Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and validation of a portable membrane inlet mass spectrometry method for the measurement of monoaromatic hydrocarbons in water from a river canal
Brankica
Kartalović
 *,
Djordje
Vujić
,
Daria
Ilić
*,
Djordje
Vujić
,
Daria
Ilić
 and
Boris
Brkić
and
Boris
Brkić
 *
*
BioSense Institute, University of Novi Sad, Dr Zorana Đinđića 1, 21 101 Novi Sad, Serbia. E-mail: brankica.kartalovic@biosense.rs; boris.brkic@biosense.rs
First published on 25th July 2024
Abstract
This work reports the validation of an analytical method for the determination of monoaromatic hydrocarbons such as benzene, toluene, and xylene (BTX) using a portable membrane inlet mass spectrometer (MIMS) with a quadrupole mass analyser. In this study of BTX in river canal water that is used for irrigation, we present a detailed analytical method for rapid, self-contained, field-transportable screening and quantitative analysis for environmental monitoring. The validation study showed that in the analytical range of 10–250 μg L−1, the correlation coefficient for all the analytes was greater than 0.99, the accuracy was in the range of 95.32–104.30%, the precision was less than 10%, and the selectivity was satisfactory. The LOD and LOQ values for benzene, toluene, and xylene were 4.88, 7.43, and 7.46 μg L−1 and 16.27, 24.77, and 27.85 μg L−1, respectively. The method was benchmarked against a lab-based GC-MS method, which confirmed its accuracy for the target compounds.
1. Introduction
In a world increasingly affected by disasters and armed conflicts with the potential to damage the environment and cause great loss of human life as well as social and economic disruptions, environmental pollution has emerged as a global problem. The introduction of harmful substances in all segments of the environment – water, air, and soil – represents a danger to the entire living world. Causes of this range from natural disasters (earthquakes, volcanic eruptions, floods) to disasters caused by human means. Therefore, it is urgent to develop portable instruments that can quickly and reliably provide information about pollution sites and prevent its spreading.The first membrane inlet mass spectrometer (MIMS) was developed in the early 1960s with main principles described in the work of Kotiaho et al.1,2 A low-impact mass spectrometry technique such as MIMS can be used to identify gaseous and volatile organic compounds (VOCs) ranging from solid and liquid to gaseous samples.3,4 Since its first appearance, MIMS has been widely used for the analysis of soil and water, as well as industrial pollutants, including in forensics.4–6 Notably, it has been shown that the portability of the instrument is very important in environmental monitoring.
One of the key aspects of such instruments is their real-time measurement capability. In their review paper, Galuszka et al.7 reported that some official methods from the US Environmental Protection Agency (EPA) are based on portable instruments for the determination of pentachlorophenol in soil as well as for the determination of 26 elements in soil and sediments using X-ray fluorescence (XRF) spectrometers. Furthermore, an official method for metal determination in air using a portable instrument was reported by Galuszka et al.7 and in the official NIOSH method.8
Our study focused on BTX aromatic hydrocarbons, namely, benzene, toluene, and xylene, which are considered as a group of organic water pollutants. BTX can be considered as carriers of pollution from anthropogenic sources, as described by Šoštarić.9 The last decade of research has found that these compounds can cause skin irritation, irritation of the central nervous system, and can damage the respiratory organs, while long-term exposure can cause diseases and illnesses such as cancer organ diseases, as reported by Fayemiwo.10
Given the fact that BTEX (benzene, toluene, ethylbenzene, xylene) are found in low concentrations in water, as well as the fact that they have low limit values according to current regulations, it was necessary to develop sensitive and reliable methods for their detection. If we look at different standardized methods, we can see that most of them require a preconcentration of the samples, i.e. the analytes of interest. Many methods for BTX detection use gas chromatography (GC) coupled with other techniques, such as purge and trap,11–14 static headspace (HS),11,14–16 and headspace solid-phase microextraction.11,14–16 The liquid–liquid extraction technique is often used for extraction, but it is time consuming and requires large quantities of solvent (EPA method 551.1).17 Many of these techniques have certain disadvantages, such as a long preparation time or solvent consumption. The headspace technique does not require the use of solvents, and mostly native samples can be used for the analysis. However, because of the injection of large amounts of gas into the column, the formation of broad peaks may occur. To avoid this, it is necessary to reduce or eliminate the use of solvents for sample preparation, as well as to protect the environment and the operator from exposure to toxic organic solvents. For these reasons, some methods have been developed that require little or no solvents, namely solid-phase microextraction (SPME), liquid-phase microextraction (LPME), and direct aqueous injection (DAI).18–20
These methods are important alternatives to the traditional sample preparation methods, and can overcome the disadvantages of conventional extraction methods, such as operation time, large amount of solvent extraction, and the need for specialized apparatus. This was the main reason behind the development of the solid-phase microextraction (SPME) method. SPME enables a preconcentration of the analytes from the gas phase onto fibres before injection into the GC/ECD or MS. This method is simple, reliable, and can achieve the desired level of sensitivity (ng L−1) for the analysis of environmental samples.21 In addition to the gas chromatography technique for the detection of BTX in water, the liquid chromatography technique also offers reliable results.
