCB) is a worthy option. However, it might also retain specific analytes, such as aromatic compounds and/or planar pesticides, due to π–π interactions.49 To mitigate this problem, small quantities of GCB were tested (2.5, 5 and 7.5 mg), with the latter being able to remove enough pigment while maintaining acceptable method accuracy and precision.
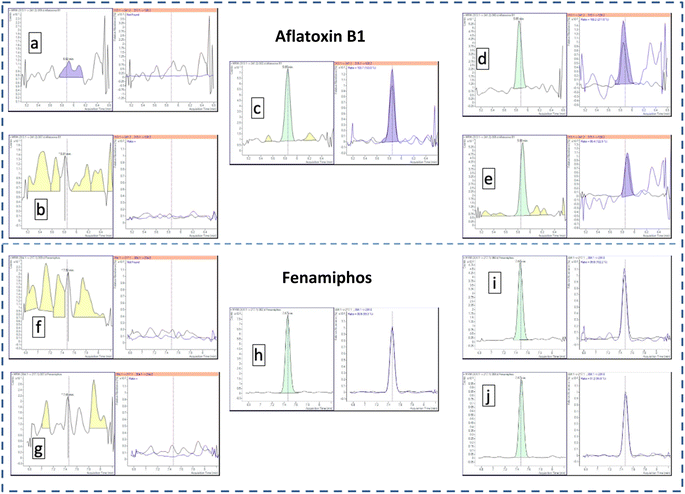
In this study, the final combination of PSA (25 mg) and GCB (7.5 mg) plus 150 mg of MgSO4 was the most effective for removing matrix co-extracts while maintaining acceptable recoveries and avoiding significant damage to the LC-TQ-MS/MS system. For instance, cyprodinil presented recoveries ranging from 71% to 83% and proper precision (RSD < 18%) despite the use of GCB. These results were also verified by Ly et al.50 who used GCB in green tea extraction and obtained satisfactory results for this pesticide. Fig. 3 represents a total ion chromatogram of two injections of the same vial, at the beginning and the end of a work list of over 100 injections and 16 h difference between those two injections, of fenamiphos and aflatoxin B1. No significant loss in precision was verified when comparing those two injections of both analytes.
Sample preparation optimization
Furthermore, the wide range of polarities, acidities and solubilities of pesticides and mycotoxins makes it challenging to develop and validate an appropriate analytical method for simultaneous determination. Additionally, representative food matrices belonging to the same food group (SANTE 11312/2021) are often used for optimization of time, reagents, and other parameters. However, in the case of dry medicinal plants, which contain a larger number of secondary metabolites (such as flavonoids, saponins and alkaloids), using a single representative matrix may present weaknesses in quantification due to differences in analytical signal suppression and enhancement in the LC-TQ-MS/MS system.For matrices with low water content, it is recommended to add water to increase the extraction efficiency. Therefore, a slurry was prepared with cold water (8 °C) for matrix rehydration (≈30 minutes) to facilitate the extraction of the analytes and prevent matrix components from being extracted and interfering with the instrumental analysis.
Analytical method validation
The validation data summarized in Table 2 show the linear range, matrix effect, LOQ, and LOD. Tables 3 and 4 demonstrate recoveries, precision (RSDr) and intermediate precision (RSDWR) for M. officinalis and M. sylvestris obtained from the method validation procedure for all spike levels studied.| Pesticide/Mycotoxin | Melissa officinalis | Malva sylvestris | ||||||
|---|---|---|---|---|---|---|---|---|
| Linear range (µg kg−1) | ME (%) | LOD (µgkg−1) | LOQ (µg kg−1) | Linear range (µg kg−1) | ME (%) | LOD (µg kg−1) | LOQ (µg kg−1) | |
| a Mycotoxins. b n.f.r.: not fulfill requirements of SANTE document. | ||||||||
| Acephate | 5–1000 | −74 | 5 | 10 | 5–1000 | −80 | 1 | 20 |
| Acetamiprid | 5–1000 | −10 | 5 | 10 | 1–1000 | 50 | 1 | 10 |
| Acetochlor | 10–1000 | −5 | 5 | 20 | 10–1000 | −16 | 5 | 10 |
| Aflatoxin B1 | 5–500 | −70 | 1 | 5 | 5–500 | −20 | 1 | 5 |
| Aflatoxin B2 | 5–500 | −71 | 3 | 5 | 10–500 | −17 | 1 | 20 |
| Aflatoxin G1 | 5–500 | −53 | 3 | 5 | 5–1000 | 31 | 4 | 5 |
| Aflatoxin G2 | 5–500 | −12 | 3 | 5 | 5–1000 | 9 | 1 | 5 |
| Aldicarb sulfone | 1–500 | −24 | 5 | 10 | 5–1000 | −29 | 1 | 10 |
| Aldicarb sulfoxide | 5–500 | −77 | 1 | 10 | 5–500 | −76 | 5 | 10 |
| Atrazine | 5–1000 | −17 | 1 | 10 | 5–500 | −10 | 1 | 10 |
| Azamethiphos | 5–500 | 0 | 1 | 10 | 5–1000 | −4 | 1 | 10 |
| Azinphos-methyl | 10–500 | 23 | 5 | 10 | 50–1000 | −15 | 1 | 50 |
| Azoxystrobin | 5–500 | 55 | 1 | 10 | 10–500 | 29 | 1 | 10 |
| Bifenazate | 10–500 | 11 | 1 | 10 | 10–500 | 11 | 1 | n.f.r.b |
| Bitertanol | 5–1000 | 20 | 1 | 10 | 5–1000 | −12 | 1 | 20 |
| Boscalid | 10–500 | −8 | 1 | 10 | 5–500 | −10 | 1 | 10 |
| Bupirimate | 5–500 | −26 | 1 | 10 | 5–500 | −21 | 1 | 10 |
| Buprofezin | 5–500 | −32 | 1 | 10 | 5–500 | −37 | 1 | 10 |
| Cadusafos | 10–500 | 8 | 1 | 10 | 5–1000 | 47 | 1 | 10 |
| Carbaryl | 5–500 | −22 | 1 | 10 | 5–1000 | −18 | 1 | 10 |
| Carbendazin | 5–500 | −67 | 1 | 10 | 10–1000 | −75 | 1 | 70 |
| Carbofuran | 5–500 | −11 | 1 | 10 | 10–500 | 7 | 1 | 10 |
| Carpropamid | 5–500 | −13 | 5 | 10 | 5–500 | −28 | 1 | 20 |
| Chlorantraniliprol | 5–500 | −5 | 5 | 20 | 5–1000 | 6 | 5 | 20 |
| Chlorfenvinphos | 10–500 | 13 | 1 | 10 | 5–500 | 3 | 5 | 10 |
| Chlorpyrifos | 5–500 | −3 | 5 | 10 | 5–1000 | −19 | 5 | 10 |
| Clofentezine | 10–1000 | −25 | 8 | 10 | 10–1000 | −7 | 8 | 10 |
| Clomazone | 10–500 | −14 | 1 | 10 | 5–1000 | −13 | 1 | 10 |
| Clothianidin | 10–1000 | −14 | 8 | 10 | 5–1000 | −2 | 5 | 10 |
| Cyazofamid | 50–500 | −29 | 5 | 10 | 10–500 | −36 | 5 | 20 |
| Cyproconazol | 10–500 | 7 | 5 | 10 | 5–1000 | −14 | 5 | 10 |
| Cyprodinil | 5–500 | −48 | 5 | 10 | 10–500 | −48 | 5 | 10 |
| Demeton-S-methyl sulfone | 5–1000 | 9 | 5 | 10 | 5–1000 | 21 | 1 | 10 |
| Demeton-S-methyl sulfoxide | 10–1000 | −72 | 5 | 10 | 5–1000 | −71 | 1 | 20 |
| Deoxynivalenol | 250–5000 | −67 | 50 | 250 | 250–5000 | −65 | 250 | 500 |
| Diacetoxyscirpenol | 250–5000 | −15 | 25 | 250 | 500–2500 | 21 | 250 | 500 |
| Diazinon | 10–500 | −12 | 1 | 10 | 5–1000 | 2 | 1 | 10 |
| Diethofencarb | 10–500 | 17 | 1 | 10 | 50–1000 | n.f.r.b | 1 | n.f.r.b |
| Difenoconazole | 5–1000 | −15 | 1 | 10 | 10–1000 | −7 | 1 | 10 |
| Diflubenzuron | 50–1000 | −18 | 10 | n.f.r.b | 50–1000 | 15 | 10 | 70 |
| Dimethoate | 1–1000 | −23 | 1 | 10 | 1–1000 | −3 | 1 | 10 |
| Dimethomorph | 10–500 | 42 | 1 | 10 | 50–500 | 50 | 1 | 10 |
| Diniconazol | 5–500 | −8 | 5 | 10 | 5–1000 | −10 | 1 | 10 |
| Diphenylamine | 5–1000 | −17 | 1 | 10 | 5–500 | −34 | 5 | 10 |
| Diuron | 10–500 | −42 | 5 | 10 | 5–1000 | −8 | 1 | 10 |
| DMST | 10–1000 | −7 | 10 | 50 | 10–1000 | 5 | 10 | 50 |
| Epoxiconazol | 10–1000 | −4 | 1 | 10 | 10–1000 | 15 | 1 | 10 |
| Ethion | 10–500 | 58 | 1 | 10 | 10–500 | −5 | 1 | 10 |
| Ethiprole | 10–500 | −15 | 1 | 10 | 10–500 | −7 | 5 | 10 |
| Ethoprophos | 10–500 | 10 | 1 | 10 | 5–500 | −7 | 5 | 10 |
| Etofenprox | 10–500 | −10 | 1 | 10 | 5–1000 | −6 | 1 | 10 |
| Etoxazol | 10–1000 | −19 | 1 | 10 | 5–1000 | −44 | 1 | 10 |
| Fenamidone | 5–500 | 3 | 1 | 10 | 5–500 | 5 | 1 | 10 |
| Fenamiphos | 10–500 | 78 | 1 | 10 | 5–1000 | 29 | 1 | 20 |
| Fenarimol | 5–1000 | −17 | 5 | 20 | 5–500 | 15 | 1 | 50 |
| Fenazaquin | 5–500 | 30 | 1 | 10 | 5–1000 | −29 | 1 | 10 |
| Fenbuconazol | 5–500 | −2 | 1 | 10 | 5–500 | 7 | 5 | 20 |
| Fenhexamid | 10–500 | −37 | 1 | 10 | 10–500 | 9 | 1 | 20 |
| Fenobucarb | 10–500 | −19 | 1 | 10 | 10–500 | −32 | 1 | 10 |
| Fenoxycarb | 10–500 | −31 | 5 | 20 | 10–1000 | −40 | 1 | 20 |
| Fenpropimorph | 5–1000 | −13 | 1 | 10 | 5–1000 | −14 | 1 | 10 |
| Fenpyroximate | 5–500 | −4 | 1 | 10 | 5–1000 | −16 | 1 | 10 |
| Fensulfothion | 50–1000 | 39 | 5 | 50 | 50–1000 | 23 | 10 | 50 |
| Fluazifop-butyl | 10–500 | −5 | 1 | 10 | 5–1000 | 7 | 1 | 10 |
| Fludioxonil | 5–1000 | −17 | 5 | 10 | 10–500 | −20 | 1 | 10 |
| Flufenoxuron | 10–500 | −42 | 1 | 10 | 50–1000 | −24 | 20 | 50 |
| Fluquinconazol | 10–1000 | −25 | 10 | 20 | 5–500 | −25 | 1 | 20 |
| Flusilazol | 5–500 | −8 | 1 | 10 | 5–500 | −26 | 1 | 10 |
| Flutolanil | 50–1000 | −2 | 1 | 50 | 5–1000 | −10 | 1 | 20 |
| Flutriafol | 10–1000 | −9 | 8 | 10 | 5–500 | 2 | 1 | 10 |
| Fosthiazate | 10–1000 | 7 | 1 | 10 | 5–1000 | −6 | 1 | 10 |
| Fumonisin B1 | 250–5000 | 51 | 250 | 500 | 250–5000 | −1 | 250 | n.f.r. |
| Fumonisin B2 | 250–5000 | −16 | 250 | 500 | 250–5000 | −31 | 500 | n.f.r. |
| Furalaxyl | 10–500 | 18 | 1 | 10 | 5–500 | 20 | 1 | 20 |
| Furathiocarb | 5–1000 | 8 | 1 | 10 | 5–1000 | 4 | 1 | 10 |
| Halofenozide | 50–1000 | −23 | 10 | 50 | 10–1000 | −33 | 10 | n.f.r.b |
| Haloxyfop-2-ethoxyethyl | 5–1000 | −8 | 1 | 10 | 5–1000 | −41 | 1 | n.f.r.b |
| Hexaconazol | 5–1000 | −16 | 5 | 10 | 5–1000 | −31 | 5 | 10 |
| Hexytiazox | 5–1000 | −6 | 1 | 10 | 1–1000 | −42 | 1 | 10 |
| Imazalil | 10–1000 | −26 | 10 | 20 | 10–1000 | −22 | 5 | 10 |
| Imazapic | 5–500 | −32 | 5 | 10 | 10–500 | 5 | 5 | 10 |
| Imazetapyr | 5–1000 | 3 | 1 | 10 | 5–1000 | 35 | 5 | 10 |
| Imidacloprid | 5–1000 | 31 | 1 | 10 | 10–1000 | 98 | 8 | 10 |
| Indoxacarb | 5–1000 | 34 | 1 | 20 | 5–1000 | 97 | 5 | 10 |
| Iprovalicarb | 5–1000 | 20 | 1 | 10 | 5–1000 | −6 | 1 | 10 |
| Isoxaflutole | 5–1000 | −30 | 5 | 50 | 50–1000 | −20 | 10 | 50 |
| Kresoxim-methyl | 5–1000 | −16 | 1 | 10 | 5–1000 | −16 | 5 | 20 |
| Linuron | 50–1000 | −40 | 10 | 50 | 50–1000 | −32 | 20 | 50 |
| Lufenuron | 10–500 | −58 | 8 | 10 | 5–500 | −84 | 5 | 10 |
| Malathion | 5–1000 | 14 | 1 | 10 | 5–1000 | 11 | 5 | 20 |
| Mecarbam | 5–1000 | 5 | 5 | 10 | 5–1000 | 4 | 1 | 10 |
| Mepanipyrim | 5–500 | −44 | 1 | 20 | 10–1000 | −17 | 10 | 50 |
| Metalaxyl | 5–1000 | 23 | 1 | 20 | 10–1000 | 33 | 1 | 10 |
| Metconazole | 5–1000 | −75 | 5 | 20 | 5–1000 | −29 | 5 | 20 |
| Methamidophos | 50–1000 | −77 | 10 | 50 | 10–1000 | −76 | 5 | 70 |
| Methidathion | 10–1000 | −5 | 10 | 20 | 50–1000 | −31 | 5 | n.f.r.b |
| Methiocarb | 5–1000 | −33 | 5 | 20 | 5–1000 | −30 | 5 | 20 |
| Methomyl | 5–1000 | −10 | 1 | 10 | 5–500 | −21 | 1 | 10 |
| Methoxyfenozide | 5–1000 | 31 | 1 | 10 | 5–500 | 15 | 1 | 20 |
| Monocrotophos | 1–1000 | −62 | 1 | 10 | 10–1000 | −57 | 1 | 20 |
| Myclobutanil | 5–1000 | −45 | 1 | 10 | 5–1000 | −1 | 1 | 10 |
| Nitenpyram | 10–1000 | −76 | 5 | 50 | 50–1000 | −82 | 1 | 70 |
| Ochratoxin A | 10–1000 | 20 | 8 | 10 | 10–1000 | 6 | 10 | 20 |
| Ofurace | 10–500 | 46 | 1 | 10 | 5–1000 | 59 | 1 | 10 |
| Omethoate | 5–1000 | −76 | 1 | 10 | n.f.r.b | −76 | n.f.r.b | n.f.r.b |
| Oxadixyl | 10–500 | 5 | 5 | 10 | 5–1000 | −10 | 1 | 10 |
| Oxamyl | 5–1000 | −20 | 1 | 10 | 5–1000 | −35 | 1 | 10 |
| Paclobutrazol | 10–1000 | 2 | 10 | 20 | 5–500 | −4 | 1 | 10 |
| Penconazole | 5–500 | −22 | 1 | 10 | 10–1000 | −29 | 1 | 10 |
| Pencycuron | 5–1000 | 6 | 1 | 10 | 5–1000 | 64 | 1 | 10 |
| Pendimethalin | 5–500 | −4 | 5 | 10 | 5–1000 | −21 | 1 | 10 |
| Phenothrin | 50–1000 | −19 | 20 | 50 | 50–1000 | 2 | 20 | 50 |
| Phenthoate | 5–500 | −28 | 5 | 10 | 5–500 | −21 | 1 | 10 |
| Phosalone | 50–1000 | −4 | 10 | 50 | 50–1000 | 2 | 20 | 50 |
| Phosmet | 50–1000 | −8 | 5 | 50 | 5–1000 | −21 | 5 | 20 |
| Picoxystrobin | 5–500 | −18 | 1 | 10 | 10–1000 | −22 | 1 | 10 |
| Piperonyl butoxide | 5–1000 | −25 | 1 | 10 | 5–1000 | −27 | 1 | 10 |
| Pirimicarb | 5–1000 | −21 | 1 | 10 | 5–1000 | −17 | 1 | 10 |
| Pirimiphos-ethyl | 1–1000 | −15 | 1 | 10 | 5–1000 | −25 | 1 | 10 |
| Pirimiphos-methyl | 5–1000 | −20 | 1 | 10 | 5–1000 | −15 | 1 | 10 |
| Prochloraz | 1–1000 | 11 | 1 | 10 | 5–1000 | 6 | 1 | 10 |
| Profenofos | 5–500 | −29 | 1 | 10 | 5–1000 | −45 | 1 | 20 |
