Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Post transition metal substituted Keggin-type POMs as thin film chemiresistive sensors for H2O and CO2 detection†
Abigail A.
Seddon
 a,
Nathan S.
Hill
a,
Nathan S.
Hill
 b,
Osama
El-Zubir
d,
Andrew
Houlton
d,
R. John
Errington
b,
Osama
El-Zubir
d,
Andrew
Houlton
d,
R. John
Errington
 a,
Pablo
Docampo
c and
Elizabeth A.
Gibson
a,
Pablo
Docampo
c and
Elizabeth A.
Gibson
 *a
*a
aEnergy Materials Laboratory, Chemistry, School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, UK. E-mail: Elizabeth.gibson@newcastle.ac.uk
bSchool of Mathematics, Statistics, and Physics, Newcastle University, Newcastle upon Tyne, UK
cSchool of Chemistry, University of Glasgow, Glasgow, UK
dChemical Nanoscience Labs, Chemistry, School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, UK. E-mail: osama.el-zubir@newcastle.ac.uk; andrew.houlton@newcastle.ac.uk
First published on 16th January 2024
Abstract
Chemiresitive sensing allows the affordable and facile detection of small molecules such as H2O and CO2. Herein, we report a novel class of Earth-abundant post transition metal substituted Keggin polyoxometalates (POMs) for chemiresistive sensing applications, with conductivities up to 0.01 S cm−1 under 100% CO2 and 65% Relative Humidity (RH).
Recently, the use of POM anions as chemiresistive gas sensors has been explored.1 Gas sensing is important for a wide range of applications such as the environmental and workplace monitoring of pollutants such as H2S and ammonia,2,3 sensing of H2(g) for the widespread implementation of hydrogen fuel sources,4 and within medical diagnostics for the identification of diseases.5–7 Chemiresistive gas sensors are one of the most commercialised gas sensing technologies as they are simple to produce and utilise cheap and widely available materials.1
A chemiresistor is a material which, in response to changes in the chemical environment, will change its electrical resistance.18 The direct interaction between the analyte and the material by hydrogen bonding, covalent bonding, or alternate intermolecular interactions is essential.8 Simply, a basic device consists of a pair of interdigitated electrodes bridged by the sensing material. The difference in resistance between the two electrodes in the absence and presence of an analyte can be recorded. Traditional inorganic semiconductor materials including TiO2 and SnO2 and organic conductive polymers such as polyaniline are used for chemiresistive sensing applications.9,10 The performance of inorganic semiconductor gas-sensitive materials is limited by rapid electron–hole recombination. POMs efficiently accept and store multiple electrons and protons, and these properties may reduce charge recombination between the electrodes. POMs can also be functionalised through their structure, elemental composition and cations, so their sensitivity to specific small molecules can be tuned.11 Recent examples include a resistive humidity sensor based on a Keggin H3PMo12O40-polypyrrole nanocomposite which was prepared by co-electrodeposition.12 The sensor showed a rapid response and recovery time (1.9/1.1 seconds respectively at 98% RH), a sensing range of 11–98% RH, excellent durability, and repeatability with little hysteresis. POMs have been reported as efficient proton conductors,13–15 as they have a decreased effective surface charge density as their negative charge is delocalised over peripheral oxygen atoms. A landmark study is that of Bourlinos and coworkers, who combined the acid-salt of [PW12O40]3− with a bulky PEG-containing quaternary ammonium cation, producing a liquid which exhibits high proton conductivity (10−3 S cm−1).15 Chemiresistive sensors for CO2 detection have been evidenced in the literature, a Cu3HIB2 MOF has been reported to detect CO2 with a limit of 400–2500 ppm, independent of humidity between 10–80%.16 There are currently no reports of a similar system using POMs. In this communication, the conductivity of films with a novel series of post transition metal substituted POMs are measured to assess if they would be beneficial to use in sensing devices.
POMs were synthesised in acetonitrile via base degradation of Na3PW12O40 with methanolic TBA(OH) to yield the lacunary species, followed by a substitution reaction with the appropriate post-TM salt as outlined in the (ESI†).17–21 Simple chemiresistive devices were fabricated with two interdigitated indium tin oxide (ITO) coated electrodes bridged by the sensing material (Fig. S1, ESI†). The conductivity of the thin films was measured from 0–100% RH, displaying a large reversible increase in response with humidity from 2 × 10−6 S m−1 to 0.01 S m−1, with a detection limit of 20–80% RH. Additionally, a significant increase in conductivity was observed when these devices were measured in the presence of CO2, indicating their use as chemiresistive sensors for the detection of H2O and CO2.
The device assembly is described in detail in the ESI.† Briefly, interdigitated electrodes were fabricated from ITO/glass substrates, with a channel etched between two electrodes. Thin films of POM were deposited on the electrodes, the film thickness was measured using atomic force microscopy (AFM), and the resistance was measured using 2-point current–voltage (I–V) sweeps. Two-point probe measurements are a useful tool to measure the conductivity of a material on a substrate with a low current output. Films of polyaniline (PANI) were also fabricated and measured as a reference for proton conduction. Measurements were undertaken in a humidity-controlled chamber, from 0–100% RH. Films of polymethyl methacrylate (PMMA, see ESI† for details) were also measured as a reference for film morphology, thickness, and for a reference in humidity measurements as its conductivity should not vary with humidity, proving differences in humidity observed were not due to water adsorbed on the surface.22 The conductivity of the films was calculated using these data following the procedure outlined in the ESI.†
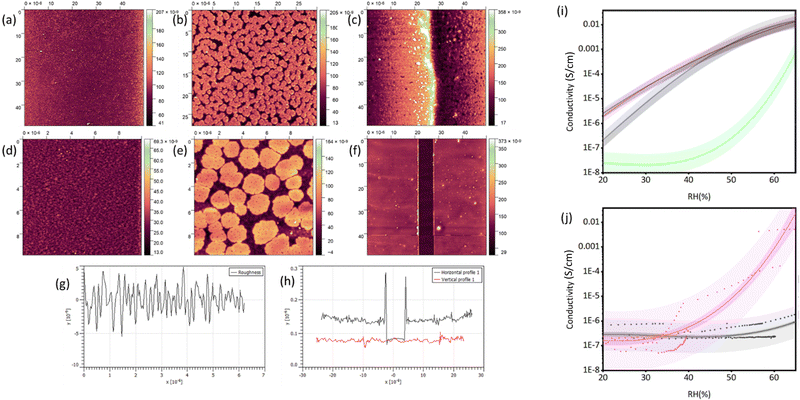
The AFM data on both the ITO electrode and on the glass are shown in Fig. 1 and Fig. S3–S6 (ESI†). These reveal the morphology of the film, average roughness (Ra) and film thickness, which was obtained by a line scan across a scratched mark. Some variation between samples in terms of roughness and particle size were observed as the substituent in the POM was changed. The film thickness varied significantly with the heterometal (Table 1). Films of (TBA)6[NaPW11O39] were the roughest and least homogeneous, with Rq an order of magnitude higher than for (TBA)4[BiPW11O39] (Table 1). The substituted POM films were smooth, with measured Ra values from 2.5 to 7.99 nm (Table 1). The same fabrication methodology was used for all POM films, so any differences in thickness and roughness are likely to be due to the different solubilities of the POMs, which influence the packing or interaction of the POM on the surface thus the way the film dries. Again, very small aggregates were observed on the ITO covered areas, and larger aggregates were observed in the ITO-free channel. This is likely due to the difference in surface charge between glass and ITO. The ITO surface is more positively charged,23 so attracts the POM anions resulting in larger aggregates.
| POM | Film thickness (nm) | R a roughness (nm) | R q roughness (nm) | Conductivity at 0% RH (S cm−2) |
|---|---|---|---|---|
| (TBA)6[NaPW11O39] | 147.7 ± 15.1 | 21.51 ± 1.28 | 29.47 ± 1.34 | 4.38 × 10−8 |
| (TBA)4[BiPW11O39] | 54.5 ± 3.3 | 2.50 ± 0.04 | 3.07 ± 0.05 | 1.22 × 10−7 |
| (TBA)5[PbPW11O39] | 878.4 ± 4.9 | 5.22 ± 0.06 | 6.48 ± 0.07 | 2.21 × 10−7 |
| (TBA)4[SbPW11O39] | 64.5 ± 2.3 | 7.99 ± 0.05 | 6.14 ± 0.06 | 1.93 × 10−8 |
As expected, the PMMA films were uniform and smooth, and they had a thickness of ca. 200 nm (Fig. S3, ESI†).16 For the thin film of (TBA)6[NaPW11O39], both large (2.5 μm) and small (< 500 nm) aggregates of POM were observed on the surface of the electrode (Fig. S4(c), ESI†) leading to a relatively high roughness (Ra = 21.51 ± 1.28 nm). In Fig. S4(b) (ESI†), the formation of lamellae can be observed on the part of the film over the ITO (non-active area). The film thickness was over 100 nm. Compared to (TBA)6[NaPW11O39] in Fig. S4 (ESI†), the (TBA)4[BiPW11O39] POM produced significantly smoother and more homogenous films (Fig. 1). Aggregates were still observed on the electrode surface (Fig. 1(b)), but they were more consistent in size and distribution, roughly 1.5 μm in diameter. The POM aggregates were larger in size in the laser-etched electrode channel than on the remaining ITO coated areas (Fig. 1(a) and (d)). The film was also thinner, with the scratch height being around 50 nm. The thickest film, over 800 nm, was observed for (TBA)5[PbPW11O39] (Fig. S5(e), ESI†). These films were more homogenous than for (TBA)4[BiPW11O39] but contained many large aggregates on the surface causing increased roughness (Fig. S5(a) and (f), ESI†). Films of (TBA)4[SbPW11O39] (Fig. S6, ESI†) were similar in morphology to (TBA)6[NaPW11O39] (Fig. S4, ESI†), with many inhomogeneous aggregates of varying size on the electrode surface. The film thickness, 64.5 nm, was closer to that of (TBA)4[BiPW11O39].
Conductivity measurements were firstly benchmarked with a blank electrode (or glass), thick (200 nm) and thin (100 nm) films of PANI and PMMA (Fig. S7, ESI†). As expected, glass and PMMA showed no change with an increase in humidity, but the conductivity of PANI did change above ca. 40% humidity from 1 × 10−6 S m−1 at 35% RH to 0.003 S m−1 at 100% RH. This was expected as PANI is a known proton-conducting material.24–27 Next, the measurements were performed with the POM electrodes and the data is presented in Fig. 1(i). Notably, the substituents play an important role on the conductivities observed at varying levels of RH in air. In Fig. 1(j) and (k), the conductivity of the films varies, with a different response observed depending on the substituent of the POM. The films of (TBA)6[NaPW11O39], (TBA)4[BiPW11O39], and (TBA)5[PbPW11O39] show responsiveness over a wider humidity range, and conductivity increases proportionally with humidity. POMs in solution exhibit proton conductivity via outer sphere proton-coupled electron-transfer mechanism.28 In solid-state, proton conductivity occurs by either vehicular proton migration or proton-hopping for a high number of water molecules, or via the Grotthuss transfer mechanism for pseudoanhydrous or totally anhydrous proton transfer activity.29,30 Overall, the highest conductivity at the highest humidity for the best performing devices was around 0.01 S cm−1 at 65% RH, which is significantly higher than previous literature reports (10−3 S cm−1),15 whilst operating at comparable humidity ranges. High proton conductivity arises via the Lewis acid centres, Na+/Bi3+/Pb2+, and the POM acts as a Lewis base support. Differences observed in proton conductivity are related to the strength of the Lewis acid centre. Lewis acidity decreases from Bi(III) to Pb(II) and the Sb(III).31,32 This has been shown previously with a crystalline composite of an Al3+-oxocluster with a wheel-shaped POM, [H7P8W48O184]33−.33 Researchers reported ultrahigh proton conductivity of >10−2 S cm−1, which was attributed to the synergistic effect of the Lewis acid–Lewis base pairs which compensate for charge migration. This allows direct proton transfer from a Lewis acid (proton-donor) to a Lewis base (proton-acceptor) via the Grotthuss mechanism.34 Consequentially, the use of substituted POMs as humidity sensing materials is demonstrated, whereby choice of substituent effects the response of the device. As the Lewis acidity of the substituent is increased, the sensitivity to RH is increased, especially in the mid-range.
At humidities above 40%, the conductivity of the (TBA)4[BiPW11O39] POM film increased substantially, up to 0.015 S m−2 at 65% humidity, when the air flowing over the sample was replaced with CO2 (Fig. 1(j)). None of the other POM films measured showed a response to CO2. Under N2 rather than CO2, at the same humidity, the conductivity was significantly lower, at around 10−6 S m−2. The greater sensitivity of the POM to humidity in the presence of CO2 indicates some interaction between the Bi in the POM and the CO2. It is widely accepted that Lewis-acidic metals such as Cu(II), Pb(II), Bi(III), and Zn(II) promote CO2 reduction.32,35–38 The Bi(III)-POM is more readily reduced, or more readily accepts electron density, than the Pb(II)-POM species (Fig. S8–S11, ESI†),39 therefore increasing sensitivity to CO2. This demonstrates the potential use of substituted POM films as chemiresistive sensing materials for CO2 detection. The different conductivity for (TBA)4[BiPW11O39] in humid air vs. humid N2 (Fig. 1i–k) could be due to interactions with O2 as well as CO2 whereby the Lewis acidic site coordinates with Lewis-basic molecular oxygen.40,41 Further work is needed to compare the selectivity of the system towards different small molecules and how this might be tuned by the substituents (e.g. Lewis acid–base).
In summary, a series of substituted lacunary Keggin POMs have been deposited onto interdigitated electrodes for the chemiresistive sensing of H2O and CO2, with clear differences observed in both film morphology and activity as the heterometal substituent is varied. This demonstrates the use of POMs in sensing devices, with a large increase in conductivity observed as humidity is increased. The observed response in reversible, with films being stable to multiple cycles of exposure to humidity and CO2, as shown in Fig. S13 (ESI†). Further to this, (TBA)4[BiPW11O39] thin-film electrodes were tested as CO2 sensors, again with promising results. A significant increase in current response was observed when the film was exposed to CO2 in the presence of H2O. However further work is needed to assess the detection limits of CO2, and the dependence of humidity on the response observed. This demonstrates the use of POMs within chemiresistive sensing technologies for the detection of small molecules as affordable and Earth-abundant materials with applications in product storage, medical testing, and workplace monitoring.
We are grateful to EPSRC for funding (EPRS1309X1, EP/S031170).
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. Song and T. Wang, ACS Sens., 2022, 7, 3634–3643 CrossRef CAS PubMed.
- G. Barandun, M. Soprani, S. Naficy, M. Grell, M. Kasimatis, K. L. Chiu, A. Ponzoni and F. Güder, ACS Sens., 2019, 4, 1662–1669 CrossRef CAS PubMed.
- B. K. S. Reddy and P. H. Borse, J. Electrochem. Soc., 2021, 168, 057521 CrossRef CAS.
- W. T. Koo, H. J. Cho, D. H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I. D. Kim, ACS Nano, 2020, 14, 14284–14322 CrossRef CAS PubMed.
- N. Nasiri and C. Clarke, Sensors, 2019, 19, 462–479 CrossRef.
- T. Kuchmenko, A. Shuba, R. Umarkhanov and L. Lvova, Chemosensors, 2021, 9, 116–132 CrossRef CAS.
- S. S. Shetty, A. Jayarama, I. Karunasagar and R. Pinto, Mater. Today Proc., 2022, 55, 122–126 CrossRef CAS.
- F.-G. Bănică, Chemical Sensors and Biosensors, John Wiley & Sons, Ltd, 2012, pp. 217–245 Search PubMed.
- B. Mondal and P. K. Gogoi, ACS Appl. Electron. Mater., 2022, 4, 59–86 CrossRef CAS.
- B. K. S. Reddy and P. H. Borse, J. Electrochem. Soc., 2021, 168, 057521 CrossRef CAS.
- A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605–7622 RSC.
- J. Miao, Y. Chen, Y. Li, J. Cheng, Q. Wu, K. W. Ng, X. Cheng, R. Chen, C. Cheng and Z. Tang, ACS Appl. Nano Mater., 2018, 1, 564–571 CrossRef CAS.
- T. Iwano, K. Shitamatsu, N. Ogiwara, M. Okuno, Y. Kikukawa, S. Ikemoto, S. Shirai, S. Muratsugu, P. G. Waddell, R. J. Errington, M. Sadakane and S. Uchida, ACS Appl. Mater. Interfaces, 2021, 13, 19138–19147 CrossRef CAS PubMed.
- N. Ogiwara, T. Iwano, T. Ito and S. Uchida, Coord. Chem. Rev., 2022, 462, 214524 CrossRef CAS.
- A. B. Bourlinos, K. Raman, R. Herrera, Q. Zhang, L. A. Archer and E. P. Giannelis, J. Am. Chem. Soc., 2004, 126, 15358–15359 CrossRef CAS PubMed.
- R. J. Errington, in Advances in Inorganic Chemistry, eds. R. van Eldik and L. Cronin, Academic Press, 2017, vol. 69, pp. 287–336 Search PubMed.
- T. Izuagie, PhD thesis, Newcastle University, 2017.
- R. J. Errington, in Comprehensive Coordination Chemistry II, ed. E. Constable, J. A. McClaverty and T. J. Meyer, Elsevier, Amsterdam, 2003, vol. 2, pp. 759–773 Search PubMed.
- R. J. Errington, in Polyoxometalate Molecular Science, ed. J. Borrás-Almenar, A. M. E. Coronado and M. Pope, Springer, Netherlands, II., 2003, vol. 1, pp. 55–78 Search PubMed.
- R. J. Errington, in Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, ed. M. T. Pope and A. Muller, Springer, Dordrecht, Dordrecht, 2001, vol. 1, pp. 1–22 Search PubMed.
- S. D. Wu and A. H. Chiou, Sci. Rep., 2021, 11, 1–12 CrossRef CAS PubMed.
- Md. R. Akanda, A. M. Osman, M. K. Nazal and Md. A. Aziz, J. Electrochem. Soc., 2020, 167, 037534 CrossRef.
- L. Yue, Y. Zheng, Y. Xie, S. Liu, S. Guo, B. Yang and T. Tang, RSC Adv., 2016, 6, 68599–68605 RSC.
- W. W. Focke and G. E. Wnek, Conduction mechanisms in polyaniline (emeraldine salt), Elsevier, Sequoia S.A., 1988, vol. 256 Search PubMed.
- Z. Yang, D. H. Coutinho, R. Sulfstede, K. J. Balkus and J. P. Ferraris, J. Membr. Sci., 2008, 313, 86–90 CrossRef CAS.
- J. Stejskal, O. E. Bogomolova, N. V. Blinova, M. Trchová, I. Šeděnková, J. Prokeš and I. Sapurina, Polym. Int., 2009, 58, 872–879 CrossRef CAS.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 RSC.
- A. Martinelli, J. M. Otero-Mato, M. N. Garaga, K. Elamin, S. M. H. Rahman, J. W. Zwanziger, U. Werner-Zwanziger and L. M. Varela, J. Am. Chem. Soc., 2021, 143, 13895–13907 CrossRef CAS PubMed.
- Y. Zhou, J. Yang, H. Su, J. Zeng, S. P. Jiang and W. A. Goddard, J. Am. Chem. Soc., 2014, 136, 4954–4964 CrossRef CAS PubMed.
- P. Yang, M. Alsufyani, A. H. Emwas, C. Chen and N. M. Khashab, Angew. Chem., Int. Ed., 2018, 57, 13046–13051 CrossRef CAS PubMed.
- T. Ogawa, H. Ohashi, T. Tamaki and T. Yamaguchi, Phys. Chem. Chem. Phys., 2013, 15, 13814–13817 RSC.
- C. J. Chang, Y. A. Lai, Y. C. Chu, C. K. Peng, H. Y. Tan, C. W. Pao, Y. G. Lin, S. F. Hung, H. C. Chen and H. M. Chen, J. Am. Chem. Soc., 2023, 145, 6953–6965 CrossRef CAS PubMed.
- J. Ramler and C. Lichtenberg, Chem. – Eur. J., 2020, 26, 10250–10258 CrossRef CAS PubMed.
- B. Chen and R. Neumann, Eur. J. Inorg. Chem., 2018, 791–794 CrossRef CAS.
- Q. Wang, Z. Miao, Y. Zhang, T. Yan, L. Meng and X. Wang, ACS Catal., 2022, 12, 4016–4025 CrossRef CAS.
- H. Agarwala, X. Chen, J. R. Lyonnet, B. A. Johnson, M. Ahlquist and S. Ott, Angew. Chem., Int. Ed., 2023, 62, e202218728 CrossRef CAS PubMed.
- S. Fukuzumi, K. Ohkubo, Y. M. Lee and W. Nam, Chem. – Eur. J., 2015, 21, 17548–17559 CrossRef CAS PubMed.
- Q. Yang, W. Xu, S. Gong, G. Zheng, Z. Tian, Y. Wen, L. Peng, L. Zhang, Z. Lu and L. Chen, Nat. Commun., 2020, 11, 5478, DOI:10.1038/s41467-020-19309-4.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05660k |
| This journal is © The Royal Society of Chemistry 2024 |