Open Access Article
Open Access ArticleA reductively convertible nickel phthalocyanine precursor as a biological thiol-responsive turn-on photoacoustic contrast agent†
Kohei
Nogita
a,
Takaya
Sugahara
a,
Koji
Miki
 *a,
Huiying
Mu
*a,
Huiying
Mu
 a,
Minoru
Kobayashi
a,
Minoru
Kobayashi
 b,
Hiroshi
Harada
b,
Hiroshi
Harada
 b and
Kouichi
Ohe
b and
Kouichi
Ohe
 *a
*a
aDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: kojimiki@scl.kyoto-u.ac.jp; ohe@scl.kyoto-u.ac.jp
bLaboratory of Cancer Cell Biology, Graduate School of Biostudies, Kyoto University, Yoshida Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan
First published on 15th January 2024
Abstract
A nickel phthalocyanine precursor bearing poly(ethylene glycol) as a turn-on contrast agent for photoacoustic imaging was prepared. The water-soluble polymeric chains were smoothly eliminated through thiol-mediated reductive aromatization in cancer cells, enabling the detection of endogenous biological thiols in vitro and in vivo.
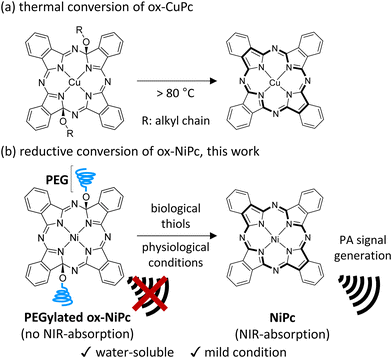
Photoacoustic (PA) imaging has attracted attention as a reliable method to visualize cancer tissues in vivo because of its advantages, such as high spatial resolution, deeper tissue visualization, and low invasiveness.1 Metallophthalocyanine (MPc) is a photostable near-infrared (NIR)-absorbing dye and is considered one of the most promising photosensitizers for PA imaging because of its high molar extinction coefficient and excellent photothermal conversion efficiency.2 Although MPc-containing nanoparticles3 and water-soluble MPcs4 have been used for PA cancer imaging, they generate PA signals as background noise in normal tissues. Several ‘activatable’ contrast agents based on MPc, which can enhance PA signal intensity in response to tumour-specific biological compounds, have been developed to visualize cancer tissue more clearly.5 Despite the activatable nature of such MPc agents, generating background PA signals by unreacted MPcs still needs to be suppressed for efficient imaging with high contrast. Therefore, a ‘turn-on’ PA photosensitizer that generates PA signals only in cancer tissues is ideal;6 however, to our knowledge, there is no example of a turn-on PA contrast agent based on MPc. Modified MPcs with two alkoxy substituents at the α-position of pyrroles (ox-MPc) were reported as thermally convertible MPc precursors (Fig. 1a).7 Considering that the transformation of ox-MPc to MPc is a formal reduction reaction, reductive aromatization is a more suitable conversion method.8 We envisioned that biological thiols such as glutathione (GSH) overexpressed in many cancer cell lines can be utilized for the reductive aromatization of ox-MPcs under physiological conditions.9
Herein, we report the turn-on PA contrast agent ox-NiPc-PEG consisting of ox-NiPc and water-soluble poly(ethylene glycol) (PEG) (Fig. 1b). Because the introduction of two alkoxy groups on the 18π aromatic core structure of NiPc causes a hypsochromic shift in the absorption peak of ox-NiPc-PEG, no PA signal is generated under NIR pulsed laser irradiation for PA imaging. The conversion of ox-NiPc-PEG to NiPc by GSH proceeded under physiological conditions, resulting in strong PA signal generation. PA imaging using ox-NiPc-PEG enabled the detection of GSH in xenograft tumour-bearing mice.
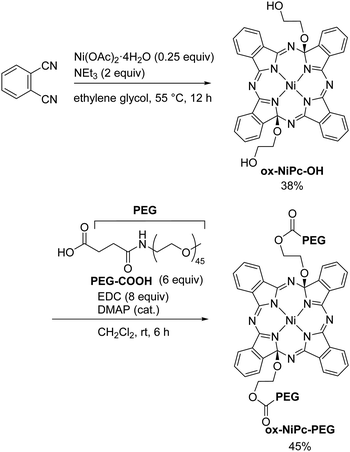
The water-soluble NiPc precursor ox-NiPc-PEG was synthesized from phthalonitrile in two steps viaox-NiPc-OH with two hydroxy groups at the side chain terminal (Scheme 1). The structure of ox-NiPc-OH was confirmed by X-ray crystallographic analysis (Fig. 2). The crystal structure clarified that two hydroxyethoxy groups on the α-carbons of diagonal pyrrole extended to the convex face of the significantly distorted NiPc macrocycle having sp3-hybridized carbons.7,10 The formation of the thermodynamically less stable trans-isomer was not observed (Fig. S4 and Table S1, ESI†). ox-NiPc-OH with a distorted structure could dissolve well in common organic solvents, such as toluene, THF, acetone, CH2Cl2, and CHCl3. To enhance the water solubility of ox-NiPc, ox-NiPc-PEG was synthesized from ox-NiPc-OH by condensation with PEG derivative PEG-COOH11 in 45% yield. ox-NiPc-PEG dissolved in water at the concentration of 500 μM or below (Fig. S3, ESI†).
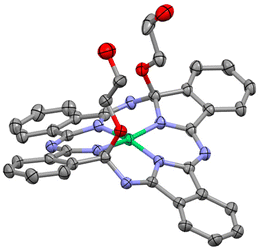 | ||
| Fig. 2 ORTEP drawing of ox-NiPc-OH (50% ellipsoids). Hydrogen atoms and solvent molecules are omitted for clarity. | ||
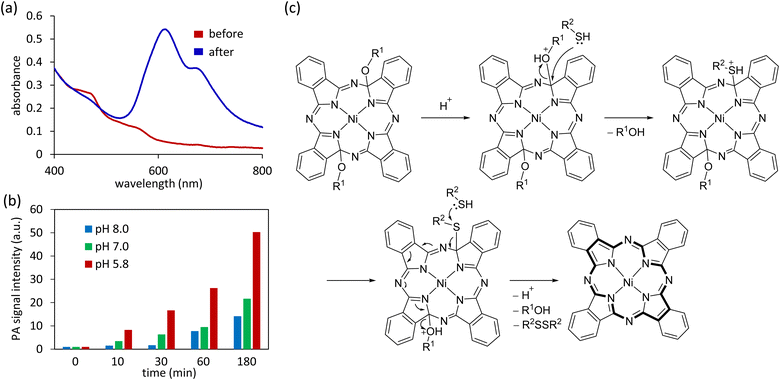
Next, we examined the reactivity of ox-NiPcs with reductants. The reaction of ox-NiPc-PEG with GSH in phosphate buffer solution (PBS) at room temperature gave a blue solid. This solid was identified as NiPc by MALDI-TOF mass spectrometry, IR absorption spectrometry, and elemental analysis (Fig. S5, S6 and Table S2, ESI†). When ox-NiPc-OH was treated with GSH at room temperature for 24 h, NiPc was obtained in 98% isolated yield. The UV-vis absorption spectrum of an aqueous solution of ox-NiPc-PEG showed no absorption signals in the NIR region because of the non-aromatic nature of the distorted NiPc core (Fig. 3a, red line). After the reaction of ox-NiPc-PEG with GSH in aqueous solution, a new absorption peak at 606 nm appeared (Fig. 3a, blue line). The absorption of the Q band of NiPc, which is generally observed at approximately 670 nm, was broadened and blue-shifted, indicating that NiPc formed H-aggregates in water.12 These results showed that ox-NiPc can be quantitatively transformed to NiPc by reduction and utilized as a biological thiol-responsive photosensitizing molecule under physiological conditions.
Next, we examined the dependency of pH on the GSH-mediated reduction of ox-NiPc-PEG. The conversion of ox-NiPc-PEG to NiPc was accelerated in acidic PBS, and the resulting NiPc aggregates generated strong PA signals under NIR pulsed laser irradiation (Fig. 3b). This result suggests that the protonation of the alkoxy groups attached to pyrrole α-carbons is crucial in the reductive aromatization of ox-NiPc-PEG.8 A plausible reaction mechanism is shown in Fig. 3c. Upon protonation, the alcohol is eliminated by a nucleophilic attack of biological thiols on the sp3-hybridized carbon. The subsequent nucleophilic attack of biological thiol to thioether affords an 18π aromatic structure of the NiPc core together with a disulfide and alcohols.
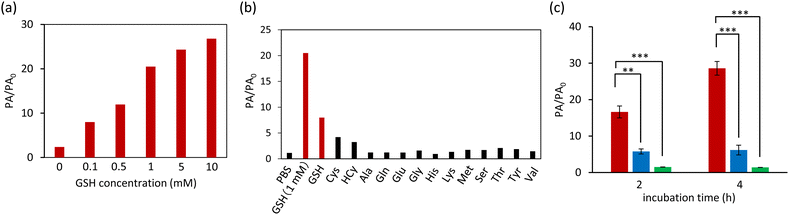
To evaluate the GSH-mediated turn-on of the PA signal, we investigated the increase in the PA signal intensity of ox-NiPc-PEG under various GSH concentrations (Fig. 4a). The PA signal intensity was dependent on the GSH concentration. By contrast, the PA signal intensity of ox-NiPc-PEG scarcely increased in the absence of GSH. When ox-NiPc-PEG was treated with GSH at the intracellular concentrations, the PA signal intensity was 4.9- and 6.3-fold higher than when treated with Cys and Hcy, respectively (Fig. 4b). These results indicate that NiPc was produced more effectively upon treatment with GSH at the intracellular concentration. We confirmed that no 1O2 was generated from NiPc, which is produced from ox-NiPc-PEG under NIR photoirradiation.12 Therefore, the oxidative stress to normal cells by 1O2 is expected to be avoidable during PA imaging using the NiPc precursor (Fig. S7, ESI†).
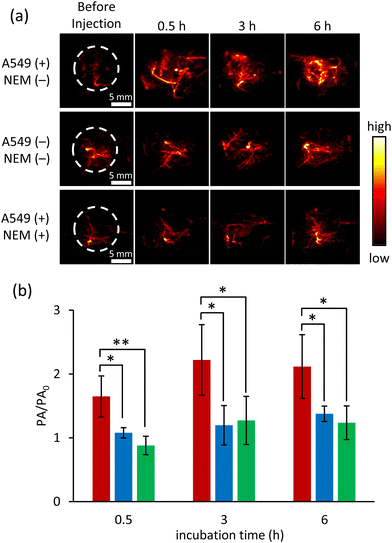
Having confirmed that the reduction of ox-NiPc-PEG can be monitored by the PA signal output, we next evaluated the PA signal intensity of ox-NiPc-PEG in GSH-overexpressing human lung cancer cell line A549.13 After incubation with ox-NiPc-PEG (10 μM), the PA signal intensity was significantly increased in A549 cells. After 4 h, the PA signal intensity in A549 cells was 4.6-fold stronger than that in the normal cell line HEK293 (Fig. 4c, red and blue). By a control experiment using N-ethylmaleimide (NEM) as a trapping agent of biological thiols in A549 cells, we confirmed that the increase in PA signal intensity was due to the formation of NiPc (Fig. 4c, green). It was confirmed that there was no cytotoxicity of ox-NiPc-PEG for cancer cells and normal cells when the concentration of ox-NiPc-PEG was less than 50 μM, using an MTT assay (Fig. S8, ESI†). These results indicate that ox-NiPc-PEG is suitable for use as a PA contrast agent to visualize the reductive environment of the tumour site in vivo.
Finally, we conducted PA imaging in living mice to verify the applicability of ox-NiPc-PEG as a biological thiol-responsive PA contrast agent in vivo (Fig. S9, ESI†). A549 cells were xenografted into the right legs of nude mice to prepare tumour-bearing mice. Eight prepared mice were divided into two groups for in vivo experiments through intratumoral administration. For the first group, saline (30 mL) was pre-treated at the tumour site in the right leg and the normal muscle in the left leg. After 30 min pre-treatment, ox-NiPc-PEG was administered intratumorally in the right leg and subcutaneously in the left leg, respectively. For the second group, a pre-treatment of NEM (2.0 mM in 30 μL saline) was conducted in tumour sites before the intratumoral administration of ox-NiPc-PEG. The PA tomography system was used to obtain horizontal and vertical view images at the indicated time points of post-injection of ox-NiPc-PEG (Fig. 5a), and the PA signal intensities at the administration sites were evaluated under NIR pulsed laser irradiation (Fig. 5b and Fig. S10–S12, ESI†). After the injection of ox-NiPc-PEG, PA signal intensity in the tumour site significantly increased in three hours (p < 0.05) in good contrast to the non-tumour site, demonstrating its sensitivity to distinguish tumour sites from normal muscle. Furthermore, the NEM-pretreated tumours exhibited weak PA signals before and after injection, suggesting that ox-NiPc-PEG can selectively detect biological thiols in living mice.
In conclusion, we have developed a turn-on PA contrast agent based on reductive aromatization for cancer imaging. A water-soluble NiPc precursor ox-NiPc-PEG was converted to NiPc by biological thiols, generating strong PA signals under NIR pulsed laser irradiation. Because the biological thiol-mediated transformation proceeded smoothly under acidic conditions, the protonation of the alkoxy groups was considered to be crucial in the reductive aromatization. The results obtained in vitro and in vivo demonstrated that the increase in PA signal intensity was mainly due to the reduction of ox-NiPc-PEG by GSH overexpressed in cancer cells. Because biological thiol-mediated reductive aromatization proceeds under mild physiological conditions, it is suitable for constructing a π-conjugated system of photosensitizer in vivo.
This work was supported by JST, the establishment of university fellowships towards the creation of science technology innovation, Grant Number JPMJFS2123. K. M. appreciates the financial supports from The Asahi Glass Foundation and The Uehara Memorial Foundation. We acknowledged Prof. Teruyuki Kondo and Associate Prof. Yu Kimura in Kyoto University for PA imaging. A part of this study was conducted through the CORE Program of the Radiation Biology Center, Kyoto University.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 16, 639–650 CrossRef; (b) R. E. Borg and J. Rochford, Photochem. Photobiol., 2018, 94, 1175–1209 CrossRef CAS PubMed; (c) W. Choi, B. Park, S. Choi, D. Oh, J. Kim and C. Kim, Chem. Rev., 2023, 123, 7379–7419 CrossRef CAS.
- (a) E.-Y. Park, D. Oh, S. Park, W. Kim and C. Kim, APL Bioeng., 2021, 5, 031510 CrossRef CAS; (b) D. Li, S. Cai, P. Wang, H. Cheng, B. Cheng, Y. Zhang and G. Liu, Adv. Healthcare Mater., 2023, 2300263 CrossRef CAS; (c) B.-D. Zheng, J. Ye, Y.-Y. Huang and M. T. Xiao, Biomater. Sci., 2021, 9, 7811–7825 RSC.
- (a) K. Strokov, A. H. Schäfer, U. Dobrindt and A. Galstyan, ACS Appl. Bio Mater., 2020, 3, 3751–3760 CrossRef CAS; (b) X. Li, S. Yu, Y. Lee, T. Guo, N. Kwon, D. Lee, S. C. Yeom, Y. Cho, G. Kim, J.-D. Huang, S. Choi, K. T. Nam and J. Yoon, J. Am. Chem. Soc., 2019, 141, 1366–1372 CrossRef CAS; (c) A. Shaukat, E. Anaya-Plaza, S. Julin, V. Linko, T. Torres, A. de la Escosura and M. A. Kostiainen, Chem. Commun., 2020, 56, 7341–7344 RSC.
- (a) X. Li, E.-Y. Park, Y. Kang, N. Kwon, M. Yang, S. Lee, W. J. Kim, C. Kim and J. Yoon, Angew. Chem., Int. Ed., 2020, 59, 8630–8634 CrossRef CAS PubMed; (b) S. Li, L. Zhao, R. Chang, R. Xing and X. Yan, Chem. – Eur. J., 2019, 25, 13429–13435 CrossRef CAS; (c) X. Li, C. Kim, S. Lee, D. Lee, H.-M. Chung, G. Kim, S.-H. Heo, C. Kim, K.-S. Hong and J. Yoon, J. Am. Chem. Soc., 2017, 139, 10880–10886 CrossRef CAS.
- (a) C. Xie, X. Zhen, Y. Lyu and K. Pu, Adv. Mater., 2017, 29, 1703693 CrossRef PubMed; (b) K. Nogita, K. Miki, N. Imaizumi, M. Oe, H. Mu and K. Ohe, J. Photochem. Photobiol., A, 2023, 438, 114547 CrossRef CAS; (c) K. Miki, N. Imaizumi, K. Nogita, M. Oe, H. Mu, W. Huo, H. Harada and K. Ohe, Bioconjugate Chem., 2021, 32, 1773–1781 CrossRef CAS PubMed.
- (a) Z. Zhao, C. B. Swartchick and J. Chan, Chem. Soc. Rev., 2022, 51, 829–868 RSC; (b) J. Huang and K. Pu, Angew. Chem., Int. Ed., 2020, 59, 11717–11731 CrossRef CAS; (c) Y. Liu, L. Teng, B. Yin, H. Meng, X. Yin, S. Huan, G. Song and X.-B. Zhang, Chem. Rev., 2022, 122, 6850–6918 CrossRef CAS; (d) Y. Zeng, T. Dou, L. Ma and J. Ma, Adv. Sci., 2022, 9, 2202384 CrossRef CAS.
- (a) Y. Kikukawa, T. Fukuda, A. Fuyuhiro, N. Ishikawa and N. Kobayashi, Chem. Commun., 2011, 47, 8518–8520 RSC; (b) T. Fukuda, Y. Kikukawa, R. Tsuruya, A. Fuyuhiro, N. Ishikawa and N. Kobayashi, Inorg. Chem., 2011, 50, 11832–11837 CrossRef CAS PubMed; (c) T. Fukuda and N. Ishikawa, Dyes Pigm., 2014, 109, 151–154 CrossRef CAS.
- J. L. Marshall, D. Lehnherr, B. D. Lindner and R. R. Tykwinski, ChemPlusChem, 2017, 82, 967–1001 CrossRef CAS PubMed.
- (a) R. Kaushik, N. Nehra, V. Novakova and P. Zimcik, ACS Omega, 2023, 8, 98–126 CrossRef CAS; (b) W. Xie, J. Jiang, D. Shu, Y. Zhang, S. Yang and K. Zhang, Molcules, 2023, 28, 4252 CrossRef CAS; (c) J. Dai, C. Ma, P. Zhang, Y. Fu and B. Shen, Dyes Pigm., 2020, 177, 108321 CrossRef CAS; (d) H. S. Jung, X. Chen, K. Seung and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC; (e) P. Anees, J. Joseph, S. Sreejith, N. V. Menon, Y. Kang, S. W.-K. Yu, A. Ajayaghosh and Y. Zhao, Chem. Sci., 2016, 7, 4110–4116 RSC; (f) H. Mu, K. Miki, T. Kubo, K. Otsuka and K. Ohe, Chem. Commun., 2021, 57, 1818–1821 RSC.
- (a) C. D. Molek, J. A. Halfen, J. C. Loe and R. W. McGaff, Chem. Commun., 2001, 2644–2645 RSC; (b) Y. Y. Karabach, M. N. Kopylovich, K. V. Luzyanin, M. F. C. Guedes da Silva, V. Yu. Kukushkin and A. J. L. Pombeiro, Inorg. Chem. Acta, 2017, 455, 696–700 CrossRef CAS.
- M. E. Ourailidou, P. Dockerty, M. Witte, G. J. Poelarends and F. J. Dekker, Org. Biomol. Chem., 2015, 13, 3648–3653 RSC.
- No generation of 1O2 from NiPc was reported. See: G. F. M. Pereira and T. T. Tasso, Inorg. Chim. Acta, 2021, 519, 120271 CrossRef CAS.
- K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Nat. Chem., 2017, 9, 279–286 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, MTT assay, spectroscopic, and NMR spectra. CCDC 2303413. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05628g |
| This journal is © The Royal Society of Chemistry 2024 |