| Prometryn | 5–1000 | −22 | 1 | 10 | 5–1000 | −20 | 1 | 10 |
| Propamocarb | 10–500 | −36 | 1 | n.f.r.b | 10–500 | −61 | 1 | 70 |
| Propanil | 1–1000 | −26 | 10 | 50 | 5–1000 | −21 | 5 | 50 |
| Prophan | 10–1000 | −33 | 8 | 10 | 50–1000 | −8 | 20 | 50 |
| Propiconazol | 5–500 | −13 | 1 | 10 | 5–500 | 5 | 1 | 10 |
| Propyzamide | 10–1000 | −19 | 8 | 10 | 5–1000 | 22 | 5 | 10 |
| Pyraclostrobin | 5–1000 | −27 | 1 | 10 | 5–1000 | −25 | 1 | 10 |
| Pyrazophos | 10–500 | 35 | 5 | 10 | 5–1000 | 50 | 1 | 10 |
| Pyridaben | 5–1000 | −9 | 1 | 10 | 5–1000 | −58 | 1 | 10 |
| Pyrimethanil | 5–500 | −38 | 1 | 10 | 5–1000 | −31 | 1 | 10 |
| Pyriproxyfen | 5–500 | −24 | 1 | 10 | 5–1000 | −30 | 1 | 10 |
| Quinalphos | 1–1000 | −42 | 1 | 10 | 5–500 | −18 | 5 | 10 |
| Quinoxyfen | 1–1000 | −16 | 1 | 10 | 5–1000 | −15 | 1 | 10 |
| Simazine | 5–500 | −35 | 1 | 10 | 10–1000 | −23 | 5 | 10 |
| Spinosyn A | 5–1000 | −39 | 1 | 10 | 5–1000 | −25 | 1 | 20 |
| Spinosyn D | 5–1000 | −35 | 5 | 10 | 5–1000 | −35 | 5 | 10 |
| Spirodiclofen | 5–500 | 24 | 5 | 50 | 5–500 | −46 | 5 | 10 |
| Spiromesifen | 5–500 | 19 | 5 | 20 | 10–1000 | −58 | 10 | 20 |
| Spiroxamine | 1–1000 | −11 | 1 | 10 | 1–1000 | −11 | 1 | 10 |
| Tau-fluvalinate | 50–1000 | 19 | 50 | 70 | 50–1000 | −55 | 10 | 50 |
| Tebuconazol | 5–500 | 8 | 5 | 10 | 5–500 | 14 | 1 | 10 |
| Tebufenozide | 5–1000 | −12 | 1 | 20 | 5–500 | −14 | 1 | 10 |
| Tebufenpyrad | 5–500 | −15 | 5 | 10 | 5–1000 | −35 | 1 | 10 |
| Terbutryn | 5–1000 | −37 | 1 | 10 | 5–1000 | −20 | 1 | 10 |
| Tetrachlorvinphos | 10–1000 | −13 | 10 | 50 | 50–1000 | −58 | 10 | 50 |
| Tetraconazole | 5–500 | 0 | 5 | 10 | 10–1000 | −4 | 5 | 10 |
| Tetramethrin | 10–1000 | −4 | 8 | 10 | 10–500 | −13 | 5 | 10 |
| Thiacloprid | 1–500 | −8 | 1 | 10 | 5–500 | 2 | 1 | 10 |
| Thiamethoxam | 5–1000 | −7 | 5 | 70 | 5–500 | 17 | 1 | 10 |
| Thiodicarb | 5–500 | −2 | 5 | 10 | 10–1000 | 55 | 1 | n.f.r.b |
| Toxin T2 | 50–2500 | −12 | 25 | 500 |
250–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
3 | 5 | 1000 |
| Triadimefon | 10–1000 | 9 | 8 | 10 | 10–1000 | 49 | 1 | 10 |
| Triadimenol | 10–1000 | 21 | 8 | n.f.r.b | 1–500 | 23 | 1 | 70 |
| Triazophos | 5–500 | 20 | 1 | 10 | 10–1000 | −22 | 1 | 20 |
| Trifloxystrobin | 5–500 | −8 | 1 | 10 | 5–1000 | −45 | 1 | 10 |
| Triflumizol | 5–1000 | −40 | 1 | 10 | 10–1000 | −40 | 1 | 10 |
| Triticonazol | 1–1000 | 4 | 1 | 10 | 5–500 | −6 | 1 | 10 |
| Zearalenone | 25–2500 | −31 | 25 | 250 |
250–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
−35 | 25 | 500 |
| Zoxamide | 5–500 | −19 | 5 | 10 | 5–500 | −21 | 5 | 10 |
| Concentration 1 | Concentration 2 | Concentration 3 | Concentration 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | |||||
| Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | |||||||||
| a Mycotoxins. b n.f.r.: not fulfill requirements of SANTE document. | ||||||||||||||||
| Acephate | 82 (16) | 76 (15) | 79 (15) | 0.214 | 82 (8) | 74 (11) | 78 (11) | 0.156 | 81 (5) | 80 (20) | 81 (14) | 0.949 | 91 (8) | 85 (10) | 88 (9) | 0.109 |
| Acetamiprid | 99 (15) | 85 (7) | 92 (14) | 0.071 | 87 (14) | 76 (6) | 81 (13) | 0.075 | 95 (16) | 83 (11) | 89 (16) | 0.102 | 83 (16) | 79 (5) | 81 (12) | 0.496 |
| Acetochlor | 90 (27) | 81 (12) | 86 (22) | 0.424 | 91 (14) | 78 (12) | 84 (15) | 0.139 | 88 (14) | 87 (16) | 88 (15) | 0.926 | 87 (9) | 82 (15) | 84 (12) | 0.526 |
| Aflatoxin B1 | 60 (60) | 114 (26) | 87 (48) | 0.000 | 99 (18) | 95 (19) | 97 (17) | 0.785 | 88 (16) | 104 (13) | 96 (17) | 0.112 | 84 (17) | 97 (8) | 91 (14) | 0.138 |
| Aflatoxin B2 | 77 (19) | 31 (0) | 54 (47) | 0.000 | 83 (12) | 95 (15) | 89 (15) | 0.196 | 87 (18) | 92 (8) | 90 (13) | 0.406 | 83 (7) | 89 (8) | 86 (8) | 0.056 |
| Aflatoxin G1 | n.f.r. | n.f.r. | n.f.r. | n.f.r. | 86 (19) | 83 (18) | 84 (18) | 0.543 | 85 (9) | 88 (6) | 87 (8) | 0.418 | 91 (14) | 99 (5) | 95 (11) | 0.083 |
| Aflatoxin G2 | n.f.r. | n.f.r. | n.f.r. | n.f.r. | 102 (19) | 79 (6) | 90 (20) | 0.035 | 97 (15) | 81 (11) | 89 (16) | 0.093 | 80 (12) | 73 (6) | 77 (11) | 0.060 |
| Aldicarb sulfone | 98 (15) | 91 (14) | 95 (15) | 0.302 | 98 (8) | 91 (11) | 95 (10) | 0.064 | 106 (5) | 113 (16) | 110 (12) | 0.361 | 103 (14) | 106 (11) | 105 (12) | 0.547 |
| Aldicarb sulfoxide | 94 (19) | 81 (15) | 87 (18) | 0.212 | 83 (13) | 75 (16) | 79 (14) | 0.334 | 80 (14) | 75 (5) | 77 (11) | 0.224 | 81 (4) | 82 (8) | 81 (6) | 0.762 |
| Atrazine | 100 (10) | 89 (12) | 95 (12) | 0.163 | 91 (8) | 83 (11) | 87 (10) | 0.083 | 88 (4) | 78 (12) | 83 (10) | 0.019 | 83 (9) | 76 (9) | 79 (10) | 0.052 |
| Azamethiphos | 85 (15) | 75 (3) | 80 (13) | 0.114 | 87 (11) | 75 (7) | 81 (12) | 0.060 | 86 (12) | 78 (22) | 82 (17) | 0.217 | 96 (7) | 84 (15) | 90 (13) | 0.155 |
| Azinphos-methyl | 88 (16) | 81 (16) | 85 (16) | 0.431 | 92 (18) | 102 (6) | 97 (13) | 0.196 | 80 (8) | 74 (4) | 77 (7) | 0.141 | 96 (14) | 105 (4) | 101 (11) | 0.087 |
| Azoxystrobin | 95 (13) | 99 (6) | 97 (10) | 0.493 | 91 (9) | 81 (8) | 86 (10) | 0.060 | 85 (10) | 78 (18) | 81 (14) | 0.236 | 83 (18) | 71 (5) | 77 (16) | 0.091 |
| Bifenazate | 78 (9) | 86 (9) | 82 (10) | 0.081 | 84 (16) | 91 (1) | 88 (11) | 0.283 | 72 (3) | 69 (8) | 71 (6) | 0.260 | 86 (15) | 99 (8) | 93 (13) | 0.072 |
| Bitertanol | 85 (13) | 81 (16) | 83 (14) | 0.505 | 85 (13) | 77 (15) | 81 (14) | 0.076 | 91 (7) | 87 (20) | 89 (14) | 0.490 | 90 (8) | 96 (20) | 93 (15) | 0.480 |
| Boscalid | 96 (12) | 107 (12) | 101 (13) | 0.119 | 94 (7) | 99 (5) | 96 (6) | 0.256 | 87 (3) | 80 (11) | 84 (9) | 0.108 | 83 (8) | 80 (20) | 81 (14) | 0.764 |
| Bupirimate | 98 (9) | 91 (11) | 94 (10) | 0.094 | 89 (7) | 84 (15) | 86 (12) | 0.303 | 85 (3) | 80 (18) | 82 (13) | 0.409 | 80 (8) | 74 (10) | 77 (10) | 0.117 |
| Buprofezin | 90 (11) | 79 (19) | 85 (16) | 0.165 | 86 (5) | 76 (19) | 81 (14) | 0.153 | 83 (2) | 75 (18) | 79 (13) | 0.177 | 74 (15) | 86 (16) | 80 (17) | 0.196 |
| Cadusafos | 96 (10) | 98 (8) | 97 (9) | 0.735 | 91 (6) | 101 (14) | 96 (12) | 0.053 | 87 (5) | 77 (16) | 82 (13) | 0.141 | 87 (9) | 83 (16) | 85 (13) | 0.496 |
| Carbaryl | 93 (11) | 78 (18) | 85 (16) | 0.089 | 89 (11) | 83 (7) | 86 (10) | 0.197 | 85 (10) | 77 (11) | 81 (12) | 0.061 | 78 (18) | 87 (2) | 83 (13) | 0.159 |
| Carbendazim | 79 (9) | 72 (14) | 75 (12) | 0.258 | 74 (11) | 77 (19) | 76 (15) | 0.517 | 72 (15) | 80 (11) | 76 (14) | 0.137 | 71 (6) | 74 (6) | 72 (6) | 0.35 |
| Carbofuran | 111 (9) | 100 (13) | 106 (12) | 0.082 | 111 (11) | 99 (8) | 105 (11) | 0.073 | 118 (8) | 108 (16) | 113 (13) | 0.080 | 113 (11) | 103 (5) | 108 (9) | 0.083 |
| Carpropamid | 84 (18) | 94 (17) | 89 (18) | 0.306 | 82 (14) | 95 (19) | 88 (18) | 0.161 | 83 (12) | 96 (12) | 89 (14) | 0.115 | 97 (13) | 103 (12) | 100 (12) | 0.387 |
| Chlorantraniliprole | 56 (41) | 39 (37) | 48 (43) | 0.247 | 82 (18) | 86 (19) | 84 (18) | 0.579 | 96 (15) | 85 (19) | 90 (17) | 0.240 | 92 (9) | 77 (17) | 85 (15) | 0.074 |
| Chlorfenvinphos | 92 (18) | 108 (10) | 100 (16) | 0.134 | 94 (13) | 101 (4) | 97 (10) | 0.156 | 80 (9) | 79 (5) | 80 (7) | 0.749 | 85 (16) | 85 (5) | 85 (12) | 0.931 |
| Chlorpyrifos | 101 (15) | 92 (19) | 96 (17) | 0.339 | 97 (20) | 106 (19) | 102 (19) | 0.335 | 76 (10) | 72 (8) | 74 (9) | 0.336 | 84 (12) | 79 (8) | 82 (11) | 0.272 |
| Clofentezine | 83 (17) | 99 (7) | 91 (15) | 0.065 | 100 (9) | 110 (14) | 105 (13) | 0.170 | 78 (14) | 73 (10) | 75 (13) | 0.370 | 91 (15) | 79 (10) | 85 (15) | 0.054 |
| Clomazone | 88 (15) | 80 (6) | 84 (12) | 0.193 | 90 (8) | 83 (7) | 87 (9) | 0.060 | 85 (5) | 77 (16) | 81 (12) | 0.216 | 83 (16) | 75 (15) | 79 (16) | 0.388 |
| Clothianidin | 89 (18) | 77 (8) | 83 (16) | 0.076 | 98 (7) | 85 (22) | 91 (16) | 0.145 | 99 (11) | 87 (14) | 93 (14) | 0.07 | 90 (11) | 79 (9) | 84 (12) | 0.134 |
| Cyazofamid | 103 (16) | 100 (14) | 101 (15) | 0.765 | 83 (13) | 76 (10) | 79 (12) | 0.132 | 88 (9) | 76 (19) | 82 (15) | 0.172 | 95 (8) | 82 (19) | 88 (15) | 0.095 |
| Cyproconazole | 102 (10) | 105 (10) | 103 (10) | 0.641 | 82 (12) | 81 (10) | 81 (10) | 0.808 | 87 (4) | 80 (13) | 84 (10) | 0.159 | 83 (13) | 72 (5) | 78 (12) | 0.057 |
| Cyprodinil | 83 (10) | 79 (4) | 81 (8) | 0.475 | 77 (6) | 72 (13) | 75 (10) | 0.407 | 71 (6) | 75 (10) | 73 (9) | 0.476 | 73 (4) | 79 (8) | 76 (8) | 0.063 |
| Demeton-S-methyl sulfone | 103 (17) | 95 (3) | 99 (13) | 0.254 | 96 (8) | 89 (12) | 93 (11) | 0.176 | 94 (4) | 83 (18) | 89 (13) | 0.095 | 96 (20) | 80 (9) | 88 (19) | 0.075 |
| Demeton-S-methyl sulfoxide | 86 (9) | 97 (17) | 91 (15) | 0.163 | 90 (18) | 78 (4) | 84 (16) | 0.083 | 79 (12) | 75 (20) | 77 (16) | 0.455 | 79 (12) | 73 (11) | 76 (12) | 0.359 |
| Deoxynivalenol | n.f.r. | n.f.r. | n.f.r. | n.f.r. | 89 (9) | 101 (16) | 95 (15) | 0.092 | 86 (14) | 97 (5) | 91 (12) | 0.077 | 84 (9) | 78 (2) | 81 (8) | 0.054 |
| Diacetoxyscirpenol | 67 (17) | 128 (161) | 98 (148) | 0.000 | 85 (15) | 90 (1) | 88 (11) | 0.353 | 81 (11) | 76 (1) | 79 (8) | 0.144 | 97 (10) | 99 (9) | 98 (9) | 0.697 |
| Diazinon | 95 (10) | 101 (8) | 98 (10) | 0.266 | 94 (5) | 99 (10) | 97 (8) | 0.322 | 92 (4) | 84 (6) | 88 (7) | 0.052 | 87 (8) | 84 (6) | 86 (7) | 0.467 |
| Diethofencarb | 91 (14) | 79 (9) | 85 (14) | 0.124 | 89 (10) | 80 (7) | 85 (10) | 0.127 | 87 (7) | 88 (7) | 88 (7) | 0.648 | 81 (15) | 88 (7) | 84 (12) | 0.175 |
| Difenoconazole | 94 (16) | 81 (13) | 87 (16) | 0.073 | 91 (10) | 83 (3) | 87 (9) | 0.051 | 81 (6) | 72 (15) | 76 (12) | 0.094 | 80 (12) | 73 (1) | 76 (9) | 0.095 |
| Diflubenzuron | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r. | n.f.r. |
| Dimethoate | 103 (16) | 87 (10) | 95 (16) | 0.073 | 100 (11) | 91 (9) | 95 (11) | 0.180 | 98 (9) | 85 (13) | 91 (13) | 0.091 | 89 (17) | 88 (3) | 88 (12) | 0.799 |
| Dimethomorph | 96 (20) | 81 (15) | 89 (19) | 0.059 | 101 (9) | 94 (12) | 98 (11) | 0.185 | 87 (15) | 77 (12) | 82 (15) | 0.110 | 91 (17) | 81 (17) | 86 (17) | 0.278 |
| Diniconazole | 89 (19) | 78 (9) | 83 (16) | 0.159 | 88 (10) | 83 (13) | 86 (11) | 0.141 | 86 (8) | 76 (11) | 81 (11) | 0.071 | 83 (14) | 72 (8) | 78 (13) | 0.097 |
| Diphenylamine | 86 (19) | 83 (1) | 84 (13) | 0.590 | 88 (17) | 78 (16) | 83 (17) | 0.196 | 90 (16) | 78 (15) | 84 (17) | 0.112 | 82 (11) | 76 (14) | 79 (13) | 0.183 |
| Diuron | 83 (19) | 81 (10) | 82 (15) | 0.687 | 81 (13) | 72 (12) | 76 (13) | 0.220 | 78 (12) | 74 (15) | 76 (13) | 0.567 | 82 (12) | 70 (11) | 76 (14) | 0.095 |
| DMST | 64 (57) | 90 (21) | 77 (40) | 0.188 | 88 (34) | 62 (27) | 75 (36) | 0.072 | 97 (16) | 83 (14) | 90 (17) | 0.149 | 107 (13) | 93 (15) | 100 (15) | 0.099 |
| Epoxiconazole | 113 (19) | 93 (7) | 103 (17) | 0.058 | 94 (20) | 82 (4) | 88 (16) | 0.148 | 86 (9) | 74 (19) | 80 (16) | 0.116 | 89 (8) | 81 (13) | 85 (11) | 0.161 |
| Ethion | 84 (16) | 79 (15) | 81 (15) | 0.502 | 83 (20) | 95 (8) | 89 (15) | 0.130 | 77 (11) | 78 (0) | 77 (8) | 0.831 | 84 (16) | 78 (0) | 81 (12) | 0.300 |
