Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pillar[6]MaxQ functions as an in vivo sequestrant for rocuronium and vecuronium†
Wanping
Zhang‡
a,
Emmanuel A.
Bazan-Bergamino‡
b,
Anton P.
Doan
b,
Xiangjun
Zhang
*a and
Lyle
Isaacs
 *b
*b
aCollege of Pharmacy, Chongqing Medical University, Chongqing 400016, P. R. China. E-mail: xiangjunzhang@cqmu.edu.cn
bDepartment of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, USA. E-mail: LIsaacs@umd.edu
First published on 28th March 2024
Abstract
The binding affinity of pillar[6]MaxQ toward a panel of neuromuscular blockers and neurotransmitters was measured in phosphate buffered saline by isothermal titration calorimetry and 1H NMR spectroscopy. In vivo efficacy studies showed that P6MQ sequesters rocuronium and vecuronium and reverses their influence on the recovery of the train-of-four (TOF) ratio.
The development of supramolecular chemistry can be traced from early work on the fundamentals of non-covalent interactions, through the use of non-covalent interactions to build up multicomponent self-assembled systems, toward the development of supramolecular systems with application in chemistry, analytical chemistry, materials science, and medicine.1 Macrocycles have long played a starring role in supramolecular chemistry due to their inherent preorganization which can result in high binding affinity and high selectivity in organic solvents and in water. Some of the most popular macrocyclic hosts include cyclodextrins, calixarenes, resorcinarenes and related species, cucurbituril (CB[n]) type receptors, cyclophanes, molecular baskets, and most recently pillararenes.2 Macrocycles that display good water solubility and biocompatibility are well suited for a variety of biomedical applications. For example, β-cyclodextrin derivatives are used as solubilizing excipients to formulate a variety of insoluble drugs for human use.3 The Smith group has used tetralactam macrocycles as the basis of squaraine rotaxanes for imaging applications, whereas the Kim group used cucurbituril derived supramolecular hydrogels for stem cell therapy.4 The Guo group has used azo-calixarene derivatives for numerous applications including hypoxia imaging.5 Our group has demonstrated the use of CB[n]-type receptors – with their ultratight binding properties2d – as in vivo sequestrants to counteract the effects of a variety of biologically active substances.6 Perhaps the most stunning real-world application of a macrocyclic host is the clinical use of the γ-cyclodextrin derivative sugammadex to alleviate the post-surgical side effects of the neuromuscular blocking agents (NMBA) rocuronium (roc) and vecuronium (vec).1e,7 Subsequently, a variety of hosts including CB[n]-type, calixarenes, pillararenes, and organic frameworks have been investigated as reversal agents for neuromuscular blockers.8
In 2020, we reported the design and synthesis of sulfated pillar[n]arene derivatives dubbed pillar[n]MaxQ (PnMQ, Fig. 1) and showed that PnMQ display ultratight binding toward hydrophobic (di)cations in sodium phosphate buffered water with high selectivity for tertiary and quaternary ammonium ions including the neuromuscular blocking agents roc and vec.9 More recently, we showed that P6MQ displays good in vitro and in vivo biocompatibility according to a variety of assays (cytotoxicity, in vivo maximum tolerated dose, Ames test, hERG ion channel inhibition), binds tightly to a panel of drugs of abuse, and functions as an in vivo sequestrant for methamphetamine and fentanyl.10 Recently, other groups have reported the synthesis of sulfated versions of other macrocyclic arenes11 and studied their potential as reversal agents for cisatracurium and hexadimethrine.12 Herein, we report our study of the interaction of P6MQ with a broad panel of neuromuscular blockers and neurotransmitters (Fig. 1) in the more biologically relevant phosphate buffered saline (PBS) and in vivo efficacy studies demonstrating that P6MQ reverses the effects of roc and vec.
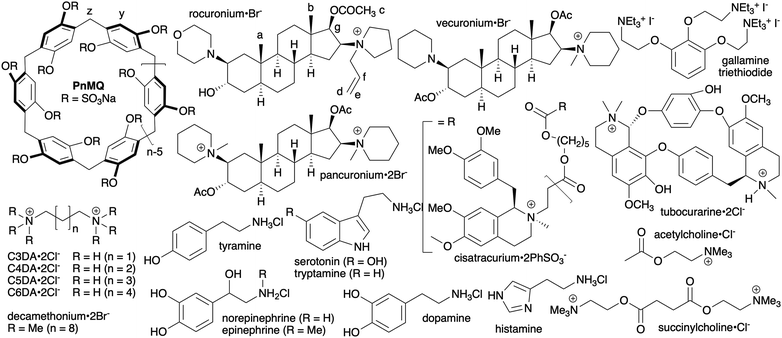 | ||
| Fig. 1 Chemical structures of P6MQ, neuromuscular blockers, neurotransmitters, and competitive guests used in this study. | ||
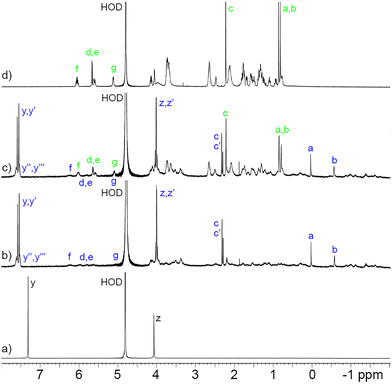
Before proceeding to measure the binding constants for the P6MQ·guest complexes, we first set out to glean their geometrical features. Previously, based on analysis of complexation induced changes in 1H NMR chemical shift, we deduced that P6MQ binds the hydrophobic regions of guests in its central hydrophobic cavity, whereas pendant cationic groups reside near the anionic sulfate groups.9,10,13 As an informative example, Fig. 2 shows the 1H NMR spectra recorded for P6MQ and roc. The 1H NMR of P6MQ (Fig. 2a) shows singlets for Hy and Hz which is consistent with a racemic mixture of planar chiral forms (pR and pS)14 of the depicted D6-symmetric conformation where the top and bottom sulfated portals are chemically equivalent. Fig. 2d shows the spectrum of roc with key resonances assigned. Upon formation of the P6MQ·roc complex (Fig. 2b), we observe that the resonances for the axial steroidal methyl groups (Ha and Hb) are shifted significantly upfield whereas the resonances for the acetoxy CH3 (Hc) and the pendant allyl group (Hd–Hg) undergo only very small changes in chemical shift. This pattern of chemical shift changes indicates that the hydrophobic steroidal skeleton is bound within the hydrophobic cavity of P6MQ, whereas the cationic units reside outside the cavity near the sulfate groups. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 P6MQ
2 P6MQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) roc ratio (Fig. 2c) we observe separate resonances for P6MQ·roc and uncomplexed roc, which indicates guest exchange is slow on the chemical shift timescale, which is commonly observed for tight complexes. Interestingly, for the P6MQ·roc complex (Fig. 2b) we observe splitting of Hz into an AB quartet (Hz, Hz′) due to the binding of the unsymmetrical guest which desymmetrizes the top and bottom portals of P6MQ as shown in the MMFF minimized molecular model presented in Fig. 3. Additionally, Hy splits into two sets of two singlets (Hy, Hy′ and Hy′′, Hy′′′) which we attribute to the presence of two diastereomeric complexes (pR-P6MQ·roc and pS-P6MQ·roc). The 1H NMR spectra for the complexes of P6MQ with vec, cisatracurium, gallamine triethiodide, decamethonium, succinylcholine, and acetylcholine display related complexation induced changes in chemical shift and are shown in the ESI.†
roc ratio (Fig. 2c) we observe separate resonances for P6MQ·roc and uncomplexed roc, which indicates guest exchange is slow on the chemical shift timescale, which is commonly observed for tight complexes. Interestingly, for the P6MQ·roc complex (Fig. 2b) we observe splitting of Hz into an AB quartet (Hz, Hz′) due to the binding of the unsymmetrical guest which desymmetrizes the top and bottom portals of P6MQ as shown in the MMFF minimized molecular model presented in Fig. 3. Additionally, Hy splits into two sets of two singlets (Hy, Hy′ and Hy′′, Hy′′′) which we attribute to the presence of two diastereomeric complexes (pR-P6MQ·roc and pS-P6MQ·roc). The 1H NMR spectra for the complexes of P6MQ with vec, cisatracurium, gallamine triethiodide, decamethonium, succinylcholine, and acetylcholine display related complexation induced changes in chemical shift and are shown in the ESI.†
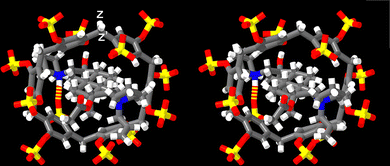 | ||
| Fig. 3 Cross-eyed stereoview of an MMFF minimized geometry of the P6MQ·roc complex. Color code: C, grey; H, white; N, blue; O, red; H-bonds, red-yellow stripped. | ||
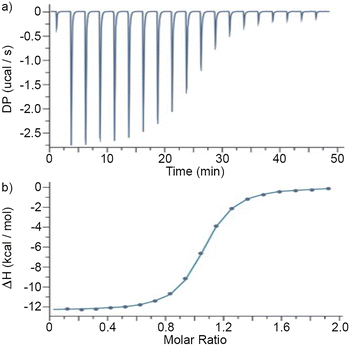
Next, we turned our attention to the measurement of the P6MQ·guest binding constants. Given the tight binding previously observed for P6MQ complexes, we decided to use isothermal titration calorimetry (ITC) as our main analytical tool because Ka values in the 103–107 M−1 range can be accurately measured by direct titrations and higher Ka values by competitive ITC titrations.15 The measurements were performed in the more competitive and biologically relevant PBS (pH 7.4) buffer. For the weaker complexes between P6MQ and all eight neurotransmitters and two NMBAs (cisatracurium and tubocurarine) we performed direct titrations in triplicate (ESI†) and report the average Ka, ΔH and Wiseman c-values in Table 1. For the tighter complexes with the NMBAs where appropriate Wiseman c-values could not be achieved in direct titrations,15b we performed competitive ITC titrations. For this purpose, we employed C3DA–C6DA, which bind with increasing Ka and different ΔH values as the chain length increases, thereby allowing the selection of an appropriate competitor for a given NMBA. The Ka and ΔH values for the P6MQ·competitor complexes were determined by direct titrations (Table 1) and used as known inputs to analyze the competitive ITC titrations. Fig. 4a shows a plot of DP versus time recorded when a solution of P6MQ (100 μM) and C6DA (1.00 mM) in the cell was titrated with a solution of roc (1.00 mM) from the syringe. Integration of the peaks in Fig. 4a allowed the construction of Fig. 4b which shows a plot of ΔH versus molar ratio. Fig. 4b was fitted to a competitive binding model to extract the Ka (2.53 × 1011 M−1) and ΔH (−22.03 kcal mol−1) values for the P6MQ·roc complex (Table 1). Please note that Fig. 4b plateaus at ≈−12.5 kcal mol−1 which represents the difference in ΔH values between the competing P6MQ·C6DA and P6MQ·roc complexes. The remaining tight complexes were measured analogously (Table 1). As expected, the stoichiometry of the P6MQ·guest complexes given in Table 1 are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as determined by the ITC n-value. The cavity of P6MQ is not large enough to comfortably form ternary complexes with even the smallest guests. The Ka values range from 103–1011 M−1 with large and negative ΔH values consistent with complexation driven by the non-classical hydrophobic effect.16P6MQ binds the steroidal NMBAs roc and vec with picomolar affinity which is 10
1 as determined by the ITC n-value. The cavity of P6MQ is not large enough to comfortably form ternary complexes with even the smallest guests. The Ka values range from 103–1011 M−1 with large and negative ΔH values consistent with complexation driven by the non-classical hydrophobic effect.16P6MQ binds the steroidal NMBAs roc and vec with picomolar affinity which is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold stronger than sugammadex which is used clinically.1e Importantly, P6MQ displays ≥105-fold selectivity for roc and vec relative to acetylcholine which is also present in the neuromuscular junction. P6MQ displays even higher levels of discrimination against the other neurotransmitters which suggests that P6MQ has great potential as an in vivo reversal agent for NMBAs.
000-fold stronger than sugammadex which is used clinically.1e Importantly, P6MQ displays ≥105-fold selectivity for roc and vec relative to acetylcholine which is also present in the neuromuscular junction. P6MQ displays even higher levels of discrimination against the other neurotransmitters which suggests that P6MQ has great potential as an in vivo reversal agent for NMBAs.
| K a (M−1) | ΔH (kcal mol−1) | c | |
|---|---|---|---|
| Conditions: PBS, 298.15 K.a Measured by direct ITC titration.b Measured by competitive ITC titration using a mixture of P6MQ and C3Da in the cell.c Measured by competitive ITC titration using a mixture of P6MQ and C4DA in the cell.d Measured by competitive ITC titration using a mixture of P6MQ and C6DA in the cell. | |||
| Rocuroniumd | (2.536 ± 0.004) × 1011 | −22.03 ± 0.02 | 90 |
| Vecuroniumd | (4.41 ± 0.01) × 1011 | −18.60 ± 0.01 | 150 |
| Decamethoniumd | (1.327 ± 0.001) × 1011 | −16.77 ± 0.01 | 49 |
| Gallaminec | (8.11 ± 0.44) × 109 | −13.53 ± 0.04 | 230 |
| Succinylcholinec | (9.20 ± 0.13) × 108 | −15.23 ± 0.02 | 26 |
| Pancuroniumc | (2.18 ± 0.11) × 108 | −11.60 ± 0.04 | 61 |
| Tubocurarinea | (5.03 ± 0.10) × 105 | −12.27 ± 0.05 | 50 |
| Cisatracuriuma | (3.89 ± 0.39) × 105 | −15.17 ± 0.26 | 39 |
| C3DAa | (5.95 ± 0.32) × 104 | −4.79 ± 0.06 | 15 |
| C4DAa | (3.53 ± 0.05) × 106 | −6.04 ± 0.01 | 350 |
| C5DAb | (3.43 ± 0.65) × 107 | −10.27 ± 0.15 | 55 |
| C6DAb | (2.73 ± 0.03) × 108 | −9.48 ± 0.03 | 45 |
| Acetylcholinea | (2.55 ± 0.15) × 106 | −9.29 ± 0.06 | 130 |
| Histaminea | (3.77 ± 0.20) × 105 | −15.47 ± 0.14 | 38 |
| Tyraminea | (3.19 ± 0.05) × 105 | −9.60 ± 0.03 | 5 |
| Dopaminea | (8.02 ± 0.76) × 104 | −9.40 ± 0.26 | 8 |
| Tryptaminea | (5.35 ± 0.11) × 104 | −9.40 ± 0.07 | 5 |
| Epinephrinea | (4.39 ± 0.17) × 104 | −9.44 ± 0.14 | 5 |
| Norepinephrinea | (9.06 ± 0.14) × 103 | −8.64 ± 0.05 | 5 |
| Serotonina | (9.04 ± 0.26) × 103 | −9.88 ± 0.11 | 5 |
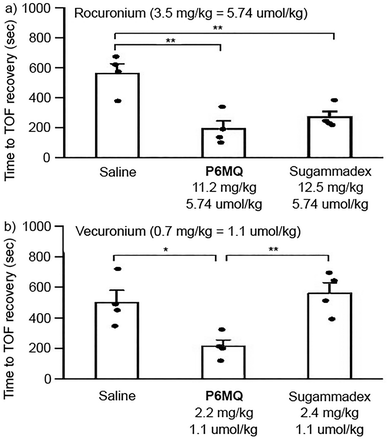
Given the exceptionally tight P6MQ·roc and P6MQ·vec complexes and the discrimination against neurotransmitters, we decided to perform in vivo efficacy studies. All studies were approved by the Institutional Animal Care and Use Committee at Chongqing Medical University (Approval number: IACUC-CQMU-2022-0025). A total of 12 male and 12 female Sprague-Dawley rats (220–320 g) were anesthetized with isoflurane and equipped with intravenous lines along with subcutaneous electrodes to stimulate the femoral nerve supramaximally and the response of the quadriceps femoris was monitored using the Veryark-TOF (Guangxi VERYARK Technology Co., Ltd, China). The femoral nerve was continuously stimulated for 10 minutes before recalibration of the Veryark-TOF and administration of rocuronium (3.5 mg kg−1, 5.74 μmol kg−1) or vecuronium (0.7 mg kg−1, 1.1 μmol kg−1). These doses of roc and vec were previously determined as twice the ED90 which is the dose required to decrease the twitch height by 90%.17 The rats were continuously ventilated and then the reversal agent (saline, P6MQ (5.74 μmol kg−1 for roc; 1.1 μmol kg−1 for vec), or sugammadex (5.74 μmol kg−1 for roc; 1.1 μmol kg−1 for vec)) were administered 30 seconds later. Please note that equimolar quantities of NMBA and reversal agent were administered. The time required for the train-of-four ratio to return to 90% of baseline T1 were determined and are plotted as a function of treatment group in Fig. 5. Fig. 5a shows that both P6MQ and sugammadex display comparable efficacy (p = 0.278) at accelerating the recovery of the train-of-four (TOF ratio to 90% of baseline when roc is used as NMBA relative to saline as placebo). In contrast, when vec (1.1 μmol kg−1) is used as NMBA (Fig. 5b), an equimolar dose of P6MQ (1.1 μmol kg−1) is effective at accelerating the recovery of the TOF ratio to 90% whereas an equimolar dose of sugammadex (1.1 μmol kg−1) is not effective.
In summary, we have established that P6MQ forms tight complexes with several NMBAs (roc, vec, decamethonium, gallamine triethiodide, succinylcholine, and pancuronium) in vitro and functions as a highly effective in vivo reversal agent for roc and vec. This work, when combined with our earlier work on the reversal of drugs of abuse using P6MQ10 and related work on sulfated macrocyclic arenes,12 further establishes the high potential of P6MQ in a variety of biomedical applications.
L. I., E. A. B.-B., and A. P. D. thank the University of Maryland for financial support. X. Z. and W. Z. thank the Chongqing Medical University for financial support.
Conflicts of interest
L. I. holds equity in Reversal Therapeutics (National Harbor, Maryland, USA) and Clear Scientific (Cambridge, Massachusetts, USA). The other authors have no conflicts to declare.Notes and references
-
(a) D. J. Cram, Angew. Chem., Int. Ed. Engl., 1988, 27, 1009–1020 CrossRef
; (b) J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef
; (c) J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094–11125 CrossRef CAS PubMed
; (d) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703–6712 RSC
; (e) A. Bom, M. Bradley, K. Cameron, J. K. Clark, J. Van Egmond, H. Feilden, E. J. MacLean, A. W. Muir, R. Palin, D. C. Rees and M.-Q. Zhang, Angew. Chem., Int. Ed., 2002, 41, 265–270 CrossRef CAS
.
-
(a) M. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1917 CrossRef CAS PubMed
; (b) F. Diederich, Angew. Chem., Int. Ed. Engl., 1988, 27, 362–386 CrossRef
; (c) J. H. Jordan and B. C. Gibb, Chem. Soc. Rev., 2015, 44, 547–585 RSC
; (d) K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394–418 RSC
; (e) T. Ogoshi, T.-A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed
; (f) H. Yin, X. Zhang, J. Wei, S. Lu, D. Bardelang and R. Wang, Theranostics, 2021, 11, 1513–1526 CrossRef CAS PubMed
; (g) V. Böhmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 713–745 CrossRef
; (h) T. J. Finnegan, V. W. L. Gunawardana and J. D. Badjic, Chem. – Eur. J., 2021, 27, 13280–13305 CrossRef CAS PubMed
.
- R. A. Rajewski and V. J. Stella, J. Pharm. Sci., 1996, 85, 1142–1169 CrossRef CAS PubMed
.
-
(a)
F. M. Roland and B. D. Smith, Supramol. Chem. Water, 2019, pp. 501–524 DOI:10.1002/9783527814923.ch13
; (b) J. Yeon, S. J. Kim, H. Jung, H. Namkoong, J. Tang, B. W. Hwang, K. Oh, K. Kim, Y. C. Sung and S. K. Hahn, Adv. Healthcare Mater., 2015, 4, 237–244 CrossRef PubMed
.
- Y.-C. Pan, J.-H. Tian and D.-S. Guo, Acc. Chem. Res., 2023, 56, 3626–3639 CrossRef CAS PubMed
.
-
(a) C.-L. Deng, S. L. Murkli and L. D. Isaacs, Chem. Soc. Rev., 2020, 49, 7516–7532 RSC
; (b) S. Ganapati and L. Isaacs, Isr. J. Chem., 2018, 58, 250–263 CrossRef CAS PubMed
.
- J. M. Adam, D. J. Bennett, A. Bom, J. K. Clark, H. Feilden, E. J. Hutchinson, R. Palin, A. Prosser, D. C. Rees, G. M. Rosair, D. Stevenson, G. J. Tarver and M.-Q. Zhang, J. Med. Chem., 2002, 45, 1806–1816 CrossRef CAS PubMed
.
-
(a) X. Zhang, Q. Cheng, L. Li, L. Shangguan, C. Li, S. Li, F. Huang, J. Zhang and R. Wang, Theranostics, 2019, 9, 3107–3121 CrossRef CAS PubMed
; (b) U. Hoffmann, M. Grosse-Sundrup, K. Eikermann-Haerter, S. Zaremba, C. Ayata, B. Zhang, D. Ma, L. Isaacs and M. Eikermann, Anesthesiology, 2013, 119, 317–325 CrossRef CAS PubMed
; (c) Y. Chai, L. Chen, Y. Zhang, L. Zhao, Z. Meng, J. Chen, C. Li and Q. Meng, Chin. Chem. Lett., 2022, 33, 3003–3006 CrossRef CAS
; (d) H.-K. Liu, F. Lin, S.-B. Yu, Y. Wu, S. Lu, Y.-Y. Liu, Q.-Y. Qi, J. Cao, W. Zhou, X. Li, H. Wang, D.-W. Zhang, Z.-T. Li and D. Ma, J. Med. Chem., 2022, 65, 16893–16901 CrossRef CAS PubMed
; (e) A. J. Selinger, N. A. Cavallin, A. Yanai, I. Birol and F. Hof, Angew. Chem., Int. Ed., 2022, 61, e202113235 CrossRef CAS PubMed
; (f) Y. Wu, Y.-Y. Liu, H.-K. Liu, S.-B. Yu, F. Lin, W. Zhou, H. Wang, D.-W. Zhang, Z.-T. Li and D. Ma, Chem. Sci., 2022, 13, 9243–9248 RSC
.
- W. Xue, P. Y. Zavalij and L. Isaacs, Angew. Chem., Int. Ed., 2020, 59, 13313–13319 CrossRef CAS PubMed
.
- A. T. Brockett, W. Xue, D. King, C.-L. Deng, C. Zhai, M. Shuster, S. Rastogi, V. Briken, M. R. Roesch and L. Isaacs, Chemistry, 2023, 9, 881–900 CrossRef CAS PubMed
.
- X.-N. Han, Y. Han and C.-F. Chen, Chem. Soc. Rev., 2023, 52, 3265–3298 RSC
.
-
(a) Y. Zhao, L. Chen, C. Junyi, J. Li, Q. Meng, A. C.-H. Sue and C. Li, Chem. Commun., 2023, 59, 5858–5861 RSC
; (b) R. Wang, W.-B. Li, J.-Y. Deng, H. Han, F.-Y. Chen, D.-Y. Li, L.-B. Jing, Z. Song, R. Fu, D.-S. Guo and K. Cai, Angew. Chem., Int. Ed., 2023, e202317402, DOI:10.1002/anie.202317402
.
-
(a) C.-L. Deng, M. Cheng, P. Y. Zavalij and L. Isaacs, New J. Chem., 2022, 46, 995–1002 RSC
; (b) D. King, C.-L. Deng and L. Isaacs, Tetrahedron, 2023, 145, 133607 CrossRef CAS
.
- J.-F. Chen, J.-D. Ding and T.-B. Wei, Chem. Commun., 2021, 57, 9029–9039 RSC
.
-
(a) T. Wiseman, S. Williston, J. F. Brandts and L.-N. Lin, Anal. Biochem., 1989, 179, 131–137 CrossRef CAS PubMed
; (b) J. Broecker, C. Vargas and S. Keller, Anal. Biochem., 2011, 418, 307–309 CrossRef CAS PubMed
; (c) A. Velazquez-Campoy and E. Freire, Nat. Protocols, 2006, 1, 186–191 CrossRef CAS PubMed
.
- E. Persch, O. Dumele and F. Diederich, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS PubMed
.
- M. Eikermann, S. Zaremba, A. Malhotra, A. S. Jordan, C. Rosow and N. L. Chamberlin, Br. J. Anaesth., 2008, 101, 344–349 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization, ITC thermograms, 1H NMR spectra of P6MQ·guest complexes, and details of in vivo studies. See DOI: https://doi.org/10.1039/d4cc00772g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |