A novel pH-activated AIEgen probe for dynamic lysosome tracking and high-efficiency photodynamic therapy†
Yingcai
Hu
 a,
Sheng-Yan
Yin
a,
Ting
Deng
*b and
Jishan
Li
a,
Sheng-Yan
Yin
a,
Ting
Deng
*b and
Jishan
Li
 *a
*a
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China. E-mail: jishanli@hnu.edu.cn; Fax: +86-731-88821848
bInstitute of Applied Chemistry, School of Science, Central South University of Forestry and Technology, Changsha 410004, China. E-mail: muzi_dt@hotmail.com
First published on 20th February 2024
Abstract
A novel AIEgen molecular probe (N-3QL) with typical AIE effects, good biocompatibility, lysosome targeting, pH activation, excellent photostability, and high brightness was synthesized using two simple synthetic steps. Spectroscopic and cytotoxicity experiments indicate that N-3QL can not only be used for the dynamic monitoring of cancer cell lysosomes, but also for photodynamic therapy (PDT) ablation of cancer cells.
As an important organelle, lysosomes can participate in many cellular activities such as cell proliferation and apoptosis. Lysosomes contain about 60 types of hydrolases, which can degrade unnecessary macromolecules (such as polypeptides, polysaccharides, and nucleic acids) under acidic conditions for recycling cellular waste.1,2 Endocytosis and autophagy pathways of lysosomes make it an important organelle for cellular homeostatic catabolism. Therefore, lysosomes have been considered as “garbage disposal stations” for a long time.3,4 Moreover, lysosomes play important roles in signal transduction, plasma membrane repair, cholesterol homeostasis, and mineralization.5 Therefore, the destruction of lysosomes will cause cellular dysfunction and ultimately lead to cell death. Furthermore, in addition to the pH difference in the extracellular fluid, the lysosomal pH in cancer cells (pHlys 3.8–4.7) is lower than that in normal cells (pHlys 4.5–6.0).6 This allows cancer cells to maximize the enzymatic activity of extracellular proteases, rapidly degrade the extracellular matrix, and accelerate cancer cell invasion and metastasis.7,8 Moreover, cancer cells have additional lysosomes, which can be used as the target of photosensitizers (PSs) and have higher utilization value for photodynamic therapy (PDT).9 Moreover, as soon as the lysosome is destroyed, it causes the release of protons and hydrolases, which triggers subcellular organelle dysfunction and leads to apoptosis and necrosis. However, the photosensitivity of traditional PSs is always “on,” which can damage normal tissues.10 Therefore, the development of target-activated PSs for the long-term visualization of lysosomes is extremely promising for exploring lysosome physiological function research and high-efficiency PDT.
To date, many commercial fluorescent probes, such as lysosome green, neutral red, and acridine orange, have been used for detecting lysosomes.11 However, there are certain problems with such probes, such as small Stokes shift, severe photobleaching, and aggregation-induced quenching (ACQ), and their applications in living organisms are limited.12–16 Moreover, traditional PSs that act as protagonists in PDT have similar problems. ACQ considerably affects and reduces the generation ability of reactive oxygen species (ROS), which in turn considerably limits the effect of PDT.17 After the discovery of aggregation-induced emission (AIE) in 2001, which not only solved the ACQ problem of traditional fluorescent dyes, but also gave high photostability, good biocompatibility, excellent ROS generation ability, and highly tunable structures, AIE gradually became a hot field of biomedical fluorescence imaging and PDT-related research.18–23 The rapid development of PDT has been promoted by the large number of suborganelle-targeting AIE photosensitizers.23,24
Although a few AIE PSs targeting lysosomes have been reported, their photosensitivity gets activated and damages normal tissues.2,24,25 Recently, a study proposed pH-activated lysosome-targeted AIE PSs, which use a low pH value to change the aggregation state of PSs and enhance photosensitivity for PDT. Similarly, it retains good photosensitivity in the normal state and is prone to “false positive” reactions, which do not belong to true pH-activated AIE PSs.26 Therefore, it is urgent to develop a probe that can target the lysosomes of cancer cells and is activated by pH. This can greatly reduce the damage to normal tissues. We herein developed a pH-activated AIE molecular probe for the dynamic tracking of lysosomes and high efficiency PDT ablation of cancer cells (Scheme 1). This pH-activated molecular probe of N-3QL was synthesized using two simple synthetic steps: (1) Fe-catalyzed synthesis of 2-vinylquinolines via sp3 C–H functionalization and subsequent C–N cleavage and (2) the Heck reaction. When the N-3QL molecule target enters the lysosome, it will be protonated to N-3QL+ with a strong push–pull electronic structure under the action of reduced pH, thus resulting in red-shifted fluorescence emission and turn-on photosensitivity. This behavior helps in realizing the high-fidelity dynamic tracing of lysosomes owing to its excellent photostability and highly efficient PDT ablation of cancer cells due to the excellent 1O2-generation ability of N-3QL+.
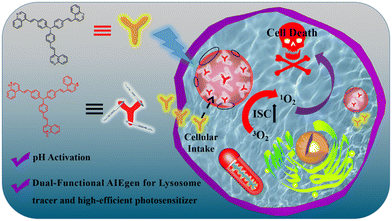 | ||
| Scheme 1 Schematic illustration of lysosome fluorescence tracking and PDT ablation of cancer cells using a pH-activated AIE molecular probe of N-3QL. | ||
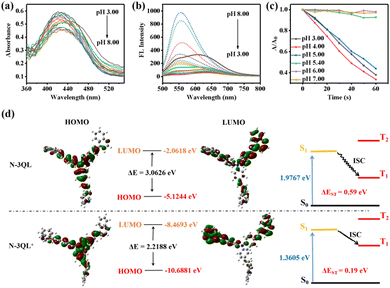
Scheme S1 (ESI†) shows the synthetic steps of N-3QL and the obtained product of N-3QL is characterized using MS, 1H NMR, and 13C NMR spectra (Fig. S1–S3 in ESI†). We then investigated the UV absorption and the fluorescence emission of N-3QL at different pHs (Fig. 1a and b). Fig. 1a shows that there is no obvious red shift in the UV absorption of N-3QL under different pHs. However, in the fluorescence emission spectra under different pHs (Fig. 1b), the fluorescence intensity gradually weakens with a reduction in the pH value. When the pH is 5.40, two groups of emission peaks appear, and the emission peaks of N-3QL are observed at 550/625 nm. The above-mentioned results indicate that the pKa of the N-3QL molecule is 5.52 (Fig. S4 in ESI†), which agrees with the lysosomal pH range of 3.8–4.7 in cancer cells. Moreover, with reduction in the pH value, the fluorescence intensity gradually weakened. The PL change of N-3QL was primarily attributed to the protonation of quinoline of N-3QL, which enhanced the electron-withdrawing ability of the pyridine moiety and enhanced the twisted intramolecular charge transfer (TICT) effect. The molecules in the excited state decayed to the ground state via a non-radiative pathway, and the fluorescence intensity considerably reduced. However, N-3QL still has good fluorescence intensity at low pH values (Fig. S5 in ESI†). We thus report that N-3QL has AIE properties and has good stability in cell culture media containing serum (Fig. S6 and S7 in ESI†).
Next, we examined the effect of different pH values on the photosensitivity of N-3QL (Fig. 1c and Fig. S8 in ESI†). The experimental results in Fig. 1c and Fig. S4 (ESI†) demonstrated that singlet oxygen (1O2) is produced after the degradation of the indicator 9,10-anthracyl (methylene) dimalonic acid (ABDA) when the pH is below 5.40. The reason is that the pKa of N-3QL is ∼5.40; thus, it is protonated to N-3QL+ at a low pH, which enhances the push–pull ability of the molecule and promotes the opening of photosensitive properties. This in turn provides a theoretical basis for pH activation.
The increase in the highest occupied molecular orbital (HOMO) energy or the decrease of the lowest unoccupied molecular orbital (LUMO) energy, i.e., the decrease of the HOMO–LUMO energy gap (ΔE) of the PS molecules, will cause a red-shifted emission in the fluorescent molecules and reduce the energy level difference (ΔEST) between the singlet state (S1) and triplet state (T1) of the molecules, thus promoting the intersystem crossing (ISC) process and enhancing their photosensitive efficiency.27 To understand the altered properties of N-3QL at a low pH, we performed density functional theory (DFT) calculations at the TD-B3LYP/6-31G(d) level (Fig. 1d). The HOMO and LUMO of the N-3QL molecule are −5.1244 and −2.0618 eV with a difference ΔE of 3.0626 eV; however, after protonation, the HOMO and LUMO changed to −10.6881 and −8.4693 eV with a difference ΔE of 2.2188 eV. The reduction in ΔE confirms the fluorescence emission red-shift. Furthermore, we determined that, after the protonation of N-3QL, S1 decreased from 1.9767 to 1.3605 eV, indicating that its fluorescence emission was red-shifted after protonation, which agreed with the above-mentioned experimental results.
Because the photosensitivity is related to ΔEST, the smaller the ΔEST, the better the photosensitivity. Therefore, we determined the ΔEST of the two before and after protonation and reported that the EST values before and after protonation of N-3QL were 0.59 and 0.19 eV, respectively. This demonstrated that the protonation of N-3QL improves the electronic push–pull performance of the PSs, which improves the photosensitivity and matches the experimental results illustrated in Fig. 1c. The calculation results support the design idea that N-3QL can be converted into a high-efficiency PS under the action of reduced pH conditions in lysosomes.
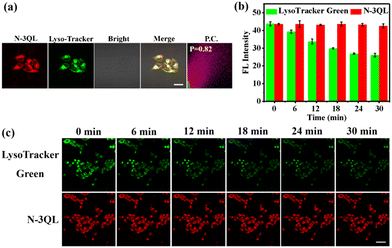
The above-mentioned buffer studies demonstrated that N-3QL can be converted into a highly efficient PS at a low pH. Because the cell-killing ability depended on the degree of its entry into the cell lysosome, a colocalization imaging experiment was performed to examine the distribution of N-3QL in cells. As can be seen in Fig. 2a and Fig. S9 (ESI†), after the incubation of HeLa cells with N-3QL for 0.5 h, strong red fluorescence emission was observed using confocal laser scanning microscopy (CLSM), which demonstrated that N-3QL can be effectively taken up by the cells. Furthermore, a yellow colocalization area of the red fluorescence (obtained from N-3QL) and the green fluorescence (obtained from LysoTracker Green and MitoTracker Green) was observed. Moreover, the Pearson's coefficients were 0.82 and 0.38, respectively, which indicated that N-3QL could target cancer cell lysosomes and confirmed the basis for lysosomal targeted activation. N-3QL selectively targets lysosomes possibly because its pKa value agrees with those of lysosomes and they have triphenylamine groups.26,28 We compared the difference between different cells by obtaining the fluorescence values in different bands (Fig. S10 in ESI†). The results demonstrated that N-3QL could distinguish normal cells from cancer cells to a certain extent, which was attributed to the difference in the lysosomal pH between normal cells and cancer cells.
Subsequently, we examined the intracellular photostability of N-3QL by comparing with the commercial LysoTracker Green (Fig. 2b and c). As per CLSM, the fluorescence intensity of N-3QL did not considerably decrease within 30 min; however, the LysoTracker Green intensity decreased by 40.4%, which indicated that N-3QL has satisfactory photostability and could be used for the fluorescence tracking of cellular lysosomes.
Owing to its high photostability, N-3QL was used to track the movement of intracellular lysosomes (Fig. 3 and Movie S1 in ESI†). During the observation period of 0–30 min, fluorescence imaging was performed at different time points (Fig. 3). We used different colors to represent the movement of lysosomes at different time points. In the combined images, the spatial distribution of lysosomes can be clearly identified using N-3QL fluorescence. Moreover, Movie S1 (ESI†) demonstrates a series of lysosomal fluorescence images taken at different times, which can clearly help in determining and monitoring the movement of lysosomes.
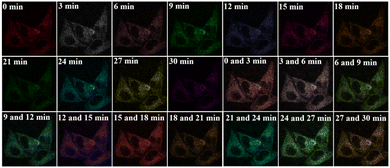 | ||
| Fig. 3 Real-time tracking of lysosomes (HeLa cell) with N-3QL (time points: 0, 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30 min). The merged images resulting from two different time points. | ||
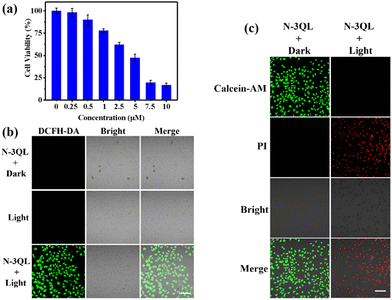
The phototoxicity and biocompatibility of N-3QL were also examined using a tumor cell line of HeLa and a normal cell line of L02 as models (Fig. 4a and Fig. S11a and b in ESI†). The CCK-8 experiment results illustrated in Fig. 4a indicated that the cell mortality of HeLa cells was proportional to the concentration of N-3QL. In particular, when the concentration of N-3QL was 5 μM, the cell viability of HeLa was <50%, and even when the concentration of N-3QL was only increased to 7.5 μM, the survival rate of HeLa cells was less than 20%. In contrast, even when the concentration of N-3QL reached 150 μM, the survival rate of normal cells (L02) was still about 50% (Fig. S11a, ESI†). Moreover, in the absence of light irradiation, the IC50 value of N-3QL is 750 μM (Fig. S11b, ESI†), indicating the good biocompatibility of N-3QL. Furthermore, cell viability experiments also showed that there was no significant effect on cell health or viability when the cells were irradiated with 405 nm light for 15 min (Fig. S11c in ESI†). Together all the above results indicate that N-3QL has high toxicity to cancer cells, which may be attributed to the ability to efficiently enter cells and target lysosomes as well, thereby further activating phototoxicity.
Then, the 1O2-generation ability of N-3QL in HeLa cells was investigated using DCFH-DA as an 1O2 indicator. As can be seen in Fig. 4b, no green fluorescence signal of the indicator was observed for the control groups, including those treated without N-3QL and those treated with N-3QL but without light irradiation. However, bright green fluorescence emission that can be attributed to the indicator can be observed for the experimental group, which was treated with N-3QL and light irradiation. All these results indicated that N-3QL was protonated in lysosomes and produced extremely toxic 1O2 under light irradiation. These results agreed with the experimental results of 1O2 assays in vitro (Fig. 1c).
Next, the cell killing mechanism of N-3QL was examined using the Calcein-AM/PI fluorescence imaging method. As can be seen in Fig. 4c, only the green fluorescence emission of Calcein-AM was observed for the “N-3QL + dark” group, while the red fluorescence emission of the PI channel was observed for the “N-3QL + light” group, indicating that N-3QL can kill HeLa cells only under the condition of light irradiation. These results agreed with the results of DCFH-DA fluorescence imaging. Furthermore, flow cytometry was used to examine the apoptosis of HeLa cells treated with N-3QL and light irradiation (Fig. S12 in ESI†). The experimental results illustrated in Fig. S12 (ESI†) demonstrated that the cell viability of all control groups was >81%. However, for the “N-3QL + light” group, the survival rate of HeLa cells was only 46.4% (Q4 region), while 43.3% were in an early apoptotic stage (Q3 region), 9.58% were in a middle apoptotic stage (Q2 region), and 0.73% were in a late apoptotic stage (Q1 region).
The above-mentioned experimental results have uniformly proved that N-3QL can produce a large amount of 1O2 with the assistance of light irradiation via effective protonation in lysosomes, and then achieve effective induction of cancer cell apoptosis.
In summary, we synthesized a novel pH-activated AIE photosensitizer of N-3QL and successfully applied it for dynamic lysosome tracking and high-efficiency PDT ablation of cancer cells. N-3QL can target cancer cell lysosomes and protonate into N-3QL+ with a strong push–pull electronic structure under acidic lysosomal conditions. It can not only achieve long-term high-fidelity tracking of lysosomes, but also generate a large amount of 1O2 to kill cancer cells. Theoretical calculations and in vitro/vivo experiments fully demonstrate that N-3QL is an excellent dual-functional AIE photosensitizer. This successful study will then expand the exploration of AIEgen's physiological functions in lysosomes and provide useful insights for improving the PDT efficiency of tumors.
This work was financially supported by the National Natural Science Foundation of China (22274039, 22376224).
Conflicts of interest
There are no conflicts to declare.Notes and references
- Z. Radisavljevic, J. Cell. Biochem., 2019, 120, 12123–12127 Search PubMed.
- W. Huang, Y. Zhang, X. Tan, N. Wang, J. Wang, M. He, J. Peng, J. Hu, Y. Zhao and S. Wang, Sens. Actuators, B, 2021, 335, 129698 Search PubMed.
- D. W. Lamming and L. Bar-Peled, Traffic, 2019, 20, 27–38 Search PubMed.
- J. E. Oyarzún, J. Lagos, M. C. Vázquez, C. Valls, C. De la Fuente, M. I. Yuseff, A. R. Alvarez and S. Zanlungo, Biochim. Biophys. Acta, Mol. Basis Dis., 2019, 1865, 1076–1087 Search PubMed.
- D. S. Leeman, K. Hebestreit, T. Ruetz, A. E. Webb, A. McKay, E. A. Pollina, B. W. Dulken, X. Zhao, R. W. Yeo, T. T. Ho, S. Mahmoudi, K. Devarajan, E. Passegué, T. A. Rando, J. Frydman and A. Brunet, Science, 2018, 359, 1277–1283 Search PubMed.
- G. Kroemer and M. Jäättelä, Nat. Rev. Cancer, 2005, 5, 886–897 Search PubMed.
- J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622–632 Search PubMed.
- J. Yin, L. Huang, L. Wu, J. Li, T. D. James and W. Lin, Chem. Soc. Rev., 2021, 50, 12098–12150 Search PubMed.
- V. Ramu, S. Gautam, P. Kondaiah and A. R. Chakravarty, Inorg. Chem., 2019, 58, 9067–9075 Search PubMed.
- Y. Hu, S.-Y. Yin, W. Liu, Z. Li, Y. Chen and J. Li, Aggregate, 2022, 3, e256 Search PubMed.
- M. Grossi, M. Morgunova, S. Cheung, D. Scholz, E. Conroy, M. Terrile, A. Panarella, J. C. Simpson, W. M. Gallagher and D. F. O'Shea, Nat. Commun., 2016, 7, 10855 Search PubMed.
- Y. Ke, J. Cao, J. Gong and N. Fu, Sens. Actuators, B, 2022, 352, 131015 Search PubMed.
- X. Wang, L. Wang, T. Jin, K. Sun and J. Yang, Sens. Actuators, B, 2023, 375, 132935 Search PubMed.
- Q. Xiao, H. Lin, J. Wu, X. Pang, Q. Zhou, Y. Jiang, P. Wang, W. Leung, H. Lee, S. Jiang, S. Q. Yao, L. Gao, G. Liu and C. Xu, J. Med. Chem., 2020, 63, 4896–48907 Search PubMed.
- Z. Li, J. Zou and X. Chen, Adv. Mater., 2022, 35, 2209529 Search PubMed.
- L. Gao, W. Wang, X. Wang, F. Yang, L. Xie, J. Shen, M. A. Brimble, Q. Xiao and S. Q. Yao, Chem. Soc. Rev., 2021, 50, 1219–1250 Search PubMed.
- X. Li, C. Y. Kim, S. Lee, D. Lee, H. M. Chung, G. Kim, S.-H. Heo, C. Kim, K.-S. Hong and J. Yoon, J. Am. Chem. Soc., 2017, 139, 10880–10886 Search PubMed.
- X. Li, C. Y. Kim, S. Lee, D. Lee, H. M. Chung, G. Kim, S.-H. Heo, C. Kim, K.-S. Hong and J. Yoon, J. Am. Chem. Soc., 2017, 139, 10880–10886 Search PubMed.
- S. Naghibi, T. Chen, A. Jamshidi Ghahfarokhi and Y. Tang, Aggregate, 2021, 2, e41 Search PubMed.
- Y. Zhou, J. Hua, D. Ding and Y. Tang, Biomaterials, 2022, 286, 121605 Search PubMed.
- C. Xiang, M. Dirak, Y. Luo, Y. Peng, L. Cai, P. Gong, P. Zhang and S. Kolemen, Mater. Chem. Front., 2022, 6, 1515–1521 Search PubMed.
- H. Li, H. Kim, J. Han, V.-N. Nguyen, X. Peng and J. Yoon, Aggregate, 2021, 2, e51 Search PubMed.
- Y. Shi, D. Zhu, D. Wang, B. Liu, X. Du, G. Wei and X. Zhou, Coord. Chem. Rev., 2022, 471, 214725 Search PubMed.
- J. Zhuang, H. Yang, Y. Li, B. Wang, N. Li and N. Zhao, Chem. Commun., 2020, 56, 2630 Search PubMed.
- Y. Dai, F. He, H. Ji, X. Zhao, S. Misal and Z. Qi, ACS Sens., 2020, 5, 225 Search PubMed.
- Y. Dai, F. He, H. Ji, X. Zhao, S. Misal and Z. Qi, ACS Sens., 2020, 5, 225 Search PubMed.
- C. Zhang, Y. Zhao, D. Li, J. Liu, H. Han, D. He, X. Tian, S. Li, J. Wu and Y. Tian, Chem. Commun., 2019, 55, 1450–1453 Search PubMed.
- R. Wang, X. Li and J. Yoon, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc06247c |
| This journal is © The Royal Society of Chemistry 2024 |