| Ethiprole | 95 (14) | 97 (6) | 96 (11) | 0.689 | 90 (19) | 103 (17) | 97 (18) | 0.238 | 90 (17) | 93 (7) | 92 (13) | 0.632 | 91 (10) | 82 (19) | 86 (15) | 0.113 |
| Ethoprophos | 101 (13) | 86 (7) | 94 (13) | 0.060 | 96 (9) | 84 (12) | 90 (12) | 0.080 | 89 (6) | 81 (14) | 85 (11) | 0.106 | 88 (12) | 80 (14) | 84 (13) | 0.175 |
| Etofenprox | 93 (8) | 86 (11) | 89 (10) | 0.124 | 89 (6) | 86 (5) | 87 (6) | 0.215 | 75 (8) | 83 (6) | 79 (9) | 0.072 | 82 (11) | 82 (6) | 82 (9) | 0.990 |
| Etoxazole | 89 (10) | 75 (16) | 82 (15) | 0.101 | 84 (4) | 79 (11) | 81 (8) | 0.112 | 81 (4) | 75 (15) | 78 (11) | 0.302 | 80 (11) | 75 (15) | 78 (13) | 0.306 |
| Fenamidone | 94 (14) | 101 (19) | 97 (17) | 0.573 | 88 (7) | 97 (11) | 92 (10) | 0.069 | 91 (15) | 93 (7) | 92 (11) | 0.638 | 86 (12) | 95 (7) | 90 (11) | 0.130 |
| Fenamiphos | 91 (14) | 94 (1) | 93 (10) | 0.560 | 88 (8) | 84 (11) | 86 (9) | 0.381 | 83 (8) | 81 (10) | 82 (9) | 0.581 | 82 (15) | 73 (17) | 78 (16) | 0.113 |
| Fenarimol | 98 (47) | 89 (1) | 94 (34) | 0.610 | 88 (13) | 101 (16) | 94 (16) | 0.077 | 92 (13) | 86 (19) | 89 (16) | 0.188 | 85 (8) | 81 (4) | 83 (7) | 0.076 |
| Fenazaquin | 89 (11) | 94 (6) | 91 (9) | 0.427 | 85 (8) | 100 (16) | 93 (15) | 0.056 | 81 (9) | 77 (14) | 79 (12) | 0.443 | 85 (11) | 76 (14) | 81 (14) | 0.098 |
| Fenbuconazole | 115 (15) | 104 (5) | 110 (13) | 0.199 | 95 (15) | 109 (19) | 102 (18) | 0.280 | 89 (7) | 86 (10) | 88 (9) | 0.419 | 91 (20) | 86 (10) | 88 (16) | 0.509 |
| Fenhexamid | 107 (19) | 108 (15) | 107 (16) | 0.892 | 99 (11) | 85 (20) | 92 (16) | 0.071 | 87 (9) | 74 (15) | 80 (14) | 0.072 | 87 (10) | 78 (9) | 83 (11) | 0.062 |
| Fenobucarb | 86 (17) | 75 (18) | 81 (18) | 0.070 | 93 (4) | 86 (12) | 89 (9) | 0.136 | 92 (5) | 89 (17) | 90 (12) | 0.696 | 81 (17) | 73 (16) | 77 (17) | 0.334 |
| Fenoxycarb | 74 (43) | 11 (444) | 42 (121) | 0.002 | 94 (15) | 87 (18) | 91 (16) | 0.498 | 83 (18) | 73 (11) | 78 (16) | 0.140 | 80 (18) | 71 (11) | 76 (16) | 0.132 |
| Fenpropimorph | 84 (15) | 76 (6) | 80 (13) | 0.112 | 77 (5) | 74 (5) | 76 (5) | 0.213 | 77 (3) | 73 (8) | 75 (7) | 0.093 | 77 (5) | 75 (9) | 76 (7) | 0.316 |
| Fenpyroximate | 88 (12) | 92 (4) | 90 (9) | 0.406 | 87 (16) | 103 (12) | 95 (16) | 0.079 | 78 (15) | 74 (11) | 76 (13) | 0.543 | 83 (20) | 73 (11) | 78 (17) | 0.287 |
| Fensulfothion | −41 (0) | 941 (3) | 450 (113) | 0.000 | −20 (0) | 493 (2) | 236 (113) | 0.000 | 97 (15) | 105 (8) | 101 (12) | 0.234 | 92 (16) | 108 (8) | 100 (14) | 0.071 |
| Fluazifop-butyl | 92 (13) | 95 (8) | 94 (10) | 0.652 | 101 (11) | 109 (13) | 105 (12) | 0.318 | 87 (8) | 74 (14) | 80 (13) | 0.064 | 89 (15) | 74 (14) | 81 (17) | 0.071 |
| Fludioxonil | 85 (16) | 92 (17) | 88 (17) | 0.349 | 100 (18) | 100 (13) | 100 (15) | 0.983 | 84 (19) | 89 (13) | 86 (16) | 0.372 | 79 (18) | 89 (13) | 84 (16) | 0.130 |
| Flufenoxuron | 105 (9) | 89 (18) | 97 (16) | 0.072 | 96 (14) | 87 (7) | 91 (12) | 0.151 | 95 (12) | 85 (12) | 90 (13) | 0.058 | 81 (14) | 72 (8) | 77 (13) | 0.125 |
| Fluquinconazole | 82 (13) | 78 (17) | 80 (15) | 0.592 | 102 (15) | 103 (6) | 102 (11) | 0.757 | 90 (14) | 79 (12) | 84 (14) | 0.103 | 77 (11) | 68 (12) | 73 (13) | 0.120 |
| Flusilazole | 96 (12) | 112 (12) | 104 (14) | 0.107 | 90 (7) | 85 (15) | 88 (12) | 0.320 | 87 (3) | 84 (4) | 86 (4) | 0.258 | 83 (8) | 72 (19) | 77 (15) | 0.081 |
| Flutolanil | 98 (15) | 108 (7) | 103 (12) | 0.124 | 90 (7) | 89 (15) | 90 (11) | 0.857 | 88 (8) | 80 (13) | 84 (11) | 0.135 | 86 (14) | 80 (13) | 83 (13) | 0.191 |
| Flutriafol | 98 (17) | 107 (10) | 103 (14) | 0.303 | 87 (17) | 100 (16) | 94 (17) | 0.226 | 87 (9) | 88 (10) | 87 (9) | 0.889 | 82 (9) | 90 (10) | 86 (10) | 0.088 |
| Fosthiazate | 92 (12) | 81 (8) | 87 (12) | 0.067 | 91 (12) | 81 (17) | 86 (15) | 0.146 | 87 (11) | 76 (8) | 81 (12) | 0.014 | 92 (17) | 77 (10) | 84 (17) | 0.066 |
| Fumonisin B1 | 378 (2) | 378 (2) | 378 (1) | n.f.r. | 152 (3) | 152 (3) | 152 (3) | n.f.r. | 77 (4) | 78 (3) | 78 (3) | 0.083 | 94 (5) | 93 (5) | 94 (5) | 0.724 |
| Fumonisin B2 | 351 (1) | 351 (1) | 351 (1) | 0.819 | 147 (3) | 149 (3) | 148 (3) | 0.085 | 77 (5) | 78 (3) | 78 (4) | 0.356 | 75 (3) | 83 (11) | 79 (10) | 0.107 |
| Furalaxyl | 93 (9) | 79 (19) | 86 (16) | 0.055 | 91 (5) | 85 (13) | 88 (10) | 0.266 | 88 (6) | 77 (13) | 82 (12) | 0.081 | 88 (11) | 75 (10) | 81 (13) | 0.054 |
| Furathiocarb | 93 (12) | 98 (7) | 96 (10) | 0.437 | 88 (11) | 85 (17) | 87 (14) | 0.668 | 84 (8) | 75 (16) | 80 (13) | 0.100 | 88 (17) | 75 (16) | 82 (18) | 0.08 |
| Halofenozide | 143 (23) | 441 (60) | 292 (81) | 0.000 | 151 (27) | 161 (81) | 156 (59) | 1.000 | 91 (10) | 93 (18) | 92 (14) | 0.697 | 106 (9) | 115 (15) | 110 (13) | 0.096 |
| Haloxyfop-2-ethoxyethyl | 93 (19) | 102 (11) | 98 (15) | 0.188 | 83 (11) | 93 (12) | 88 (13) | 0.051 | 80 (10) | 79 (15) | 79 (12) | 0.913 | 79 (18) | 79 (15) | 79 (16) | 0.939 |
| Hexaconazole | 92 (16) | 77 (14) | 84 (17) | 0.12 | 84 (11) | 73 (13) | 78 (13) | 0.069 | 83 (6) | 77 (10) | 80 (9) | 0.140 | 78 (8) | 74 (17) | 76 (13) | 0.565 |
| Hexytiazox | 87 (15) | 95 (11) | 91 (13) | 0.368 | 81 (13) | 87 (19) | 84 (17) | 0.516 | 81 (10) | 73 (15) | 77 (13) | 0.064 | 76 (15) | 73 (15) | 75 (15) | 0.451 |
| Imazalil | 90 (42) | −268 (−3) | −89 (−211) | 0.000 | 84 (16) | 95 (12) | 90 (15) | 0.086 | 70 (9) | 69 (9) | 70 (9) | 0.809 | 80 (12) | 90 (7) | 85 (11) | 0.127 |
| Imazapic | 88 (13) | 97 (8) | 93 (11) | 0.141 | 83 (6) | 97 (19) | 90 (17) | 0.154 | 81 (6) | 75 (11) | 78 (9) | 0.197 | 81 (3) | 75 (11) | 78 (9) | 0.127 |
| Imazetapyr | 83 (13) | 78 (19) | 81 (16) | 0.318 | 81 (4) | 78 (8) | 79 (6) | 0.103 | 77 (4) | 72 (17) | 75 (12) | 0.288 | 74 (14) | 79 (2) | 77 (10) | 0.203 |
| Imidacloprid | 95 (12) | 83 (12) | 89 (14) | 0.099 | 114 (16) | 119 (12) | 117 (14) | 0.441 | 100 (12) | 105 (8) | 102 (10) | 0.518 | 97 (8) | 107 (10) | 102 (10) | 0.069 |
| Indoxacarb | 106 (9) | 92 (20) | 99 (16) | 0.078 | 88 (19) | 98 (18) | 93 (19) | 0.067 | 81 (14) | 75 (16) | 78 (15) | 0.291 | 84 (18) | 76 (16) | 80 (17) | 0.183 |
| Iprovalicarb | 95 (13) | 101 (18) | 98 (16) | 0.519 | 92 (15) | 109 (17) | 100 (18) | 0.060 | 87 (10) | 95 (8) | 91 (10) | 0.078 | 92 (16) | 95 (8) | 94 (12) | 0.666 |
| Isoxaflutole | −30 (0) | 903 (59) | 437 (139) | 0.000 | 95 (41) | 105 (143) | 100 (106) | 1.000 | 84 (16) | 85 (21) | 85 (18) | 0.896 | 98 (16) | 93 (9) | 96 (13) | 0.548 |
| Kresoxim-methyl | 74 (18) | 72 (20) | 73 (18) | 0.716 | 90 (13) | 97 (10) | 94 (12) | 0.121 | 78 (14) | 77 (12) | 77 (13) | 0.811 | 82 (21) | 77 (12) | 79 (17) | 0.577 |
| Linuron | 111 (76) | 20 (216) | 66 (121) | 0.000 | 80 (26) | 78 (40) | 79 (33) | 0.884 | 73 (19) | 75 (19) | 74 (18) | 0.816 | 84 (13) | 85 (18) | 84 (15) | 0.862 |
| Lufenuron | 94 (15) | 96 (9) | 95 (12) | 0.730 | 88 (16) | 94 (3) | 91 (11) | 0.381 | 82 (6) | 79 (12) | 81 (9) | 0.369 | 83 (20) | 79 (12) | 81 (16) | 0.608 |
| Malathion | 91 (15) | 97 (9) | 94 (13) | 0.380 | 85 (9) | 95 (8) | 90 (10) | 0.051 | 85 (11) | 77 (7) | 81 (10) | 0.111 | 84 (15) | 79 (7) | 81 (12) | 0.44 |
| Mecarbam | 79 (12) | 92 (13) | 85 (14) | 0.098 | 84 (4) | 88 (4) | 86 (5) | 0.078 | 81 (11) | 88 (8) | 85 (10) | 0.289 | 82 (19) | 89 (8) | 86 (14) | 0.424 |
| Mepanipyrim | 86 (18) | 92 (11) | 89 (14) | 0.189 | 85 (18) | 100 (13) | 92 (17) | 0.062 | 77 (15) | 78 (8) | 78 (12) | 0.622 | 86 (18) | 80 (8) | 83 (14) | 0.374 |
| Metalaxyl | 116 (8) | 117 (18) | 117 (14) | 0.905 | 114 (16) | 110 (15) | 112 (15) | 0.252 | 96 (8) | 92 (14) | 94 (11) | 0.249 | 94 (11) | 84 (17) | 89 (15) | 0.188 |
| Metconazole | 320 (136) | 123 (17) | 222 (141) | 0.000 | 97 (13) | 86 (14) | 91 (14) | 0.078 | 85 (9) | 77 (13) | 81 (12) | 0.264 | 80 (6) | 72 (15) | 76 (12) | 0.186 |
| Methamidophos | 84 (17) | 73 (15) | 79 (17) | 0.191 | 83 (12) | 90 (17) | 86 (15) | 0.294 | 72 (8) | 79 (12) | 76 (11) | 0.064 | 81 (12) | 93 (10) | 87 (13) | 0.102 |
| Methidathion | 17 (422) | 17 (422) | 17 (406) | n.f.r.b | 88 (16) | 76 (5) | 82 (14) | 0.057 | 84 (4) | 84 (6) | 84 (5) | 0.811 | 75 (15) | 76 (15) | 76 (14) | 0.853 |
| Methiocarb | 87 (20) | 85 (3) | 86 (14) | 0.751 | 88 (14) | 99 (19) | 93 (17) | 0.152 | 81 (8) | 88 (9) | 84 (9) | 0.098 | 80 (12) | 88 (9) | 84 (11) | 0.113 |
| Methomyl | 90 (11) | 81 (7) | 85 (11) | 0.136 | 89 (9) | 81 (8) | 85 (10) | 0.066 | 88 (4) | 80 (12) | 84 (10) | 0.071 | 85 (13) | 74 (5) | 80 (13) | 0.066 |
| Methoxyfenozide | 99 (15) | 103 (7) | 101 (11) | 0.601 | 91 (9) | 109 (18) | 100 (17) | 0.060 | 89 (8) | 79 (12) | 84 (12) | 0.140 | 90 (14) | 78 (12) | 84 (15) | 0.109 |
| Monocrotophos | 85 (12) | 73 (18) | 79 (16) | 0.159 | 90 (9) | 77 (15) | 84 (14) | 0.090 | 87 (4) | 81 (15) | 84 (11) | 0.263 | 91 (19) | 81 (8) | 86 (16) | 0.181 |
| Myclobutanil | 96 (18) | 81 (11) | 88 (17) | 0.129 | 92 (7) | 81 (12) | 86 (12) | 0.077 | 88 (2) | 82 (18) | 85 (12) | 0.350 | 84 (9) | 74 (14) | 79 (13) | 0.111 |
| Nitenpyram | −267 (0) | −78 (−35) | −173 (−58) | 0.000 | −134 (0) | −20 (−145) | −77 (−81) | 0.000 | 100 (19) | 99 (7) | 100 (14) | 0.933 | 92 (19) | 98 (9) | 95 (15) | 0.200 |
| Ochratoxin A | 53 (0) | 53 (0) | 53 (0) | n.f.r. | 39 (15) | 40 (4) | 39 (10) | 0.772 | 106 (19) | 109 (8) | 107 (14) | 0.686 | 81 (18) | 89 (5) | 85 (13) | 0.220 |
| Ofurace | 99 (13) | 93 (17) | 96 (15) | 0.244 | 90 (12) | 76 (11) | 83 (14) | 0.067 | 89 (8) | 82 (17) | 85 (13) | 0.300 | 99 (9) | 88 (18) | 93 (15) | 0.171 |
| Omethoate | 102 (7) | 98 (19) | 100 (14) | 0.629 | 84 (4) | 80 (20) | 82 (14) | 0.466 | 79 (8) | 76 (12) | 78 (10) | 0.194 | 84 (13) | 73 (8) | 78 (13) | 0.057 |
| Oxadixyl | 93 (16) | 81 (9) | 87 (15) | 0.109 | 91 (13) | 80 (13) | 86 (14) | 0.115 | 87 (7) | 80 (10) | 83 (9) | 0.055 | 88 (8) | 84 (11) | 86 (10) | 0.266 |
| Oxamyl | 96 (12) | 82 (12) | 89 (14) | 0.098 | 94 (8) | 89 (14) | 92 (11) | 0.328 | 89 (3) | 83 (11) | 86 (8) | 0.120 | 93 (6) | 89 (11) | 91 (9) | 0.389 |
| Paclobutrazol | 109 (15) | 100 (19) | 105 (17) | 0.354 | 99 (12) | 92 (14) | 96 (13) | 0.429 | 89 (5) | 83 (13) | 86 (10) | 0.102 | 83 (5) | 75 (13) | 79 (11) | 0.053 |
| Penconazole | 94 (14) | 77 (19) | 86 (18) | 0.071 | 85 (9) | 79 (11) | 82 (10) | 0.127 | 85 (8) | 74 (17) | 80 (14) | 0.080 | 79 (12) | 70 (6) | 75 (11) | 0.086 |
| Pencycuron | 83 (14) | 87 (8) | 85 (11) | 0.511 | 89 (15) | 102 (13) | 95 (15) | 0.075 | 87 (14) | 94 (4) | 90 (10) | 0.192 | 88 (13) | 93 (4) | 91 (9) | 0.352 |
| Pendimethalin | 83 (19) | 79 (5) | 81 (14) | 0.588 | 72 (11) | 71 (3) | 72 (8) | 0.630 | 82 (17) | 78 (15) | 80 (15) | 0.544 | 88 (6) | 79 (15) | 83 (12) | 0.165 |
| Phenothrin | n.f.r.b | 49 (72) | n.f.r.b | n.f.r.b | 87 (12) | 88 (6) | 87 (9) | 0.774 | 81 (18) | 97 (16) | 89 (18) | 0.133 | 91 (6) | 98 (16) | 95 (12) | 0.315 |
| Phenthoate | 91 (15) | 95 (15) | 93 (15) | 0.520 | 85 (9) | 91 (16) | 88 (13) | 0.195 | 85 (11) | 81 (11) | 83 (11) | 0.412 | 84 (15) | 83 (11) | 83 (13) | 0.862 |
| Phosalone | 43 (113) | 15 (215) | 29 (146) | 0.000 | 115 (12) | 105 (18) | 110 (15) | 0.390 | 93 (8) | 85 (14) | 89 (12) | 0.219 | 81 (20) | 88 (13) | 85 (17) | 0.433 |
| Phosmet | 84 (17) | 102 (14) | 93 (18) | 0.080 | 92 (10) | 99 (13) | 96 (12) | 0.293 | 80 (19) | 83 (12) | 81 (15) | 0.655 | 84 (15) | 84 (12) | 84 (13) | 0.968 |
| Picoxystrobin | 103 (11) | 110 (13) | 106 (12) | 0.201 | 87 (8) | 89 (7) | 88 (7) | 0.617 | 84 (10) | 82 (4) | 83 (7) | 0.311 | 86 (17) | 81 (4) | 84 (13) | 0.403 |
| Piperonyl butoxide | 94 (11) | 103 (11) | 98 (12) | 0.129 | 88 (8) | 96 (9) | 92 (9) | 0.067 | 84 (7) | 76 (9) | 80 (9) | 0.063 | 83 (14) | 76 (9) | 79 (12) | 0.202 |
| Pirimicarb | 94 (11) | 87 (9) | 90 (11) | 0.067 | 93 (7) | 82 (9) | 88 (10) | 0.06 | 89 (3) | 82 (11) | 86 (8) | 0.090 | 85 (3) | 82 (14) | 83 (10) | 0.458 |
| Pirimiphos-ethyl | 94 (12) | 84 (10) | 89 (12) | 0.128 | 89 (4) | 81 (9) | 85 (8) | 0.077 | 87 (1) | 77 (15) | 82 (12) | 0.057 | 83 (9) | 77 (16) | 80 (13) | 0.269 |
| Pirimiphos-methyl | 95 (13) | 96 (19) | 96 (16) | 0.926 | 92 (4) | 80 (19) | 86 (14) | 0.091 | 87 (3) | 77 (13) | 82 (11) | 0.051 | 84 (10) | 73 (11) | 79 (12) | 0.070 |
| Prochloraz | 78 (11) | 93 (14) | 86 (15) | 0.053 | 79 (9) | 90 (13) | 85 (13) | 0.103 | 76 (9) | 81 (4) | 78 (7) | 0.211 | 72 (13) | 80 (4) | 76 (11) | 0.103 |
| Profenofos | 93 (11) | 83 (8) | 88 (11) | 0.096 | 83 (14) | 105 (17) | 94 (19) | 0.058 | 77 (19) | 71 (7) | 74 (15) | 0.273 | 77 (16) | 72 (7) | 75 (12) | 0.299 |
| Prometryn | 89 (13) | 80 (4) | 85 (11) | 0.082 | 89 (5) | 83 (11) | 86 (9) | 0.079 | 85 (2) | 80 (12) | 82 (9) | 0.284 | 82 (6) | 78 (13) | 80 (10) | 0.274 |
| Propamocarb | 262 (7) | 218 (4) | 240 (11) | 0.000 | 212 (4) | 171 (5) | 191 (12) | 0.000 | 181 (2) | 157 (16) | 169 (13) | 0.059 | 176 (9) | 147 (6) | 162 (12) | 0.002 |
| Propanil | 50 (193) | 28 (252) | 39 (210) | 1.000 | 104 (41) | 75 (81) | 89 (59) | 0.368 | 102 (13) | 97 (13) | 99 (13) | 0.597 | 85 (17) | 102 (13) | 93 (17) | 0.059 |
| Propham | 96 (11) | 102 (11) | 99 (11) | 0.340 | 102 (12) | 105 (5) | 104 (9) | 0.567 | 93 (17) | 94 (8) | 94 (13) | 0.963 | 94 (12) | 95 (8) | 95 (10) | 0.840 |
| Propiconazole | 89 (10) | 76 (19) | 82 (16) | 0.152 | 84 (14) | 65 (14) | 74 (19) | 0.025 | 79 (8) | 70 (17) | 74 (13) | 0.150 | 83 (10) | 75 (17) | 79 (14) | 0.136 |
| Propyzamide | 88 (17) | 82 (19) | 85 (18) | 0.597 | 90 (10) | 82 (16) | 86 (14) | 0.342 | 88 (18) | 76 (16) | 82 (18) | 0.247 | 82 (15) | 75 (15) | 78 (15) | 0.104 |
| Pyraclostrobin | 98 (13) | 109 (9) | 104 (12) | 0.062 | 95 (13) | 102 (2) | 98 (9) | 0.201 | 76 (11) | 82 (7) | 79 (9) | 0.069 | 84 (6) | 83 (7) | 84 (6) | 0.643 |
| Pyrazophos | 103 (12) | 112 (8) | 107 (11) | 0.097 | 92 (15) | 101 (2) | 96 (11) | 0.151 | 82 (14) | 78 (5) | 80 (11) | 0.384 | 83 (18) | 78 (5) | 81 (14) | 0.410 |
| Pyridaben | 91 (16) | 79 (19) | 85 (18) | 0.223 | 78 (12) | 86 (11) | 82 (12) | 0.101 | 73 (15) | 71 (5) | 72 (11) | 0.711 | 78 (17) | 71 (5) | 74 (13) | 0.190 |
| Pyrimethanil | 84 (13) | 77 (10) | 81 (12) | 0.293 | 80 (6) | 73 (11) | 77 (9) | 0.099 | 77 (3) | 71 (13) | 74 (10) | 0.071 | 73 (11) | 70 (18) | 71 (14) | 0.622 |
| Pyriproxyfen | 87 (12) | 96 (7) | 91 (10) | 0.137 | 82 (8) | 83 (11) | 82 (9) | 0.862 | 79 (8) | 78 (13) | 79 (10) | 0.869 | 82 (4) | 78 (13) | 80 (9) | 0.371 |
| Quinalphos | 86 (20) | 76 (15) | 81 (18) | 0.241 | 83 (20) | 75 (9) | 79 (16) | 0.341 | 81 (9) | 87 (9) | 84 (9) | 0.223 | 90 (7) | 87 (9) | 89 (8) | 0.452 |
| Quinoxyfen | 97 (15) | 108 (4) | 102 (12) | 0.100 | 87 (11) | 97 (11) | 92 (12) | 0.146 | 83 (15) | 70 (4) | 77 (14) | 0.055 | 73 (6) | 69 (4) | 71 (6) | 0.198 |
| Simazine | 88 (18) | 77 (12) | 82 (16) | 0.289 | 89 (10) | 75 (16) | 82 (15) | 0.076 | 90 (7) | 81 (15) | 86 (12) | 0.157 | 87 (2) | 81 (8) | 84 (7) | 0.082 |
| Spinosyn A | 79 (16) | 74 (13) | 77 (14) | 0.408 | 72 (8) | 72 (4) | 72 (6) | 0.748 | 71 (6) | 73 (2) | 72 (5) | 0.221 | 74 (6) | 72 (14) | 73 (10) | 0.646 |
| Spinosyn D | 70 (16) | 74 (19) | 72 (17) | 0.502 | 78 (18) | 84 (6) | 81 (13) | 0.348 | 70 (9) | 74 (10) | 72 (10) | 0.394 | 72 (6) | 74 (10) | 73 (8) | 0.442 |
| Spirodiclofen | 77 (53) | 66 (23) | 71 (42) | 0.517 | 72 (36) | 81 (17) | 77 (27) | 0.395 | 79 (18) | 78 (16) | 78 (17) | 0.896 | 84 (7) | 79 (16) | 81 (12) | 0.270 |
| Spiromesifen | 98 (7) | 97 (19) | 97 (14) | 0.877 | 101 (17) | 99 (16) | 100 (16) | 0.798 | 85 (11) | 96 (19) | 90 (16) | 0.213 | 107 (17) | 96 (19) | 102 (18) | 0.248 |
| Spiroxamine | 89 (11) | 81 (10) | 85 (11) | 0.212 | 83 (4) | 78 (8) | 81 (7) | 0.087 | 82 (2) | 77 (11) | 80 (8) | 0.123 | 81 (2) | 77 (19) | 79 (13) | 0.588 |
| Tau-fluvalinate | 64 (114) | 334 (52) | 199 (95) | 0.000 | 112 (63) | 247 (33) | 180 (56) | 0.000 | 76 (30) | 123 (17) | 100 (33) | 0.010 | 85 (15) | 85 (12) | 85 (13) | 0.999 |
| Tebuconazole | 97 (12) | 85 (13) | 91 (14) | 0.068 | 79 (13) | 89 (4) | 84 (11) | 0.089 | 85 (3) | 81 (1) | 83 (3) | 0.017 | 83 (7) | 76 (19) | 79 (14) | 0.324 |
| Tebufenozide | 115 (29) | 143 (3) | 129 (21) | 0.056 | 111 (5) | 107 (2) | 109 (4) | 0.276 | 101 (5) | 93 (16) | 97 (12) | 0.251 | 95 (9) | 83 (11) | 89 (12) | 0.051 |
| Tebufenpyrad | 94 (18) | 106 (7) | 100 (14) | 0.133 | 87 (12) | 92 (1) | 89 (9) | 0.320 | 83 (13) | 71 (7) | 77 (13) | 0.074 | 80 (16) | 71 (7) | 75 (14) | 0.081 |
| Terbutryn | 91 (11) | 81 (13) | 86 (13) | 0.142 | 88 (4) | 81 (10) | 85 (8) | 0.069 | 85 (2) | 80 (12) | 83 (8) | 0.167 | 82 (6) | 77 (9) | 80 (8) | 0.157 |
| Tetrachlorvinphos | 94 (9) | 90 (18) | 92 (14) | 0.473 | 91 (32) | 57 (31) | 74 (39) | 0.039 | 86 (12) | 89 (13) | 88 (12) | 0.472 | 98 (16) | 89 (13) | 94 (15) | 0.302 |
| Tetraconazole | 97 (14) | 81 (14) | 89 (17) | 0.067 | 85 (10) | 73 (14) | 79 (14) | 0.078 | 93 (11) | 91 (13) | 92 (12) | 0.61 | 84 (10) | 81 (14) | 82 (12) | 0.354 |
| Tetramethrin | 98 (12) | 90 (20) | 94 (16) | 0.309 | 84 (13) | 95 (8) | 90 (12) | 0.113 | 86 (4) | 81 (13) | 83 (10) | 0.312 | 84 (15) | 80 (13) | 82 (14) | 0.548 |
| Thiabendazole | 45 (16) | 39 (8) | 42 (15) | 0.070 | 38 (4) | 36 (8) | 37 (7) | 0.144 | 37 (4) | 39 (17) | 38 (12) | 0.348 | 38 (8) | 38 (12) | 38 (10) | 0.952 |
| Thiacloprid | 88 (16) | 76 (8) | 82 (15) | 0.085 | 81 (14) | 70 (12) | 76 (15) | 0.059 | 78 (14) | 74 (26) | 76 (20) | 0.526 | 82 (15) | 71 (13) | 76 (15) | 0.222 |
| Thiamethoxam | 139 (24) | 42 (35) | 90 (63) | 0.000 | 118 (42) | 71 (24) | 94 (46) | 0.091 | 104 (28) | 96 (33) | 100 (30) | 0.155 | 100 (7) | 89 (17) | 95 (13) | 0.177 |
| Thiodicarb | 97 (13) | 81 (13) | 89 (16) | 0.103 | 89 (11) | 82 (19) | 85 (15) | 0.381 | 84 (8) | 74 (16) | 79 (13) | 0.101 | 86 (7) | 77 (19) | 81 (14) | 0.176 |
| Toxin T2 | n.f.r. | 486 (52) | n.f.r. | n.f.r. | 946 (27) | 729 (30) | 838 (31) | 0.000 | 82 (14) | 73 (8) | 78 (12) | 0.101 | 98 (7) | 88 (17) | 93 (13) | 0.135 |
| Triadimefon | 102 (17) | 86 (2) | 94 (16) | 0.067 | 90 (8) | 82 (16) | 86 (13) | 0.236 | 85 (12) | 75 (12) | 80 (13) | 0.170 | 85 (5) | 79 (20) | 82 (14) | 0.364 |
| Triadimenol | 142 (186) | −604 (−4) | −231 (−185) | 0.000 | 66 (106) | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | −89 (−15) | n.f.r.b | n.f.r.b | 41 (62) | −53 (−18) | −6 (−829) | 0.000 |
| Triazophos | 91 (12) | 98 (20) | 94 (16) | 0.215 | 88 (8) | 85 (7) | 87 (7) | 0.429 | 86 (9) | 87 (9) | 86 (9) | 0.747 | 91 (6) | 86 (9) | 89 (8) | 0.365 |
| Trifloxystrobin | 86 (11) | 86 (8) | 86 (9) | 0.954 | 84 (17) | 83 (16) | 84 (16) | 0.859 | 77 (11) | 73 (6) | 75 (9) | 0.270 | 87 (9) | 83 (9) | 85 (9) | 0.080 |
| Triflumizole | 91 (12) | 78 (14) | 84 (15) | 0.074 | 83 (5) | 73 (18) | 78 (14) | 0.119 | 83 (4) | 85 (15) | 84 (10) | 0.606 | 81 (5) | 77 (20) | 79 (14) | 0.517 |
| Triticonazole | 92 (16) | 96 (11) | 94 (13) | 0.532 | 94 (9) | 106 (9) | 100 (10) | 0.056 | 85 (7) | 90 (4) | 88 (7) | 0.055 | 91 (13) | 90 (4) | 91 (9) | 0.936 |
| Zearalenone | −5131 (0) | −5131 (0) | −5131 (0) | n.f.r. | 105 (11) | 86 (44) | 96 (30) | 0.225 | 107 (8) | 112 (19) | 109 (14) | 0.554 | 93 (19) | 88 (19) | 91 (18) | 0.636 |
| Zoxamide | 86 (16) | 93 (15) | 90 (15) | 0.388 | 84 (14) | 71 (19) | 77 (18) | 0.174 | 81 (16) | 77 (8) | 79 (13) | 0.440 | 87 (10) | 80 (11) | 84 (11) | 0.237 |
| Concentration 1 | Concentration 2 | Concentration 3 | Concentration 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | Average recovery (%) (RSDr (%)) (n = 7) | Average recovery (%) (RSDWR (%)) (n = 14) | P value | |||||
| Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | Analyst 1 | Analyst 2 | |||||||||
| a Mycotoxins. b n.f.r.: not fulfill requirements of SANTE document. | ||||||||||||||||
| Acephate | 76 (20) | 84 (9) | 80 (15) | 0.164 | 84 (13) | 101 (12) | 93 (15) | 0.061 | 86 (16) | 90 (13) | 88 (14) | 0.619 | 94 (17) | 108 (10) | 101 (15) | 0.063 |
| Acetamiprid | 107 (9) | 102 (5) | 105 (7) | 0.273 | 94 (6) | 89 (11) | 92 (9) | 0.396 | 86 (17) | 78 (5) | 82 (14) | 0.295 | 95 (11) | 98 (2) | 97 (8) | 0.367 |
| Acetochlor | 106 (17) | 95 (11) | 100 (15) | 0.127 | 99 (8) | 98 (16) | 99 (12) | 0.811 | 87 (6) | 83 (9) | 85 (8) | 0.371 | 99 (5) | 97 (10) | 98 (8) | 0.446 |
| Aflatoxin B1 | 51 (21) | 84 (15) | 67 (31) | 0.001 | 76 (8) | 71 (19) | 73 (14) | 0.353 | 88 (18) | 76 (14) | 82 (18) | 0.237 | 95 (10) | 91 (12) | 93 (11) | 0.403 |
| Aflatoxin B2 | −111 (-11) | −120 (-20) | −115 (-17) | 0.438 | 9 (220) | 8 (195) | 8 (203) | 0.883 | 57 (18) | 62 (10) | 59 (15) | 0.167 | 75 (18) | 75 (6) | 75 (13) | 0.985 |
| Aflatoxin G1 | 66 (58) | 83 (38) | 75 (47) | 0.191 | 78 (13) | 84 (16) | 81 (15) | 0.223 | 96 (14) | 85 (14) | 90 (15) | 0.143 | 84 (15) | 85 (11) | 85 (12) | 0.832 |
| Aflatoxin G2 | n.f.r . | 39 (228) | n.f.r . | n.f.r . | 90 (20) | 85 (11) | 88 (16) | 0.434 | 89 (19) | 88 (7) | 89 (14) | 0.801 | 85 (11) | 96 (11) | 90 (12) | 0.119 |
| Aldicarb sulfone | 90 (16) | 93 (18) | 91 (16) | 0.726 | 90 (13) | 84 (13) | 87 (13) | 0.416 | 99 (4) | 98 (9) | 98 (7) | 0.728 | 103 (6) | 111 (5) | 107 (7) | 0.063 |
| Aldicarb sulfoxide | 101 (17) | 86 (20) | 94 (20) | 0.180 | 90 (13) | 106 (13) | 98 (15) | 0.087 | 86 (16) | 91 (19) | 89 (17) | 0.616 | 83 (5) | 94 (13) | 89 (12) | 0.106 |
| Atrazine | 104 (13) | 114 (8) | 109 (11) | 0.184 | 107 (12) | 116 (3) | 111 (9) | 0.155 | 90 (10) | 83 (2) | 87 (8) | 0.118 | 87 (10) | 82 (2) | 84 (7) | 0.224 |
| Azamethiphos | 80 (16) | 89 (14) | 85 (16) | 0.298 | 89 (20) | 102 (6) | 95 (15) | 0.135 | 87 (19) | 96 (3) | 91 (14) | 0.214 | 87 (12) | 98 (3) | 93 (10) | 0.054 |
| Azinphos-methyl | 75 (49) | 123 (57) | 99 (60) | 0.059 | 81 (51) | 168 (19) | 124 (46) | 0.008 | 82 (17) | 93 (18) | 88 (18) | 0.294 | 92 (14) | 79 (19) | 85 (18) | 0.120 |
| Azoxystrobin | 95 (19) | 110 (4) | 103 (14) | 0.089 | 93 (11) | 102 (6) | 98 (9) | 0.063 | 90 (9) | 98 (4) | 94 (8) | 0.054 | 93 (12) | 96 (1) | 94 (8) | 0.509 |
| Bifenazate | 23 (48) | −10 (−52) | 6 (301) | 0.000 | 42 (12) | 11 (97) | 26 (68) | 0.000 | 56 (15) | 16 (56) | 36 (63) | 0.000 | 56 (16) | 19 (27) | 38 (53) | 0.000 |
| Bitertanol | 83 (18) | 79 (11) | 81 (15) | 0.518 | 89 (19) | 81 (12) | 85 (17) | 0.281 | 95 (16) | 85 (9) | 90 (14) | 0.195 | 94 (16) | 92 (5) | 93 (11) | 0.712 |
| Boscalid | 87 (10) | 110 (24) | 98 (23) | 0.052 | 83 (11) | 75 (14) | 79 (13) | 0.219 | 88 (8) | 82 (13) | 85 (11) | 0.282 | 91 (15) | 80 (6) | 85 (13) | 0.061 |
| Bupirimate | 102 (10) | 96 (7) | 99 (9) | 0.224 | 98 (12) | 89 (5) | 93 (10) | 0.119 | 93 (9) | 93 (5) | 93 (7) | 0.907 | 93 (8) | 88 (3) | 91 (7) | 0.143 |
| Buprofezin | 101 (15) | 114 (5) | 108 (12) | 0.091 | 111 (15) | 111 (5) | 111 (11) | 0.968 | 86 (9) | 103 (24) | 94 (20) | 0.172 | 106 (16) | 110 (3) | 108 (11) | 0.528 |
| Cadusafos | 100 (17) | 112 (7) | 106 (13) | 0.133 | 96 (13) | 107 (12) | 102 (13) | 0.254 | 103 (13) | 113 (8) | 108 (11) | 0.081 | 103 (19) | 116 (2) | 109 (14) | 0.136 |
| Carbaryl | 99 (18) | 104 (6) | 102 (13) | 0.592 | 96 (11) | 108 (7) | 102 (10) | 0.087 | 106 (15) | 113 (3) | 109 (10) | 0.318 | 99 (11) | 110 (5) | 105 (10) | 0.071 |
| Carbendazim | 65 (54) | 48 (11) | 57 (45) | 0.263 | 53 (40) | 75 (11) | 64 (30) | 0.067 | 62 (11) | 90 (4) | 76 (20) | 0.000 | 82 (11) | 91 (3) | 86 (9) | 0.060 |
| Carbofuran | 118 (11) | 115 (5) | 116 (8) | 0.632 | 115 (5) | 107 (10) | 111 (8) | 0.139 | 113 (10) | 104 (5) | 108 (9) | 0.093 | 116 (13) | 104 (2) | 110 (11) | 0.117 |
| Carpropamid | −40 (0) | 34 (24) | −3 (−1288) | 0.000 | 108 (10) | 92 (19) | 100 (16) | 0.050 | 88 (9) | 75 (17) | 81 (15) | 0.133 | 100 (8) | 97 (5) | 98 (7) | 0.211 |
| Chlorantraniliprole | 78 (52) | 56 (26) | 67 (47) | 0.199 | 78 (20) | 87 (19) | 82 (19) | 0.488 | 84 (10) | 91 (13) | 87 (12) | 0.172 | 97 (12) | 93 (9) | 95 (10) | 0.390 |
| Chlorfenvinphos | 97 (19) | 102 (9) | 100 (14) | 0.599 | 92 (8) | 100 (9) | 96 (9) | 0.066 | 89 (11) | 80 (19) | 84 (16) | 0.340 | 93 (16) | 108 (4) | 101 (13) | 0.074 |
| Chlorpyrifos | 112 (5) | 101 (18) | 107 (13) | 0.174 | 102 (14) | 103 (16) | 102 (15) | 0.915 | 83 (7) | 90 (13) | 87 (11) | 0.116 | 81 (16) | 81 (6) | 81 (11) | 0.995 |
| Clofentezine | 90 (20) | 79 (16) | 84 (19) | 0.096 | 76 (12) | 76 (18) | 76 (15) | 0.998 | 75 (11) | 93 (18) | 84 (19) | 0.066 | 89 (19) | 79 (19) | 84 (19) | 0.289 |
| Clomazone | 83 (20) | 73 (13) | 78 (18) | 0.271 | 92 (6) | 98 (8) | 95 (7) | 0.192 | 93 (10) | 105 (14) | 99 (13) | 0.209 | 105 (9) | 111 (12) | 108 (11) | 0.352 |
| Clothianidin | 109 (8) | 95 (12) | 102 (12) | 0.051 | 101 (11) | 88 (13) | 94 (14) | 0.055 | 92 (19) | 78 (6) | 85 (17) | 0.049 | 89 (17) | 84 (5) | 87 (13) | 0.419 |
| Cyazofamid | 76 (47) | 41 (54) | 59 (58) | 0.136 | 91 (17) | 93 (19) | 92 (17) | 0.746 | 96 (10) | 105 (7) | 101 (10) | 0.091 | 99 (11) | 107 (10) | 103 (11) | 0.277 |
| Cyproconazole | 92 (19) | 94 (7) | 93 (14) | 0.866 | 94 (9) | 103 (9) | 99 (10) | 0.101 | 90 (9) | 90 (6) | 90 (8) | 0.946 | 94 (10) | 88 (12) | 91 (11) | 0.310 |
| Cyprodinil | 75 (17) | 71 (10) | 73 (14) | 0.469 | 71 (5) | 73 (7) | 72 (6) | 0.401 | 70 (9) | 72 (8) | 71 (8) | 0.678 | 71 (18) | 78 (5) | 75 (13) | 0.142 |
| Demeton-S-methyl sulfone | 94 (17) | 93 (6) | 94 (13) | 0.836 | 93 (9) | 99 (4) | 96 (7) | 0.146 | 101 (10) | 107 (5) | 104 (8) | 0.147 | 104 (8) | 103 (4) | 104 (6) | 0.780 |
| Demeton-S-methyl sulfoxide | 77 (60) | 79 (16) | 78 (42) | 0.913 | 87 (10) | 102 (15) | 95 (15) | 0.128 | 89 (16) | 105 (20) | 97 (20) | 0.118 | 96 (11) | 107 (3) | 102 (9) | 0.074 |
| Deoxynivalenol | 202 (358) | 1010 (67) | 606 (131) | 0.147 | 657 (68) | 109 (283) | 383 (122) | 0.015 | 25 (11) | 32 (14) | 28 (18) | 0.038 | 26 (8) | 25 (9) | 26 (9) | 0.155 |
| Diacetoxyscirpenol | 2 (291) | −4 (-167) | −1 (-728) | 0.036 | 16 (60) | 17 (20) | 16 (41) | 0.699 | 23 (7) | 23 (8) | 23 (7) | 0.547 | 22 (12) | 25 (4) | 24 (11) | 0.038 |
| Diazinon | 98 (12) | 108 (15) | 103 (14) | 0.330 | 99 (6) | 107 (15) | 103 (12) | 0.226 | 108 (19) | 113 (9) | 111 (14) | 0.509 | 93 (9) | 108 (15) | 100 (15) | 0.106 |
| Diethofencarb | 82 (37) | 39 (27) | 61 (51) | 0.013 | 109 (20) | 98 (18) | 104 (19) | 0.140 | 89 (14) | 95 (6) | 92 (11) | 0.357 | 100 (13) | 94 (5) | 97 (10) | 0.155 |
| Difenoconazol | 91 (17) | 81 (6) | 86 (14) | 0.109 | 94 (10) | 96 (7) | 95 (8) | 0.594 | 89 (10) | 100 (5) | 94 (10) | 0.063 | 105 (17) | 101 (4) | 103 (12) | 0.641 |
| Diflubenzuron | 49 (168) | 14 (560) | 32 (253) | 0.347 | 91 (44) | 54 (92) | 73 (66) | 0.147 | 89 (11) | 101 (13) | 95 (13) | 0.170 | 95 (14) | 115 (19) | 105 (19) | 0.092 |
| Dimethoate | 101 (11) | 100 (9) | 101 (9) | 0.831 | 93 (7) | 98 (6) | 95 (7) | 0.161 | 88 (18) | 92 (3) | 90 (12) | 0.464 | 89 (18) | 91 (2) | 90 (12) | 0.816 |
| Dimethomorph | 93 (12) | 106 (8) | 100 (12) | 0.125 | 102 (12) | 110 (6) | 106 (10) | 0.197 | 91 (12) | 87 (4) | 89 (9) | 0.398 | 94 (13) | 86 (3) | 90 (10) | 0.189 |
| Diniconazole | 91 (13) | 83 (14) | 87 (14) | 0.129 | 91 (16) | 80 (7) | 85 (14) | 0.061 | 85 (8) | 79 (9) | 82 (9) | 0.189 | 89 (10) | 82 (11) | 85 (11) | 0.275 |
| Diphenylamine | 115 (13) | 96 (15) | 106 (16) | 0.114 | 98 (11) | 87 (10) | 92 (12) | 0.071 | 89 (11) | 84 (8) | 86 (10) | 0.388 | 86 (10) | 86 (8) | 86 (9) | 0.944 |
| Diuron | 86 (17) | 74 (7) | 80 (16) | 0.096 | 90 (8) | 93 (7) | 91 (7) | 0.248 | 92 (9) | 102 (5) | 97 (8) | 0.055 | 90 (9) | 89 (20) | 90 (15) | 0.850 |
| DMST | 86 (16) | 100 (18) | 93 (19) | 0.110 | 105 (20) | 97 (13) | 101 (17) | 0.532 | 97 (9) | 98 (16) | 97 (13) | 0.963 | 94 (13) | 82 (10) | 88 (13) | 0.088 |
| Epoxiconazol | 89 (5) | 102 (17) | 96 (14) | 0.088 | 96 (10) | 111 (14) | 104 (14) | 0.061 | 97 (6) | 100 (15) | 98 (12) | 0.587 | 89 (15) | 103 (9) | 96 (14) | 0.059 |
| Ethion | 87 (14) | 108 (17) | 98 (19) | 0.051 | 92 (8) | 104 (14) | 98 (13) | 0.063 | 89 (9) | 96 (5) | 93 (8) | 0.155 | 88 (13) | 94 (6) | 91 (10) | 0.170 |
| Ethiprole | 86 (14) | 79 (19) | 82 (16) | 0.393 | 102 (12) | 86 (17) | 94 (16) | 0.093 | 95 (6) | 97 (17) | 96 (13) | 0.735 | 102 (14) | 103 (5) | 103 (10) | 0.827 |
| Ethoprophos | 98 (16) | 112 (9) | 105 (14) | 0.213 | 87 (13) | 86 (8) | 87 (10) | 0.952 | 88 (9) | 81 (20) | 84 (15) | 0.427 | 98 (10) | 106 (10) | 102 (11) | 0.200 |
| Etofenprox | 95 (19) | 87 (20) | 91 (19) | 0.478 | 107 (18) | 98 (20) | 103 (19) | 0.452 | 86 (9) | 80 (15) | 83 (13) | 0.366 | 85 (15) | 87 (6) | 86 (11) | 0.821 |
| Etoxazole | 92 (16) | 88 (3) | 90 (11) | 0.464 | 89 (9) | 86 (5) | 87 (7) | 0.177 | 86 (11) | 88 (2) | 87 (8) | 0.572 | 87 (6) | 87 (3) | 87 (4) | 0.817 |
| Fenamidone | 97 (12) | 108 (8) | 102 (11) | 0.111 | 100 (7) | 97 (20) | 98 (14) | 0.761 | 93 (11) | 92 (13) | 93 (12) | 0.689 | 91 (11) | 86 (4) | 88 (9) | 0.164 |
| Fenamiphos | 105 (12) | 98 (11) | 102 (11) | 0.352 | 91 (13) | 91 (5) | 91 (9) | 0.948 | 87 (10) | 91 (3) | 89 (8) | 0.475 | 90 (14) | 92 (2) | 91 (9) | 0.654 |
| Fenarimol | 76 (35) | 48 (24) | 62 (39) | 0.097 | 89 (11) | 102 (15) | 96 (15) | 0.118 | 76 (19) | 74 (10) | 75 (15) | 0.739 | 89 (16) | 80 (10) | 85 (15) | 0.117 |
| Fenazaquin | 89 (18) | 84 (7) | 86 (14) | 0.499 | 89 (16) | 94 (7) | 91 (12) | 0.384 | 84 (14) | 93 (3) | 88 (11) | 0.062 | 83 (15) | 91 (2) | 87 (11) | 0.161 |
| Fenbuconazol | 65 (27) | 40 (47) | 53 (42) | 0.025 | 90 (19) | 110 (16) | 100 (20) | 0.158 | 98 (19) | 106 (11) | 102 (15) | 0.403 | 98 (6) | 99 (21) | 98 (15) | 0.908 |
| Fenhexamid | 81 (34) | 78 (20) | 79 (27) | 0.854 | 95 (15) | 94 (19) | 94 (16) | 0.910 | 93 (10) | 103 (13) | 98 (13) | 0.099 | 99 (14) | 104 (15) | 102 (15) | 0.684 |
| Fenobucarb | 89 (20) | 103 (7) | 96 (16) | 0.116 | 99 (13) | 111 (9) | 105 (12) | 0.107 | 91 (10) | 106 (16) | 98 (15) | 0.084 | 101 (7) | 113 (13) | 107 (12) | 0.074 |
| Fenoxycarb | 72 (55) | 52 (0) | 62 (47) | 0.231 | 81 (19) | 73 (14) | 77 (17) | 0.191 | 86 (15) | 79 (18) | 82 (16) | 0.457 | 94 (13) | 84 (19) | 89 (17) | 0.259 |
| Fenpropimorph | 100 (13) | 107 (7) | 103 (11) | 0.341 | 96 (6) | 105 (12) | 100 (11) | 0.139 | 93 (5) | 96 (4) | 94 (5) | 0.401 | 86 (11) | 95 (2) | 90 (9) | 0.068 |
| Fenpyroximate | 85 (17) | 93 (4) | 89 (12) | 0.252 | 83 (11) | 94 (8) | 88 (11) | 0.058 | 85 (15) | 95 (5) | 90 (12) | 0.151 | 85 (7) | 91 (9) | 88 (9) | 0.174 |
| Fensulfothion | 104 (13) | 101 (19) | 103 (16) | 0.752 | 100 (14) | 99 (9) | 100 (12) | 0.871 | 113 (14) | 101 (11) | 107 (14) | 0.079 | 119 (5) | 111 (6) | 115 (7) | 0.068 |
| Fluazifop-butyl | 89 (13) | 99 (10) | 94 (12) | 0.117 | 87 (9) | 99 (9) | 93 (11) | 0.066 | 88 (10) | 93 (18) | 90 (14) | 0.361 | 87 (12) | 98 (14) | 93 (14) | 0.195 |
| Fludioxonil | 108 (14) | 92 (15) | 100 (16) | 0.085 | 90 (18) | 89 (15) | 89 (16) | 0.817 | 97 (11) | 98 (12) | 97 (11) | 0.853 | 95 (9) | 95 (8) | 95 (8) | 0.992 |
| Flufenoxuron | −22 (0) | 57 (34) | 18 (245) | 0.000 | 105 (17) | 101 (12) | 103 (15) | 0.522 | 78 (11) | 79 (12) | 78 (11) | 0.911 | 96 (14) | 98 (19) | 97 (16) | 0.833 |
| Fluquinconazol | 89 (53) | 65 (40) | 77 (51) | 0.308 | 94 (15) | 94 (20) | 94 (17) | 0.968 | 93 (17) | 89 (17) | 91 (16) | 0.715 | 94 (14) | 86 (12) | 90 (14) | 0.125 |
| Flusilazol | 84 (19) | 104 (15) | 94 (20) | 0.055 | 94 (18) | 104 (10) | 99 (15) | 0.172 | 88 (8) | 78 (10) | 83 (11) | 0.057 | 96 (10) | 85 (14) | 91 (13) | 0.157 |
| Flutolanil | 56 (39) | 25 (22) | 41 (54) | 0.016 | 81 (19) | 99 (18) | 90 (20) | 0.106 | 85 (13) | 80 (17) | 82 (15) | 0.502 | 97 (18) | 84 (16) | 91 (19) | 0.066 |
| Flutriafol | 99 (16) | 87 (9) | 93 (15) | 0.118 | 93 (9) | 84 (12) | 89 (11) | 0.122 | 87 (11) | 90 (5) | 88 (8) | 0.509 | 91 (15) | 82 (7) | 87 (13) | 0.223 |
| Fosthiazate | 105 (15) | 95 (7) | 100 (12) | 0.172 | 98 (6) | 94 (10) | 96 (8) | 0.269 | 89 (13) | 90 (4) | 89 (9) | 0.788 | 91 (13) | 89 (4) | 90 (9) | 0.722 |
| Fumonisin B1 | 156 (2) | 163 (14) | 159 (10) | 0.490 | 72 (17) | 61 (0) | 67 (15) | 0.047 | 49 (39) | 30 (1) | 40 (41) | 0.044 | 23 (31) | 15 (1) | 19 (32) | 0.030 |
| Fumonisin B2 | 186 (4) | 186 (1) | 186 (3) | 0.996 | 86 (8) | 72 (1) | 79 (11) | 0.002 | 51 (8) | 36 (0) | 44 (19) | 0.000 | 26 (28) | 18 (1) | 22 (29) | 0.032 |
| Furalaxyl | 81 (33) | 124 (5) | 102 (28) | 0.003 | 79 (14) | 131 (4) | 105 (27) | 0.000 | 83 (10) | 89 (2) | 86 (8) | 0.146 | 95 (11) | 89 (2) | 92 (9) | 0.154 |
| Furathiocarb | 92 (19) | 108 (6) | 100 (15) | 0.118 | 92 (9) | 103 (10) | 97 (11) | 0.151 | 88 (9) | 100 (13) | 94 (13) | 0.072 | 87 (12) | 98 (11) | 93 (12) | 0.086 |
| Halofenozide | −177 (0) | −177 (0) | −177 (0) | n.f.r.b | −88 (0) | 127 (34) | 19 (603) | 0.000 | 91 (20) | 103 (20) | 97 (20) | 0.161 | 103 (15) | 97 (20) | 100 (17) | 0.544 |
| Haloxyfop-2-ethoxyethyl | 87 (18) | 92 (12) | 89 (15) | 0.464 | 87 (10) | 91 (9) | 89 (9) | 0.312 | 86 (7) | 98 (11) | 92 (11) | 0.072 | 93 (8) | 102 (8) | 97 (9) | 0.151 |
| Hexaconazol | 97 (15) | 95 (12) | 96 (13) | 0.677 | 97 (6) | 93 (13) | 95 (10) | 0.464 | 90 (12) | 85 (6) | 88 (9) | 0.393 | 86 (10) | 84 (8) | 85 (9) | 0.670 |
| Hexytiazox | 100 (18) | 81 (17) | 91 (20) | 0.059 | 90 (15) | 87 (19) | 88 (16) | 0.712 | 86 (8) | 83 (9) | 85 (8) | 0.507 | 86 (14) | 92 (9) | 89 (12) | 0.327 |
| Imazalil | 96 (18) | 83 (5) | 89 (16) | 0.101 | 83 (9) | 88 (8) | 85 (9) | 0.337 | 85 (9) | 93 (5) | 89 (9) | 0.087 | 86 (9) | 89 (4) | 87 (7) | 0.530 |
| Imazapic | 111 (7) | 75 (10) | 93 (22) | 0.000 | 91 (8) | 82 (16) | 87 (13) | 0.083 | 86 (20) | 71 (10) | 78 (19) | 0.114 | 81 (18) | 74 (6) | 77 (14) | 0.239 |
| Imazetapyr | 94 (14) | 86 (12) | 90 (13) | 0.118 | 84 (5) | 89 (8) | 86 (7) | 0.119 | 72 (17) | 79 (4) | 76 (12) | 0.220 | 78 (16) | 79 (3) | 78 (11) | 0.844 |
| Imidacloprid | 100 (13) | 92 (15) | 96 (14) | 0.428 | 101 (18) | 88 (12) | 95 (17) | 0.058 | 99 (8) | 89 (14) | 94 (12) | 0.062 | 89 (19) | 92 (21) | 91 (19) | 0.807 |
| Indoxacarb | 82 (17) | 102 (18) | 92 (21) | 0.091 | 87 (12) | 90 (14) | 89 (12) | 0.543 | 93 (14) | 107 (18) | 100 (17) | 0.054 | 101 (8) | 109 (8) | 105 (9) | 0.124 |
| Iprovalicarb | 72 (13) | 76 (14) | 74 (13) | 0.381 | 79 (15) | 92 (10) | 86 (14) | 0.050 | 81 (11) | 97 (10) | 89 (13) | 0.055 | 94 (15) | 101 (1) | 98 (11) | 0.223 |
| Isoxaflutole | 281 (110) | 203 (94) | 242 (103) | 0.324 | 168 (35) | 225 (85) | 196 (71) | 0.474 | 96 (18) | 106 (21) | 101 (19) | 0.076 | 100 (15) | 92 (10) | 96 (13) | 0.392 |
| Kresoxim-methyl | 86 (17) | 93 (19) | 89 (18) | 0.468 | 92 (13) | 95 (17) | 94 (15) | 0.646 | 100 (19) | 117 (6) | 108 (15) | 0.067 | 96 (11) | 109 (7) | 103 (11) | 0.069 |
| Linuron | −14 (0) | 55 (29) | 21 (183) | 0.000 | 93 (13) | 101 (16) | 97 (15) | 0.187 | 118 (12) | 119 (7) | 119 (9) | 0.803 | 113 (10) | 116 (10) | 114 (10) | 0.636 |
| Lufenuron | 86 (20) | 97 (11) | 91 (17) | 0.169 | 93 (15) | 84 (17) | 89 (16) | 0.130 | 81 (14) | 81 (9) | 81 (11) | 0.931 | 92 (11) | 84 (6) | 88 (10) | 0.124 |
| Malathion | 103 (15) | 104 (12) | 104 (13) | 0.962 | 81 (13) | 91 (14) | 86 (14) | 0.200 | 84 (6) | 79 (17) | 82 (12) | 0.378 | 106 (8) | 114 (5) | 110 (8) | 0.148 |
| Mecarban | 91 (19) | 89 (14) | 90 (16) | 0.732 | 98 (11) | 87 (14) | 92 (14) | 0.103 | 93 (12) | 101 (11) | 97 (12) | 0.096 | 99 (7) | 100 (9) | 100 (7) | 0.741 |
| Mepanipyrin | 56 (44) | 186 (31) | 121 (66) | 0.002 | 59 (31) | 78 (40) | 69 (39) | 0.147 | 77 (13) | 77 (14) | 77 (13) | 0.919 | 91 (7) | 101 (10) | 96 (10) | 0.101 |
| Metalaxyl | 109 (11) | 100 (4) | 105 (9) | 0.111 | 96 (5) | 94 (6) | 95 (5) | 0.438 | 89 (15) | 88 (2) | 88 (11) | 0.850 | 88 (13) | 87 (2) | 88 (9) | 0.857 |
| Metconazol | 92 (20) | 110 (8) | 101 (16) | 0.102 | 90 (15) | 103 (5) | 96 (13) | 0.060 | 86 (18) | 96 (6) | 91 (14) | 0.134 | 88 (14) | 97 (5) | 92 (11) | 0.131 |
| Methamidophos | 51 (107) | 166 (79) | 109 (104) | 0.063 | 104 (15) | 101 (20) | 102 (17) | 0.711 | 44 (18) | 64 (11) | 54 (24) | 0.003 | 78 (17) | 93 (14) | 86 (17) | 0.071 |
| Methidathion | −73 (−89) | 65 (65) | −4 (−2073) | 0.005 | 92 (18) | 97 (17) | 94 (17) | 0.616 | 89 (18) | 84 (15) | 86 (16) | 0.309 | 104 (13) | 113 (10) | 109 (12) | 0.212 |
| Methiocarb | 56 (43) | 17 (62) | 37 (74) | 0.005 | 112 (17) | 113 (13) | 112 (15) | 0.957 | 86 (9) | 78 (8) | 82 (10) | 0.073 | 98 (13) | 103 (16) | 100 (14) | 0.563 |
| Methomyl | 103 (13) | 113 (7) | 108 (11) | 0.146 | 100 (14) | 104 (13) | 102 (13) | 0.679 | 97 (19) | 107 (18) | 102 (19) | 0.119 | 102 (10) | 105 (19) | 103 (15) | 0.841 |
| Methoxyfenozide | 89 (31) | 164 (8) | 127 (35) | 0.001 | 89 (17) | 105 (18) | 97 (19) | 0.089 | 85 (13) | 86 (19) | 85 (16) | 0.748 | 98 (9) | 107 (14) | 103 (13) | 0.274 |
| Monocrotophos | 94 (13) | 104 (11) | 99 (13) | 0.241 | 75 (14) | 87 (17) | 81 (17) | 0.077 | 91 (13) | 99 (8) | 95 (11) | 0.133 | 89 (10) | 96 (7) | 93 (9) | 0.143 |
| Myclobutanil | 99 (17) | 82 (10) | 91 (17) | 0.060 | 103 (6) | 92 (15) | 98 (12) | 0.130 | 96 (15) | 112 (8) | 104 (14) | 0.075 | 91 (12) | 91 (4) | 91 (9) | 0.895 |
| Nitenpyran | 290 (41) | 871 (28) | 580 (61) | 0.002 | 141 (92) | 666 (37) | 404 (82) | 0.002 | 93 (47) | 244 (46) | 169 (67) | 0.011 | 109 (14) | 187 (70) | 148 (66) | 0.183 |
| Ochratoxin A | 101 (8) | 109 (4) | 105 (7) | 0.106 | 70 (11) | 69 (15) | 70 (13) | 0.760 | 81 (14) | 73 (8) | 77 (12) | 0.137 | 70 (5) | 71 (10) | 70 (7) | 0.648 |
| Ofurace | 97 (19) | 95 (13) | 96 (16) | 0.783 | 92 (9) | 85 (3) | 88 (8) | 0.059 | 91 (17) | 77 (5) | 84 (15) | 0.079 | 90 (12) | 81 (5) | 85 (11) | 0.127 |
| Omethoate | 980 (72) | 697 (58) | 839 (68) | 0.377 | 210 (83) | 739 (16) | 475 (65) | 0.001 | 96 (20) | 107 (19) | 102 (20) | 0.228 | 89 (4) | 84 (18) | 86 (13) | 0.447 |
| Oxadixyl | 90 (17) | 83 (5) | 86 (13) | 0.318 | 88 (7) | 88 (8) | 88 (7) | 0.945 | 90 (17) | 93 (1) | 92 (12) | 0.639 | 94 (11) | 92 (3) | 93 (8) | 0.666 |
| Oxamyl | 91 (16) | 80 (6) | 85 (14) | 0.086 | 82 (14) | 88 (9) | 85 (12) | 0.337 | 84 (5) | 90 (12) | 87 (9) | 0.331 | 85 (10) | 89 (4) | 87 (7) | 0.241 |
| Paclobutrazol | 105 (15) | 119 (12) | 112 (15) | 0.163 | 98 (11) | 111 (9) | 105 (12) | 0.054 | 96 (6) | 107 (9) | 102 (9) | 0.063 | 93 (10) | 108 (14) | 100 (14) | 0.063 |
| Penconazol | 92 (17) | 102 (11) | 97 (14) | 0.086 | 90 (9) | 92 (6) | 91 (7) | 0.715 | 92 (9) | 96 (6) | 94 (8) | 0.303 | 88 (9) | 98 (3) | 93 (8) | 0.073 |
| Pencycuron | 94 (17) | 106 (11) | 100 (15) | 0.103 | 91 (10) | 107 (13) | 99 (14) | 0.063 | 95 (11) | 108 (12) | 101 (13) | 0.113 | 99 (7) | 106 (4) | 103 (6) | 0.052 |
| Pendimethalin | 85 (18) | 80 (18) | 83 (17) | 0.689 | 88 (11) | 92 (11) | 90 (11) | 0.517 | 86 (9) | 94 (6) | 90 (9) | 0.081 | 81 (7) | 88 (13) | 84 (11) | 0.080 |
| Phenothrin | 113 (18) | 107 (14) | 110 (16) | 0.548 | 97 (13) | 113 (13) | 105 (15) | 0.082 | 83 (12) | 90 (9) | 86 (11) | 0.154 | 88 (15) | 82 (7) | 85 (13) | 0.339 |
| Phenthoate | 416 (266) | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | n.f.r.b | 516 (207) | 4543 (67) | 2530 (120) | 0.027 | 141 (216) | n.f.r.b | n.f.r.b | n.f.r.b |
| Phosalone | 86 (46) | −67 (−205) | 9 (1328) | 0.029 | 100 (17) | 106 (13) | 103 (15) | 0.510 | 86 (10) | 98 (15) | 92 (14) | 0.057 | 93 (9) | 105 (9) | 99 (11) | 0.052 |
| Phosmet | −79 (0) | 31 (71) | −24 (−247) | 0.000 | 85 (17) | 93 (16) | 89 (16) | 0.241 | 94 (15) | 87 (4) | 91 (12) | 0.157 | 106 (13) | 90 (15) | 98 (16) | 0.072 |
| Picoxystrobin | 101 (17) | 115 (2) | 108 (13) | 0.068 | 90 (11) | 102 (15) | 96 (15) | 0.176 | 95 (15) | 104 (18) | 99 (17) | 0.419 | 107 (15) | 112 (4) | 109 (11) | 0.467 |
| Piperonyl butoxide | 92 (16) | 103 (6) | 97 (13) | 0.173 | 91 (8) | 97 (9) | 94 (9) | 0.140 | 86 (8) | 95 (11) | 91 (10) | 0.189 | 89 (10) | 96 (12) | 93 (11) | 0.137 |
| Pirimicarb | 101 (12) | 97 (5) | 99 (9) | 0.420 | 92 (5) | 97 (8) | 95 (7) | 0.356 | 85 (15) | 96 (4) | 91 (12) | 0.071 | 86 (11) | 91 (8) | 88 (10) | 0.171 |
| Pirimiphos-ethyl | 98 (13) | 104 (6) | 101 (10) | 0.405 | 97 (3) | 99 (4) | 98 (3) | 0.245 | 92 (7) | 99 (12) | 95 (10) | 0.070 | 104 (18) | 101 (2) | 103 (12) | 0.650 |
| Pirimiphos-methyl | 102 (13) | 104 (9) | 103 (11) | 0.761 | 97 (4) | 105 (7) | 101 (7) | 0.011 | 99 (13) | 110 (6) | 104 (11) | 0.135 | 91 (10) | 105 (14) | 98 (14) | 0.097 |
| Prochloraz | 86 (19) | 105 (9) | 95 (17) | 0.074 | 85 (8) | 87 (7) | 86 (7) | 0.728 | 85 (10) | 92 (4) | 89 (8) | 0.128 | 90 (10) | 89 (5) | 90 (8) | 0.907 |
| Profenofos | 100 (19) | 88 (12) | 94 (17) | 0.193 | 91 (17) | 91 (18) | 91 (17) | 0.956 | 92 (6) | 102 (9) | 97 (9) | 0.074 | 88 (14) | 103 (11) | 96 (14) | 0.094 |
| Prometryn | 97 (12) | 101 (4) | 99 (9) | 0.445 | 94 (7) | 96 (3) | 95 (5) | 0.477 | 88 (8) | 95 (2) | 91 (7) | 0.105 | 88 (10) | 92 (2) | 90 (7) | 0.305 |
| Propamocarb | 44 (73) | −4 (−707) | 20 (197) | 0.030 | 52 (39) | 5 (74) | 29 (99) | 0.002 | 59 (16) | 18 (8) | 38 (58) | 0.000 | 86 (19) | 107 (15) | 97 (20) | 0.063 |
| Propanil | 71 (59) | −80 (0) | −5 (−1818) | 0.000 | 67 (70) | 73 (45) | 70 (56) | 0.811 | 105 (17) | 95 (19) | 100 (18) | 0.306 | 86 (18) | 80 (16) | 83 (17) | 0.491 |
| Propham | 77 (18) | 88 (20) | 82 (20) | 0.269 | 84 (16) | 90 (16) | 87 (16) | 0.475 | 96 (18) | 89 (8) | 92 (14) | 0.188 | 103 (8) | 89 (19) | 96 (15) | 0.157 |
| Propiconazol | 82 (15) | 88 (13) | 85 (14) | 0.258 | 84 (11) | 96 (17) | 90 (16) | 0.229 | 84 (10) | 76 (5) | 80 (10) | 0.082 | 90 (7) | 98 (14) | 94 (12) | 0.100 |
| Propyzamide | 93 (20) | 102 (12) | 98 (16) | 0.361 | 84 (20) | 102 (20) | 93 (22) | 0.196 | 92 (16) | 79 (9) | 85 (15) | 0.055 | 100 (9) | 110 (5) | 105 (8) | 0.056 |
| Pyraclostrobin | 88 (16) | 71 (13) | 79 (18) | 0.056 | 88 (10) | 93 (10) | 91 (10) | 0.375 | 83 (8) | 94 (11) | 89 (11) | 0.132 | 93 (7) | 97 (3) | 95 (5) | 0.127 |
| Pyrazophos | 275 (11) | 296 (7) | 286 (10) | 0.097 | 236 (14) | 257 (2) | 246 (10) | 0.151 | 205 (14) | 195 (4) | 200 (10) | 0.384 | 205 (18) | 193 (5) | 199 (13) | 0.410 |
| Pyridaben | 88 (18) | 84 (10) | 86 (14) | 0.649 | 87 (5) | 89 (5) | 88 (5) | 0.146 | 82 (7) | 89 (8) | 85 (9) | 0.058 | 82 (9) | 89 (8) | 85 (10) | 0.097 |
| Pyrimethanil | 85 (16) | 73 (4) | 79 (14) | 0.064 | 78 (5) | 77 (5) | 78 (5) | 0.367 | 77 (8) | 73 (3) | 75 (7) | 0.161 | 78 (13) | 74 (2) | 76 (9) | 0.475 |
| Pyriproxyfen | 96 (16) | 88 (6) | 92 (13) | 0.331 | 90 (8) | 91 (7) | 90 (7) | 0.569 | 82 (8) | 91 (10) | 87 (10) | 0.114 | 85 (8) | 92 (3) | 88 (7) | 0.089 |
| Quinalphos | 95 (19) | 101 (16) | 98 (17) | 0.457 | 90 (12) | 97 (17) | 94 (15) | 0.171 | 99 (17) | 105 (8) | 102 (13) | 0.443 | 95 (8) | 102 (7) | 98 (8) | 0.100 |
| Quinoxyfen | 95 (16) | 89 (11) | 92 (14) | 0.483 | 81 (13) | 93 (11) | 87 (13) | 0.087 | 83 (9) | 88 (5) | 85 (8) | 0.147 | 80 (8) | 84 (5) | 82 (7) | 0.252 |
| Simazine | 98 (15) | 96 (10) | 97 (12) | 0.714 | 92 (9) | 89 (9) | 90 (9) | 0.480 | 87 (14) | 89 (3) | 88 (10) | 0.586 | 89 (13) | 88 (3) | 89 (9) | 0.863 |
| Spinosyn A | 92 (16) | 79 (14) | 86 (16) | 0.142 | 89 (8) | 84 (7) | 86 (8) | 0.097 | 92 (10) | 85 (3) | 89 (8) | 0.069 | 93 (9) | 91 (5) | 92 (7) | 0.727 |
| Spinosyn D | 100 (17) | 84 (16) | 92 (19) | 0.139 | 94 (10) | 93 (13) | 93 (11) | 0.789 | 88 (6) | 86 (5) | 87 (6) | 0.443 | 92 (11) | 89 (5) | 90 (9) | 0.412 |
| Spirodiclofen | 78 (17) | 86 (17) | 82 (17) | 0.324 | 80 (15) | 99 (12) | 90 (17) | 0.055 | 91 (11) | 99 (8) | 95 (10) | 0.206 | 93 (9) | 98 (5) | 95 (7) | 0.169 |
| Spiromesifen | 74 (33) | 83 (23) | 79 (28) | 0.359 | 78 (6) | 91 (17) | 85 (15) | 0.127 | 80 (15) | 77 (9) | 78 (12) | 0.675 | 88 (13) | 78 (10) | 83 (13) | 0.152 |
| Spiroxamine | 104 (12) | 97 (5) | 101 (10) | 0.208 | 97 (4) | 100 (3) | 98 (4) | 0.067 | 91 (8) | 96 (2) | 93 (6) | 0.153 | 89 (12) | 96 (2) | 92 (9) | 0.179 |
| Tau-fluvalinate | 161 (41) | 80 (119) | 121 (74) | 0.091 | 102 (10) | 103 (5) | 102 (8) | 0.720 | 93 (17) | 91 (16) | 92 (16) | 0.754 | 79 (20) | 98 (16) | 89 (20) | 0.103 |
| Tebuconazol | 97 (17) | 110 (6) | 104 (13) | 0.110 | 92 (23) | 112 (7) | 102 (18) | 0.069 | 86 (7) | 86 (20) | 86 (15) | 0.904 | 97 (8) | 108 (19) | 102 (15) | 0.282 |
| Tebufenozide | 98 (12) | 95 (9) | 97 (10) | 0.444 | 104 (5) | 107 (17) | 105 (12) | 0.667 | 93 (8) | 102 (7) | 97 (8) | 0.108 | 101 (5) | 107 (4) | 104 (5) | 0.081 |
| Tebufenpyrad | 95 (18) | 95 (12) | 95 (15) | 0.974 | 90 (15) | 101 (11) | 95 (14) | 0.236 | 97 (15) | 107 (6) | 102 (12) | 0.156 | 88 (10) | 101 (7) | 95 (11) | 0.067 |
| Terbutryn | 100 (12) | 101 (3) | 100 (9) | 0.746 | 94 (4) | 95 (4) | 95 (4) | 0.465 | 88 (8) | 94 (3) | 91 (7) | 0.090 | 86 (11) | 92 (2) | 89 (8) | 0.202 |
| Tetrachlorvinphos | 77 (99) | 72 (110) | 74 (100) | 0.858 | 68 (44) | 64 (25) | 66 (35) | 0.759 | 82 (17) | 83 (16) | 83 (16) | 0.912 | 82 (14) | 78 (9) | 80 (11) | 0.384 |
| Tetraconazol | 100 (18) | 115 (9) | 108 (15) | 0.106 | 100 (10) | 112 (11) | 106 (12) | 0.158 | 97 (10) | 110 (10) | 103 (12) | 0.104 | 91 (17) | 83 (2) | 87 (13) | 0.223 |
| Tetramethrin | 97 (14) | 97 (16) | 97 (14) | 0.969 | 90 (14) | 104 (12) | 97 (15) | 0.124 | 83 (9) | 92 (9) | 88 (10) | 0.091 | 88 (12) | 100 (10) | 94 (12) | 0.068 |
| Thiabendazol | 26 (82) | 37 (7) | 31 (50) | 0.226 | 33 (49) | 58 (12) | 45 (39) | 0.009 | 43 (14) | 65 (9) | 54 (24) | 0.001 | 57 (14) | 68 (6) | 62 (14) | 0.012 |
| Thiacloprid | 96 (13) | 88 (11) | 92 (12) | 0.157 | 89 (9) | 90 (8) | 90 (8) | 0.762 | 84 (10) | 92 (1) | 88 (8) | 0.059 | 96 (11) | 90 (4) | 93 (9) | 0.135 |
| Thiamethoxan | 96 (21) | 113 (4) | 104 (16) | 0.076 | 86 (13) | 93 (17) | 90 (15) | 0.437 | 91 (6) | 97 (10) | 94 (9) | 0.229 | 93 (9) | 99 (10) | 96 (10) | 0.093 |
| Thiodicarb | 86 (14) | 77 (6) | 82 (12) | 0.141 | 78 (9) | 74 (5) | 76 (8) | 0.183 | 70 (9) | 70 (5) | 70 (7) | 0.970 | 65 (13) | 74 (20) | 70 (18) | 0.202 |
| Thiophanate-methyl | 40 (28) | 23 (55) | 32 (45) | 0.044 | 54 (12) | 37 (15) | 46 (23) | 0.002 | 72 (18) | 85 (18) | 78 (19) | 0.097 | 73 (6) | 72 (18) | 72 (13) | 0.935 |
| Toxin T2 | 15 (40) | 17 (47) | 16 (43) | 0.623 | 24 (25) | 22 (19) | 23 (22) | 0.585 | 109 (4) | 109 (6) | 109 (5) | 0.896 | 87 (3) | 84 (3) | 86 (3) | 0.157 |
| Triadimefon | 97 (17) | 83 (10) | 90 (16) | 0.129 | 97 (5) | 104 (14) | 100 (11) | 0.325 | 90 (9) | 91 (14) | 91 (11) | 0.945 | 92 (11) | 105 (4) | 99 (10) | 0.052 |
| Triadimenol | 193 (92) | −32 (−66) | 80 (210) | 0.018 | 96 (19) | 73 (19) | 84 (23) | 0.071 | 93 (19) | 98 (13) | 95 (16) | 0.587 | 96 (20) | 77 (11) | 86 (20) | 0.075 |
| Triazophos | 90 (16) | 103 (7) | 97 (13) | 0.094 | 82 (17) | 77 (7) | 80 (13) | 0.449 | 90 (14) | 88 (18) | 89 (15) | 0.832 | 108 (13) | 97 (5) | 102 (11) | 0.065 |
| Trifloxystrobin | 89 (19) | 84 (10) | 86 (15) | 0.497 | 98 (10) | 89 (16) | 94 (14) | 0.104 | 88 (7) | 98 (15) | 93 (13) | 0.081 | 94 (7) | 95 (12) | 94 (10) | 0.818 |
| Triflumizole | 97 (18) | 92 (4) | 95 (13) | 0.554 | 94 (9) | 92 (7) | 93 (8) | 0.527 | 86 (7) | 93 (5) | 89 (7) | 0.122 | 89 (7) | 96 (4) | 92 (7) | 0.097 |
| Triticonazole | 90 (20) | 104 (16) | 97 (19) | 0.113 | 89 (11) | 82 (6) | 85 (10) | 0.121 | 83 (9) | 83 (7) | 83 (8) | 0.987 | 91 (12) | 84 (5) | 87 (10) | 0.138 |
| Zearalenone | 45 (109) | −180 (-45) | −67 (-198) | 0.001 | 23 (123) | −17 (-50) | 3 (975) | 0.011 | 29 (8) | 1 (577) | 15 (101) | 0.000 | 22 (12) | 8 (22) | 15 (49) | 0.000 |
| Zoxamide | 86 (17) | 96 (11) | 91 (15) | 0.092 | 92 (9) | 95 (8) | 93 (8) | 0.584 | 89 (13) | 95 (12) | 92 (13) | 0.129 | 94 (7) | 96 (5) | 95 (6) | 0.475 |
For all pesticides and mycotoxins, the criterion for linearity was r2 ≥ 0.99 and the deviation of back-calculated concentration to be within ± 20% of the assigned concentration. If this value was not achieved, the t-test was applied to r2 to prove linearity. If the value of tr for analytical curve regression was greater than or equal to the critical (tabulated) bilateral t-value, for a confidence level of 95% and (Nx – 2) degrees of freedom, the range was considered linear, rejecting the null hypothesis H0: r = 0 (there is no correlation between x and y).
Although most pesticides presented a linear range of 5–1000 or 5–500 µg kg−1, 63% of analytes presented different linear ranges for M. officinalis and M. sylvestris, especially at the first analytical curve concentration. Mycotoxins of Group 1 presented a linear range of 5–500 µg kg−1 for all aflatoxins and 10–1000 µg kg−1 for ochratoxin A, while mycotoxins of Group 2 presented 250–5000 µg kg−1 for both fumonisins, deoxynivalenol, and diacetoxyscirpenol, 50–2500 µg kg−1 for toxin T2, and 25–500 µg kg−1 for zearalenone.
Method selectivity was evaluated in two different ways, in terms of the matrix effect calculated from the slope of the analytical curves obtained from solutions in a blank matrix extract and in organic solvent (at 1 ng mL−1 for pesticides and mycotoxins of group 1, and 50 ng mL−1 for mycotoxins of group 2). Afterwards, by comparing the selected chromatograms from the blank matrix extract and from solutions in organic solvent.
This evaluation verified the absence of analytes in the matrix by comparing the peak shape, ion ratio, and resolution in the solvent and matrix extract. These calculations and observations were performed automatically using the Mass Hunter Workstation Quantitative Analysis software, version 10.0. Fig. 3 presents an example of the selectivity obtained from the extracted chromatograms of aflatoxin B1 and fenamiphos.
Matrix effects can be described as an increase or decrease in the analytical signal due to co-extractives from the matrix when compared with the detection response for the analytes in organic solvent.51Table 1 presents the matrix effects for all analytes in M. officinalis and M. sylvestris.
Analytes with more polar characteristics presented a higher negative matrix effect. For instance, acephate presented a matrix effect of −74% and −80%, methamidophos −77% and −76%, and omethoate −76% and −76% for M. officinalis and M. sylvestris, respectively. Wu X and Ding Z52 demonstrated that early and late eluting pesticides were observed with strong signal suppression. The suppression effects of the initially eluting pesticides can be explained by the co-elution of polar coexisting compounds in the reversed-phase column, which can affect the ionization efficiency of the target analyte. Additionally, in the initial part of the chromatographic run, the low organic content may affect ESI ionization, leading to high signal suppression.53
Although more polar pesticides presented a similar matrix effect in both matrices, other compounds presented very different matrix effects in each medicinal plant. Fig. 4 shows the analytes with the highest dissimilar matrix effects. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of the analytes ranges from 0.5 to 7.02, indicating that both more polar and nonpolar analytes may experience different matrix effects in the two plants studied. For example, spirodiclofen showed a 24% signal enhancement in M. officinalis while it showed a 46% signal suppression in M. sylvestris, indicating that a representative matrix-matched calibration would lead to inaccurate quantification of the analyte. No analyte had a matrix effect within the ± 20% range nor was the same matrix effect observed for an analyte in both matrices. Therefore, individual analytical curves for each matrix were used. Table 2 presents a summary of the analytes' matrix effect for M. officinalis and M. sylvestris.
Kow of the analytes ranges from 0.5 to 7.02, indicating that both more polar and nonpolar analytes may experience different matrix effects in the two plants studied. For example, spirodiclofen showed a 24% signal enhancement in M. officinalis while it showed a 46% signal suppression in M. sylvestris, indicating that a representative matrix-matched calibration would lead to inaccurate quantification of the analyte. No analyte had a matrix effect within the ± 20% range nor was the same matrix effect observed for an analyte in both matrices. Therefore, individual analytical curves for each matrix were used. Table 2 presents a summary of the analytes' matrix effect for M. officinalis and M. sylvestris.
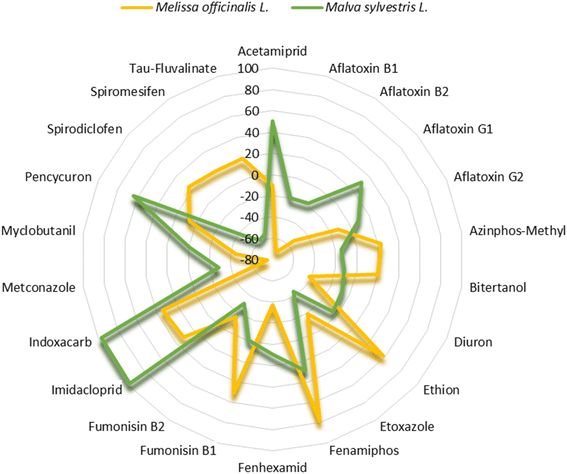 | ||
| Fig. 4 Percentage of analytes presenting a matrix effect in the ranges of ± 20%, between ± 20% and ± 50% and higher than ± 50%. | ||
Method accuracy was determined by assessing trueness (as recovery) and precision (as repeatability and as reproducibility – RSDr and RSDWR, respectively). M. officinalis and M. sylvestris were spiked at 12, 20, 50, and 75 µg kg−1 for pesticides, 2, 5, 10 and 20 µg kg−1 for mycotoxins of group 1, and 100, 250, 500 and 1000 µg kg−1 for mycotoxins of group 2, with sevens replicates at each level. As shown in Tables 3 and 4, the recovery percentages obtained (70–120%) and the standard deviations associated with the replicates showed RSD < 20%, which are acceptable according to the SANTE document 11312/2021 (ref. 35) for the 157 pesticide residues and mycotoxins in M. officinalis and the 152 in M. sylvestris.
The LOD and LOQ were established as the lowest tested solution with a S/N > 3 and the lowest spiked concentration with acceptable accuracy and precision (RSDr and RSDWR), respectively, fulfilling the requirements of SANTE document 11312/2021 (ref. 35) for a quantitative method. When the data were analyzed, 117 pesticides presented an LOQ at 10 µg kg−1, and 15, 14 and 2 pesticides presented an LOQ at 20, 50, and 70 µg kg−1, respectively, for M. officinalis. For M. sylvestris, 99 pesticides presented an LOQ at 10 µg kg−1, and 20, 14 and 6 pesticides presented an LOQ at 20, 50, and 70 µg kg−1, respectively, showing that most pesticides met the accuracy and precision requirements at the lowest spiked level.
In some cases, such as diflubenzuron, propamocarb, and triadimenol, an LOQ (70 µg kg−1) was achieved in M. sylvestris that did not fulfill validation requirements (n.f.r.) for M. officinalis. Conversely, analytes validated in M. officinalis but not in M. sylvestris included bifenazate, diethofencarb, fumonisin B1 and B2, halofenozide, haloxyfop-2-ethoxyethyl, methidathion, omethoate and thiodicarb. Most of these analytes had recovery fluctuations between all 14 replicates, leading to a low precision, indicating the method was not repeatable nor reproducible for these analytes in this specific matrix. For mycotoxins, all four aflatoxins presented an LOQ at 5 µg kg−1 and ochratoxin A at 10 µg kg−1.
In this study, two different medicinal herbs, from distinct families and genera, with different pharmacological parts were used for method validation. When comparing the two matrices for all compounds, it is evident that significant deviation in results can occur due to the unique matrix effect caused by each matrix on each analyte. The matrix-matched calibration for both matrices presented similar matrix effects for 111 analytes. Most mycotoxins presented a difference higher than 20% in matrix effect between the two matrices. Fenazaquin, fenhexamid, imazapic, and propyzamid showed signal suppression in M. officinalis while in M. sylvestris, an enhancement in the analytical signal was observed. More polar compounds, such as acephate, methamidophos, and omethoate, presented the same matrix effect in both matrices, indicating that a representative matrix could be used without compromising the results.
Commercial sample
Imidacloprid residues (13 µg kg−1) were found in a M. officinalis sample. However, there is no MRL for this pesticide, meaning there should be no residues in medicinal herbs sold in the country. Only one sample of M. sylvestris showed residues of methyl pirimiphos, at a concentration of 11.6 µg kg−1, which is within the MRL (4000 µg kg−1) set by Brazilian legislation.32Sample comparisons were carried out with herbarium reference material (SMDB) and via anatomical analysis of samples that showed pesticide residues. These evaluations were carried out in the herbarium of the Botanical Garden (SMDB) and in the Laboratory of Plant Taxonomy (Biology Department/UFSM). The sample sold as M. officinalis was not confirmed to be this species but was compatible with species of Lamiaceae and Verbenaceae. Thus, the consumer used a species other than M. officinalis, and in addition to not having its pharmacological properties, they were also exposed to pesticide residue. The Malva sylvestris sample was identified as partially compatible with Malva sp., mostly mixed with other Malvaceae species.
Despite the limited sampling, the results obtained suggest the non-application of pesticides or the conscious use of pesticides on the medicinal herbs analyzed. In China, in green tea samples analyzed by Y. Huang et al.,54 67% of the samples contained some pesticide residue, and the majority contained more than five pesticides.
Regarding the presence of mycotoxins, none of those studied were detected in the analyzed samples, indicating correct drying and storage. In the study by N. Pallarés et al.,55 224 samples of herbal medicines and their infusions were analyzed. The results revealed that aflatoxins B2, G1, and G2 as well as zearalenone, were detected in infusions with incidences ≤ 6% and at concentrations below the limit of quantification up to 82.2 µg L−1. Even though in this study the majority of samples were not positive for the target compounds, investigations need to continue so that more data can be collected to guide national public policies.
Conclusion
This study presents the first reported method for the determination of over 160 mycotoxins/pesticides in medicinal herbs. The developed approach involves a rapid, simple, and effective extraction applying QuEChERS coupled with dSPE clean-up and LC-TQ-MS/MS quantification, which proved to be sufficiently sensitive to meet the diverse analytical requirements for multi-mycotoxin and multi-pesticide analysis. Through a comprehensive clean-up study, it was determined that a combination of GCB, PSA, and MgSO4 provided the optimal conditions for the simultaneous determination of mycotoxins and pesticides. Validation of the method was conducted using two complex matrices, M. officinalis and M. sylvestris, demonstrating that the majority of analytes met the criteria outlined in the EU SANTE/11312/2021 method validation guidelines. The method demonstrates reliable recoveries, as well as excellent accuracy and precision. Additionally, quality controls were implemented for both the extraction process and equipment injection to identify any potential method deviations during the analysis of commercial samples. Analysis of forty-two commercial samples from Southern Brazil revealed the presence of imidacloprid in M. officinalis and methyl pirimiphos in M. sylvestris underscoring the efficacy of the method for routine analysis of medicinal plants.Importantly, this method addresses a significant gap in the literature, as specific analytical methods for mycotoxins and pesticides in M. officinalis and M. sylvestris are currently limited. Consequently, this method represents a valuable tool for monitoring programs aimed at generating data on residue and contaminants in medicinal plants, thereby aiding in the establishment of maximum residue levels (MRLs) and facilitating risk assessment procedures.
Data availability
At this moment, the raw data generated from this study are only available from computers located at the Center of Research and Analysis of Residues and Contaminants (CEPARC) – Chemistry Department – Federal University of Santa Maria, Santa Maria, Brazil. However, the great majority of secondary data obtained are already present in the tables, figures and text submitted here.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge Agilent Technologies; The Brazilian Ministry of Science, Technology, and Innovation (MCTI); The Ministry of Agriculture, Livestock and Food Supply (MAPA); The Studies and Projects Finance Organization (FINEP); The National Council for Scientific and Technological Development (CNPq); The Coordination for the Improvement of Higher-Level Personnel (CAPES); Rio Grande do Sul State Research Support Foundation (FAPERGS) – PPSUS 2020 call and Federal University of Santa Maria (UFSM).References
- C. S. P. Della Pasqua, R. D. Iwamoto, E. Antunes, A. A. Borghi, A. C. H. F. Sawaya and E. C. T. Landucci, J. Ethnopharmacol., 2019, 231, 50–56, DOI:10.1016/j.jep.2018.11.012.
- M. K. Purkait, D. Haldar and P. Duarah, in Advances in Extraction and Applications of Bioactive Phytochemicals, Elsevier, 2023, pp. 197–217, DOI:10.1016/B978-0-443-18535-9.00002-8.
- B. Motahareh, H. Shahin, M. Masoud and S. Tabandeh, J. Herb. Med., 2022, 31, 100532, DOI:10.1016/j.hermed.2021.100532.
- W. A. Abdel-Naime, J. R. Fahim, M. A. Fouad and M. S. Kamel, S. Afr. J. Bot., 2019, 124, 228–234, DOI:10.1016/j.sajb.2019.05.011.
- M. Meneses, A. L. Antonio and S. Cabo Verde, Appl. Radiat. Isot., 2021, 168, 109391, DOI:10.1016/j.apradiso.2020.109391.
- A. Irfan, M. Imran, M. Khalid, M. Sami Ullah, N. Khalid, M. A. Assiri, R. Thomas, S. Muthu, M. A. Raza Basra, M. Hussein, A. G. Al-Sehemi and M. Shahzad, J. Saudi Chem. Soc., 2021, 25, 101277, DOI:10.1016/j.jscs.2021.101277.
- M. Seddighfar, S. M. Mirghazanfari and M. Dadpay, J. Integr. Med., 2020, 18, 181–188, DOI:10.1016/j.joim.2020.02.003.
- L. Luo, L. Dong, Q. Huang, S. Ma, P. Fantke, J. Li, J. Jiang, M. Fitzgerald, J. Yang, Z. Jia, J. Zhang, H. Wang, Y. Dai, G. Zhu, Z. Xing, Y. Liang, M. Li, G. Wei, J. Song, J. Wei, C. Peng, H. Zhang, W. Zhang, S. Wang, K. Mizuno, A. A. G. Marco, L. Wu, J. Xu, C. Xiong and S. Chen, Chemosphere, 2021, 262, 127477, DOI:10.1016/j.chemosphere.2020.127477.
- N. S. Shaban, K. A. Abdou and N. E.-H. Y. Hassan, Beni-Suef Univ. J. Basic Appl. Sci., 2016, 5, 102–106, DOI:10.1016/j.bjbas.2015.10.001.
- I. Sedova, M. Kiseleva and V. Tutelyan, Toxins, 2018, 10, 444, DOI:10.3390/toxins10110444.
- S. Agriopoulou, E. Stamatelopoulou and T. Varzakas, Foods, 2020, 9, 137, DOI:10.3390/toxins13060399.
- D. Pickova, V. Ostry, J. Toman and F. Malir, Toxins, 2021, 13, 399, DOI:10.3390/toxins13060399.
- O. M. Areo, O. A. Abafe, S. Gbashi and P. B. Njobeh, Food Control, 2023, 143, 109255, DOI:10.1016/j.foodcont.2022.109255.
- Brasil. Ministério da Saúde, Agência Nacional de Vigilância Sanitária, RDC no 105 de 31 de agosto de 2016. Altera a Resolução da Diretoria Colegiada - RDC no 26, de 13 de maio de 2014, que dispõe sobre o registro de medicamentos fitoterápicos e o registro e a notificação de produtos tradicionais fitoterápicos, 2016, available at, https://www.gov.br/saude/pt-br/composicao/sectics/pnpmf/orientacao-ao-prescritor/Publicacoes/resolucao-rdc-no-26-de-13-de-maio-de-2014.pdf/view Search PubMed.
- P. Eyring, M. Tienstra, H. Mol, S. S. Herrmann, P. H. Rasmussen, H. L. Frandsen and M. E. Poulsen, Food Chem., 2021, 356, 129653, DOI:10.1016/j.foodchem.2021.129653.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, J. AOAC Int., 2003, 86, 412–431, DOI:10.1093/jaoac/86.2.412.
- S. J. Lehotay, K. Mastovská and A. R. Lightfield, J. AOAC Int., 2005, 88, 615–629 CrossRef CAS PubMed.
- G. Martínez-Domínguez, R. Romero-González and A. Garrido Frenich, Food Chem., 2016, 197, 907–915, DOI:10.1016/j.foodchem.2015.11.070.
- H. G. J. Mol, P. Plaza-Bolaños, P. Zomer, T. C. de Rijk, A. A. M. Stolker and P. P. J. Mulder, Anal. Chem., 2008, 80, 9450–9459, DOI:10.1016/j.foodcont.2022.109255.
- Y. Sapozhnikova and S. J. Lehotay, Anal. Chim. Acta, 2013, 758, 80–92, DOI:10.1016/j.aca.2012.10.034.
- M. Leite, A. Freitas, J. Barbosa and F. Ramos, Food Chem. Adv., 2023, 2, 100145, DOI:10.1016/j.focha.2022.100145.
- J. Rubert, Z. Dzuman, M. Vaclavikova, M. Zachariasova, C. Soler and J. Hajslova, Talanta, 2012, 99, 712–719, DOI:10.1016/j.talanta.2012.07.010.
- I. Rodríguez-Cañás, J. M. González-Jartín, R. Alvariño, A. Alfonso, M. R. Vieytes and L. M. Botana, Food Chem., 2023, 408, 135182, DOI:10.1016/B978-0-12-805392-8.00016-5.
- I. Rodríguez, J. M. González, A. M. Botana, M. J. Sainz, M. R. Vieytes, A. Alfonso and L. M. Botana, in Liquid Chromatography, Elsevier, 2017, pp. 479–514, DOI:10.1016/B978-0-12-805392-8.00016-5.
- M. Anastassiades, E. Scherbaum, B. Taşdelen and D. štajnbaher, in Pesticide Chemistry, Wiley, 2007, pp. 439–458, DOI:10.1002/9783527611249.ch46.
- L. Zhang, X.-W. Dou, C. Zhang, A. Logrieco and M.-H. Yang, Toxins, 2018, 10, 65, DOI:10.3390/toxins10020065.
- Y.-B. Luo, H.-B. Zheng, X.-Y. Jiang, X. Li, H.-F. Zhang, F.-P. Zhu, Y.-Q. Pang and Y.-Q. Feng, Chin. J. Anal. Chem., 2015, 43, 1538–1544, DOI:10.1016/S1872-2040(15)60870-2.
- X. Xu, X. Xu, M. Han, S. Qiu and X. Hou, Food Chem., 2019, 276, 419–426, DOI:10.1016/j.foodchem.2018.10.051.
- Y.-B. Luo, Z.-G. Shi, Q. Gao and Y.-Q. Feng, J. Chromatogr. A, 2011, 1218, 1353–1358, DOI:10.1016/j.foodchem.2018.10.051.
- X.-T. Peng, L. Jiang, Y. Gong, X.-Z. Hu, L.-J. Peng and Y.-Q. Feng, Talanta, 2015, 132, 118–125, DOI:10.1016/j.talanta.2014.08.069.
- E. Rutkowska, B. Łozowicka and P. Kaczynski, Food Chem., 2019, 279, 20–29, DOI:10.1016/j.foodchem.2018.11.130.
- Brasil. Agência Nacional de Vigilância Sanitária, Farmacopéia Brasileira, plantas medicinais, Anvisa 6a edição, 2019, pp. 1–744, available at https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira Search PubMed.
- Codex Alimentarius, Recommended Methods of Sampling for the Determination of Pesticide Residues for Compliance with Maximum Residue Levels (MRLs), 1999, available in: <, https://apeda.gov.in/apedawebsite/Announcements/CODEX_method_for_sampling_for_determination_of_pesticides.pdf & gt, accessed August 30th, 2021 Search PubMed.
- R. C. da Silva, I. D. dos Santos, J. P. Neu, R. D. Wouters, M. E. Z. Fontana, P. D. R. Balbinot, R. Wagner and I. R. Pizzutti, Food Chem., 2022, 394, 133513, DOI:10.1016/j.foodchem.2022.133513.
- European Commission, Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed Document no SANTE/11312/2021 v.2, European Commission Directorate-General for Health and Food Safety, 2024, available at https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727%26LabID=100%26Lang=EN Search PubMed.
- J. V. Dias, M. da G. P. Nunes, I. R. Pizzutti, B. Reichert, A. A. Jung and C. D. Cardoso, Food Chem., 2019, 293, 83–91, DOI:10.1016/j.foodchem.2019.04.088.
- O. Lacina, M. Zachariasova, J. Urbanova, M. Vaclavikova, T. Cajka and J. Hajslova, J. Chromatogr. A, 2012, 1262, 8–18, DOI:10.1016/j.chroma.2012.08.097.
- L. P. da Silva, F. Madureira, E. de Azevedo Vargas, A. F. Faria and R. Augusti, Food Chem., 2019, 270, 420–427, DOI:10.1016/j.foodchem.2018.07.126.
- M. Kresse, H. Drinda, A. Romanotto and K. Speer, J. Chromatogr. B, 2019, 1117, 86–102, DOI:10.1016/j.jchromb.2019.04.013.
- A.-K. Rausch, R. Brockmeyer and T. Schwerdtle, Anal. Bioanal. Chem., 2021, 413, 3041–3054, DOI:10.1007/s00216-021-03239-1.
- J. V. Dias, R. C. da Silva, I. R. Pizzutti, I. D. dos Santos, M. Dassi and C. D. Cardoso, J. Food Compos. Anal., 2019, 82, 103242, DOI:10.1016/j.jfca.2019.103242.
- X. Xu, X. Xu, M. Han, S. Qiu and X. Hou, Food Chem., 2019, 276, 419–426, DOI:10.1016/j.foodchem.2018.10.051.
- T. W. Na, H.-J. Seo, S.-N. Jang, H. Kim, H. Yun, H. Kim, J. Ahn, H. Cho, S.-H. Hong, H. J. Kim and S. H. Lee, J. Chromatogr. A, 2022, 1676, 463257, DOI:10.1016/j.chroma.2022.463257.
- Y. Sapozhnikova, P. Zomer, A. Gerssen, A. Nuñez and H. G. J. Mol, Food Control, 2020, 116, 107323, DOI:10.1016/j.foodcont.2020.107323.
- B. Reichert, A. de Kok, I. R. Pizzutti, J. Scholten, C. D. Cardoso and M. Spanjer, Anal. Chim. Acta, 2018, 1004, 40–50, DOI:10.1016/j.aca.2017.11.077.
- M. Cladière, G. Delaporte, E. Le Roux and V. Camel, Food Chem., 2018, 242, 113–121, DOI:10.1016/j.foodchem.2017.08.108.
- K. Russo, D. Lucchetti, D. Triolone, P. di Giustino, M. Mancuso, D. Delfino and B. Neri, Phytochem. Lett., 2021, 46, 153–161, DOI:10.1016/j.phytol.2021.10.002.
- L. Izzo, A. Narváez, L. Castaldo, A. Gaspari, Y. Rodríguez-Carrasco, M. Grosso and A. Ritieni, J. Dairy Sci., 2022, 105, 2948–2962, DOI:10.3168/jds.2021-21123.
- S. Bhattacharyya, R. Poi, M. Baskey Sen, D. Kumar Hazra, R. Ghosh, S. Mandal and R. Karmakar, Microchem. J., 2022, 179, 107444, DOI:10.1016/j.microc.2022.107444.
- T.-K. Ly, T.-D. Ho, P. Behra and T.-T. Nhu-Trang, Food Chem., 2020, 326, 126928, DOI:10.1016/j.foodchem.2020.126928.
- C. S. Vareli, I. R. Pizzutti, L. Gebler, C. D. Cardoso, D. S. H. Gai and M. E. Z. Fontana, Talanta, 2018, 184, 202–209, DOI:10.1016/j.talanta.2018.03.009.
- X. Wu and Z. Ding, Food Chem., 2023, 405, 134755, DOI:10.1016/j.foodchem.2022.134755.
- W. Li, Y. Liu, J. Duan, C. P. Saint and D. Mulcahy, J. Chromatogr. A, 2015, 1389, 76–84, DOI:10.1016/j.chroma.2015.02.044.
- Y. Huang, T. Shi, X. Luo, H. Xiong, F. Min, Y. Chen, S. Nie and M. Xie, Food Chem., 2019, 275, 255–264, DOI:10.1016/j.foodchem.2018.09.094.
- N. Pallarés, H. Berrada, M. Fernández-Franzón and E. Ferrer, Plant Foods Hum. Nutr., 2020, 75, 362–368, DOI:10.1007/s11130-020-00820-4.
| This journal is © The Royal Society of Chemistry 2024 |