Open Access Article
Open Access ArticleHarnessing acetogenic bacteria for one-carbon valorization toward sustainable chemical production
Jiyun
Bae
a,
Chanho
Park
a,
Hyunwoo
Jung
a,
Sangrak
Jin
a and
Byung-Kwan
Cho
 *abc
*abc
aDepartment of Biological Sciences, Korea Advanced Institute of Science and Technology, Daejeon 34141, Republic of Korea
bKAIST Institute for the BioCentury, Korea Advanced Institute of Science and Technology, Daejeon 34141, Republic of Korea. E-mail: bcho@kaist.ac.kr
cGraduate School of Engineering Biology, Korea Advanced Institute of Science and Technology, Daejeon 34141, Republic of Korea
First published on 8th July 2024
Abstract
The pressing climate change issues have intensified the need for a rapid transition towards a bio-based circular carbon economy. Harnessing acetogenic bacteria as biocatalysts to convert C1 compounds such as CO2, CO, formate, or methanol into value-added multicarbon chemicals is a promising solution for both carbon capture and utilization, enabling sustainable and green chemical production. Recent advances in the metabolic engineering of acetogens have expanded the range of commodity chemicals and biofuels produced from C1 compounds. However, producing energy-demanding high-value chemicals on an industrial scale from C1 substrates remains challenging because of the inherent energetic limitations of acetogenic bacteria. Therefore, overcoming this hurdle is necessary to scale up the acetogenic C1 conversion process and realize a circular carbon economy. This review overviews the acetogenic bacteria and their potential as sustainable and green chemical production platforms. Recent efforts to address these challenges have focused on enhancing the ATP and redox availability of acetogens to improve their energetics and conversion performances. Furthermore, promising technologies that leverage low-cost, sustainable energy sources such as electricity and light are discussed to improve the sustainability of the overall process. Finally, we review emerging technologies that accelerate the development of high-performance acetogenic bacteria suitable for industrial-scale production and address the economic sustainability of acetogenic C1 conversion. Overall, harnessing acetogenic bacteria for C1 valorization offers a promising route toward sustainable and green chemical production, aligning with the circular economy concept.
1 Introduction
The imminent threat of climate change is pressing humanity to reduce global greenhouse gas (GHG) emissions.1 A circular carbon economy has emerged as a promising framework in the quest for sustainable solutions to mitigate climate change.2 The circular carbon economy aims to minimize carbon emissions by closing the carbon loop, wherein carbon is continuously recycled and reused rather than being released into the atmosphere. This initiative has driven the development of sustainable carbon-negative manufacturing technologies for chemical production.One strategy to achieve this goal is adopting carbon capture and utilization (CCU) technologies, which capture carbon dioxide (CO2) at the point of emission or after emission and utilize it as a feedstock for producing value-added compounds such as commodity chemicals and fuels.3 By decreasing both direct CO2 emissions and the reliance of the chemical industry on fossil fuels as a carbon source, CCU offers routes to carbon-negative manufacturing and a circular economy.4 Among various CCU technologies, biocatalysts represent a greener alternative to chemical catalyst-based CCUs, offering several advantages.5 They are biodegradable, safe, and nontoxic, operating under mild conditions, which leads to less energy-intensive processes, unlike chemical catalysts that operate under extreme conditions and contain toxic, harmful compounds. Therefore, conforming to 10 of the 12 principles of green chemistry,6 biocatalysts play a vital role in developing sustainable and green CCU technologies.
One promising approach for a greener CCU involves harnessing acetogenic bacteria (acetogens) as biocatalysts for sustainable chemical production from waste carbon sources. Acetogens possess a unique capability to convert one-carbon (C1) compounds, such as CO2, CO, formate, or methanol, into value-added chemicals via the Wood–Ljungdahl pathway (WLP), known as the most energetically efficient CO2 fixation pathway in nature.7,8 Given the natural abundance, low production cost, and availability of C1 compounds as industrial waste by-products, leveraging acetogens to valorize these substrates offers a sustainable and green alternative for chemical synthesis.9,10 With advancements in genetic tools developed for acetogens, over 50 different chemicals have been produced from C1 compounds.7,11,12 While some of these chemicals have already achieved industrially relevant performance levels, they are limited to short-chain compounds such as ethanol. Because of the growing interest in longer-chain compounds owing to their higher market value, it is imperative to realize industrial-scale production of these energy-demanding high-value chemicals from C1 substrates. However, this remains challenging because of the inherent energetic limitations of acetogens, which operate at the thermodynamic limit of life.13,14 In pursuit of addressing this challenge, numerous studies have recently been conducted, aiming to fully exploit the potential of acetogens as green chemical production platforms.
This review overviews acetogens and their potential as sustainable, green chemical production platforms. Recent efforts to overcome the challenges hindering their industrial-scale application have focused on improving their energetics regarding ATP and redox availability. Furthermore, promising technologies that leverage low-cost and sustainable energy sources such as electricity and light are discussed to enhance both the performance and sustainability of the acetogenic C1 conversion process. Finally, emerging technologies for the development of high-performance strains and the economic sustainability of acetogenic C1 bioconversion are reviewed to accelerate advancements in this field.
2 One-carbon valorization via acetogenic bacteria
2.1 The Wood–Ljungdahl pathway and energy conservation system
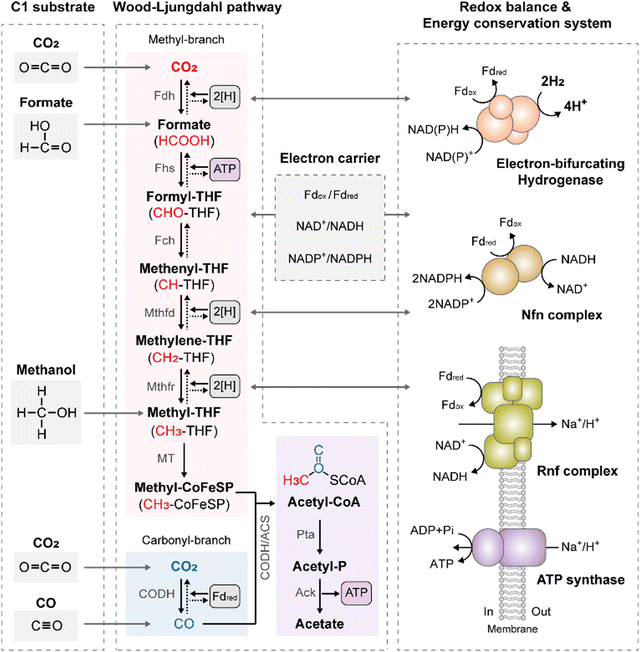
Acetogens are strictly anaerobic bacteria capable of fixing CO2via the WLP, recognized as the most energetically efficient carbon fixation pathway in nature, as it requires only one mole of adenosine triphosphate (ATP) for carbon fixation.15 In the WLP, two moles of CO2 are reduced to one mole of two-carbon (C2) acetyl-CoA through a series of stepwise reactions catalyzed by metalloenzymes, with tetrahydrofolate (THF) as a C1-carrier (Fig. 1). These reactions occur in two branches: the methyl-branch and carbonyl-branch, which generate methyl and carbonyl groups, respectively, contributing to the formation of acetyl-CoA.16 In the methyl branch, CO2 is first reduced to formate by formate dehydrogenase (Fdh). Subsequently, formate is activated to formyl-THF by formyl-THF synthetase (Fhs), driven by ATP hydrolysis. Formyl-THF is further reduced to methyl-THF through a series of reduction reactions catalyzed by formyl-THF cyclohydrolase (Fch), methenyl-THF dehydrogenase (Mthfd), and methylene-THF reductase (Mthfr). Finally, methyl-THF condenses with CO derived from the carbonyl branch and CoA on CO dehydrogenase/acetyl-CoA synthase (CODH/ACS), resulting in acetyl-CoA (Fig. 1).16,17This stepwise reduction of two CO2 molecules to acetyl-CoA via the WLP requires one ATP molecule and eight electrons. These electrons are provided by intracellular electron carriers such as ferredoxin (Fd) and nicotinamide adenine dinucleotide (NADH), which serve as reducing equivalents. As CO2 is fully oxidized, H2 or CO must be utilized as an energy source to provide these reducing equivalents. When H2 is the energy source, electron-bifurcating hydrogenase (Hyd) oxidizes H2, yielding reduced Fd (Fdred) and NADH.18 Despite the higher redox potential of H2 (E°′ = −414 mV) compared to that of Fd (E°′ = −500 mV), reduction of Fd with H2 becomes possible through the energy bifurcation mechanism of Hyd. This mechanism couples the exergonic electron flow from H2 to NAD+ (E°′ = −320 mV) with the endergonic electron flow from H2 to Fd.13 On the other hand, when CO is utilized as the energy source, direct reduction of Fd with CO is feasible due to the low redox potential of the CO2/CO couple (E°′ = −520 mV). CODH oxidizes CO to CO2 and produces Fdred.13 The reducing equivalents generated by Hyd or CODH are then supplied to the WLP. In certain acetogens, NADPH serves as a cofactor for the WLP operation. NADPH is generated from the obtained Fdred and NADH through the action of NADH-dependent Fdred: NADP+ oxidoreductase (Nfn) or Sporomusa-type Nfn (Stn) complex (Fig. 1).19,20
The resulting acetyl-CoA is converted to acetate via acetyl-phosphate, providing one ATP molecule via substrate-level phosphorylation (SLP) in the acetate kinase reactions to compensate for one ATP molecule consumed in the WLP. This results in a net-zero ATP yield for the overall process.21 However, in energy-limited environments where acetogens grow on gaseous substrates, they must generate additional ATP to sustain life. This is accomplished through an energy conservation system in which Fd-driven membrane-bound respiratory enzymes and ATP synthases cooperate to synthesize chemiosmotic ATP (Fig. 1). Acetogens possess one of two types of respiratory enzymes found in acetogens: Rnf (Rhodobacter nitrogen fixation) and energy-converting hydrogenase (Ech), which are classified based on their final electron acceptor. The Rnf complex is an ion-translocating Fd: NAD+ oxidoreductase that transfers electrons from Fdred to NAD+.22,23 The energy released in this exergonic reaction is used to generate a transmembrane proton (H+) or sodium (Na+) gradient. The Rnf complex plays a crucial role in the acetogenic metabolism, serving both as a redox-balancing and energy-conserving module.24 In contrast, the Ech complex acts as an Fd:H+ oxidoreductase, transferring electrons from Fdred to H+ to establish a transmembrane H+ gradient. This ion gradient drives ATP formation via ATP synthase, leading to chemiosmotic ion gradient-driven phosphorylation (Fig. 1). Given that the overall WLP process yields net-zero ATP, the energy conservation system is primarily responsible for ATP production during acetogenic C1 conversion.13 Although the final ATP yields depend on the energy systems present in acetogens,14,25 for example, the Rnf-containing, Na+-dependent acetogen Acetobacterium woodii yields 1 mole of ATP per 3.3 Na+ translocation. This resulted in 0.3 moles of ATP per mole of acetate from CO2/H2.
2.2 Upgrading one-carbon to multicarbon chemicals via acetogens
Acetogens comprise over 100 species isolated from diverse habitats (e.g., soils, sediments, sludge, and the intestinal tracts of numerous animals), exhibiting both phylogenetic and metabolic versatility. They are capable of growing on a wide range of substrates, including sugars, carboxylic acids, aldehydes, and CO2.16,24 Despite their extensive diversity, only a subset of acetogenic species is currently the focus of active investigation and application as biocatalysts for C1 bioconversion (Table 1). This selective attention is due to their notable features, which include the ability to utilize diverse C1 compounds (e.g., CO2, CO, formate, and methanol), genetic accessibility to available genome sequences and genetic tools, and relatively well-characterized physiology compared with other less-explored acetogens. These ten representative acetogens and their characteristics are listed in Table 1.| Acetogenic bacteria | C1 substrates | Temp. (°C) | pH | Natural product | Genetic tools | Ref. |
|---|---|---|---|---|---|---|
| Acetobacterium woodii | CO2, methanol, formate | 30 | 7.0–7.4 | Acetate | 26 | 27 and 28 |
| Butyribacterium methylotrophicum | CO2, CO, methanol, formate | 37 | 7.5 | Acetate, ethanol, lactate, butyrate, butanol | 29 | 30 |
| Clostridium autoethanogenum | CO2, CO | 37 | 5.8–6.0 | Acetate, ethanol, lactate, 2,3-butanediol | 31 | 32 |
| Clostridium carboxidivorans | CO2, CO | 37 | 5.0–7.0 | Acetate, ethanol, butyrate, butanol, caproate, hexanol | 33 | 34 and 35 |
| Clostridium ljungdahlii | CO2, CO | 37 | 6.0 | Acetate, ethanol, lactate, 2,3-butanediol | 36 | 32 |
| Clostridium sp. AWRP | CO2, CO | 37 | 6.0 | Acetate, ethanol, 2,3-butanediol | 37 | 38 |
| Eubacterium limosum | CO2, CO, methanol, formate | 37 | 7.0 | Acetate, lactate, butyrate, butanol, caproate | 39 | 40–42 |
| Sporomusa ovata | CO2, methanol, formate | 34 | 6.3 | Acetate | 43 | 44 and 45 |
| Moorella thermoacetica | CO2, CO, methanol, formate | 55 | 6.5–6.8 | Acetate | 46 | 47 |
| Thermoanaerobacter kivui | CO2, CO, formate | 66 | 6.4 | Acetate | 48 | 49 |
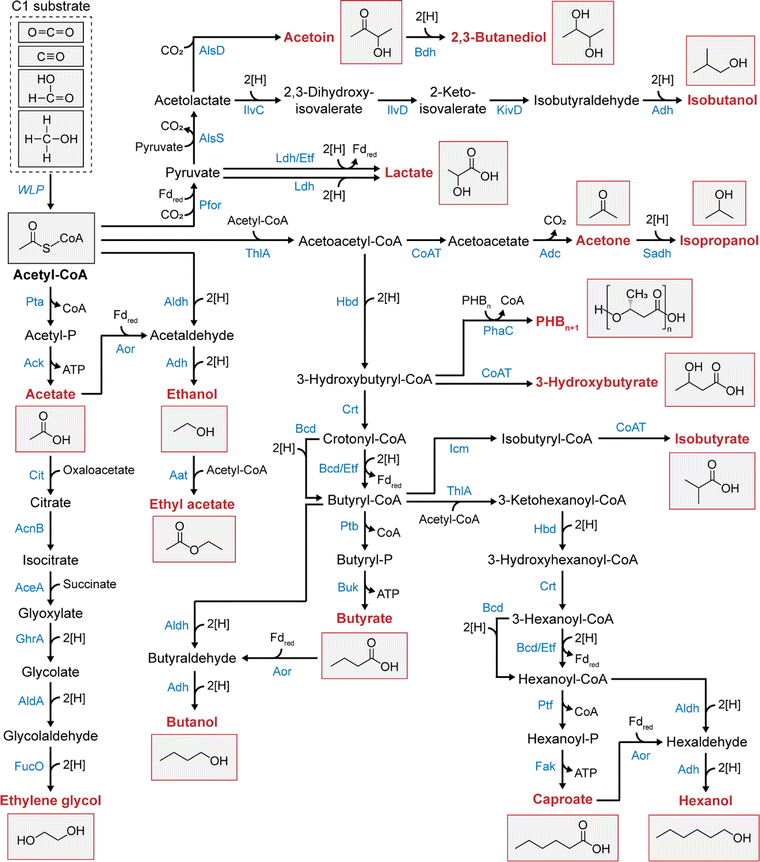
Depending on their genetic and metabolic features, acetogens can convert C1 substrates into various multicarbon chemicals other than acetate (Table 1 and Fig. 2). Acetogens such as A. woodii, Sporomusa ovata, Moorella thermoacetica, and Thermoanaerobacter kivui have been reported to exclusively produce acetate as the sole end-product of acetogenic C1 conversion,27,28,44,45,47,49 while others are capable of producing ethanol, lactate, 2,3-butanediol, butyrate, butanol, caproate, and hexanol. Among them, ethanol production from waste gases utilizing Clostridium autoethanogenum has already been commercialized by LanzaTech.7Clostridium carboxidivorans stands out as an intriguing acetogen due to its broad product profile, encompassing acetate, ethanol, butyrate, butanol, caproate, and hexanol.34 Of particular interest are the longest-chain compounds, caproate and hexanol (C6 compounds), which exhibit significantly higher energy densities than short-chain acids and alcohols. This feature makes them promising platform chemicals for producing biodiesel and jet fuels.50,51 A recently discovered acetogenic species, Clostridium luticellarii, has been found to produce a novel compound within the acetogen isobutyrate from CO2/H2, adding to the diversity of chemicals generated by acetogens.52,53
Despite the promising capabilities of the Clostridium species in producing various acids and alcohols, their utilization as C1 substrates is limited to gaseous forms (CO2 and CO). Expanding the substrate range, Butyribacterium methylotrophicum and Eubacterium limosum emerge as promising biocatalysts with the ability to efficiently utilize liquid C1 substrates (methanol and formate) alongside C1 gases.30,40 Methanol and formate are promising liquid C1 substrates, bypassing the gas–liquid mass transfer issue posed by gaseous substrates and providing higher energy efficiency than CO2/H2 or CO.54 These substrates can be directly incorporated into the methyl branch of the WLP, where reducing equivalents can be generated by operating the WLP in the reverse direction (Fig. 1).30,40B. methylotrophicum and E. limosum are known for butyrate production, and in some instances, they can produce more reduced butanol when supplied with methanol.
Extensive research has focused on metabolic engineering to expand the product spectrum of acetogens with recent advancements in genetic tools developed for acetogens (Table 1). Table 2 lists the chemicals produced by engineered acetogens reported within the last five years. Fifteen chemicals were tested and produced during this period in acetogens starting with acetyl-CoA as the precursor (Fig. 2). A. woodii and M. thermoacetica, which were initially capable of producing only acetate, were engineered to synthesize ethanol, lactate, acetone, isopropanol, butyrate, isobutanol, poly-3-hydroxybutyrate (PHB), and caproate (Table 2). These were achieved by introducing the corresponding synthesis pathways from other organisms or disrupting endogenous genes without pathway introduction. The latter approach was particularly intriguing as it unraveled novel metabolic traits of acetogens. For instance, disrupting methylene-THF reductase in A. woodii enables the production of ethanol and lactate alongside formate, molecular hydrogen, and acetate.55 Similarly, deleting hydrogenases in A. woodii produces lactate from fructose or methyl groups + CO.56 Although these compounds are not produced solely on C1 substrates, these findings suggest the potential to engineer strains that convert fructose or even C1 compounds into reduced products, such as ethanol or lactate. However, numerous efforts have focused on introducing heterologous pathways in A. woodii, leading to acetone synthesis,57 isopropanol,58,59 butyrate,60 isobutanol,61 PHB,62 and caproate.63 In comparison, only the ethanol,64 acetone,65,66 and isopropanol67 pathways have been demonstrated in M. thermoacetica.
| Chemical | Acetogenic bacteria | Substrate | Product titer (g L−1) | Productivity | Production scale | Ref. |
|---|---|---|---|---|---|---|
| Ethanol (C2H6O) | M. thermoacetica | Fructose/CO2 | 0.810 | — | Serum bottle | 64 |
| A. woodii | Fructose | 0.138 | — | Resting cell | 55 | |
| Ethylene glycol (C2H6O2) | C. autoethanogenum | Fructose | 0.394 | — | Serum bottle | 68 |
| Lactate (C3H6O3) | A. woodii | Fructose | 0.522 | — | Resting cell | 55 |
| A. woodii | Glycine betaine + 10% CO | 1.838 | — | Resting cell | 56 | |
| A. woodii | CO2/H2 | 1.693 | — | Serum bottle | 69 | |
| Acetone (C3H6O) | A. woodii | CO2/H2 | 1.249 | — | Serum bottle | 57 |
| M. thermoacetica | CO2/H2 | 0.232 | — | Serum bottle | 65 | |
| M. thermoacetica | CO/H2 | 0.192 | 0.09 g gDCW−1 h−1 | Serum bottle | 66 | |
| C. autoethanogenum | CO/H2/CO2/N2 (50/10/30/10) | — | 2.5 g L−1 h−1 | 2-L CSTR | 70 | |
| E. limosum | MeOH | 0.092 | — | Serum bottle | 71 | |
| Isopropanol (C3H8O) | A. woodii | CO2/H2 | 0.871 | — | 2-L CSTR | 58 |
| A. woodii | CO2/H2 | 0.834 | — | Serum bottle | 59 | |
| M. thermoacetica | CO/H2 | 0.120 | 0.03 g gDCW−1 h−1 | Serum bottle | 67 | |
| C. autoethanogenum | CO/H2/CO2/N2 (50/10/30/10) | — | 3 g L−1 h−1 | 2-L CSTR | 70 | |
| C. ljungdahlii | CO/CO2/H2/N2 (56/20/9/15) | 13.4 | — | 2-L CSTR | 72 | |
| C. ljungdahlii | CO/CO2/H2 (70/20/10) | 2.4 | — | 2-L CSTR | 73 | |
| Acetoin (C4H8O2) | E. limosum | CO/CO2/H2 (44/22/2) | 0.070 | — | Serum bottle | 41 |
| 2,3-Butanediol (C4H10O2) | E. limosum | CO/CO2/H2 (66/22/2) | 1.370 | 0.0026 g L−1 h−1 | 1-L gas-lift reactor | 74 |
| Butyrate (C4H8O2) | A. woodii | CO2/MeOH | 0.015 | — | Serum bottle | 60 |
| C. ljungdahlii | CO2/CO | 1.010 | — | Serum bottle | 75 | |
| Butanol (C4H10O) | B. methylotrophicum | MeOH/formate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
0.111 | — | Serum bottle | 30 |
| E. limosum | MeOH | 0.044 | — | Serum bottle | 71 | |
| Isobutanol (C4H10O) | A. woodii | Fructose | 0.007 | — | Serum bottle | 61 |
| C. ljungdahlii | CO/CO2/H2 (50/5/45) | 0.007 | — | Serum bottle | 61 | |
| C. ljungdahlii | CO/CO2/H2 (55/5/30) | 0.130 | — | 3-L CSTR | 76 | |
| 3-Hydroxybutyrate (C4H8O3) | C. ljungdahlii | CO/CO2/H2/N2 (56/20/9/15) | 3.0 | — | 2-L CSTR | 72 |
| C. autoethanogenum | CO/CO2/H2 (50/40/10) | 14.6 | 1.5 g L−1 h−1 | 1.5-L CSTR | 77 | |
| Poly-3-hydroxybutyrate ([C4H6O2]n) | C. autoethanogenum | CO/CO2/H2 (50/20/20) | 0.027 | 0.00113 g L−1 h−1 | Continuous reactor | 78 |
| A. woodii | CO2/H2 | 0.024 | — | Serum bottle | 62 | |
| Ethyl acetate (C4H8O2) | C. autoethanogenum | CO | 0.018 | — | Serum bottle | 79 |
| Caproate (C6H12O2) | A. woodii | CO2/H2 | 0.181 | — | Serum bottle | 63 |
| Hexanol (C6H14O) | C. ljungdahlii | CO2/H2 | 0.393 | — | 3.7-L CSTR | 80 |
Engineering E. limosum and B. methylotrophicum have also been successful, albeit restricted to the chemical production of C3 or C4, including acetone,71 acetoin,41 2,3-butanediol,74 and butanol30,71 (Table 2). On the other hand, Clostridium species have been actively engineered to produce various non-native chemicals and fuels, including ethylene glycol,68 acetone,70 isopropanol,72,73 butyrate,75 isobutanol,61,76 3-hydroxybutyrate (3-HB),72 PHB,78 ethyl acetate,79 and hexanol80 (Table 2). Of these, ethylene glycol68 and ethyl acetate79 production demonstrated in C. autoethanogenum is the first report of acetogens, as they are novel compounds not naturally produced in acetogens. Notably, acetone,70 isopropanol,70 and 3-HB77 have recently been produced at relatively high levels from C1 gases, reaching productivities of up to 2.5 g L−1 h−1, 3 g L−1 h−1, and 1.5 g L−1 h−1, respectively, in C. autoethanogenum. These results demonstrate the potential of the acetogenic C1 conversion as a platform for the sustainable production of chemicals and fuels.
2.3 Challenges in acetogenic C1 conversion
Engineered acetogens have demonstrated the ability to produce various multicarbon chemicals; however, their product titers and productivities remain limited, typically reaching only mg L−1 or mg L−1 h−1 scales on C1 substrates (Table 2). Although high-level production of 3-HB, acetone, and isopropanol has been achieved in C. autoethanogenum, there is still room for improvement in higher carbon production, given the increasing interest for longer-chain products, such as butyrate, caproate, and hexanol due to their substantially higher market value (e.g., butyrate has 3–5 times higher value than acetate).51,53,81 While C. carboxidivorans can naturally produce caproate and hexanol, its product titer, yield, and selectivity are insufficient for large-scale production due to its predominant production of C2 compounds (acetate and ethanol) with small amounts of C4 and C6 products.34,35 Despite engineering efforts in A. woodii and C. ljungdahlii showing potential for caproate and hexanol production, respectively, titers remain very low (Table 2).63,80 Overcoming these limitations in upgrading C1 to value-added multicarbon chemicals is crucial for ultimately replacing the fossil-based chemical industry with an acetogenic C1 conversion platform.3 Overcoming energetic limitations in acetogenic C1 conversion
3.1 Impacts of electron donors on the feasibility of acetogenic C1 conversion
The low product titers and yields in acetogens are attributable to the thermodynamic feasibility of acetogenic C1 conversion, which requires the substrate to provide sufficient energy for cellular maintenance and growth.82 The ATP yield varies depending on the electron donors utilized (H2, CO, or methanol) and the final products, which range from −0.4 ATP to +4.4 ATP per mol of product (Table 3).14,83 Notably, employing H2 as an electron donor presents the highest ATP demands, as it results in negative ATP yields. Thus, producing chemicals other than acetate (e.g., ethanol, lactate, acetone, and butanol) under CO2/H2 conditions poses significant challenges. For instance, while acetate production from CO2/H2 is feasible with 0.3 ATP/acetate, ethanol or butanol production may not be viable because of negative ATP yields (Table 3). Under a CO2/H2 environment, acetogens operate their metabolism at the thermodynamic limit of feasibility.13 Due to the inherent energetic limitations, engineering acetogens to redirect carbon flow from acetate to other chemicals often leads to ATP shortage unless the chemical biosynthetic pathway generates ATP. Consequently, only the biosynthesis pathways generating ATP can serve as significant products (e.g., acetate and ethanol). In contrast, ATP-demanding products (negative ATP yields) can only be produced at a higher yield if generated concurrently with other by-products that yield ATP, leading to a low product yield and selectivity.| Product | Equation | Electron donor | Metabolic pathway | ATP yield (mol ATP/mol product) |
|---|---|---|---|---|
| Acetate | 2CO2 + 4H2 → CH3COOH + 2H2O | H2 | Ack | 0.3 |
| 4CO + 2H2O → CH3COOH + 2CO2 | CO | Ack | 1.5 | |
| 4CH3OH + 2CO2 → 3CH3COOH + 2H2O | MeOH | Ack | 0.8 | |
| Ethanol | 2CO2 + 6H2 → C2H5OH + 3H2O | H2 | Aldh-Adh | −0.1 |
| Aor-Adh | 0.3 | |||
| 6CO + 3H2O → C2H5OH + 4CO2 | CO | Aldh-Adh | 1.7 | |
| Aor-Adh | 2.1 | |||
| 6CH3OH → 3C2H5OH + 3H2O | MeOH | Aldh-Adh | 0.7 | |
| Aor-Adh | 1.1 | |||
| Lactate | 3CO2 + 6H2 → C3H6O3 + 3H2O | H2 | Ldh/Etf | −0.1 |
| 6CO + 3H2O → C3H6O3 + 3CO2 | CO | Ldh/Etf | 1.7 | |
| 6CH3OH + 3CO2 → 3C3H6O3 + 3H2O | MeOH | Ldh/Etf | 0.7 | |
| Acetone | 3CO2 + 8H2 → C3H6O + 5H2O | H2 | Adc | −0.4 |
| 8CO + 3H2O → C3H6O + 5CO2 | CO | Adc | 2.0 | |
| 4CH3OH + CO2 → C3H6O + CH3COOH + 3H2O | MeOH | Adc | 0.5 |
In contrast, utilizing CO and methanol as electron donors yielded positive ATP yields for all products (Table 3), as they are more reduced substrates than CO2/H2. The utilization of these reduced substrates has been shown to favor the production of more reduced products (e.g., butyrate, ethanol, and butanol) by altering the metabolism of acetogens and thereby shifting the product profiles towards reduced products.30,40,84E. limosum exhibited butyrate production when grown with methanol but not with other C1 substrates such as CO, CO2, or formate.84 Moreover, butanol production in E. limosum was only demonstrated when methanol and formate were utilized as substrates at a ratio of 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, resulting in butyrate as the major product.40 Similarly, supplying both CO and methanol induced butanol formation and ethanol and lactate production in B. methylotrophicum, whereas methanol/CO2 and only CO conditions did not trigger butanol formation.30 These findings emphasize the significance of supplying CO or methanol as electron donors, as they play a critical role in shifting the product spectrum towards more reduced products and determining the feasibility of desired chemical production.
1, resulting in butyrate as the major product.40 Similarly, supplying both CO and methanol induced butanol formation and ethanol and lactate production in B. methylotrophicum, whereas methanol/CO2 and only CO conditions did not trigger butanol formation.30 These findings emphasize the significance of supplying CO or methanol as electron donors, as they play a critical role in shifting the product spectrum towards more reduced products and determining the feasibility of desired chemical production.