AlSalka et al.18 developed and optimised a method for a high-performance liquid chromatography system equipped with a photo diode array detector (HPLC-DAD) for the simple and reliable determination of BTEX without using a high-cost special technique or large volume of solvents. Wittkamp and Tilotta22 described a method for extracting BTEX from a water solution into a solid phase before direct detection by Raman spectroscopy. This represented an example of liquid-phase microextraction (LPME) based on the solidification of floating organic microdrops followed by gas chromatography (detection by flame ionization) for the preconcentration and determination of benzene, toluene, ethylbenzene, and xylene (BTEX) in water samples.23 Common to all the listed techniques and methods is the requirement for preparation of the samples and analysis in the laboratory, which is not the case for portable instruments and on-site analysis. This important advantage of the MIMS in relation to GC-MS is emphasized. Allard and Lauritsen21 presented MIMS as an analytical tool capable of analyzing a range of compounds in water without needing special sample preparation and monitoring chemical processes in real time, unlike traditional VOC analysis using gas chromatography (GC) followed by electron-capture detection (ECD) or mass spectrometry (MS) or other noted techniques.
Therefore, over the years, MIMS has gained increasing importance due to its advantages over stationary chromatography,9 and the possibility for detecting compounds of interest in the field, right at the scene of an accident or incident.
A number of works24–29 on MIMS have shown its suitability for rapid detection in environmental monitoring. The development of portable instruments is important for environmental protection.24–26 Furthermore, the in-field use of portable analytical equipment can provide the required intelligence and information from the scene of an incident,24–27 as well as rapid, and highly detailed results that may be crucial for preventing major environmental disasters. The improvement of portable instruments over the years has led to the enhancement of quantitative analysis in the field, as shown by Duff et al.,27 Fiorentin et al.,30 and Brkić et al.28 Monitoring of the environment has been the subject of study of many notable works detailing the development of portable instruments for the rapid analysis of air, water, and soil. For example, Brennwald et al.29 developed a portable mass spectrometric system for the continuous on-site analysis of dissolved gases in groundwater, including He, Ar, Kr, N2, and O2. It is important to highlight that this system did not require any purification or other preparation of the sampled gases and, therefore, enabled maintenance-free and autonomous operation.
When it comes to environmental monitoring, the importance of the rapid identification of VOCs in water with fast response times and high sensitivity was highlighted by Wu et al.31 To address this, they developed a helical membrane inlet for time-of-flight mass spectrometry (TOF-MS), and then used this system for determining analytes in complex matrices. On the other hand, Brkić et al.28 developed a portable MIMS system for the lab-based water-quality monitoring of organic compounds present in highly toxic nuclear waste ponds. To enhance the operation of the MIMS instrument, Armaković et al.32 performed a computational study to assess the possibility of applying polydimethylsiloxane (PDMS) and its structurally similar derivatives (PDMS-HDT and PDMS-TMT) towards the identification of target naphthenic acids using a portable mass spectrometer.
This paper reports a validation study of a portable MIMS system for the in-field analysis of BTX in river canal water. Our MIMS system consisted of a quadrupole mass spectrometer with an open ion source, connected to a sample probe with a sheet PDMS membrane inlet as described by Aleksić et al.33 Our laboratory tests were performed on deionized water (DI) enriched with a known concentration of the analytes of interest and real water samples taken from the Danube–Tisa–Danube (DTD) canal, known as the hydrosystem DTD with (Google, n.d.) coordinates at a latitude/longitude of 44° 57′ 22.0314′′ N/21° 16′ 20.5314′′ E.34 Selected-ion monitoring (SIM) was performed for the target compounds to determine the linearity, precision, and accuracy of the results.
We achieved satisfactory limits of detection (LODs) and limits of quantification (LOQs) as shown in the Results and discussion section.
2. Experimental section
2.1 Concept
The details of the compounds of interest are presented in Table 1, including their molecular weights, target ions, and maximum residue levels (MRLs) allowed in drinking and irrigation water. The MRL limits are expressed in micrograms per litre of water (μg L−1) as determined by the regulations of the Republic of Serbia,35,36 where class I and II can be used for irrigation.Our testing, calibration, and validation of the MIMS portable analytical system for water-quality monitoring was carried out in accordance with the relevant guidance documents.37,38 Following the guide points for the validation and verification of quantitative and qualitative test methods and the analytical quality control requirements, we clearly demonstrate that BTX from water could be successfully quantified with a high level of confidence in the results.
2.2 Chemicals and supplies
All the chemicals used in the experiments were of analytical grade purity. Anhydrous sodium sulfate powder, HPLC grade methane, and HPLC grade hexane were obtained from Sigma-Aldrich, Australia. The analytical standards for the BTX mix were obtained from Dr Ehrenstorfer GmbH, Denmark.The analytical standards for BTX contained three volatile organic compounds that are commonly tested in operational environmental laboratories using GC-MS. Water matrices were used during the method development. The DI water used as a blank did not contain organic contaminants. A stock standard solutions of BTX was prepared in methanol. Working standard solutions with concentrations ranging from 10 to 250 μg L−1, were prepared by a step-by-step dilution with DI water. For quality control, DI water was used as well as water samples from the Danube–Tisza–Danube canal.21 Real canal water samples were used to test the application of the developed method.
2.3 Experimental set-up
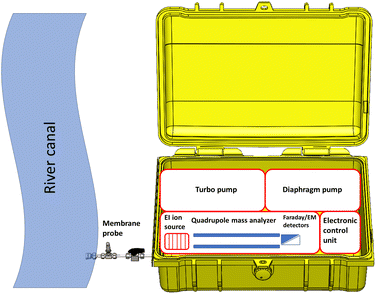
The experimental set-up section describes in depth the MIMS and GC-MS instruments that were used. Sample analysis and the validation plan are also described in detail. The set-up of the MIMS system is illustrated in the schematic diagram in Fig. 1.All the water analyses that were necessary for the validation study were performed using our proprietary portable MIMS instrument. The system comprised a membrane sample inlet, vacuum chamber, quadrupole mass spectrometer (QMS) with an electronic control unit (ECU) and a vacuum system with a diaphragm and turbo pumps. The mass spectrometer featured an open electron impact (EI) ion source with yttriated iridium filaments, a 100 mm long single quadrupole mass analyzer (Pfeiffer QMG 250 M3) and a dual detector (Faraday cup and secondary electron multiplier) with a mass range from 1–300 amu. The ECU enabled efficient operation specifically designed for partial pressure analysis below 5 × 10−4 mbar.
The MIMS system delivered a unit resolution, rapid response times (≤0.5 s), and precise measurements at low concentration levels (parts per billion) throughout the entire working mass range, as explained by Ilić et al.39 The QMS was mounted inside the vacuum chamber with the ECU closely coupled to the QMS flange, as described by Brkić et al.28 The vacuum system consisted of a diaphragm pump (MVP 030-3DC) and a turbomolecular pump (HiPace 80) purchased from Pfeiffer Vacuum GmbH (Asslar, Germany). It provided a base pressure of 3 × 10−6 torr, and was monitored by a digital cold cathode pressure gauge (model: MPT 200) supplied by Pfeiffer Vacuum GmbH. The MIMS system weighed 25 kg, and its dimensions (height × width × length) were 25 × 50 × 60 cm, respectively.
The membrane for the inlet was made of polydimethylsiloxane (PDMS). The membrane was 6.5 mm in diameter with a thickness of 120 microns. The membrane probe assembly consisted of 10 cm stainless steel tubing coupled with a membrane sheet supported by a 6.35 mm Swagelok stainless steel vacuum fitting union. The non-sterile PDMS membrane sheeting was provided by Technical Products, Inc. of Georgia, USA. The PDMS membrane sheet thickness was 0.12 mm, while the sampling area was 33.2 mm2. The membrane was supported by a 0.8 mm thick stainless steel porous frit with 20 μm porosity.
To ensure that the MIMS instrument operated according to its specification, prior to every analysis, validation checks were performed. Special care was taken to avoid cross-contamination between the analyzed samples. An external laptop with PV MassSpec software V23.06 by Pfeiffer Vacuum GmbH was used for spectral analysis and raw data collection. The raw spectral data were processed using in-house built software written in R language for automated determination of the analyte concentrations. Analyte identification was done by observing the characteristic ions that did not overlap with the mass fragments from other compounds. Calibration curves were created based on the intensity of the observed ions. Using MIMS, water could be analyzed in the field in near real-time.
GC-MS headspace calibration was done with aromatic mix, purchased from Sigma-Aldrich, St. Louis, MO, USA. A series of solutions in the range of 10–250 μg mL−1 were made using DI water in a total volume of 5 mL.
2.4 Sample analysis
The developed water analysis method was based on a method that was first demonstrated for oil-in-water monitoring by Brkić et al.40 During the field tests for the river canal water, the membrane sample probe was connected to the water flow system via a 1/4 inch flexible PA hose. The field method was evaluated against the laboratory method for the quantitative analysis of the target compounds. The assessment was based on the validation study testing residues of benzene, toluene, and xylene in water using two instruments and two different techniques.DI water and water from the DTD canal were used for the validation study. Laboratory verification was performed with DI water spiked with a known concentration of the analyte of interest using BTX liquid reference standards. Appropriate volumes of each liquid stock solution were injected with high-precision micropipettes (Dr Ehrenstorfer, Denmark) into a glass aquarium in amounts that provided concentrations of 10, 25, 50, 100, and 250 μg L−1 for each analyte of interest. The aquarium was filled with water to provide a total volume of 20 L. It was covered with a lid with the water temperature controlled at 25 °C to achieve thermodynamic equilibrium and stable conditions. To ensure the homogeneity of the sample, pumps were used to constantly mix the contents of the aquarium. Before starting the analysis, all the glassware was carefully washed to remove all traces and possible interference of other volatile compounds. Reference standards were tested from the lowest concentration to highest. This was done to reduce cross-contamination.
During the tests, the membrane probe was directly inserted into the aquarium, and it was used for sampling the prepared water with known standards for different concentrations. The membrane probe and subsequently the membrane were kept at ambient temperature throughout the measurements. The sample was introduced directly into the vacuum system.28,39,41
2.5 Validation plan
The aim of the validation plan was to confirm that the method developed using MIMS can be used in the field with a high level of confidence for the quantitative real-time analysis of BTX from canal water. Validation plans included a definition of the analytical range, confirmation of the selectivity of the method, linearity verification (R2 > 0.99), precision (<10%), recovery (80–110%), and determination of the limit of detection (LOD) and limit of quantification (LOQ).To investigate the linearity of the MIMS, we used five-point calibration. For the laboratory tests, we used standard reference materials and DI water. Real water samples from the canal and DI water with and without the addition of a known analyte concentration were used for quality control and verification of the accuracy of the method. Measurements were performed under the same conditions four times at two levels, to determine the precision of the instrument as well as to determine the limit of detection and limit of quantification. Accuracy checks were done with water from the DTD canal, with a known concentration of the analyte added, to consider the influence of the matrix on the results. In this study, the LOQ represents the lowest concentration that could be determined with acceptable accuracy, while the LOD was the lowest concentration that could be proven, but not determined with appropriate accuracy.
3. Results and discussion
3.1 BTX experimental plan
The analysis of BTX in water and the total time needed for the on-site analysis was ∼10 min, similar to in the work of Hu et al.42 On the other hand, Duff et al.27 used SPME fibres with a portable MIMS to achieve rapid on-site analysis. They reported an analysis time of 6.5 min.During the validation study, all the tests were performed in the analytical range from 10–250 μg L−1 to confirm the linearity, specificity, selectivity, accuracy, and precision of the method, and to determine the detection and quantification limits.
3.2 Method linearity
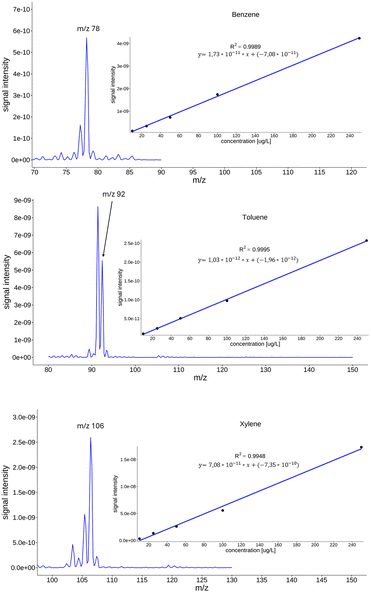
In the reported analytical range from 10–250 μg L−1, the linearity was assessed using five calibration points, and demonstrated a satisfactory correlation factor R2 > 0.99 for all the compounds of interest. Given the fact that the range of linearity depends on the nature of the analyte, the influence of the matrix, the type of detector, and the type of portable instrument, different authors report different correlation factors for their research.Brkić et al. reported correlation factors > 0.98 for groups of alcohols, ketones, and aldehydes detected in nuclear waste lakes,28 while Wu et al.31 reported correlation factors for benzene > 0.99 and for xylene > 0.98. Fig. 2 shows the intensities of the characteristic ions and calibration curves for benzene, toluene, and xylene in water obtained using a portable MIMS. The results were obtained under controlled laboratory conditions.
3.3 Method selectivity
In our study, the selectivity of the method was confirmed. For instance, Fig. 3 shows that the developed analytical method was able to distinguish the analyte(s) of interest from endogenous components in the matrix or other components in the sample, without interference. Fig. 3a displays the selectivity of the method when using DI water, while Fig. 3b illustrates the influence of the matrix on the selectivity of the method. In this instance, water from the canal was purged with nitrogen and used as a blank. After recording and enriching the canal water, it was observed that the selectivity of the method was at the same level as the DI water.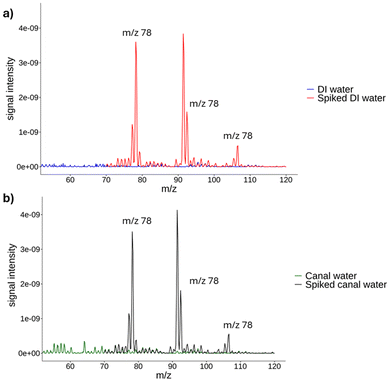 | ||
| Fig. 3 Full scan of pure and spiked samples for (a) DI water and (b) canal water using the portable MIMS. | ||
The selectivity of the method using portable MIMS was also proven by Duff et al.27 at low concentrations of benzene and xylene.
3.4 Method precision and accuracy
We conducted four replicate tests on three analytes in water, using the optimized procedures to assess the precision of the new method. The precision results are presented in Table 2, in which it can be seen that the presented approach could achieve sufficient precision to fulfil the requirements for BTX analysis in water using a portable MIMS, with a relative standard deviation (RSD) below 2.60%, indicating the high precision of the proposed method.| Replica no. | Benzene | Toluene | Xylene |
|---|---|---|---|
| 1 | 105.2 | 97.1 | 98.1 |
| 2 | 106.1 | 96.7 | 102.8 |
| 3 | 102.6 | 95.8 | 99.3 |
| 4 | 103.3 | 91.7 | 97.1 |
| RSD, % | 1.56 | 2.60 | 2.50 |
To confirm the accuracy of the method, we compared the measurement results for an enriched sample of the canal water obtained using the portable MIMS and the results from using a benchtop GC-MS. Table 3 shows the measured BTX values from the enriched water samples. It is evident that the relative differences (RD) between these techniques were within 4.89%, which indicates the accuracy and reliability of the observed portable MIMS method for the quantitative analysis of BTX from water.
| Sample no. | MIMS method | GC-MS method | Relative difference, % |
|---|---|---|---|
| Benzene | |||
| 1 | 105.2 | 100.3 | 4.89 |
| 2 | 106.1 | 101.2 | 4.84 |
| 3 | 102.6 | 100.6 | 1.99 |
| 4 | 103.3 | 100.1 | 3.2 |
| STD | 0.32 | 0.48 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Toluene | |||
| 1 | 97.1 | 100.2 | −3.09 |
| 2 | 96.7 | 98.5 | −1.83 |
| 3 | 95.8 | 99.3 | −3.52 |
| 4 | 91.7 | 95.9 | −4.38 |
| STD | 2.48 | 1.85 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Xylene | |||
| 1 | 98.10 | 100.20 | −2.1 |
| 2 | 102.80 | 101.30 | 1.48 |
| 3 | 99.30 | 99.81 | −0.5 |
| 4 | 97.10 | 102.20 | −4.99 |
| STD | 2,40 | 1,09 | |
Our validation study required verification of the recovery value as this can show the accuracy of the analytical method through the degree of agreement between the actual value and the value obtained by applying the analytical procedure a certain number of times. This can indicate the fidelity of the measurements. Depending on the substances and the matrix effect, some authors have reported recoveries ranging from 50.0–87.5%; for example, in the study of Fiorentin.30 Our results gave recoveries of 95.32% for toluene, 99.32% for xylene, and 104.30% for benzene. To determine the accuracy of the field analysis, the results obtained with the portable MIMS were compared to those obtained on a benchtop GC-MS, using the same range of BTX to be identified by both methods. Native samples were used in both techniques. Although these compounds were present in lower concentrations, they could still be observed in both instruments. All the major peaks detected using the field-based method were also detected using the benchtop GC-MS for all the materials burned. Fig. 4a shows a representative benchtop GC-MS chromatogram. The same peaks of interest were also detected in the field using the portable MIMS, as shown in Fig. 4b.
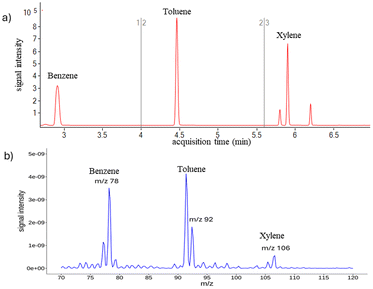 | ||
| Fig. 4 Representative benchtop gas chromatography-mass spectrometry (GC-MS) chromatogram (a) compared to a portable MIMS mass spectrum (b) for a canal water sample. | ||
3.5 Methods for obtaining the limit of detection and limit of quantification
The results for the LOQ and LOD values obtained using the MIMS are shown in Table 4.| Analyte | MIMS (LOD), μg L−1 | MIMS (LOQ), μg L−1 |
|---|---|---|
| Benzene | 4.88 | 16.27 |
| Toluene | 7.43 | 24.77 |
| Xylene | 7.46 | 24.85 |
To provide an estimate of the LOQ and LOD values for the target compounds, an analytical standard of 10 mg L−1 was used, which was added to the water sample at a defined concentration range. After performing ten-times measurements of the first point of calibration, the LOD and LOQ were calculated. Our results show that the LOQ values obtained by validation were significantly lower than the MRL values for irrigation water and slightly above the MRL values for drinking water, when considering the regulations of the Republic of Serbia. Comparing the LOD values of different authors, we can see that the LOD value depends not only on the matrix and the type of compound, but also on the instrument itself.
In the work of Wu et al.,31 who used a helical membrane inlet single photon ionization time-of-flight mass spectrometry (SPI-TOFMS) method, the reported LOQ values for benzene and xylene were respectively 0.014 and 0.036 μg L−1. These are significantly lower than the LOQ values obtained in our study. Fiorentin et al.30 reported detection limits in the range of 0.01–0.1 mg mL−1 for some drugs (with a higher molecular weight than BTX). Hu et al.42 reported limits of detection in the low parts-per-trillion levels (≤5.25 ng L−1) for the determination of persistent organic pollutants (polychlorinated biphenyls, organochlorine pesticides, and polycyclic aromatic hydrocarbons) in a total analysis time only of 30 min.
It is, therefore, important to emphasize that the use of portable instrumentation is not intended to replace laboratory analysis, but rather to obtain an in-field alert report of the polluted site to allow taking actions to protect the environment. This will require immediate quantitative validation of the results. In such situations, the concentrations of pollutants are generally high, in contrast to environmental monitoring, where the concentrations of pollutants are at residual, expected levels.
4. Conclusions and future work
In this study, we used a portable MIMS instrument to develop and validate a method for the rapid quantitative analysis of BTX in river canal irrigation water within a defined analytical range. The results confirmed the linearity, precision, selectivity, measurement, and accuracy of the method.Future work will involve pilot tests in the DTD hydrocanal with the aim of collecting data on the loading of irrigation water with monoaromatic hydrocarbons and improving the quality of the environment. Real-time quantitative analysis will also be performed not only for BTX, but also for other dissolved hydrocarbons of interest. Considering the results obtained for the LOQ and LOD (μg L−1 level), in the following period, work will be done on improving the sensitivity of our proprietary portable MIMS device with an effort to allow it to be used for drinking water monitoring as well as the rapid screening of drinking water following accidental situations.
Data availability
Data for this article, including original data for figure creation are available at University of Novi Sad at https://open.uns.ac.rs/handle/123456789/32772.Author contributions
B. K. – conceptualization, methodology, investigation, formal analysis, visualization, writing – original draft, writing – review and editing. D. I. – formal analysis, visualization. D. V. – methodology, visualization. B. B. – supervision, conceptualization, methodology, writing – original draft, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Science Fund of the Republic of Serbia, # grant no. 7335, sustainable environmental monitoring and prediction of pollutants spread – EnviLife. This work is also supported through ANTARES project that has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement SGA-CSA no. 739570 under FPA no. 664387, https://doi.org/10.3030/739570. Brankica Kartalović, Daria Ilić and Boris Brkić acknowledge the financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant no. 451-03-47/2023-01/200358).References
- T. Kotiaho, F. R. Lauritsen, T. K. Choudhury, R. G. Cooks and G. T. Tsao, Anal. Chem., 1991, 63, 875A–883A CrossRef CAS.
- T. Kotiaho and F. R. Lauritsen, Compr. Anal. Chem., 2002, 37, 531–557 CAS.
- S. Nalbantoglu, Mol. Med., 2019 DOI:10.5772/intechopen.88563.
- S. Sajid, I. Aryal, S. F. Chaudhri, F. R. Lauritsen, M. G. Jørgensen, H. Jenssen and B. K. Prabhala, Water, 2023, 15, 184 CrossRef.
- R. C. Johnson, R. G. Cooks, T. M. Allen, M. E. Cisper and P. H. Hemberger, Mass Spectrom. Rev., 2000, 19, 1–37 CrossRef CAS PubMed.
- S. Giannoukos, B. Brkić, S. Taylor and N. France, J. Am. Soc. Mass Spectrom., 2015, 26, 231–239 CrossRef CAS PubMed.
- A. Galuszka, Z. M. Migaszewski and J. Namiesnik, Environ. Res., 2015, 140, 593–603 CrossRef CAS PubMed.
- NIOSH Method 7702, Lead by Field Portable XRF, 1998 Search PubMed.
- A. Šoštarić, Mechanisms of Scavenging Monoaromatic Hydrocarbons (BTEX) from Ambient Air by Wet Deposition, University of Belgrade, 2017 Search PubMed.
- O. M. Fayemiwo, M. O. Daramola and K. Moothi, Water SA, 2017, 43(4), 602–613 CrossRef CAS.
- A. Serrano and M. Gallego, J. Chromatogr. A, 2004, 1045(1–2), 181–188 CrossRef CAS PubMed.
- E. Martínez, S. Lacorte, I. Llobet, P. Viana and D. Barceló, J. Chromatogr. A, 2002, 959(1–2), 181–190 CrossRef PubMed.
- A. D. Nikolaou, S. K. Golfinopoulos, M. N. Kostopoulou, G. A. Kolokythas and T. D. Lekkas, Water Res., 2002, 36, 2883–2890 CrossRef CAS PubMed.
- S. Lacorte, L. Olivella, M. Rosell, M. Figueras, A. Ginebreda and D. Barceló, Chromatographia, 2002, 56, 739–744 CrossRef CAS.
- Z. Wang, K. Li, M. Fingas, L. Sigouin and L. Ménard, J. Chromatogr. A, 2002, 971(1–2), 173–184 CrossRef CAS PubMed.
- A. Alonso, M. A. Fernández-Torroba, M. T. Tena and B. Pons, Chromatographia, 2003, 57, 369–378 CrossRef CAS.
- EPA Method 551.1, Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection, 1995 Search PubMed.
- Y. AlSalka, F. Karabeta and S. Hashem, Anal. Methods, 2010, 2, 1026–1035 RSC.
- A. Sarafraz-Yazdi, A. H. Amiri and Z. Es'haghi, Chemosphere, 2008, 71(4), 671–676 CrossRef CAS PubMed.
- E. Baltussen, C. Cramers and P. Sandra, Anal. Bioanal. Chem., 2002, 373, 3–22 CrossRef CAS PubMed.
- S. Allard and F. R. Lauritsen, TrAC, Trends Anal. Chem., 2023, 165, 117141 CrossRef CAS.
- B. Wittkamp and D. Tilotta, Anal. Chem., 1995, 67(3), 600–605 CrossRef CAS.
- H. Faraji, M. Tajbakhsh and M. Helalizadeh, Anal. Methods, 2012, 4, 3372–3380 RSC.
- R. Lam, C. Lennard, G. Kingsland, P. Johnstone, A. Symons, L. Wythes, J. Fewtrell, D. O'Brien and V. Spikmans, J. Forensic Sci., 2018, 50(6), 672–681 Search PubMed.
- D. L. Bartley, J. E. Slaven, M. C. Rose, M. E. Andrew and M. Harper, J. Occup. Environ. Hyg., 2007, 4(12), 931–942 CrossRef CAS PubMed.
- A. Galuszka, Z. M. Migaszewski and J. Namiesnik, Environ. Res., 2015, 140, 593–603 CrossRef CAS PubMed.
- D. Duff, C. Lennard, Y. Li, C. Doyle, K. J. Edge, I. Holland, K. Lothridge, P. Johnstone, P. Beylerian and V. Spikmans, Environ. Sci. Pollut. Res. Int., 2023, 30(40), 93088–93102 CrossRef CAS PubMed.
- B. Brkić, N. France, S. Giannoukos and S. Taylor, Analyst, 2018, 143, 3722–3728 RSC.
- M. S. Brennwald, M. Schmidt, J. Oser and R. Kipfer, Environ. Sci. Technol., 2016, 50(24), 13455–13463 CrossRef CAS PubMed.
- T. R. Fiorentin, B. K. Logan, D. M. Martin, T. Browne and E. F. Rieders, Forensic Sci. Int., 2020, 313, 110342 CrossRef CAS PubMed.
- C. Wu, W. Liu, J. Jiang, Y. Wang, K. Hou and H. Li, Talanta, 2019, 192, 46–51 CrossRef CAS PubMed.
- S. Armaković, Đ. Vujić and B. Brkić, J. Mol. Liq., 2022, 351, 118657 CrossRef.
- M. Aleksić, A. Simeon, Đ. Vujić, S. Giannoukos and B. Brkić, J. Breath Res., 2023, 17, 4 CrossRef PubMed.
- Google, Inc., Google Maps, retrieved April 9, 2024, from https://maps.app.goo.gl/XPEa3amyHvTnrPkV8 Search PubMed.
- Rule Book on Hazardous Substances in Waters (Official Gazette of the Republic of Serbia, No. 31/82) Search PubMed.
- Rule Book on the Hygienic Correctness of Drinking Water, (Official Gazette of the FRY, No. 42/98 and 44/99 and Official Gazette of the RS, No. 28/2019) Search PubMed.
- European Commision, Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed SANTE 11312/2021, (Applying from 1 January 2024), 2024, available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727, accessed on 22 January 2024 Search PubMed.
- NATA, Guidelines for the validation and verification of quantitative and qualitative test methods, Technical Note 17 – October 2013, National Association of Testing Authorities, Australia, 2013 Search PubMed.
- D. Ilić, B. Brkić and M. T. Sekulić, Sustainability, 2024, 16, 1713 CrossRef.
- B. Brkić, N. France and S. Taylor, Anal. Chem., 2011, 83(16), 6230–6236 CrossRef PubMed.
- S. Giannoukos, B. Brkić and S. Taylor, Anal. Methods, 2016, 8, 6607–6615 RSC.
- Q. Hu, S. Liu, Y. Liu, Xa. Fang, J. Xu, X. Chen, F. Zhu and G. Ouyang, Anal. Chim. Acta, 2019, 1050, 88–94 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |