Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterobifunctional rotaxanes featuring two chiral subunits – synthesis and application in asymmetric organocatalysis†
Dana
Kauerhof
,
Jan
Riebe
 ,
Christoph J.
Vonnemann‡
,
Christoph J.
Vonnemann‡
 ,
Maike
Thiele
,
Dennis
Jansen
and
Jochen
Niemeyer
,
Maike
Thiele
,
Dennis
Jansen
and
Jochen
Niemeyer
 *
*
Faculty of Chemistry (Organic Chemistry) and Centre of Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Universitätsstr. 7, 45141 Essen, Germany. E-mail: jochen.niemeyer@uni-due.de
First published on 29th January 2024
Abstract
Rotaxanes can serve as scaffolds for the generation of bifunctional catalysts. We have now generated acid–base functionalized rotaxanes featuring two chiral subunits. The mechanical bond leads to increased reaction rates and also to strongly altered enantioselectivites in comparison to the non-interlocked control catalysts.
Mechanically interlocked molecules (MIMs),1 such as catenanes and rotaxanes, have found increasing attention for the design of novel catalysts.2 Here, the mechanical bond offers exciting possibilities for catalyst design based on the flexible, but permanent connection of two or more subunits. For example, MIMs can provide unique three-dimensional environments around a catalytically active site.3 The possibility to control the co-conformation of MIMs has been used to create switchable catalysts, including ON/OFF switchability but also a switchability between different reaction modes.4 Furthermore, MIMs allow for the generation of bi- or multifunctional systems by introduction of several functional groups on the subcomponents.5 Last but not least, the mechanical bond can be used for the introduction of chirality:6 achiral subcomponents of suitable symmetry can be interlocked to give MIMs with nonclassical mechanical chirality.7,8 Alternatively, chiral subcomponents can be used, e.g. in order to transfer the chiral information via the mechanical bond (Fig. 1).9
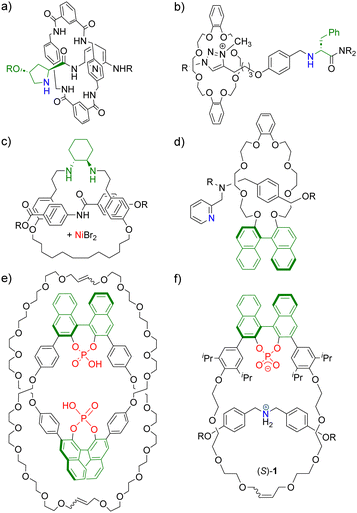 | ||
| Fig. 1 Previously reported interlocked catalysts featuring chiral threads or macrocycles from Berna (a),9a Leigh (b) and (c),9d,e Takata (d)9c and Niemeyer (e) and (f).10,11 Chiral units shown in green, catalytically active centers shown in blue/red. Examples of previously reported interlocked catalysts from ref. 9b and f not shown. | ||
Berna, Leigh and Takata have previously reported chiral interlocked catalysts that feature either chiral macrocycles or chiral threads (Fig. 1a–d). In our group, homobifunctional catenanes composed of two chiral macrocycles with 1,1′-binaphthyl phosphoric acids were used in enantioselective transfer hydrogenations (Fig. 1e).10 Furthermore, heterobifunctional rotaxanes composed of a macrocycle with a chiral 1,1′-binaphthyl phosphate and an achiral dibenzyl-ammonium-thread were used as catalysts for enantioselective Michael-additions (e.g. rotaxane (S)-1, Fig. 1f).11 Both for the catenanes and the rotaxanes, we found that the interlocked catalysts can deliver enhanced stereoselectivities in comparison to their non-interlocked counterparts. For the rotaxane-catalysts, we also found increased reaction rates.
Supported by extensive DFT-calculations, the increased rates and selectivities were attributed to a cooperativity between the functional groups, enabled by the mechanical bond, demonstrating the power of using MIMs as catalysts.11
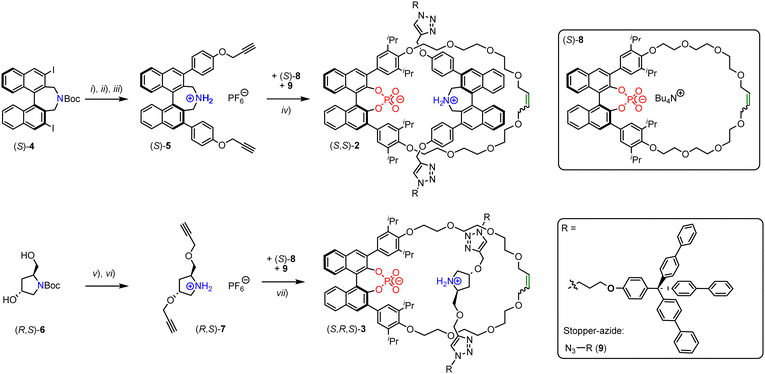
However, the use of multiple elements of chirality in MIM-based catalysts has not been investigated to the best of our knowledge. Herein, we report the synthesis and catalytic application of rotaxanes featuring both a chiral macrocycle and a chiral thread. Based on our previously reported rotaxane (S)-1 (Fig. 1f), we developed two novel rotaxanes: These contain the same 1,1′-binaphthyl phosphate macrocycle, but now feature a chiral thread either based on Maruokas 1,1′-binaphthyl azepine12 or based on 3-hydroxy-prolinol. For a first investigation of their catalytic performance, we only generated one diastereomer of each rotaxane.
The rotaxanes were generated by a CuAAc-based stoppering approach, using our previously established phosphate-ammonium passive template.11 The required ammonium-bisalkynes were synthesized as follows (Fig. 2): Starting from 3,3′-diiodinated azepine (S)-4 (available in 8 steps from BINOL13), bisalkyne (S)-5 was synthesized by Suzuki-coupling with 4-hydroxyphenylboronic acid, followed by propargylation, Boc-deprotection and anion-exchange (45% yield over three steps). Starting from commercially available Boc-protected 3-hydroxy-prolinol (R,S)-6, bisalkyne (R,S)-7 was generated by propargylation and Boc-deprotection plus anion-exchange (55% yield over two steps). To generate the rotaxanes, the 1,1′-binaphthyl phosphate based macrocycle (S)-811 was reacted with bisalkyne (S)-5 or (R,S)-7 and stopper-azide 911 to give either the azepin-based rotaxane (S,S)-2 or the hydroxyprolinol-based rotaxane (S,R,S)-3 (Fig. 2). The rotaxanes were isolated as the zwitterionic phosphate-ammonium-salts and obtained in yields of 20/37%. Due to the C2-symmetry of the phosphate-macrocycle, no orientational isomers are expected for these rotaxanes.
All precursors, threads (independently synthesized) and rotaxanes were fully characterized by standard analytical methods and the purity of the final catalyts was further demonstrated by HPLC-analyses (ESI,† Fig. S18, S29 and S32). Here, we will briefly discuss rotaxane (S,R,S)-3: In the NMR (Fig. 3), the interlocking of both subcomponents leads to distinct changes in chemical shifts, e.g. for the olefinic unit of the macrocycle (δ = 5.63 vs. 5.76 ppm for rotaxane vs. free macrocylce) and the ammonium-group on the thread (δ = 9.76 + 8.67 vs. 5.36 ppm for rotaxane vs. free thread).
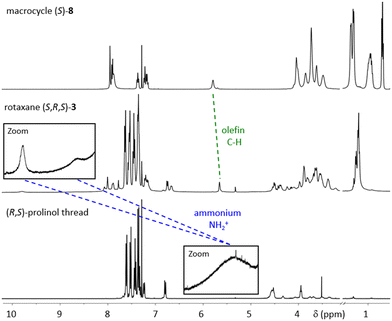 | ||
| Fig. 3 1H NMR spectra of macrocycle (S)-8, rotaxane (S,R,S)-3 and (R,S)-prolinol-thread (all: CDCl3, 400 MHz, 298 K). | ||
The strong downfield shift of the NH2+-group is indicative of the mechanointramolecular phosphate-ammonium inter-action. By high-resolution mass-spectrometry, the rotaxane can unambiguously be identified as the [M + 2H]-dication at m/z = 1253.0875 (calculated m/z = 1253.0981).
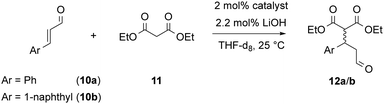
With both rotaxanes in hand, we tested their application in asymmetric catalysis of the Michael-addition of diethylmalonate 11 to cinnamaldehyde 10a. Based on our previously established protocol, the inactive ammonium-phosphate rotaxanes were first deprotonated by LiOH14 to give the active catalysts in the (ArO)2POOLi⋯HNR2 form. The reaction was carried out in tetrahydrofurane-d8 and followed by NMR over 7 days, followed by isolation and analysis of the products. Surprisingly, no conversion was found for the azepine-based rotaxane (S,S)-2, while the prolinol-based rotaxane (S,R,S)-3 gave 84% conversion after 7 days. This might be attributed to the higher basicity of the prolinol-unit in comparison of the dibenzoazepine (cf., pKa = 11.3 for pyrrolidinium, pKa = 8.5 for dibenzylammonium), according to the role of the amine as a Brønsted-base in the catalytic mechanism.11
Due to the inactivity of rotaxane 2, we decided to investigate only the prolinol-based system 3 in more detail. To obtain a full picture about the influence of the chiral subcomponents on the behaviour in catalysis, we also synthesized the diastereomeric rotaxane (R,R,S)-3, which features the same (R,S)-prolinol-based thread, but now in combination with the (R)-configured macrocycle (for synthetic details and full characterization of (R,R,S)-3 and the (R,S)-prolinol thread, see the ESI,† Section 2.3).
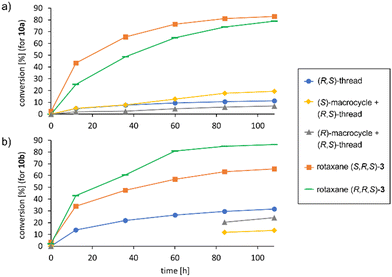
In the application in catalysis, both rotaxanes were compared to the free (R,S)-prolinol thread and the non-interlocked mixtures of (R,S)-thread with either (S)- or (R)-macrocycle.15 To further extend the scope of this investigation, we used both cinnamaldehyde 10a and its naphthyl-derivative 10b as electrophiles in the Michael-addition reaction. Interestingly, 10b has not been previously described as an electrophile for malonate addition. When generating the racemic reference product (rac)-12b, we found a complex product mixture under standard condititions (diethylmalonate nucleophile, pyrrolidine catalyst), and only when using a lithium-phosphate + dibenzylamine catalysts, the product could be obtained.
To our delight, the rotaxane-catalysts 3 cleanly convert both substrates 10a/b into the corresponding products 12a/b. Here, the rotaxanes are significantly more active (71–84% conversion after 7 days) than the thread alone (12–34% conversion) or the non-interlocked mixtures of subcomponents (7–30% conversion) (Fig. 4 shows the first 5 days). This is in line with our earlier findings for rotaxane (S)-1, which showed that the interlocked nature of the catalyst results in cooperative behaviour of the lithium-phosphate and the amine group, leading to increased reaction rates.
However, to our surprise, the incorporation of the chiral prolinol-thread did not lead to an increase in stereoselectivity. While we did observe matched/mismatched effects for the diastereomeric rotaxanes (S,R,S)-3 and (R,R,S)-3 both for 12a (−8%/29% ee) and the naphthyl-derivative 12b (−30%/0% ee), the overall stereoselectivities were not higher than those observed for our reference rotaxane (S)-1 (53%/29% ee for 12a/b), which features the same chiral macrocycle, but an achiral thread. When comparing the rotaxanes to their non-interlocked counterparts (thread or macrocycle + thread), the following trends can be observed: Firstly, the (R,S)-prolinol thread alone only gives poor enantioinduction (14%/11% ee for 12a/b). When combining the thread with the (R)-macrocycle, enantioselectivities remain largely unchanged for the non-interlocked mixture (21%/15% ee for 12a/b), but show slightly stronger changes when mechanically interlocked in rotaxane (R,R,S)-3 (29%/0% ee for 12a/b). When combining the thread with the (S)-macrocycle, the stereoselectivities of the non-interlocked mixture (36%/18% ee for 12a/b) are actually inverted in comparison to the rotaxane (S,R,S)-3 for both substrates (−8%/−30% ee for 12a/b). Thus, although absolute enantioselectivies remain low due to the inversion of the preferred product configuration, the mechanical bond does lead to strong relative changes in enantioselectivity in case of the (S)-macrocycle (Δee = 44–48%, i.e. ΔΔG≠ = 0.48–0.54 kcal mol−1) between interlocked and non-interlocked catalyst (Table 1).
| Entry | Catalyst precursor | Substrate | Conversiona (%) | eebc (%) |
|---|---|---|---|---|
| a Determined by 1H NMR after 7 days. b Determined by chiral HPLC for isolated products. c Positive ee denotes excess of the (R)-isomer, according to assignment of HPLC-peaks in ref. 16. d Data taken from ref. 11. e After 5 days. | ||||
| 1 | Rotaxane (S)-1d | 10a | 88 | 53 |
| 2 | (S)-Macrocycle + achiral threadd | 10a | 76 | 9 |
| 3 | (R,S)-Prolinol-thread | 10a | 12e | 17 |
| 4 | (S)-Macrocycle + (R,S)-thread | 10a | 30 | 37 |
| 5 | Rotaxane (S,R,S)-3 | 10a | 84 | −8 |
| 6 | (R)-Macrocycle + (R,S)-thread | 10a | 7e | 21 |
| 7 | Rotaxane (R,R,S)-3 | 10a | 82 | 30 |
| 8 | Rotaxane (S)-1 | 10b | 54 | 32 |
| 9 | (S)-Macrocycle + achiral thread | 10b | 11 | 5 |
| 10 | (R,S)-Prolinol-thread | 10b | 34 | 10 |
| 11 | (S)-Macrocycle + (R,S)-thread | 10b | 18 | 16 |
| 12 | Rotaxane (S,R,S)-3 | 10b | 71 | −30 |
| 13 | (R)-Macrocycle + (R,S)-thread | 10b | 30 | 17 |
| 14 | Rotaxane (R,R,S)-3 | 10b | 82e | 0 |
In conclusion, we could demonstrate the synthesis and application of rotaxane-organocatalysts featuring two chiral subunits. While an azepine-based rotaxane was inactive, the use of a prolinol-thread gave rise to active organocatalysts for the asymmetric Michael-addition of malonates to α,β-unsaturated aldehydes. We could show that for two different substrates, the rotaxanes show significantly increased reaction rates in comparison to their non-interlocked counterparts, indicating a cooperative behaviour of the functional groups, enabled by the mechanical bond. The observed stereoselectivities were moderate, but a strong difference between the interlocked rotaxanes and the non-interlocked control catalysts (Δee = 44–48%) also demonstrates the effect of the mechanical bond on catalyst performance.
D. K., J. R., C. J. V., M. T. and D. J. conducted the experimental work. D. K. and J. N. wrote the original and revised manuscript. J. N. conceived and supervised the study.
Funding from the Deutsche Forschungsgemeinschaft DFG (project NI1273/2-2 and Heisenberg-Professorship NI1273/4-1) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, Wiley & Sons, Hoboken, 2016 Search PubMed.
- (a) A. W. Heard, J. M. Suárez and S. M. Goldup, Nat. Rev. Chem., 2022, 6, 182–196 CrossRef PubMed; (b) C. Kwamen and J. Niemeyer, Chem. – Eur. J., 2021, 27, 175–186 CrossRef CAS PubMed; (c) D. Sluysmans and J. F. Stoddart, Trends Chem., 2019, 1, 185–197 CrossRef CAS; (d) D. A. Leigh, V. Marcos and M. R. Wilson, ACS Catal., 2014, 4, 4490–4497 CrossRef CAS; (e) A. Martinez-Cuezva, A. Saura-Sanmartin, M. Alajarin and J. Berna, ACS Catal., 2020, 10, 7719–7733 CrossRef CAS.
- (a) J. E. Lewis, M. Galli and S. M. Goldup, Chem. Commun., 2016, 52, 298–312 Search PubMed; (b) E. A. Neal and S. M. Goldup, Chem. Commun., 2014, 50, 5128–5142 RSC.
- (a) V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC; (b) S. F. M. van Dongen, S. Cantekin, J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, Chem. Soc. Rev., 2014, 43, 99–122 RSC.
- R. Mitra and J. Niemeyer, ChemCatChem, 2018, 10, 1221–1234 CrossRef CAS.
- (a) M. Krajnc and J. Niemeyer, Beilstein J. Org. Chem., 2022, 18, 508–523 CrossRef CAS PubMed; (b) J. R. J. Maynard and S. M. Goldup, Chem, 2020, 6, 1914–1932 CrossRef CAS; (c) N. Pairault and J. Niemeyer, Synlett, 2018, 689–698 CAS; (d) E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chem. Soc. Rev., 2018, 47, 5266–5311 RSC; (e) N. H. Evans, Chem. – Eur. J., 2018, 24, 3101–3112 CrossRef CAS PubMed.
- (a) A. W. Heard and S. M. Goldup, Chem, 2020, 6, 994–1006 CrossRef CAS PubMed; (b) M. Dommaschk, J. Echavarren, D. A. Leigh, V. Marcos and T. A. Singleton, Angew. Chem., Int. Ed., 2019, 58, 14955–14958 CrossRef CAS PubMed; (c) Y. Cakmak, S. Erbas-Cakmak and D. A. Leigh, J. Am. Chem. Soc., 2016, 138, 1749–1751 CrossRef CAS PubMed.
- For recent examples of MIMs with nonclassical chirality, which however were not applied in catalysis, see: (a) S. Zhang, A. Rodríguez-Rubio, A. Saady, G. J. Tizzard and S. M. Goldup, Chem, 2023, 9, 1195–1207 CrossRef CAS; (b) N. Pairault, F. Rizzi, D. Lozano, E. M. G. Jamieson, G. J. Tizzard and S. M. Goldup, Nat. Chem., 2023, 15, 781–786 CrossRef CAS PubMed; (c) A. Rodriguez-Rubio, A. Savoini, F. Modicom, P. Butler and S. M. Goldup, J. Am. Chem. Soc., 2022, 144, 11927–11932 CrossRef CAS PubMed; (d) J. R. J. Maynard, P. Gallagher, D. Lozano, P. Butler and S. M. Goldup, Nat. Chem., 2022, 14, 1038–1044 CrossRef CAS PubMed; (e) A. de Juan, D. Lozano, A. W. Heard, M. A. Jinks, J. M. Suarez, G. J. Tizzard and S. M. Goldup, Nat. Chem., 2022, 14, 179–187 CrossRef CAS PubMed.
- (a) M. Calles, J. Puigcerver, D. A. Alonso, M. Alajarin, A. Martinez-Cuezva and J. Berna, Chem. Sci., 2020, 11, 3629–3635 RSC; (b) A. Martinez-Cuezva, M. Marin-Luna, D. A. Alonso, D. Ros-Ñiguez, M. Alajarin and J. Berna, Org. Lett., 2019, 21, 5192–5196 CrossRef CAS PubMed; (c) K. Xu, K. Nakazono and T. Takata, Chem. Lett., 2016, 45, 1274–1276 CrossRef CAS; (d) S. Hoekman, M. O. Kitching, D. A. Leigh, M. Papmeyer and D. Roke, J. Am. Chem. Soc., 2015, 137, 7656–7659 CrossRef CAS PubMed; (e) V. Blanco, D. A. Leigh, V. Marcos, J. A. Morales-Serna and A. L. Nussbaumer, J. Am. Chem. Soc., 2014, 136, 4905–4908 CrossRef CAS PubMed; (f) Y. Tachibana, N. Kihara and T. Takata, J. Am. Chem. Soc., 2004, 126, 3438–3439 CrossRef CAS PubMed.
- (a) R. Mitra, M. Thiele, F. Octa-Smolin, M. C. Letzel and J. Niemeyer, Chem. Commun., 2016, 52, 5977–5980 RSC; (b) D. Jansen, J. Gramüller, F. Niemeyer, T. Schaller, M. C. Letzel, S. Grimme, H. Zhu, R. M. Gschwind and J. Niemeyer, Chem. Sci., 2020, 11, 4381–4390 RSC; (c) R. Mitra, H. Zhu, S. Grimme and J. Niemeyer, Angew. Chem., Int. Ed., 2017, 56, 11456–11459 CrossRef CAS PubMed.
- N. Pairault, H. Zhu, D. Jansen, A. Huber, C. G. Daniliuc, S. Grimme and J. Niemeyer, Angew. Chem., Int. Ed., 2020, 59, 5102–5107 CrossRef CAS PubMed.
- T. Ooi, M. Kameda and K. Maruoka, J. Am. Chem. Soc., 1999, 121, 6519–6520 CrossRef CAS.
- (a) A. Manaprasertsak, S. Tharamak, C. Schedl, A. Roller and M. Widhalm, Molecules, 2019, 24, 3844 CrossRef PubMed; (b) M. Thiele, F. Octa-Smolin, S. Thölke, C. Wölper, J. Linders, C. Mayer, G. Haberhauer and J. Niemeyer, Chem. Commun., 2021, 57, 9842–9845 RSC.
- LiOH does not significantly affect catalyst stability, as evidenced by a control experiment (see ESI† Table S1 and Fig. S36).
- The lithium phosphate macrocycle alone does not show any catalytic activity (see ref. 11) and was thus not included in the control experiments.
- Y.-L. Zhao, Y. Wang, X.-Q. Hu and P.-F. Xu, Chem. Commun., 2013, 49, 7555–7557 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Syntheses and analytical data for all catalysts, details of catalytic reactions. See DOI: https://doi.org/10.1039/d3cc05482a |
| ‡ Current address: Faculty of Chemistry and Biochemistry, Organic Chemistry I, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany. |
| This journal is © The Royal Society of Chemistry 2024 |