Despite the higher energy potential of methanol and CO compared to H2, their utilization is restricted to a few acetogens, such as carboxydotrophic acetogens capable of catalyzing and resistant to CO,85 and methylotrophic acetogens harboring a methyltransferase for methanol assimilation (Table 1).83 Hence, a fundamental solution for enhancing intracellular ATP availability, even when using H2 as an electron donor, is imperative.
3.2 Boosting chemiosmotic ATP synthesis with energy-conserving electron acceptors
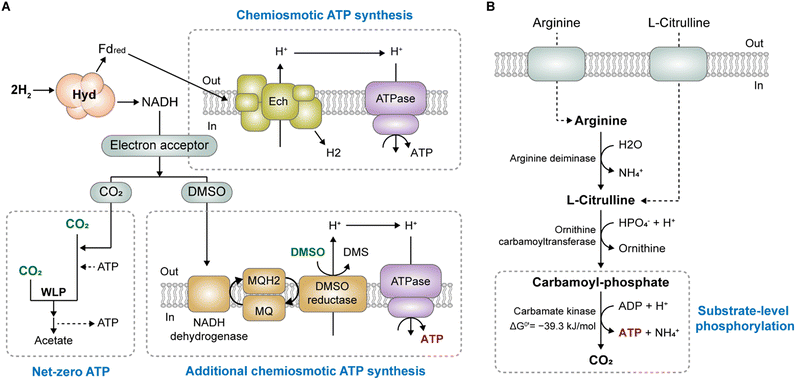
One strategy for enhancing ATP production involves boosting ion gradient-driven phosphorylation (IGP). Given that most ATP is generated through the ion gradient established by membrane-bound respiratory enzymes (Rnf or Ech), the overexpression or engineering of these enzymes could stimulate ion gradient generation, thereby augmenting IGP. However, adopting this direct approach can be challenging because membrane-bound proteins are often difficult to express in heterologous systems.86,87 Instead of directly manipulating the respiratory enzyme, a preferable alternative approach is indirectly increasing the ion gradient by supplying additional terminal electron acceptors other than CO2.Appropriate electron acceptors can facilitate IGP-dependent ATP synthesis independent of SLP through H2 oxidation. Studies have demonstrated the effectiveness of electron acceptors such as dimethyl sulfoxide (DMSO),64,65,88 thiosulfate,65 nitrate,89,90 and caffeate91–93 as energy-conserving electron acceptors for several acetogens. Enhanced ATP production increases biomass and product formation, accompanied by reduced acetate production.65,88,89
As low-cost electron acceptors, DMSO and nitrate enable cost-effective processes and successfully enhance product synthesis. For instance, M. thermoacetica can utilize DMSO alongside CO2 as an electron acceptor (Fig. 3A).88 It has been reported that DMSO increases intracellular ATP levels 2-fold and reduces acetate production by half, indicating a redirection of electron flow away from acetogenesis towards DMSO reduction in M. thermoacetica.65 The study also confirmed that DMSO acts solely as an electron acceptor, not as a carbon source, despite being an organic compound containing two methyl groups. Moreover, supplying DMSO to engineered M. thermoacetica restored both growth and acetone production under CO2/H2 conditions, which could not be achieved without DMSO supplementation because of its inability to grow solely on CO2/H2.65,66 As shown in Table 3, acetone production under CO2/H2 conditions required ATP because of the absence of ATP yields. Consequently, the engineered M. thermoacetica strain, which includes the partial deletion of the acetate pathway and introduction of the acetone production pathway, encounters challenges in CO2/H2 environments owing to ATP shortage, resulting in no growth or acetone production. However, DMSO supplementation restored the autotrophic growth and enabled acetone production. This positive effect was attributed to the increased intracellular ATP levels in the DSMO-supplemented strain.65 Since M. thermoacetica possesses DMSO reductase, menaquinone (MQ), and the NADH dehydrogenase complex, it is assumed that ATP production could be augmented through anaerobic DMSO respiration, a mechanism akin to DMSO respiration in E. coli, where H2 is the electron source.65 Through the oxidation of H2 coupled with DMSO respiration in M. thermoacetica, a proton gradient is generated, subsequently driving ATP synthase for IGP-coupled chemiosmotic ATP synthesis (Fig. 3A). However, a detailed understanding of the mechanism underlying H2 oxidation coupled with DMSO reduction has yet to be achieved.
Similarly, nitrate supplementation has been reported to enhance H2-dependent growth in C. ljungdahlii.89 With the necessary genes for nitrate assimilation, C. ljungdahlii simultaneously reduces CO2 and nitrate using electrons derived from H2. In contrast to DMSO, which is presumed to promote ion gradient generation directly, nitrate acts as an electron sink for reducing equivalents derived from the Rnf complex, thus indirectly promoting chemiosmotic ATP generation. The proposed mechanism relies on electron bifurcation by hydrogenase to couple nitrate reduction with ATP production. Since reduced Fd is utilized for chemiosmotic ATP synthesis rather than CO2 reduction, nitrate reduction yields more ATP than acetate production from CO2/H2 (1.5 ATP for nitrate and 0.63 ATP for CO2 reduction).89 The study also found a significant increase in the ATP/ADP ratio and acetyl-CoA pools, indicating that nitrate reduction is coupled to ATP generation. In order to employ this mechanism, the effect of nitrate supplementation on ethanol production by C. ljungdahlii was investigated.90 Indeed, supplying nitrate improved its growth by up to 62% and ethanol production by up to 3-fold from CO2/H2. However, this positive effect was observed only in pH-controlled bioreactors and not in serum bottle experiments. This occurred because the ammonium produced during nitrate reduction increased the pH of the medium, leading to inhibition of biomass formation. Variations in biomass, acetate, and ethanol production were observed across all nitrate-supplemented bioreactors. Because of stochastic inhibition events stemming from the interplay between undissociated acetic acid and ammonium production, this study emphasizes the need for further investigation at both physiological and bioprocessing levels to effectively harness the potential of nitrate as an electron acceptor to overcome ATP limitations.
While caffeate has been shown to enhance growth yield and ATP levels in A. woodii through caffeate respiration linked to energy conservation via Rnf and ATP synthase, its application for improving desired product synthesis has not been demonstrated.91–93 This is likely due to the toxicity of caffeine to cells and its high cost, making this approach economically less favorable.14 To leverage additional ATP production via electron acceptors, a metabolic system must be in place that couples ATP synthesis with the electron acceptor reduction. Supplying electron acceptors may not significantly affect growth and could even lead to growth inhibition, as demonstrated in M. thermoacetica, where nitrate supplementation inhibited WLP and acetogenic metabolism.94 Nevertheless, these findings suggest that supplying electron acceptors can be a beneficial strategy for producing ATP-intensive heterologous products from acetyl-CoA by decoupling ATP production from CO2 fixation and redirecting carbon flux from acetate to the desired product.
3.3 Employing SLP-coupled reactions to gain additional ATP
Another strategy for enhancing ATP availability involves leveraging additional SLP. Katsyv et al. (2020) described several reactions coupled to SLP, which have a phosphoryl group transfer potential with more negative free energy than −31.8 kJ mol−1, enabling ADP phosphorylation.14 Among these reactions, the carbamate kinase reaction (ΔG°′ = −39.3 kJ mol−1) has been reported to be effective for ATP production in several acetogens. As part of the arginine deiminase (ADI) pathway, carbamate kinase generates one ATP molecule with arginine as a supplement (Fig. 3B). The pathway was initially discovered in C. autoethanogenum, where arginine supplementation increased growth rate and abolished acetate production during autotrophic growth on syngas (a mixture of CO, CO2, and H2).95 The observed growth-stimulating effect of arginine was attributed to the increased ATP production from SLP during the catabolism of arginine by carbamate kinase in the ADI pathway.In addition, supplementation of citrulline promoted ATP production in C. carboxidivorans P7, leading to increased biomass and specific growth rate under both heterotrophic and autotrophic conditions.96 Specifically, the intracellular ATP level and biomass were improved by 80.5% and 31.6%, respectively, under syngas conditions. Moreover, the ethanol yield increased by 18.6%, and the alcohol-to-acid production ratio increased by 60.3%, likely because of the extra ATP generated from the citrulline supply.
Based on these findings, leveraging the AID pathway for SLP via the carbamate kinase reaction could be a strategy for overcoming ATP limitations in acetogens. Validating this approach, the heterologous expression of the AID pathway in A. woodii led to a 69% increase in biomass and a 60% reduction in the acetate yield per biomass.97 Further research is needed to assess the effectiveness of this strategy for producing ATP-intensive chemicals. In addition, exploring various SLP-coupled reactions for ATP synthesis in acetogens is necessary.
3.4 Utilizing acetate as a precursor for alcohol production
In addition to ATP supply through additional SLP- or IGP-coupled reactions, utilizing acetate as a precursor for the desired chemical production can be helpful for ATP production without relying on acetate kinase reactions to obtain ATP. In Clostridium species of acetogens, such as C. autoethanogenum and C. ljungdahlii, two pathways for ethanol production exist: the aldehyde:Fd oxidoreductase (Aor)-alcohol dehydrogenase (Adh) pathway and the bifunctional aldehyde/alcohol dehydrogenase pathway (Aldh-Adh) (Fig. 2). The Aor-Adh pathway is preferred to the Aldh-Adh pathway because it retains the acetate kinase reaction, enabling ATP generation during ethanol production.31,98–100 In the Aor-Adh pathway, Aor utilizes acetate as a substrate and reduces it to acetaldehyde using reduced Fd, which is then converted to ethanol by Adh using NADH. Conversely, the Aldh-Adh pathway converts acetyl-CoA into ethanol using acetaldehyde as an intermediate, resulting in ATP loss by bypassing the acetate kinase reaction (Fig. 2). Therefore, ethanol production via the Aor-Adh pathway yields more ATP than that via the Aldh-Adh pathway; for example, when CO is a substrate, providing 2.1 ATP and 1.7 ATP, respectively (Table 3). Based on this approach, introducing Aor into non-Aor-containing acetogens such as A. woodii enables ethanol production without ATP loss.Since its discovery, Aor has garnered attention as the only enzyme to catalyze a thermodynamically challenging reverse reaction: reducing nonactivated acids to aldehydes using a low-redox-potential electron donor. Additionally, its wide substrate range has expanded its applications in reducing various organic acids (e.g., acetate, butyrate, and caproate) to alcohols (e.g., ethanol, butanol, and hexanol).101 As such, Aor can be heterologously expressed in butyrate-producing acetogens, such as E. limosum and B. methylotrophicum, to produce butanol by reducing butyrate production. This would benefit ATP yield, as butanol production using the Aor route results in greater ATP acquisition than non-Aor routes (Table 3). However, the heterologous expression of Aor remains to be explored. To date, only homologous expression of Aor in acetogens has been demonstrated in C. carboxidivorans and C. autoethanogenum, where the overexpression of Aor effectively reduced acetate accumulation and improved ethanol production.33,72
In addition to the role of intracellular acetate in alcohol production, supplying exogenous acetate has been shown to affect the production of reduced acetogen products positively. Specifically, CO gas fermentation supplemented with acetate resulted in increased ethanol production by C. ljungdahlii,102C. autoethanogenum,103,104 and Clostridium sp. AWRP.105 In C. ljungdahlii, supplementation with 15 mM acetate boosted the ethanol production rate by over 2.4-fold, along with enhanced cell density and selectivity toward ethanol, achieving an ethanol-to-acetate ratio of 93.0.102 This effect was observed at a slightly acidic pH, ensuring an excess of both CO and undissociated acetic acid for optimal Aor activity, thereby promoting higher selectivity for the more reduced product, ethanol. Acetate supplementation has been proposed as a simple and effective approach to alleviate CO inhibition in Clostridium sp. AWRP.106 This is because CO oxidation is coupled with acetate reduction to ethanol, potentially lowering the intracellular CO levels below inhibitory levels, thereby mitigating CO inhibition. In this acetogen, CO fermentation with exogenous acetate enhanced the maximum cell density by 2-fold, overall alcohol production (2.9- and 2.3-fold higher titers of ethanol and 2,3-BDO, respectively), and specific growth rates (2.6-fold) compared to CO fermentation without acetate supplementation. Similarly, ethanol production in C. autoethanogenum was improved in the presence of exogenous acetate.103 A recent study found that the addition of exogenous acetate increased the concentration of undissociated acetic acid, which in turn regulated the ethanol yield and production rates, presumably to counteract the inhibition caused by undissociated acetic acid.104 These results highlight the potential of acetate as both a precursor and accelerator for alcohol production. Therefore, acetate, often regarded as a waste product, can be effectively utilized for alcohol production via the acetate kinase reaction without sacrificing ATP synthesis.
4 Redox supply for powering energetics in a sustainable manner
4.1 Optimizing redox balance for efficient acetogenic C1 conversion
The balance of the reducing equivalents is important for both growth and product synthesis in acetogens. Redox recycling enzymes, such as Rnf, Hyd, Nfn, and Aor, along with most enzymes in the WLP, are pivotal for maintaining redox balance.24,25,64,100 Particularly, during syngas fermentation and alcohol production, Aor activity is crucial for consuming reduced Fd and regulating redox balance and thus metabolic homeostasis.100 Ethanol production is predominantly governed by thermodynamics and redox rather than gene or protein expression levels in acetogens, as indicated by several studies.100,107,108 Notably, C. autoethanogenum employs metabolic oscillations to manage redox imbalances arising from co-metabolizing H2 and CO, ensuring effective homeostatic regulation.100However, the engineering of acetogens can lead to redox imbalances, resulting in low product yields and poor growth. For instance, excessive intracellular NADH levels caused by H2-supplemented mixotrophy inhibited the growth of engineered ethanol-producing M. thermoacetica.64 Although the authors applied H2-supplemented mixotrophy (CO2/H2/fructose) to enhance ethanol yield, H2 supply prevented the strain from H2 formation with reversible hydrogenase activity, which is necessary to balance the redox by oxidizing excess electrons generated from fructose catabolism. This inhibition of H2 formation leads to increased intracellular NADH levels and, hence, a redox imbalance, hampering growth and ethanol production. In contrast to the ethanol production pathway, introducing the acetone production pathway into M. thermoacetica successfully increased acetate and acetone production. Because the acetone production pathway from acetyl-CoA does not require a reducing equivalent, H2-supplemented mixotrophy provided positive effects without redox imbalance issues. Therefore, this study suggests that reversible hydrogenase activity enables M. thermoacetica to flexibly balance its intracellular redox state, emphasizing the need to fine-tune the redox balance to benefit from H2-supplemented mixotrophy.64
Balancing intracellular NADPH levels is crucial, especially for isopropanol production. The reduction of acetone to isopropanol by primary–secondary alcohol dehydrogenase is NADPH-dependent, and a redox imbalance may lead to incomplete conversion.109 Therefore, NADPH supply and balance are essential for efficient acetone reduction. For example, engineered M. thermoacetica achieved complete acetone reduction to isopropanol from C1 substrates, whereas engineered A. woodii exhibited incomplete reduction, leaving one-third of the acetone unconsumed.67 This difference is likely due to the presence of Nfn, an NADPH-dependent hydrogenase in M. thermoacetica, facilitating direct NADPH production from H2. These findings underscore the importance of the redox balance and supply in achieving successful acetogenic C1 conversion with high yields and selectivity.
The intracellular redox pool must be increased to balance redox reactions and improve energetics. This can be achieved through the addition of H2 or reduced substrates such as methanol and CO. This approach has been proven effective in increasing butyrate production in recombinant A. woodii60 and ethanol and PHB production in C. autoethanogenum78,110 because these substrates can generate additional reducing equivalents. However, employing this approach becomes challenging when considering carbon-negative production because utilizing CO as the sole carbon and energy source theoretically results in unwanted CO2 emissions with a loss of two-thirds of the carbon, lowering product yields. Given the current production method and cost of H2, acetogenic C1 conversion seems less favorable than the photoautotrophic pathway, which uses renewable light at no cost.111,112 Therefore, exploring alternative renewable redox suppliers for powering acetogenic C1 conversion is necessary to achieve complete sustainability. There is growing interest in leveraging abundant, low-cost, and renewable energy sources such as electricity and light. Abiotic electrocatalysts and photocatalysts with robust stability and high catalytic efficiency are crucial materials for sustainably powering microbial growth and chemical biosynthesis.113 This approach offers promising routes for achieving a sustainable and renewable energy supply through reducing equivalents derived from light and electricity.
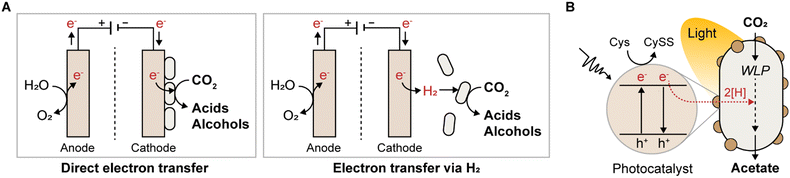
4.2 Electricity as a sustainable redox supplier
By adopting microbial electrosynthesis (MES), reducing equivalents can be supplied in unlimited quantities, either through direct electron transfer or indirectly via H2 generated from the cathode surface (Fig. 4A). Electrotropic acetogens such as S. ovata,114–118C. ljungdahlii,119–122T. kivui,123 and Clostridium scatologenes,124 are harnessed for MES because they can take up electrons to reduce CO2 into various multicarbon chemicals (Table 4). Notably, MES allows the production of reduced chemicals, such as ethanol, lactate, and butyrate, from CO2 when a sufficient redox supply is achieved,119–121,124 which is not achievable with chemoautotrophic growth on CO2/H2 owing to energetic limitations and negative ATP yields (Table 3).| Species | Cathode material | Cathode potential (vs. Ag/AgCl) | Product | Maximum titer (g L−1) | Maximum productivity | Ref. |
|---|---|---|---|---|---|---|
| S. ovata | Bio-printed carbon cloth | −0.8 V | Acetate | 3.45 | 0.68 g L−1 d−1 | 114 |
| S. ovata | Bio-printed Ti mesh | −0.8 V | Acetate | 9.4 | 0.84 g L−1 d−1 | 116 |
| S. ovata | Copper foam coated with reduced graphene oxide | −1.2 V | Acetate | — | 1697.6 mmol m−2 d−1 | 118 |
| S. ovata | Porous nickel hollow fiber | −0.9 V | Acetate | — | 247 mmol m−2 d−1 | 117 |
| S. ovata | Dual cathode (graphite rods and titanium mesh) | −0.9 V | Acetate | 11 | 0.7 g L−1 d−1 | 115 |
| T. kivui | Ni–Mo coated graphite rod | NA | Acetate | 6.0 | 3.36 g L−1 d−1 | |
| C. scatologenes | Carbon felt | −0.6 V | Acetate | 0.03 | — | 124 |
| Butyrate | 0.01 | — | ||||
| C. scatologenes | Carbon felt | −1.2 V | Acetate | 0.44 | — | 124 |
| Butyrate | 0.085 | — | ||||
| Ethanol | 0.015 | — | ||||
| C. ljungdahlii (planktonic-dominant condition) | Graphite block | −0.9 V | Acetate | 6.06 | 0.11 g L−1 d−1 | 122 |
| C. ljungdahlii (biofilm-dominant condition) | Graphite block | −0.9 V | Acetate | 1.01 | — | 122 |
| Glycine | 0.39 | — | ||||
| Ethanolamine | 0.14 | — | ||||
| C. ljungdahlii | Graphite block | −0.8 V | Acetate | 0.053 | — | 119 |
| C. ljungdahlii | Graphite block | −1.0 V | Acetate | 0.328 | — | 119 |
| Formate | 0.28 | — | ||||
| Lactate | 0.069 | — | ||||
| C. ljungdahlii | Cobalt phthalocyanine incorporated into porous cathode | −1.2 V | Acetate | 5.1 | — | 121 |
| Ethanol | 1.2 | — | ||||
| C. ljungdahlii (engineered) | Nickel phosphide modified carbon felt | −1.1 V | Acetate | 1.18 | 0.17 g L−1 d−1 | 120 |
| Butyrate | 0.67 | 0.1 g L−1 d−1 | ||||
| C. ljungdahlii | Graphite plate | −1.2 V | Acetate | 1.14 | 0.138 g L−1 d−1 | 125 |
| Mixed culture | Graphite plate | −1.0 V | Acetate | 1.8 | 0.26 g L−1 d−1 | 125 |
| Mixed culture | Graphite granules | −0.8 V | Acetate | 4.9 | — | 126 |
| Butyrate | 3.1 | — | ||||
| Isobutyrate | 1.6 | — | ||||
| Caproate | 1.2 | — | ||||
| Ethanol | 1.3 | — | ||||
| Butanol | 0.8 | — | ||||
| Isobutanol | 0.2 | — | ||||
| Hexanol | 0.2 | — | ||||
| Mixed culture | Carbon felt | −1.0 V to −1.3 V | Acetate | 5.47 | — | 127 |
| Propanol | 0.04 | — | ||||
| Butanol | 0.08 | — | ||||
| Hexanol | 0.02 | — | ||||
| Butyrate | NA | — | ||||
| Caproate | NA | — | ||||
| Mixed culture | Nickel foam–carbon felt | −1.1 V | Acetate | 16.0 | — | 128 |
| Butyrate | 2.87 | — | ||||
| Formate | NA | — |
MES has several advantages over chemoautotrophy with externally supplied H2 gas. A recent study demonstrated that CO2 fixation by S. ovata with H2 generated from the cathode (electroautotrophy) was more efficient than externally supplied H2 (chemoautotrophy).129 The measured electron efficiencies were 99% and 71% under electroautotrophic and chemoautotrophic conditions, respectively, resulting in an increase in the efficiency of reducing equivalent utilization and CO2 fixation into acetate from less than 80% with chemoautotrophy to over 95% under electroautotrophic conditions. This improvement was attributed to metabolomic rewiring and the regulation of the membrane electrochemical potential in S. ovata during MES, leading to enhanced proton transmembrane transport and boosted chemiosmotic ATP generation, consequently elevating CO2 fixation rates.129
Furthermore, recent research has emphasized the crucial role of H2 availability in MES for the metabolic activity and productivity of C. ljungdahlii, which determine the efficiency of the MES process.122 By controlling inoculation density, two distinct conditions were established in MES: planktonic-dominant (high cell density) and biofilm-dominant (low cell density), characterized by high and low H2 availability, respectively. The biofilm-dominant conditions resulted in significant glycine and ethanolamine production, likely due to a redox imbalance from low H2 availability. In contrast, increased H2 availability in the planktonic-dominant condition augmented redox cofactor pools, and thereby promoted faster turnover of redox-based metabolic activities and a larger driving force for CO2 reduction, resulting in a higher acetate titer of 6.06 g L−1, among the highest reported for MES from CO2 with pure culture Clostridium species.122
Enhancements in the production of reduced chemicals, such as ethanol and lactate, have been achieved through an additional supply of reducing equivalent in MES, facilitated by decreasing a cathodic potential (CP) and developing cathode materials to promote H2 evolution, thereby increasing CO2 conversion rates.119–121,124 Decreasing the CP from −0.6 V to −1.2 V (vs. Ag/AgCl) increased current density and product titers, including ethanol production in C. scatologenes.124 Similarly, higher acetate product titers were achieved at a CP of −1.0 V, with lactate and formate production, which were unattainable at CPs of −0.6 V and −0.8 V, yielding only acetate.119 Additionally, newly developed cathode materials accelerated H2 evolution on the electrode. For instance, modifying the cathode with nickel phosphide (Ni–P), a catalyst for the H2 evolution reaction, increased the H2 evolution rate and hence enhanced the C1-to-C4 conversion in MES using engineered butyrate-producing C. ljungdahlii.120 The butyrate production rate was 2.5-fold higher in MES with Ni–P than in MES without Ni–P. Syngas-mediated MES has recently been demonstrated using a novel porous 3D cathode incorporating cobalt phthalocyanine catalysts to produce syngas (CO and H2) from CO2. The newly developed cathode achieved stable CO production and acetate (5.1 g L−1) and ethanol (1.2 g L−1) production titers, outperforming similar MES studies using C. ljungdahlii.121 This syngas-mediated MES system provided a promising approach for CO2 conversion to high-value, energy-demanding chemicals through sustainable syngas generation from electrodes, providing more reducing equivalents than H2.
The MES performance, including product profile and production capacity, varies among acetogens owing to their distinct characteristics. Among them, with its high productivity and efficiency, S. ovata is the best biocatalyst for acetate production in MES.130,131S. ovata exhibits the highest conversion rates (51.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g m−2 d−1) in pure culture.131 Through the development of cathode materials and optimization of the MES reactor configuration to increase H2 availability,115,117,118 as well as bio-printing of synthetic biofilm to reduce the time for biofilm formation on the electrode,114,116 acetate production rates and titers have been significantly improved. The highest acetate titer reached 11 g L−1,115 with an outstanding acetate production rate of 1697.6 mmol m−2 d−1 from CO2 (Table 4).118 Despite numerous efforts, acetate production remains the predominant product in S. ovata, with no by-products other than acetate, likely due to its metabolic features and the absence of genetic tools for metabolic engineering to produce chemicals. Nevertheless, owing to its high MES productivity and efficiency, engineered S. ovata holds promise for expanding the product spectrum and outperforming other pure cultures in various chemical production processes. Recent advancements in the genetic tools available for S. ovata43 lay the groundwork for future metabolic engineering endeavors to diversify product outputs beyond acetate in S. ovata.
g m−2 d−1) in pure culture.131 Through the development of cathode materials and optimization of the MES reactor configuration to increase H2 availability,115,117,118 as well as bio-printing of synthetic biofilm to reduce the time for biofilm formation on the electrode,114,116 acetate production rates and titers have been significantly improved. The highest acetate titer reached 11 g L−1,115 with an outstanding acetate production rate of 1697.6 mmol m−2 d−1 from CO2 (Table 4).118 Despite numerous efforts, acetate production remains the predominant product in S. ovata, with no by-products other than acetate, likely due to its metabolic features and the absence of genetic tools for metabolic engineering to produce chemicals. Nevertheless, owing to its high MES productivity and efficiency, engineered S. ovata holds promise for expanding the product spectrum and outperforming other pure cultures in various chemical production processes. Recent advancements in the genetic tools available for S. ovata43 lay the groundwork for future metabolic engineering endeavors to diversify product outputs beyond acetate in S. ovata.
Using pure cultures, MES successfully converted CO2 into a C4 acid (butyrate) and a C2 alcohol (ethanol). With the aid of chain elongating microbes in mixed cultures, these short-chain carboxylic acids can be further transformed into longer-chain carboxylic acids and alcohols, such as caproate and hexanol (Table 4).125–128 Mixed culture MES has demonstrated the production of acetate, butyrate, isobutyrate, caproate, and their corresponding alcohols, including ethanol, butanol, isobutanol, and hexanol, from CO2 through chain elongation via reverse β-oxidation.126 Maintaining the culture pH around 5 in MES was crucial, as the mildly acidic pH promoted dominance of Clostridium species, enabling reverse β-oxidation for chain elongation and production of butyrate and caproate. Furthermore, the successful utilization of alternative substrates other than pure CO2 has been demonstrated in mixed-culture MES. Direct utilization of industrial CO2 with low impurities for acetate production via MES resulted in higher acetate production rates and titers (1.8 g L−1, 0.26 g L−1 d−1) than pure culture.125 The mixed culture outperformed the pure culture in chemical production from industrial CO2, likely because of its robustness and diverse functionality in coping with industrial gas impurities. Another study showed that waste C1 gas, as an alternative to pure CO2, enhanced the generation of C4 and C6 carboxylates in MES.127 Using a CO/CO2 mixture as the substrate in MES favored simultaneous acetogenesis, solventogenesis, and chain elongation, resulting in the production of acetate, butyrate, caproate, propanol, butanol, and hexanol. Recently, the highest acetate titer of 16.0 g L−1 was achieved in lab-scale fed-batch reactors, representing one of the highest acetate titers reported in the literature.128
4.3 Light-driven electrons
The integration of semiconductor nanomaterials with acetogens is another efficient strategy for facilitating sustainable chemical production from C1 substrates by harnessing light-driven electrons (Fig. 4B). Employing light-capturing photocatalysts such as cadmium sulfide nanoparticles or gold nanoparticles, several studies have demonstrated light-driven chemical production in acetogens M. thermoacetica,132,133S. ovata,134 and C. autoethanogenum.135 Developing efficient, highly biocompatible, low-cost photocatalysts is crucial to improving conversion efficiency and establishing high-performance artificial photosynthesis platforms. In this pursuit, recent advancements have focused on twinning Cd0.8Zn0.2S nanoparticles with long-range ordered homojunction, successfully utilized for the first time in constructing a biohybrid system in S. ovata.136 This system achieved a notable production yield of 49.33 mM of acetate at a rate of 8.22 mM per day, with a quantum efficiency of 16.82% under illumination, surpassing previous biotic–abiotic hybrid systems. However, current light-driven chemical production is primarily limited to acetate production, necessitating further enhancement of artificial photosynthetic systems using acetogens to broaden the range of synthesized products.Interestingly, a recent study revealed that visible light alone can stimulate autotrophic and heterotrophic ethanol production by C. autoethanogenum via photoexcitation-induced stress, even without the assistance of photocatalytic semiconductors.137 Illumination of C. autoethanogenum resulted in 1.8- and 2.2-fold increases in ethanol production from C1 gases and fructose, respectively. This study revealed, for the first time, the direct impact of light on the metabolism of acetogens in the absence of photocatalytic semiconductors, which was a previously unknown phenomenon. It was found that C. autoethanogenum activates all ethanol production pathways upon energization with visible light, partly to accelerate acetate turnover, thereby mitigating cellular exposure to multifactorial stress. Although further investigation is required to fully understand this phenomenon, unassisted stimulation of autotrophic ethanol production by visible light offers insights into the direct utilization of light as an electron source for sustainable chemical production via acetogens.
5 Emerging technologies to accelerate strain development
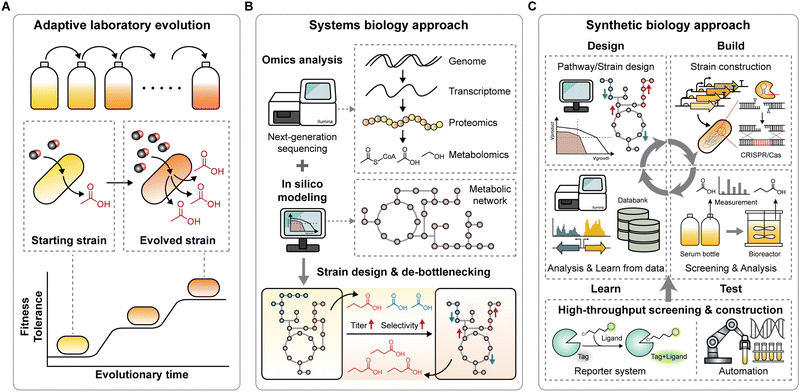
Significant advancements have been made in the acetogenic C1 conversion, resulting in an expanded product spectrum and improved energetics with increased ATP and redox availability. However, it remains challenging to understand acetogens and their limited tolerance to toxic compounds (e.g., CO and methanol) or impurities in gas mixtures remains challenging. Additionally, the oxygen-sensitive nature and slow growth of acetogens pose significant hurdles to developing efficient platform strains, impeding the transition of this system to an industrial scale. Fortunately, advanced strategies that offer practical solutions to these challenges are available.5.1 Adaptive laboratory evolution for enhancing strain tolerance and capability
Adaptive laboratory evolution (ALE) is a powerful approach for readily obtaining beneficial mutant strains through artificially applied selective pressure (Fig. 5A).138,139 Relying on the natural selection mechanism. ALE requires no genetic tools or engineering strategies to develop the strain. Among acetogens, ALE has proven successful in improving growth and production performance.Although CO and methanol are favorable electron donors because of their high energy potentials, supplying them at high concentrations can inhibit growth owing to their toxicity.41,74,140 Adopting ALE in acetogens has proven effective in obtaining exceptional strains with high tolerance to C1 substrates and production capabilities. For instance, ALE techniques adapted S. ovata to methanol, significantly increasing its acetate production rate by 6.5-fold.140 Similarly, adaptive evolution of E. limosum ATCC 8486 under syngas containing 66% CO enhanced its tolerance to high concentrations of CO, resulting in an 8-fold increase in growth rates. Remarkably, the resulting mutant strains exhibited 6.5-fold higher productivity of 2,3-butanediol than the wild-type under CO 66% syngas fed-batch fermentation.41,74
Apart from C1 substrates, ALE has been applied to improve tolerance towards impurities present in syngas, such as cyanide in C. ljungdahlii,141 benzene, toluene, and xylenes in C. autoethanogenum,142 and oxygen in S. ovata.143 These impurities inhibit cell growth and enzyme activities, lowering process productivity. However, adapted strains with improved robustness and tolerance to impurities can overcome these inhibitory effects. Enhancing the oxygen tolerance of S. ovata improved its performance in MES, in which oxygen was generated by the anode and diffused into the cathode chamber, thereby serving as an inhibitor and adapting S. ovata to tolerate up to 5% oxygen improved robustness during MES, resulting in a 1.5-fold higher acetate production compared to the wild-type.143 Moreover, yeast extract is another prohibitive supplement for economically favorable scale-up,144 which complicates experimental analysis due to its unquantified sources of vitamins, nutrients, and trace elements. However, because most acetogens rely on yeast extract for growth, efforts have been made to eliminate this requirement. Continuous bioreactor cultivation has been employed to adapt C. autoethanogenum and C. ljungdahlii, enabling their growth without yeast extract in defined media.102,145
Recently, ALE was used in C. carboxidivorans to expand its gas fermentation product spectrum from CO2/H2.146 This study demonstrated, for the first time, direct hexanol production from energy-limited CO2/H2 conditions, achieving a maximum hexanol productivity of 0.031 g L−1 h−1, the highest reported to date in the literature using C. carboxidivorans. Furthermore, traces of valerate, pentanol, heptanol, and octanol, products not typically reported to be naturally generated by pure culture acetogens, were detected. Therefore, ALE has emerged as a potent strategy for developing highly tolerant and industrially relevant acetogens and expanding the product spectrum.
5.2 Systems biology: a guide for understanding and designing improved strains
The systems biology approach serves as a guide for fundamentally understanding acetogens, unraveling associated complex regulatory systems, and designing efficient, high-performance platform strains (Fig. 5B). Recent advances in next-generation sequencing (NGS) technologies have facilitated numerous omics studies, including genomics, transcriptomics, translatomics, proteomics, and metabolomics in acetogens, which have been reviewed in several papers.147,148 These studies have significantly increased our molecular and system understanding by elucidating the regulatory mechanisms of carbon and energy metabolism in acetogens. For example, studies have revealed the role of redox control in metabolic robustness in C. autoethanogenum100 and the significance of the Rnf and Nfn complex in maintaining the redox and ATP pool at constant levels.149 Additionally, translational regulation has been revealed in A. woodii,150E. limosum,151 and C. ljungdahlii,152 confirming its control over resource allocation in energy-deprived environments, given the energy-expensive nature of translation.Omics analyses have also been applied to understand the mechanisms of MES and artificial photosynthesis systems.122,129,134–136 These studies investigated the reasons behind the superior CO2 fixation performance of MES compared to chemoautotrophic conditions in S. ovata,129 and explored the direct effects of light on metabolism in C. autoethanogenum in the absence of a photocatalyst.137 These findings provide insights for developing high-performance MES and artificial photosynthesis systems to increase the CO2-fixing efficiency.
In conjunction with the CRISPR–Cas or transposon systems, NGS has enabled functional genomic studies of acetogens. Transposon insertion sequencing in C. autoethanogenum153 and genome-wide CRISPRi screening in E. limosum154 have revealed gene essentiality and genotype–phenotype relationships under autotrophic and heterotrophic growth conditions. These datasets further expand our knowledge of acetogens and guide strain engineering.
Enabled by the growing pool of omics data, prediction models, such as genome-scale metabolic and kinetic ensemble models, have been developed for several acetogens and have provided a deeper understanding of metabolism and regulation.147 Furthermore, these models help estimate the production yields of various metabolites and pathway feasibility, aiding in the design of efficient strains and pathways. Model-guided rational strain or pathway design streamlines the selection of target metabolic pathways and offers insights into their effects on the metabolic network (Fig. 5B).148
Systematic analysis of engineered strains, based on kinetic ensemble modeling and omics data (e.g., proteomics, metabolomics), can identify potential bottlenecks in production pathways. For example, systemic analysis of engineered C. ljungdahlii identified bottlenecks in isobutanol production pathways as an NADPH limitation and thereby guided further engineering strategies such as replacing the cofactor dependency on NADPH by NADH to improve the desired chemical production.76 For efficient acetone production in C. autoethanogenum, fine-tuning enzyme levels relieved the identified pathway bottlenecks, optimizing the flux to acetone production and enhancing its productivity.70 Alternatively, thermodynamic and kinetic modeling guided pathway optimization for isopropanol production, resulting in significant improvements in the engineered strain.73
5.3 Synthetic biology and high-throughput screening for accelerating strain development
Considerable advances in synthetic biology tools have paved the way for accelerating the development of next-generation strains.155,156 CRISPR–Cas genome engineering tools represent a breakthrough technology, offering new engineering opportunities for previously difficult-to-engineer acetogens.7 CRISPR–Cas systems have been developed and applied in several acetogens, including C. autoethanogenum, C. ljungdahlii, and E. limosum, for gene disruption and transcriptional repression to efficiently manipulate carbon fluxes.39,156–158 Specifically, genes encoding phosphotransacetylase (pta), aldehyde:Fd oxidoreductase (aor1 and aor2), or bifunctional alcohol/aldehyde dehydrogenases (adhE1 and adhE2) have been targeted in C. ljungdahlii.36,159–161 This genetic manipulation redirected carbon flux towards desired products, successfully improving the production of acetate,159 ethanol,160 butyrate,161 and 3-HB.36 While these studies focused on targeting genes directly related to biochemical synthesis pathways, a recent genome-wide CRISPRi screening in E. limosum identified three novel genes (ELIM_c2976 encoding putative Fe–S binding protein, ELIM_c1759 encoding Rho protein, and ELIM_c1868 encoding putative transcription regulator) whose repression improved autotrophic growth.154 The simultaneous repression of these three genes resulted in a 1.5-fold increase in acetoin production in E. limosum.Development of efficient production strains requires exploring a large design space, which involves screening hundreds to thousands of different pathways and strains. However, the slow growth and oxygen-sensitive nature of acetogens hinder this process, making screening and strain construction time-consuming and labor-intensive. This challenge can be overcome using synthetic biology tools with high-throughput workflows for strain construction and screening (Fig. 5C).
A suitable reporter system for acetogens is an initial requirement for high-throughput screening. Commonly used systems such as GFP or mRFP require oxygen for chromophore maturation, limiting their application to acetogens growing under strictly anaerobic conditions. As an alternative, oxygen-independent reporter systems such as HaloTag, SNAP-tag, and fluorescence-activating and absorption-shifting tag (FAST) have been developed and applied to the acetogens C. ljungdahlii,162–164E. limosum,71,165A. woodii,69 and T. kivui166 to study co-culture dynamics, screen genetic bioparts, and track heterologous protein production. The deployment of these reporter systems enables high-throughput screening of acetogens.
Cell-free prototyping, a cutting-edge synthetic biology tool, allows in vitro pathway prototyping to inform in vivo design to expedite strain engineering. This technology accelerates the process by testing hundreds to thousands of enzyme variants and combinations in weeks rather than months.7 Cell-free prototyping based on the iPROBE platform has been utilized in developing efficient strains for producing 3-HB and butanol, evaluating 54 different pathway combinations for 3-HB production and optimizing 205 pathways for butanol production.77 With a strong correlation between cell-free and in vivo cellular performance, this approach significantly improved production capabilities, showing a 20-fold increase in 3-HB titer, reaching up to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g L−1 at rates of over 1.5
g L−1 at rates of over 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g L−1 h−1 in C. autoethanogenum. This result demonstrates its potential for industrial-scale applications.
g L−1 h−1 in C. autoethanogenum. This result demonstrates its potential for industrial-scale applications.
The various tools mentioned above can be combined to construct highly efficient platform strains capable of producing high-energy compounds on an industrial scale. Recent achievements in this field have demonstrated the feasibility of applying synthetic biology tools to genome-scale optimization of acetogens for sustainable and scalable biomanufacturing.70 Leveraging genome mining, genome engineering tools, omics analysis, kinetic modeling, cell-free prototyping, and high-throughput screening of nearly 300 strains, highly engineered C. autoethanogenum capable of carbon-negative production of isopropanol and acetone from industrial waste gases was developed. This successful scale-up to 120 L demonstrated a production rate of ∼3 g L−1 h−1 and ∼90% selectivity.
The design–build–test–lean (DBTL) cycle, a framework in synthetic biology for developing strains with desired functionalities, can be automated and streamlined in a biofoundry to accelerate strain development at high throughput, increasing speed, efficiency, cost-effectiveness, and consistency.167 Biofoundries utilize advanced automation, synthetic biology, and computational tools to accelerate design of new strains, and their construction, and testing. Despite the challenges posed by the requirement for anaerobic conditions and gaseous substrates, LanzaTech's world-first anaerobic biofoundry for acetogens is rapidly showing the possibilities by generating and screening thousands of strains per cycle.11 With synthetic biology technologies and automated workflows in biofoundries, engineering acetogens for industrial-scale production of high-energy compounds is now feasible. Implementing these technologies would significantly accelerate the development of sustainable and green, industrially relevant acetogenic C1 conversion platform strains.
6 Economic sustainability of acetogenic C1 conversion
To industrialize acetogenic C1 conversion and achieve a circular carbon economy, assessing its economic sustainability is crucial, which can be evaluated by the techno-economic assessment (TEA) and life-cycle analysis (LCA).168 Recent TEA and LCA studies on acetogenic C1 conversion have demonstrated its economic and environmental viability, which depends on serval factors such as feedstock costs and final products.70,82,168–170Recent TEA of CO2 utilization via biological conversion has revealed that PHB production is cost-competitive, projecting a minimum selling price (MSP) of $1.36 per kg, which falls below its market price of $2.40–3.30 per kg.171 Another assessment covering 11 products synthesized via various C1 conversion routes suggested focusing research efforts on products such as butyrate and butanol, whose production costs are near or below current market prices.168 This strategic focus on high-value market products is crucial for ensuring economic competitiveness against current fossil fuel-based production.
Although ethanol and acetone have lower market prices compared to the aforementioned C4 products (PHB, butyrate, and butanol), their production processes can still be economically viable with strain improvements and process optimization aimed at increasing production efficiency and thereby reducing costs.170 TEA and LCA studies can identify major cost contributors and research needs, guiding strain design and process optimization to enhance product yield, productivity, and titer. For instance, syngas fermentation with the best-engineered strain and optimized process achieved cost-competitive production of acetone and ethanol.170 The cash cost of production (CCOP) was reduced by over 60% compared to the start of the project, enabling biofuel production at or below DOE's target of $3 per gallon gasoline equivalent. Moreover, LCA indicated significant reductions in GHG emissions in the process, achieving reductions of over 180% for acetone and 90% for ethanol, compared to conventional processes.
Feedstock cost is a primary factor affecting the economic viability of the process.172 A comparative analysis of the economic feasibility of acetone production from various sources of syngas using M. thermoacetica identified syngas derived from a basic oxygen furnace (BOF) to be more economically feasible than syngas from natural gas and corn stover, which have higher feedstock costs.82 Another study reported that using reformed biogas is more favorable for sustainable and economically viable chemical production compared to utilizing steel mill off-gas.168 Given that steel production is a major source of GHG emissions,173 using steel mill off-gas results in higher emissions compared to reformed biogas, albeit achieving 60% lower emissions compared to conventional gasoline.174 Nevertheless, via strain and process optimization, acetone and isopropanol production from such industrial waste gases can be economically viable. This was demonstrated by achieving a negative carbon footprint of −1.78 kgCO2e per kg for the produced acetone and −1.17 kgCO2e per kg for the produced isopropanol, which is lower than traditional petrochemical production that emits substantial GHGs (2.55 kgCO2e per kg for acetone and 1.85 kgCO2e per kg for isopropanol).70
With its promises of economic sustainability, several companies have already established pilot and commercial plants for acetogenic C1 conversion (Table 5). Scaling up and commercializing gas fermentation for ethanol production have been pursued by several companies, including INEOS Bio (acquired by Jupeng Bio), Coskata (acquired by Synata Bio), Genomatica, and LanzaTech (Table 5).175,176 However, INEOS Bio and Coskata are no longer operational due to financial and operational challenges. To enhance product yields and improve process economics, White Dog Labs developed mixotrophic fermentation utilizing both syngas and sugars for the production of acetone and isopropanol.175,177
| Company | C1 conversion technology | Final product(s) | Ref. |
|---|---|---|---|
| LanzaTech | Syngas or industrial waste gas fermentation | Ethanol, 2,3-butanediol, acetone, isopropanol, and others | 178 |
| Sekisui Bio-Refinery | Syngas fermentation | Ethanol | 179 |
| ArcelorMittal | Industrial blast furnace gas fermentation | Ethanol | 180 |
| Jupeng Bio | Syngas fermentation | Ethanol | 181 |
| Genomatica | Syngas fermentation | Ethanol | 182 |
| White Dog Labs | Mixotrophic fermentation (syngas + sugars) | Acetone, isopropanol | 177 |
| Evonik, Siemens | CO2 electrolysis (Siemens) + syngas fermentation (Evonik) | Butanol, hexanol, 2-hydroxyisobutyric acid | 183 |
| VITO | Microbial electrosynthesis (CO2, electricity) | Ethanol, ethylene | 184 |
LanzaTech is a leading company in acetogenic C1 conversion. Their various pilot projects and commercial installations have demonstrated positive results (e.g., ethanol production from C1 gases) in terms of both economic and environmental benefits, thereby proving the economic sustainability of their acetogenic C1 conversion technologies.11,185 By partnering with companies across the global supply chain, including Sekisui Bio-Refinery, ArcelorMittal, Zara, and BASF, LanzaTech is paving the way for a circular carbon economy and advancing its carbon recycling technology.186
Although MES holds promise with the potential use of renewable electricity, its commercialization is hindered by technological, economic, and scalability challenges. These include the high cost and low scalability of CO2 electrolyser, as well as the low electron consumption rate of microbial biofilms.168,171,187 One suggested approach to make the MES process economically viable is to decouple the surface-dependent abiotic process for electron delivery from the volume-dependent biotic carbon fixation process.168 Additionally, research efforts to decrease electrolysis costs are also necessary. Currently, a few companies have explored MES for industrializing CO2 conversion to chemical production using acetogens (Table 5). For example, VITO has conducted research on MES for producing ethanol and ethylene.184 Joint research led by Evonik and Siemens utilizes a CO2 electrolyser and syngas fermentation of a microbial consortium of acetogens and chain-elongating bacteria to convert CO2 to butanol and hexanol.183
Methanol is another promising C1 feedstock. The cost for butanol production using methanol was estimated at $930 per t, close to the current market price. This estimate considers non-renewable methanol, which has a lower market price ($300 per t) than renewable methanol ($560 per t).168,188 Despite its economic feasibility, methanol is not currently used as a feedstock for industrial-scale acetogenic C1 conversion. Given that methylotrophic growth of acetogens can potentially achieve higher product yields, growth rates, and energy efficiency, using methanol as a feedstock is expected to become economically viable with strain improvements and process optimization.
7 Conclusions and future perspectives
Harnessing acetogenic bacteria for one-carbon valorization presents a promising route towards sustainable and green chemical production aligned with the circular economy concept. Recent advancements in genetic tools developed for acetogens have enabled the production of over 50 different chemicals from engineered acetogens.11,12 Several of these, including ethanol, 2,3-butanediol, acetone, and isopropanol, have already reached industrially relevant performance levels, with production rates in the g L−1 h−1 range and titers at the tens of g L−1 level.7,70,170 As a frontrunner in global carbon recycling, LanzaTech is actively commercializing its technology for acetogenic C1 conversion, providing actual proof of its potential value to the carbon-producing industry.81 However, they are currently limited to native products of acetogens or non-native short-chain compounds. The production of energy-demanding long-chain or non-native chemicals from C1 substrates remains a challenge, with most research focused mainly on low technology readiness levels (TRL) 2 to 3, corresponding to basic and applied research.81 The major obstacle in this regard is the inherent energetic limitations of acetogens.Overcoming the energetic limitations of acetogens is crucial for achieving high product titers, yields, and productivities sufficient for advancing to higher TRLs (TRL 7–9 for commercial development). Several studies have focused on improving energetics by increasing ATP and redox availability. These studies employed effective strategies such as enhancing ATP availability through alternative electron donors, boosting chemiosmotic or SLP-coupled ATP synthesis, and leveraging acetate as a precursor. Furthermore, the cost and sustainability of the overall process are critical considerations for developing a green chemical production platform based on acetogenic C1 conversion. The availability and cost of C1 substrates and the bioconversion process efficiency significantly influence economic viability. In this regard, efforts to utilize low-cost and sustainable redox suppliers such as electricity and light offer promising paths to enhance the energetics and sustainability of the acetogenic C1 conversion process. In particular, the electricity use of MES has advanced considerably, allowing energy-demanding chemical production from CO2. Despite its potential, its TRL currently corresponds to the lab scale.189 Efforts are underway to improve its performance and bring MES technology towards commercialization.187,190,191
The deployment of advanced technologies, including adaptive evolution, systems biology, and synthetic biology, with high-throughput workflows facilitates the design and construction of acetogenic strains with improved productivity, robustness, and efficiency. In particular, integrating biofoundries will accelerate the development of next-generation acetogenic platform strains, revealing the full potential of acetogenic C1 conversion for sustainable and green chemical synthesis.
In conclusion, acetogenic C1 conversion holds great promise for transforming the fossil-based chemical industry towards more sustainable and greener chemical production. By overcoming existing challenges and embracing emerging technologies, acetogenic C1 conversion can play a pivotal role in achieving a bio-based circular economy, reducing the reliance on fossil fuels, and creating a greener society.
Author contributions
Jiyun Bae: writing – original draft, writing – review & editing, visualization. Chanho Park: writing – original draft. Hyunwoo Jung: writing – original draft. Sangrak Jin: writing – review & editing. Byung-Kwan Cho: conceptualization, supervision, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the C1 Gas Refinery Program (2018M3D3A1A01055733 to B.-K.C.) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT. This research was also supported by a grant from the National Institute of Biological Resources (NIBR) funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202327201 and NIBR202423201).References
- C. Intergovernmental Panel on Climate, Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 2023.
- C. Schilling and S. Weiss, Nat. Biotechnol., 2021, 60, 9–11 CAS.
- J. A. Martens, A. Bogaerts, N. De Kimpe, P. A. Jacobs, G. B. Marin, K. Rabaey, M. Saeys and S. Verhelst, ChemSusChem, 2017, 10, 1039–1055 CrossRef CAS PubMed.
- G. Garcia-Garcia, M. C. Fernandez, K. Armstrong, S. Woolass and P. Styring, ChemSusChem, 2021, 14, 995–1015 CrossRef CAS PubMed.
- W. Gao, S. Liang, R. Wang, Q. Jiang, Y. Zhang, Q. Zheng, B. Xie, C. Y. Toe, X. Zhu and J. Wang, Chem. Soc. Rev., 2020, 49, 8584–8686 RSC.
- P. Lozano and E. García-Verdugo, Green Chem., 2023, 25, 7041–7057 RSC.
- N. Fackler, B. D. Heijstra, B. J. Rasor, H. Brown, J. Martin, Z. Ni, K. M. Shebek, R. R. Rosin, S. D. Simpson, K. E. Tyo, R. J. Giannone, R. L. Hettich, T. J. Tschaplinski, C. Leang, S. D. Brown, M. C. Jewett and M. Kopke, Annu. Rev. Chem. Biomol. Eng., 2021, 12, 439–470 CrossRef PubMed.
- J. Bae, Y. Song, H. Lee, J. Shin, S. Jin, S. Kang and B.-K. Cho, Chem. Eng. J., 2022, 428, 131325 CrossRef CAS.
- W. Jiang, D. Hernández Villamor, H. Peng, J. Chen, L. Liu, V. Haritos and R. Ledesma-Amaro, Nat. Chem. Biol., 2021, 17, 845–855 CrossRef CAS PubMed.
- J. Bae, S. Jin, S. Kang, B.-K. Cho and M.-K. Oh, Curr. Opin. Biotechnol, 2022, 78, 102836 CrossRef CAS PubMed.
- M. Kopke and S. D. Simpson, Curr. Opin. Biotechnol, 2020, 65, 180–189 CrossRef CAS PubMed.
- F. Liew, M. E. Martin, R. C. Tappel, B. D. Heijstra, C. Mihalcea and M. Kopke, Front. Microbiol., 2016, 7, 694 Search PubMed.
- K. Schuchmann and V. Müller, Nat. Rev. Microbiol., 2014, 12, 809–821 CrossRef CAS PubMed.
- A. Katsyv and V. Muller, Front. Bioeng. Biotechnol., 2020, 8, 621166 CrossRef PubMed.
- A. G. Fast and E. T. Papoutsakis, Curr. Opin. Chem. Eng., 2012, 1, 380–395 CrossRef.
- H. L. Drake, A. S. Gossner and S. L. Daniel, Ann. N. Y. Acad. Sci., 2008, 1125, 100–128 CrossRef CAS PubMed.
- R. K. Thauer, K. Jungermann and K. Decker, Bacteriol. Rev., 1977, 41, 100–180 CrossRef CAS PubMed.
- K. Schuchmann and V. Muller, J. Biol. Chem., 2012, 287, 31165–31171 CrossRef CAS PubMed.
- S. Wang, H. Huang, J. Kahnt, A. P. Mueller, M. Kopke and R. K. Thauer, J. Bacteriol., 2013, 195, 4373–4386 CrossRef CAS PubMed.
- F. Kremp, J. Roth and V. Muller, Sci. Rep., 2020, 10, 14872 CrossRef CAS PubMed.
- S. W. Ragsdale and E. Pierce, Biochim. Biophys. Acta, 2008, 1784, 1873–1898 CrossRef CAS PubMed.
- V. Hess, K. Schuchmann and V. Muller, J. Biol. Chem., 2013, 288, 31496–31502 CrossRef CAS PubMed.
- P. L. Tremblay, T. Zhang, S. A. Dar, C. Leang and D. R. Lovley, mBio, 2012, 4, e00406-00412 Search PubMed.
- K. Schuchmann and V. Muller, Appl. Environ. Microbiol., 2016, 82, 4056–4069 CrossRef CAS PubMed.
- H. Lee, J. Bae, S. Jin, S. Kang and B.-K. Cho, Front. Microbiol., 2022, 13, 865168 CrossRef PubMed.
- S. Hoffmeister, M. Gerdom, F. R. Bengelsdorf, S. Linder, S. Fluchter, H. Ozturk, W. Blumke, A. May, R. J. Fischer, H. Bahl and P. Durre, Metab. Eng., 2016, 36, 37–47 CrossRef CAS PubMed.
- F. Kremp, A. Poehlein, R. Daniel and V. Muller, Environ. Microbiol., 2018, 20, 4369–4384 CrossRef CAS PubMed.
- J. Moon, J. Donig, S. Kramer, A. Poehlein, R. Daniel and V. Muller, Environ. Microbiol., 2021, 23, 4214–4227 CrossRef CAS PubMed.
- X. Wang, J. Qin, C. Ma, J. Wang, X. Wang, S. Xu, J. Feng, K. Chen and P. Ouyang, ACS Sustainable Chem. Eng., 2021, 9, 12079–12090 CrossRef.
- J. R. Humphreys, S. D. Hebdon, H. Rohrer, L. Magnusson, C. Urban, Y.-P. Chen and J. Lo, Appl. Environ. Microbiol., 2022, 88, e02393-02321 CrossRef PubMed.
- F. Liew, A. M. Henstra, M. Kopke, K. Winzer, S. D. Simpson and N. P. Minton, Metab. Eng., 2017, 40, 104–114 CrossRef CAS PubMed.
- M. Kopke, C. Mihalcea, F. Liew, J. H. Tizard, M. S. Ali, J. J. Conolly, B. Al-Sinawi and S. D. Simpson, Appl. Environ. Microbiol., 2011, 77, 5467–5475 CrossRef CAS PubMed.
- C. Cheng, W. Li, M. Lin and S. T. Yang, Bioresour. Technol., 2019, 284, 415–423 CrossRef CAS PubMed.
- A. Fernandez-Naveira, M. C. Veiga and C. Kennes, Bioresour. Technol., 2019, 280, 387–395 CrossRef CAS PubMed.
- J. R. Phillips, H. K. Atiyeh, R. S. Tanner, J. R. Torres, J. Saxena, M. R. Wilkins and R. L. Huhnke, Bioresour. Technol., 2015, 190, 114–121 CrossRef CAS PubMed.
- B. M. Woolston, D. F. Emerson, D. H. Currie and G. Stephanopoulos, Metab. Eng., 2018, 48, 243–253 CrossRef CAS PubMed.
- H. J. Kwon, J. Lee, S. J. Kwon and H. S. Lee, Microb. Cell Fact., 2024, 23, 6 CrossRef CAS PubMed.
- J. Lee, J. W. Lee, C. G. Chae, S. J. Kwon, Y. J. Kim, J. H. Lee and H. S. Lee, Biotechnol. Biofuels, 2019, 12, 228 CrossRef PubMed.
- J. Shin, S. Kang, Y. Song, S. Jin, J. S. Lee, J. K. Lee, D. R. Kim, S. C. Kim, S. Cho and B. K. Cho, ACS Synth. Biol., 2019, 8, 2059–2068 CrossRef CAS PubMed.
- J. C. Wood, E. Marcellin, M. R. Plan and B. Virdis, Microb. Biotechnol., 2022, 15, 1542–1549 CrossRef CAS PubMed.
- S. Kang, Y. Song, S. Jin, J. Shin, J. Bae, D. R. Kim, J. K. Lee, S. C. Kim, S. Cho and B. K. Cho, Front. Microbiol., 2020, 11, 402 CrossRef PubMed.
- Y. Song, J. Shin, Y. Jeong, S. Jin, J.-K. Lee, D. R. Kim, S. C. Kim, S. Cho and B.-K. Cho, Sci. Rep., 2017, 7, 13694 CrossRef PubMed.
- P.-L. Tremblay and T. Zhang, Appl. Environ. Microbiol., 2023, 90, e01757-01723 Search PubMed.
- B. Möller, R. Oßmer, B. H. Howard, G. Gottschalk and H. Hippe, Arch. Microbiol., 1984, 139, 388–396 CrossRef.
- A. Poehlein, G. Gottschalk and R. Daniel, Genome Announc., 2013, 1 DOI:10.1128/genomea.00734-13.
- A. Kita, Y. Iwasaki, S. Sakai, S. Okuto, K. Takaoka, T. Suzuki, S. Yano, S. Sawayama, T. Tajima, J. Kato, N. Nishio, K. Murakami and Y. Nakashimada, J. Biosci. Bioeng., 2013, 115, 347–352 CrossRef CAS PubMed.
- L. Daniel Steven and L. Drake Harold, Appl. Environ. Microbiol., 1993, 59, 3062–3069 CrossRef PubMed.
- M. Basen, I. Geiger, L. Henke and V. Muller, Appl. Environ. Microbiol., 2018, 84, e02210-17 CrossRef PubMed.
- M. C. Weghoff and V. Muller, Appl. Environ. Microbiol., 2016, 82, 2312–2319 CrossRef CAS PubMed.
- C. M. Spirito, H. Richter, K. Rabaey, A. J. Stams and L. T. Angenent, Curr. Opin. Biotechnol, 2014, 27, 115–122 CrossRef CAS PubMed.
- B. G. Harvey and H. A. Meylemans, Green Chem., 2014, 16, 770–776 RSC.
- C. Petrognani, N. Boon and R. Ganigué, Green Chem., 2020, 22, 8389–8402 RSC.
- Q. Marien, A. Regueira and R. Ganigue, Microb. Biotechnol., 2024, 17, e14321 CrossRef CAS PubMed.
- C. A. Cotton, N. J. Claassens, S. Benito-Vaquerizo and A. Bar-Even, Curr. Opin. Biotechnol, 2020, 62, 168–180 CrossRef CAS PubMed.
- J. Moon, A. Schubert, A. Poehlein, R. Daniel and V. Muller, Environ. Microbiol. Rep., 2023, 15, 339–351 CrossRef CAS PubMed.
- J. Moon, L. M. Waschinger and V. Muller, Appl. Microbiol. Biotechnol., 2023, 107, 5491–5502 CrossRef CAS PubMed.
- L. Tarraran, F. Demichelis, V. Agostino, N. S. Vasile, J. Baker, J. Millard, N. P. Minton, C. F. Pirri, D. Fino and G. Saracco, Sustainable Chem. Pharm., 2024, 37, 101440 CrossRef CAS.
- K. Arslan, T. Schoch, F. Hofele, S. Herrschaft, C. Oberlies, F. Bengelsdorf, M. C. Veiga, P. Durre and C. Kennes, Biotechnol. J., 2022, 17, e2100515 CrossRef PubMed.
- F. Hofele, T. Schoch, C. Oberlies and P. Durre, Bioengineering, 2023, 10, 1381 CrossRef PubMed.
- N. P. Chowdhury, D. Litty and V. Muller, Int. Microbiol., 2022, 25, 551–560 CrossRef CAS PubMed.
- S. Weitz, M. Hermann, S. Linder, F. R. Bengelsdorf, R. Takors and P. Durre, Front. Bioeng. Biotechnol., 2021, 9, 657253 CrossRef PubMed.
- F. Höfele and P. Dürre, Fermentation, 2023, 9, 799 CrossRef.
- S. Wirth and P. Durre, Metab. Eng., 2021, 66, 296–307 CrossRef CAS PubMed.
- S. Kobayashi, J. Kato, K. Wada, K. Takemura, S. Kato, T. Fujii, Y. Iwasaki, Y. Aoi, T. Morita, A. Matsushika, K. Murakami and Y. Nakashimada, Front. Microbiol., 2022, 13, 897066 CrossRef PubMed.
- K. Takemura, J. Kato, S. Kato, T. Fujii, K. Wada, Y. Iwasaki, Y. Aoi, A. Matsushika, T. Morita, K. Murakami and Y. Nakashimada, J. Biosci. Bioeng., 2023, 136, 13–19 CrossRef CAS PubMed.
- J. Kato, K. Takemura, S. Kato, T. Fujii, K. Wada, Y. Iwasaki, Y. Aoi, A. Matsushika, K. Murakami and Y. Nakashimada, AMB Express, 2021, 11, 59 CrossRef CAS PubMed.
- J. Kato, T. Matsuo, K. Takemura, S. Kato, T. Fujii, K. Wada, Y. Nakamichi, M. Watanabe, Y. Aoi, T. Morita, K. Murakami and Y. Nakashimada, Biotechnol. Biofuels Bioprod., 2024, 17, 13 CrossRef CAS PubMed.
- B. Bourgade, C. M. Humphreys, J. Millard, N. P. Minton and M. A. Islam, ACS Synth. Biol., 2022, 11, 1790–1800 CrossRef CAS PubMed.
- A. Mook, M. H. Beck, J. P. Baker, N. P. Minton, P. Durre and F. R. Bengelsdorf, Appl. Microbiol. Biotechnol., 2022, 106, 1447–1458 CrossRef CAS PubMed.
- F. E. Liew, R. Nogle, T. Abdalla, B. J. Rasor, C. Canter, R. O. Jensen, L. Wang, J. Strutz, P. Chirania, S. De Tissera, A. P. Mueller, Z. Ruan, A. Gao, L. Tran, N. L. Engle, J. C. Bromley, J. Daniell, R. Conrado, T. J. Tschaplinski, R. J. Giannone, R. L. Hettich, A. S. Karim, S. D. Simpson, S. D. Brown, C. Leang, M. C. Jewett and M. Kopke, Nat. Biotechnol., 2022, 40, 335–344 CrossRef CAS PubMed.
- M. Flaiz, G. Ludwig, F. R. Bengelsdorf and P. Durre, Biotechnol. Biofuels, 2021, 14, 117 CrossRef CAS PubMed.
- D. Jia, M. He, Y. Tian, S. Shen, X. Zhu, Y. Wang, Y. Zhuang, W. Jiang and Y. Gu, ACS Synth. Biol., 2021, 10, 2628–2638 CrossRef CAS PubMed.
- J. Lo, C. Wu, J. R. Humphreys, B. Yang, Z. Jiang, X. Wang, P. Maness, N. Tsesmetzis and W. Xiong, mSystems, 2023, 8, e01274-01222 Search PubMed.
- S. Jin, S. Kang, J. Bae, H. Lee and B.-K. Cho, Chem. Eng. J., 2022, 449, 137678 CrossRef CAS.
- H. Huang, C. Chai, S. Yang, W. Jiang and Y. Gu, Metab. Eng., 2019, 52, 293–302 CrossRef CAS PubMed.
- M. Hermann, A. Teleki, S. Weitz, A. Niess, A. Freund, F. R. Bengelsdorf, P. Durre and R. Takors, Front. Bioeng. Biotechnol., 2021, 9, 647853 CrossRef PubMed.
- A. S. Karim, Q. M. Dudley, A. Juminaga, Y. Yuan, S. A. Crowe, J. T. Heggestad, S. Garg, T. Abdalla, W. S. Grubbe, B. J. Rasor, D. N. Coar, M. Torculas, M. Krein, F. E. Liew, A. Quattlebaum, R. O. Jensen, J. A. Stuart, S. D. Simpson, M. Kopke and M. C. Jewett, Nat. Chem. Biol., 2020, 16, 912–919 CrossRef CAS PubMed.
- R. de Souza Pinto Lemgruber, K. Valgepea, R. Tappel, J. B. Behrendorff, R. W. Palfreyman, M. Plan, M. P. Hodson, S. D. Simpson, L. K. Nielsen, M. Kopke and E. Marcellin, Metab. Eng., 2019, 53, 14–23 CrossRef PubMed.
- J. C. Dykstra, J. van Oort, A. T. Yazdi, E. Vossen, C. Patinios, J. van der Oost, D. Z. Sousa and S. W. M. Kengen, Microb. Cell Fact., 2022, 21, 243 CrossRef PubMed.
- I. Lauer, G. Philipps and S. Jennewein, Microb. Cell Fact., 2022, 21, 85 CrossRef CAS PubMed.
- M. Pacheco, P. Moura and C. Silva, Energies, 2023, 16, 3241 CrossRef CAS.
- S. Redl, S. Sukumara, T. Ploeger, L. Wu, T. Olshoj Jensen, A. T. Nielsen and H. Noorman, Biotechnol. Biofuels, 2017, 10, 150 CrossRef PubMed.
- F. Kremp and V. Muller, FEMS Microbiol. Rev., 2021, 45, fuaa040 CrossRef CAS PubMed.
- D. Litty and V. Muller, Microb. Biotechnol., 2021, 14, 2686–2692 CrossRef CAS PubMed.
- M. T. Allaart, M. Diender, D. Z. Sousa and R. Kleerebezem, Microb. Biotechnol., 2023, 16, 697–705 CrossRef CAS PubMed.
- K. Kim, D. Choe, D. H. Lee and B. K. Cho, Int. J. Mol. Sci., 2020, 21, 990 CrossRef CAS PubMed.
- A. Wiechmann, D. Trifunović, S. Klein and V. Müller, Biotechnol. Biofuels, 2020, 13, 208 CrossRef CAS PubMed.
- F. P. Rosenbaum, A. Poehlein, R. Daniel and V. Muller, Environ. Microbiol., 2022, 24, 2000–2012 CrossRef CAS PubMed.
- D. F. Emerson, B. M. Woolston, N. Liu, M. Donnelly, D. H. Currie and G. Stephanopoulos, Biotechnol. Bioeng., 2019, 116, 294–306 CrossRef CAS PubMed.
- C. M. Klask, N. Kliem-Kuster, B. Molitor and L. T. Angenent, Front. Microbiol., 2020, 11, 724 CrossRef PubMed.
- A. Tschech and N. Pfennig, Arch. Microbiol., 1984, 137, 163–167 CrossRef CAS.
- S. Dilling, F. Imkamp, S. Schmidt and V. Muller, Appl. Environ. Microbiol., 2007, 73, 3630–3636 CrossRef CAS PubMed.
- V. Hess, J. M. Gonzalez, A. Parthasarathy, W. Buckel and V. Muller, Appl. Environ. Microbiol., 2013, 79, 1942–1947 CrossRef CAS PubMed.
- J. M. Fröstl, C. Seifritz and H. L. Drake, J. Bacteriol., 1996, 178, 4597–4603 CrossRef PubMed.
- K. Valgepea, K. Q. Loi, J. B. Behrendorff, R. S. P. Lemgruber, M. Plan, M. P. Hodson, M. Kopke, L. K. Nielsen and E. Marcellin, Metab. Eng., 2017, 41, 202–211 CrossRef CAS PubMed.
- X. Li, R. Han, T. Bao, T. Osire, X. Zhang, M. Xu, T. Yang and Z. Rao, Biotechnol. Biofuels, 2021, 14, 204 CrossRef CAS PubMed.
- M. H. Beck, M. Flaiz, F. R. Bengelsdorf and P. Durre, Appl. Microbiol. Biotechnol., 2020, 104, 687–699 CrossRef CAS PubMed.
- C. E. Isom, M. A. Nanny and R. S. Tanner, J. Ind. Microbiol. Biotechnol., 2015, 42, 29–38 CrossRef CAS PubMed.
- Z.-Y. Liu, D.-C. Jia, K.-D. Zhang, H.-F. Zhu, Q. Zhang, W.-H. Jiang, Y. Gu and F.-L. Li, Appl. Environ. Microbiol., 2020, 86, e00730-00720 Search PubMed.
- V. Mahamkali, K. Valgepea, R. de Souza Pinto Lemgruber, M. Plan, R. Tappel, M. Kopke, S. D. Simpson, L. K. Nielsen and E. Marcellin, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 13168–13175 CrossRef CAS PubMed.
- L. S. Nissen and M. Basen, J. Biotechnol., 2019, 306, 105–117 CrossRef CAS PubMed.
- S. Schulz, B. Molitor and L. T. Angenent, Bioresour. Technol., 2023, 369, 128387 CrossRef CAS PubMed.
- H. Xu, C. Liang, X. Chen, J. Xu, Q. Yu, Y. Zhang and Z. Yuan, Biochem. Eng. J., 2020, 155, 107470 CrossRef CAS.
- M. P. Elisiario, W. Van Hecke, H. De Wever, H. Noorman and A. J. J. Straathof, Appl. Microbiol. Biotechnol., 2023, 107, 5329–5340 CrossRef CAS PubMed.
- S. J. Kwon, J. Lee and H. S. Lee, Process Biochem., 2022, 113, 47–54 CrossRef CAS.
- S. J. Kwon, J. Lee and H. S. Lee, Front. Microbiol., 2022, 13, 982442 CrossRef PubMed.
- H. Richter, B. Molitor, H. Wei, W. Chen, L. Aristilde and L. Angenent, Energy Environ. Sci., 2016, 9, 2392–2399 RSC.
- K. Valgepea, R. de Souza Pinto Lemgruber, K. Meaghan, R. W. Palfreyman, T. Abdalla, B. D. Heijstra, J. B. Behrendorff, R. Tappel, M. Kopke, S. D. Simpson, L. K. Nielsen and E. Marcellin, Cell Syst., 2017, 4, 505–515 e505 CrossRef CAS PubMed.
- C. F. Dos Santos Vieira, F. Maugeri Filho, R. Maciel Filho and A. Pinto Mariano, Bioresour. Technol., 2019, 287, 121425 CrossRef CAS PubMed.
- K. Valgepea, R. de Souza Pinto Lemgruber, T. Abdalla, S. Binos, N. Takemori, A. Takemori, Y. Tanaka, R. Tappel, M. Kopke, S. D. Simpson, L. K. Nielsen and E. Marcellin, Biotechnol. Biofuels, 2018, 11, 55 CrossRef PubMed.
- N. R. Boyle and J. A. Morgan, Metab. Eng., 2011, 13, 150–158 CrossRef CAS PubMed.
- Z. Liu, K. Wang, Y. Chen, T. Tan and J. Nielsen, Nat. Catal., 2020, 3, 274–288 CrossRef CAS.
- J. Li, Y. Tian, Y. Zhou, Y. Zong, N. Yang, M. Zhang, Z. Guo and H. Song, Trans. Tianjin Univ., 2020, 26, 237–247 CrossRef CAS.
- A. Krige, U. Rova and P. Christakopoulos, J. Environ. Chem. Eng., 2021, 9, 106189 CrossRef CAS.
- S. Bajracharya, A. Krige, L. Matsakas, U. Rova and P. Christakopoulos, Chemosphere, 2022, 287, 132188 CrossRef CAS PubMed.
- S. Bajracharya, A. Krige, L. Matsakas, U. Rova and P. Christakopoulos, Fermentation, 2024, 10, 34 CrossRef CAS.
- B. Bian, M. F. Alqahtani, K. P. Katuri, D. Liu, S. Bajracharya, Z. Lai, K. Rabaey and P. E. Saikaly, J. Mater. Chem. A, 2018, 6, 17201–17211 RSC.
- N. Aryal, L. Wan, M. H. Overgaard, A. C. Stoot, Y. Chen, P. L. Tremblay and T. Zhang, Bioelectrochemistry, 2019, 128, 83–93 CrossRef CAS PubMed.
- C. Im, K. Valgepea, O. Modin and Y. Nygård, Bioresour. Technol. Rep., 2022, 19, 101156 CrossRef CAS.
- G. Wang, Q. Huang, T.-S. Song and J. Xie, Energy Fuels, 2020, 34, 8666–8675 CrossRef CAS.
- X. Zhu, J. Jack, A. Leininger, M. Yang, Y. Bian, J. Lo, W. Xiong, N. Tsesmetzis and Z. J. Ren, Resour., Conserv. Recycl., 2022, 184, 106395 CrossRef CAS.
- S. T. Boto, B. Bardl, F. Harnisch and M. A. Rosenbaum, Green Chem., 2023, 25, 4375–4386 RSC.
- J. S. Deutzmann, F. Kracke, W. Gu and A. M. Spormann, Environ. Sci. Technol., 2022, 56, 16073–16081 CrossRef CAS PubMed.
- H. Liu, T. Song, K. Fei, H. Wang and J. Xie, Bioresour. Bioprocess., 2018, 5, 7 CrossRef.
- M. Roy, R. Yadav, P. Chiranjeevi and S. A. Patil, Bioresour. Technol., 2021, 320, 124289 CrossRef CAS PubMed.
- I. Vassilev, P. A. Hernandez, P. Batlle-Vilanova, S. Freguia, J. O. Krömer, J. Keller, P. Ledezma and B. Virdis, ACS Sustainable Chem. Eng., 2018, 6, 8485–8493 CrossRef CAS.
- N. Chu, Q. Liang, W. Zhang, Z. Ge, W. Hao, Y. Jiang and R. J. Zeng, ACS Sustainable Chem. Eng., 2020, 8, 8773–8782 CrossRef CAS.
- Y. Bian, A. Leininger, H. D. May and Z. J. Ren, Environ. Sci. Ecotechnol., 2024, 19, 100324 CrossRef CAS PubMed.
- Y. Xie, S. Ersan, X. Guan, J. Wang, J. Sha, S. Xu, J. A. Wohlschlegel, J. O. Park and C. Liu, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2308373120 CrossRef CAS PubMed.
- J. Madjarov, R. Soares, C. M. Paquete and R. O. Louro, Front. Microbiol., 2022, 13, 913311 CrossRef PubMed.
- S. Das, L. Diels, D. Pant, S. A. Patil and M. M. Ghangrekar, J. Electrochem. Soc., 2020, 167, 155510 CrossRef CAS.
- K. K. Sakimoto, A. B. Wong and P. Yang, Science, 2016, 351, 74–77 CrossRef CAS PubMed.
- H. Zhang, H. Liu, Z. Tian, D. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. Yang, Nat. Nanotechnol., 2018, 13, 900–905 CrossRef CAS PubMed.
- Y. He, S. Wang, X. Han, J. Shen, Y. Lu, J. Zhao, C. Shen and L. Qiao, ACS Appl. Mater. Interfaces, 2022, 14, 23364–23374 CrossRef CAS PubMed.
- S. Jin, Y. Jeon, M. S. Jeon, J. Shin, Y. Song, S. Kang, J. Bae, S. Cho, J. K. Lee, D. R. Kim and B. K. Cho, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020552118 CrossRef CAS PubMed.
- K. Zhang, R. Li, J. Chen, L. Chai, Z. Lin, L. Zou and Y. Shi, Appl. Catal., B, 2024, 342, 123375 CrossRef CAS.
- W. Rao, Y. Kang, H. Zheng, M. Ye, Z. Liu, T. Zhang and P.-L. Tremblay, Sustainable Energy Fuels, 2023, 7, 2462–2472 RSC.
- V. A. Portnoy, D. Bezdan and K. Zengler, Curr. Opin. Biotechnol, 2011, 22, 590–594 CrossRef CAS PubMed.
- S. Lee and P. Kim, J. Microbiol. Biotechnol., 2020, 30, 793–803 CrossRef CAS PubMed.
- P. L. Tremblay, D. Hoglund, A. Koza, I. Bonde and T. Zhang, Sci. Rep., 2015, 5, 16168 CrossRef CAS PubMed.
- F. Ostwald, M. Zwick, O. Omar, E. N. Hotz and A. Neumann, Front. Microbiol., 2018, 9, 1213 CrossRef PubMed.
- P. Piatek, L. Olsson and Y. Nygård, Bioresour. Technol. Rep., 2020, 12, 100564 CrossRef.
- X. C. Shi, P. L. Tremblay, L. Wan and T. Zhang, Sci. Total Environ., 2021, 754, 142440 CrossRef CAS PubMed.
- H. Richter, S. E. Loftus and L. T. Angenent, Environ. Technol., 2013, 34, 1983–1994 CrossRef CAS PubMed.
- M. E. Martin, H. Richter, S. Saha and L. T. Angenent, Biotechnol. Bioeng., 2016, 113, 531–539 CrossRef CAS PubMed.
- G. Antonicelli, L. Ricci, L. Tarraran, S. Fraterrigo Garofalo, A. Re, N. S. Vasile, F. Verga, C. F. Pirri, B. Menin and V. Agostino, Bioresour. Technol., 2023, 387, 129689 CrossRef CAS PubMed.
- J. K. Heffernan, V. Mahamkali, K. Valgepea, E. Marcellin and L. K. Nielsen, Curr. Opin. Biotechnol, 2022, 75, 102700 CrossRef CAS PubMed.
- Y. Song, J. Bae, J. Shin, S. Jin, S. Kang, H. Lee, S. Cho and B.-K. Cho, in One-Carbon Feedstocks for Sustainable Bioproduction, ed. A.-P. Zeng and N. J. Claassens, Springer International Publishing, Cham, 2022, pp. 57–90 DOI:10.1007/10_2021_199.
- E. Marcellin, J. B. Behrendorff, S. Nagaraju, S. DeTissera, S. Segovia, R. W. Palfreyman, J. Daniell, C. Licona-Cassani, L.-E. Quek, R. Speight, M. P. Hodson, S. D. Simpson, W. P. Mitchell, M. Köpke and L. K. Nielsen, Green Chem., 2016, 18, 3020–3028 RSC.
- J. Shin, Y. Song, S. Kang, S. Jin, J.-K. Lee, R. Kim Dong, S. Cho, V. Müller and B.-K. Cho, mSystems, 2021, 6 DOI:10.1128/msystems.00696-00621.
- Y. Song, J. Shin, S. Jin, J. K. Lee, D. R. Kim, S. C. Kim, S. Cho and B. K. Cho, BMC Genomics, 2018, 19, 837 CrossRef CAS PubMed.
- M. M. Al-Bassam, J. N. Kim, L. S. Zaramela, B. P. Kellman, C. Zuniga, J. M. Wozniak, D. J. Gonzalez and K. Zengler, Nat. Commun., 2018, 9, 4474 CrossRef PubMed.
- C. Woods, M. Humphreys Christopher, C. Tomi-Andrino, M. Henstra Anne, M. Köpke, D. Simpson Sean, K. Winzer and P. Minton Nigel, Appl. Environ. Microbiol., 2022, 88, e02479-02421 CrossRef PubMed.
- J. Shin, J. Bae, H. Lee, S. Kang, S. Jin, Y. Song, S. Cho and B. K. Cho, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2216244120 CrossRef CAS PubMed.
- B. Bourgade, N. P. Minton and M. A. Islam, FEMS Microbiol. Rev., 2021, 45, fuab008 CrossRef CAS PubMed.
- S. Jin, J. Bae, Y. Song, N. Pearcy, J. Shin, S. Kang, N. P. Minton, P. Soucaille and B. K. Cho, Int. J. Mol. Sci., 2020, 21 Search PubMed.
- S. Nagaraju, N. K. Davies, D. J. Walker, M. Kopke and S. D. Simpson, Biotechnol. Biofuels, 2016, 9, 219 CrossRef PubMed.
- H. Huang, C. Chai, N. Li, P. Rowe, N. P. Minton, S. Yang, W. Jiang and Y. Gu, ACS Synth. Biol., 2016, 5, 1355–1361 CrossRef CAS PubMed.
- P. F. Xia, I. Casini, S. Schulz, C. M. Klask, L. T. Angenent and B. Molitor, ACS Synth. Biol., 2020, 9, 2162–2171 CrossRef CAS PubMed.
- J. Lo, J. R. Humphreys, J. Jack, C. Urban, L. Magnusson, W. Xiong, Y. Gu, Z. J. Ren and P. C. Maness, Front. Bioeng. Biotechnol., 2020, 8, 560726 CrossRef PubMed.
- R. Zhao, Y. Liu, H. Zhang, C. Chai, J. Wang, W. Jiang and Y. Gu, ACS Synth. Biol., 2019, 8, 2270–2279 CrossRef CAS PubMed.
- K. Charubin, S. Modla, J. L. Caplan and E. T. Papoutsakis, mBio, 2020, 11 DOI:10.1128/mbio.02030-20.
- K. Charubin, H. Streett and E. T. Papoutsakis, Appl. Environ. Microbiol., 2020, 86, e01271-20 CrossRef PubMed.
- E. Streett Hannah, M. Kalis Katie and T. Papoutsakis Eleftherios, Appl. Environ. Microbiol., 2019, 85, e00622-00619 Search PubMed.
- Y. Song, J. Bae, S. Jin, H. Lee, S. Kang, J. Lee, J. Shin, S. Cho and B. K. Cho, Metab. Eng., 2022, 72, 215–226 CrossRef CAS PubMed.
- R. Hocq, S. Bottone, A. Gautier and S. Pflugl, Front. Bioeng. Biotechnol., 2023, 11, 1226889 CrossRef PubMed.
- D.-H. Lee, H. Kim, B.-H. Sung, B. K. Cho and S.-G. Lee, Biotechnol. Bioprocess Eng., 2023, 28, 892–904 CrossRef CAS.
- J. C. Wood, J. Grove, E. Marcellin, J. K. Heffernan, S. Hu, Z. Yuan and B. Virdis, Water Res., 2021, 201, 117306 CrossRef CAS PubMed.
- E. A. Benalcázar, B. G. Deynoot, H. Noorman, P. Osseweijer and J. A. Posada, Biofuels, Bioprod. Biorefin., 2017, 11, 861–886 CrossRef.
- T. J. Tschaplinski, P. Charania, N. L. Engle, R. J. Giannone, R. B. L. Hettich, D. M. Klingeman, S. Poudel, Z. K. Yang, S. D. Simpson and T. Abdalla, Development of a Sustainable Green Chemistry Platform for Production of Acetone and Downstream Drop-in Fuel and Commodity Products Directly From Biomass Syngas Via a Novel Energy Conserving Route in Engineered Acetogenic Bacteria, Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States), 2019.
- Z. Huang, R. G. Grim, J. A. Schaidle and L. Tao, Energy Environ. Sci., 2021, 14, 3664–3678 RSC.
- A. G. Fast, E. D. Schmidt, S. W. Jones and B. P. Tracy, Curr. Opin. Biotechnol, 2015, 33, 60–72 CrossRef CAS PubMed.
- B. J. van Ruijven, D. P. van Vuuren, W. Boskaljon, M. L. Neelis, D. Saygin and M. K. Patel, Resour., Conserv. Recycl., 2016, 112, 15–36 CrossRef.
- R. M. Handler, D. R. Shonnard, E. M. Griffing, A. Lai and I. Palou-Rivera, Ind. Eng. Chem. Res., 2015, 55, 3253–3261 CrossRef.
- L. V. Teixeira, L. F. Moutinho and A. S. Romão-Dumaresq, Biofuels, Bioprod. Biorefin., 2018, 12, 1103–1117 CrossRef CAS.
- A. Owoade, A. S. Alshami, D. Levin, S. Onaizi and Z. O. Malaibari, Biofuels, Bioprod. Biorefin., 2023, 17, 1328–1342 CrossRef CAS.
- White Dog Labs Inc., US Pat., US9938542B2, 2018 Search PubMed.
- LanzaTech, https://lanzatech.com/, (accessed 30 June 2024).
- L. Sekisui Chemical Co., Establishment of Joint Venture to Commercialize “Waste to Ethanol” Technology, https://www.sekisuichemical.com/news/2020/1364066_38530.html, (accessed 30 June 2024).
- ArcelorMittal, World's leading steel company, ArcelorMittal and LanzaTech announce first ethanol samples from commercial flagship carbon capture and utilisation facility in Ghent, Belgium, https://belgium.arcelormittal.com/en/worlds-leading-steel-company-arcelormittal-and-lanzatech-announce-first-ethanol-samples-from-commercial-flagship-carbon-capture-and-utilisation-facility-in-ghent-belgium/, (accessed 30 June 2024).
- Jupeng Bio, https://www.jupengbio.com/, (accessed 30 June 2024).
- Genomatica Inc., US Pat., US10550411B2, 2020 Search PubMed.
- Evonik, For a climate-friendly industry: Using carbon dioxide and hydrogen as raw materials for sustainable chemicals, https://corporate.evonik.com/en/media/press-releases/corporate/for-a-climate-friendly-industry-using-carbon-dioxide-and-hydrogen-as-raw-materials-for-sustainable-c-143559.html, (accessed 30 June 2024).
- VITO, https://vito.be/en/microbial-electrosynthesis, (accessed 30 June 2024).
- C. Mihalcea, F. Burton, R. Conrado and S. Simpson, in CO2 and CO as Feedstock: Sustainable Carbon Sources for the Circular Economy, ed. M. Kircher and T. Schwarz, Springer International Publishing, Cham, 2023, pp. 333–343 DOI:10.1007/978-3-031-27811-2_27.
- LanzaTech, LanzaTech Global, Inc. Begins Trading on Nasdaq Stock Exchange, https://lanzatech.com/lanzatech-global-inc-begins-trading-on-nasdaq-stock-exchange/, (accessed 30 June 2024).
- L. Jourdin, J. Sousa, N. V. Stralen and D. P. B. T. B. Strik, Appl. Energy, 2020, 279, 115775 CrossRef CAS.
- C. Hank, S. Gelpke, A. Schnabl, R. J. White, J. Full, N. Wiebe, T. Smolinka, A. Schaadt, H.-M. Henning and C. Hebling, Sustainable Energy Fuels, 2018, 2, 1244–1261 RSC.
- H. M. Fruehauf, F. Enzmann, F. Harnisch, R. Ulber and D. Holtmann, Biotechnol. J., 2020, 15, 2000066 CrossRef CAS PubMed.
- L. Jourdin and T. Burdyny, Trends Biotechnol., 2021, 39, 359–369 CrossRef CAS PubMed.
- P. Dessì, L. Rovira-Alsina, C. Sánchez, G. K. Dinesh, W. Tong, P. Chatterjee, M. Tedesco, P. Farràs, H. M. V. Hamelers and S. Puig, Biotechnol. Adv., 2021, 46, 107675 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |