Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Employing transfer-dominated branching radical telomerisation (TBRT) and atom transfer radical polymerisation (ATRP) to form complex polyester-polymethacrylate branched-linear star copolymer hybrids via orthogonal initiation†
Andrew B.
Dwyer
ab,
William
Sandy
 ab,
Faye Y.
Hern
ab,
Oliver B.
Penrhyn-Lowe
ab,
Samuel
McKeating
ab,
Sean
Flynn
ab,
Stephen
Wright
ab,
Sophie
Pate
ab,
Pierre
Chambon
ab and
Steve P.
Rannard
ab,
Faye Y.
Hern
ab,
Oliver B.
Penrhyn-Lowe
ab,
Samuel
McKeating
ab,
Sean
Flynn
ab,
Stephen
Wright
ab,
Sophie
Pate
ab,
Pierre
Chambon
ab and
Steve P.
Rannard
 *ab
*ab
aDepartment of Chemistry, University of Liverpool, Crown Street, L69 7ZD, UK. E-mail: srannard@liv.ac.uk
bMaterials Innovation Factory, University of Liverpool, Crown Street, L69 7ZD, UK
First published on 31st July 2024
Abstract
TBRT and ATRP are orthogonal initiation chemistries used in vinyl polymerisations. Here, we present the first combination of these techniques to readily create high molecular weight branched polyester macroinitiators capable of forming star copolymers from a range of methacrylate monomers.
Combinations of different polymer architectures have been of significant academic interest, generating highly interesting complex macromolecular structures using varied strategies. For example, linear-dendritic hybrids, and dendronized polymers have utilised approaches such as: (1) iterative or one pot dendron synthesis from preformed polymers;1,2 (2) dendron focal point propagation of linear polymers using step growth3 or chain growth4,5 mechanisms; (3) light branching6,7 or crosslinking;8 (4) propagation of dendritic macromonomers;9 and (5) click chemistry strategies.1,2
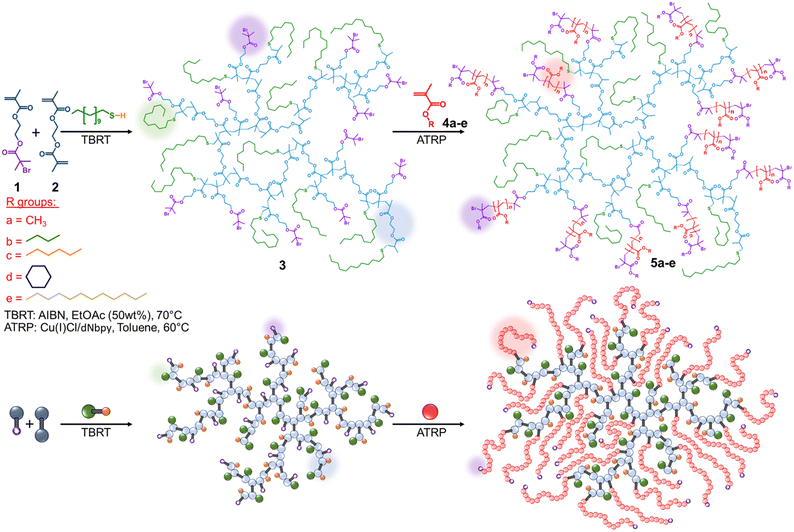
Highly branched polymers have been utilised as macroinitiators for extended hyperbranched polymerisation,10 and the formation of linear polymer arms to form core–shell structures.11–13 The use of self-condensing vinyl polymerisation14,15 to form hyperbranched macroinitiators has been a popular theme in such research, and utilises the polymerisation, or copolymerisation, of a vinyl monomer that is also able to undergo initiation of new chains; an inimer.16 The formation of macroinitiators using non-vinyl inimers17,18 has also been achieved, with the incorporation of ATRP initiator or reversible addition fragmentation chain-transfer (RAFT) agent functionality into lactone or alcohol structures, allowing the ring-opening copolymerisation of mono/bis-lactones. The resulting polyester “cores” contained numerous initiators/RAFT agents to mediate the controlled radical polymerisation of vinyl monomers.19 Here, we demonstrate the first use of the new polymerisation technique TBRT,20–26 to form branched polyester macroinitiators containing ATRP initiating functionality using conventional free radical chemistry techniques. Subsequent Cu-catalysed ATRP polymerisation yields complex star copolymer structures with poly(methacrylate) arms emanating from the branched polyester core. The versatility of the approach is demonstrated using five methacrylate monomers and the physical properties of the resulting polymers are studied.
TBRT utilises the chain-transfer controlled telomerisation of divinyl monomers to yield highly branched polymers with complete vinyl group consumption whilst avoiding gelation. The number average degree of polymerisation (DPn) derived from vinyl group propagation is maintained at <2 vinyl groups, and the extended macromolecular structure is derived from the linking chemistry between the unsaturated double bonds. Essentially, the ester functionality of a dimethacrylate becomes the dominant chemistry within the backbone of the resulting branched polymer and a polyester structure is formed. Within telomerisation nomenclature the vinyl functional monomers are termed taxogens, and chain transfer agents are known as telogens. The introduction of low concentrations of vinyl taxogens (VTs) relative to multi-vinyl taxogens (MVTs) allows new copolymer structures to be synthesised.27
The definition of “orthogonality” within synthetic chemistry has varied over the years and was initially used to describe mixed protecting group chemistries that could be selectively removed using different mechanisms.28 The ability to mix functional groups that react independently under defined but inherently different reaction conditions has since been exploited in numerous contexts across diverse chemical fields.29 We use the term “orthogonal” within the context previously presented for dendrimer synthesis and glycosylation reactions, namely “two sets of chemically distinct (orthogonal) [groups] and activation conditions”30 and “functional groups …inert to the [reaction] conditions of the other…allow[ing them] to be employed consecutively”.31
TBRT utilises conventional free radical initiation chemistry, therefore incorporation of an ATRP-functional vinyl inimer within a dimethacrylate TBRT reaction was considered a valid strategy in the creation of branched polyester macroinitiators, as ATRP requires organometallic catalysis to create the redox conditions for controlled radical polymerisation, Scheme 1. Thio-bromo “click” reactions have been widely reported under basic conditions,32 therefore, to confirm the stability of ATRP initiation groups under standard telomerisation conditions, 2-hydroxyethyl methacrylate was reacted with α-bromoisobutyryl bromide to form an inimer containing the α-bromoisobutyrate ATRP initiating functionality, 1 (2-(α-bromoisobutyryloxy)ethylmethacrylate; BBEMA)33 [ESI,† Fig. S1–S6]. The inimer was incorporated into a linear telomerisation of methyl methacrylate (MMA), using 1-dodecanethiol (DDT) as telogen and azobisisobutyronitrile (AIBN) in ethyl acetate (EtoAc; 50 wt% solids) at 70 °C (DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMA
MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio = 1.00
1 molar ratio = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.70
1.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85). The linear telomerisation was monitored by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectrometry. The resulting liquid product showed complete consumption of the vinyl functionality of MMA and 1, with no detectable vinyl resonances within the 1H NMR spectrum [ESI,† Fig. S7–S11]. Within TBRT, and branched polymers generated from the reaction of vinyl functional feedstocks, it is important to differentiate between conversion of monomer to polymer (associated with conventional chain polymerisation concepts and usually equated to the loss of vinyl groups), and vinyl group consumption. The consumption of vinyl functionality in TBRT is a direct indication of the extent of reaction as the complete conversion of MVT to polymer may nevertheless result in many unreacted pendant vinyl groups.
0.85). The linear telomerisation was monitored by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectrometry. The resulting liquid product showed complete consumption of the vinyl functionality of MMA and 1, with no detectable vinyl resonances within the 1H NMR spectrum [ESI,† Fig. S7–S11]. Within TBRT, and branched polymers generated from the reaction of vinyl functional feedstocks, it is important to differentiate between conversion of monomer to polymer (associated with conventional chain polymerisation concepts and usually equated to the loss of vinyl groups), and vinyl group consumption. The consumption of vinyl functionality in TBRT is a direct indication of the extent of reaction as the complete conversion of MVT to polymer may nevertheless result in many unreacted pendant vinyl groups.
The 13C NMR resonance associated with the tertiary bromide was readily observed within the spectrum of 1 at 55.4 ppm and was clearly preserved within the linear p([DDT-MMA]-stat-BBEMA) telomer sample. The MALDI-TOF spectrum of the linear telomer showed mass ions separated by the molecular weights of MMA and 1, strongly implying the robustness of the tertiary bromide under TBRT conditions. For example, a species with an M[H]+ = 305.52 Da was associated with a telomer comprising a degree of polymerisation (DP) of one MMA taxogen residue (added to one DDT telogen; note: S isotopes); subsequent peaks at M[Na]+ = 608.66 Da was indicative of a DP2 species containing one MMA and one 1 residue, within M[Na]+ ions ranging from 708.78–1009.10 Da representing DP3–5 telomers containing a single inimer with its preserved tertiary bromide functionality [ESI,† Fig. S11].
The TBRT of ethylene glycol dimethacrylate (EGDMA, 2) with 1 (polymer target 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio = 1
2 ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was undertaken using DDT and AIBN under identical conditions to the MMA linear telomerisation. The crude reaction showed no vinyl proton resonances (1H NMR) and the purified material confirmed the statistical copolymer/macroinitiator p([DDT-EGDMA]-stat-BBEMA), 3, with a DDT
1) was undertaken using DDT and AIBN under identical conditions to the MMA linear telomerisation. The crude reaction showed no vinyl proton resonances (1H NMR) and the purified material confirmed the statistical copolymer/macroinitiator p([DDT-EGDMA]-stat-BBEMA), 3, with a DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1
1 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [ESI,† Fig. S12–S20].
1 [ESI,† Fig. S12–S20].
For comparison, an inimer-free EGDMA/DDT TBRT was conducted; subsequent triple-detection size exclusion chromatography (TD-SEC) yielded a number average molecular weight (Mn) of 174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 g mol−1 and a weight average molecular weight (Mw) of 2
490 g mol−1 and a weight average molecular weight (Mw) of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499
499![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Table 1 [ESI,† Fig. S16]. VT incorporation in TBRT is known to yield lower molecular weight branched polymers due to the presence of non-branching units in the telomer sub-unit distribution and vinyl functionality dilution when working at constant solids content. This was also seen when using 1 (purified 3: Mn = 12
000 g mol−1, Table 1 [ESI,† Fig. S16]. VT incorporation in TBRT is known to yield lower molecular weight branched polymers due to the presence of non-branching units in the telomer sub-unit distribution and vinyl functionality dilution when working at constant solids content. This was also seen when using 1 (purified 3: Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 g mol−1; Mw = 205
290 g mol−1; Mw = 205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 g mol−1), Table 1. Differential scanning calorimetry studies of the p(DDT-EGDMA) and p([DDT-EGDMA]-stat-BBEMA), 3, showed a the influence of the pendant tertiary bromide groups; the glass transition temperature (Tg) increased by 38 °C [ESI,† Fig. S19 and S20]. The effect of VTs on TBRT copolymer Tg has been reported previously.27 The 1
330 g mol−1), Table 1. Differential scanning calorimetry studies of the p(DDT-EGDMA) and p([DDT-EGDMA]-stat-BBEMA), 3, showed a the influence of the pendant tertiary bromide groups; the glass transition temperature (Tg) increased by 38 °C [ESI,† Fig. S19 and S20]. The effect of VTs on TBRT copolymer Tg has been reported previously.27 The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of DDT
1 molar ratio of DDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
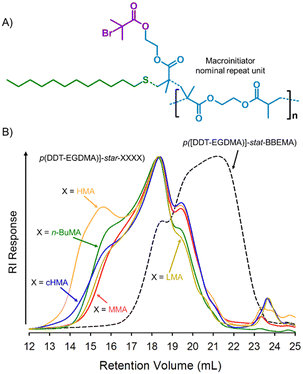
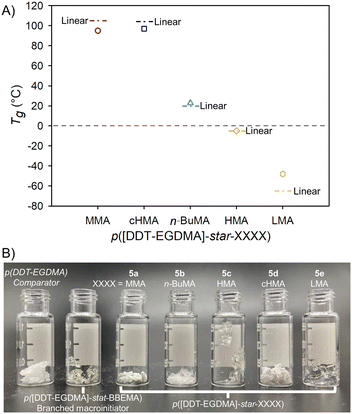
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 3 allowed the nominal repeat unit, Fig. 1A, or tertiary bromide equivalent weight, to be readily calculated as 681 g mol−1, thus enabling molar calculations of initiator (3 has a number average of 18, and weight average of 302 initiating sites). The ability of 3 to act as a macroinitiator was studied using five monomers, namely methyl, n-butyl (n-BuMA), hexyl (HMA), cyclohexyl (cHMA) and lauryl (LMA) methacrylate. All reactions were conducted in toluene, targeting arms of DPn = 50 monomer units, using a Cu(I)Cl/4,4′-dinonyl-2,2′-dipyridyl catalyst system, Scheme 1 [ESI,† Fig. S21–S30], and reaching very high/near complete, conversion. TD-SEC analysis showed a considerable increase in molecular weight for each p([DDT-EGDMA]-star-XXXX) copolymer, 5a–e, Table 1 and Fig. 1B [ESI,† Fig. S21–S25 and S31–S34]. p([DDT-EGDMA]-star-LMA), 5e, formation was examined in detail, displaying first order kinetics of ATRP, indicating steady radical concentration during arm growth [ESI,† Fig. S35]. After purification, the five star copolymers were visually different to 3 displaying Tg values that strongly correlate to literature Tg∞ values of the corresponding linear methacrylate homopolymers, Table 1 and Fig. 2A, B [ESI,† Fig. S36].
1 in 3 allowed the nominal repeat unit, Fig. 1A, or tertiary bromide equivalent weight, to be readily calculated as 681 g mol−1, thus enabling molar calculations of initiator (3 has a number average of 18, and weight average of 302 initiating sites). The ability of 3 to act as a macroinitiator was studied using five monomers, namely methyl, n-butyl (n-BuMA), hexyl (HMA), cyclohexyl (cHMA) and lauryl (LMA) methacrylate. All reactions were conducted in toluene, targeting arms of DPn = 50 monomer units, using a Cu(I)Cl/4,4′-dinonyl-2,2′-dipyridyl catalyst system, Scheme 1 [ESI,† Fig. S21–S30], and reaching very high/near complete, conversion. TD-SEC analysis showed a considerable increase in molecular weight for each p([DDT-EGDMA]-star-XXXX) copolymer, 5a–e, Table 1 and Fig. 1B [ESI,† Fig. S21–S25 and S31–S34]. p([DDT-EGDMA]-star-LMA), 5e, formation was examined in detail, displaying first order kinetics of ATRP, indicating steady radical concentration during arm growth [ESI,† Fig. S35]. After purification, the five star copolymers were visually different to 3 displaying Tg values that strongly correlate to literature Tg∞ values of the corresponding linear methacrylate homopolymers, Table 1 and Fig. 2A, B [ESI,† Fig. S36].
| Polymer | 1H NMR (CDCl3) | TD-SECc | DSCd | |||||
|---|---|---|---|---|---|---|---|---|
[EGDMA]F/[DDT]F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
[BBEMA]F/[DDT]F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Vinyl consb (%) | M n (g mol−1) | M w (g mol−1) | Đ | α | T g (°C) | |
| a Calculated by 1H NMR of purified and dried material. b Determined by 1H NMR of crude sample. c Triple-detection size exclusion chromatography using THF eluent. d Differential scanning calorimetry at a heating rate of 20 °C min−1. | ||||||||
| Branched | ||||||||
| p(DDT-EGDMA) | 1.07 | — | >99 | 174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 499![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14.3 | 0.416 | −44 |
| p([DDT-EGDMA]-stat-BBEMA) 3 | 0.95 | 1.04 | >99 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
16.7 | 0.225 | −6 |
| Branched star | ||||||||
| p([DDT-EGDMA]-star-MMA) 5a | — | — | 97 | 892![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.9 | 0.206 | 95 |
| p([DDT-EGDMA]-star-n-BuMA) 5b | — | — | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 301 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 952![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.6 | 0.232 | 23 |
| p([DDT-EGDMA]-star-HMA) 5c | — | — | >99 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 012 012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.8 | 0.250 | −5 |
| p([DDT-EGDMA]-star-cHMA) 5d | — | — | >99 | 504![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 645![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15.2 | 0.171 | 97 |
| p([DDT-EGDMA]-star-LMA) 5e | — | — | 97 | 572![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 070![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10.6 | 0.103 | −48 |
In conclusion, we demonstrate the first orthogonal synthesis of star copolymers using TBRT and ATRP leading to complex polyester-polymethacrylate branched-linear hybrids. The scope for further study is considerable and is underway to vary the number of initiating sites in the core macroinitiator, arm chemistry, and their chain length.
ABD – overseeing the investigation, supervision, conceptualisation, manuscript review, editing; WMS – investigation, data curation; FYH – methodology; OBP-L, SM, SF, SW, SP – analysis, methodology; PC – conceptualisation, manuscript review; SPR – conceptualisation, interpretation, funding, investigation, administration, resources, supervision, writing (original draft, review & editing).
The authors acknowledge the Engineering & Physical Sciences Research Council for funding (EPSRC; EP/R010544/1). WMS, OBPL and SM thank the University of Liverpool, EPSRC and Unilever for funding. Dr Megan Carr of the Materials Innovation Factory is thanked for analytical support (MALDI-TOF).
Data availability
The data supporting this article have been included within the ESI.†Conflicts of interest
SPR and PC are co-inventors on licenced patents that form the basis of Polymer Mimetics Ltd (Company number 12598928).Notes and references
- B. Helms, J. L. Mynar, C. J. Hawker and J. M. J. Fréchet, J. Am. Chem. Soc., 2004, 126(46), 15020 CrossRef CAS PubMed.
- A. Carlmark, C. Hawker, A. Hult and M. Malkoch, Chem. Soc. Rev., 2009, 38, 352 RSC.
- I. Chandrasiri, D. G. Abebe, S. Gupta, J. S. D. Williams, W. D. Rieger, B. L. Simms, M. L. Yaddehige, Y. Noh, M. E. Payne, A. W. Fortenberry, A. E. Smith, J. Ilavsky, S. M. Grayson, G. J. Schneider and D. L. Watkins, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1448 CrossRef CAS.
- D. L. Patton, P. Taranekar, T. Fulghum and R. Advincula, Macromolecules, 2008, 41, 6703 CrossRef CAS.
- S. E. R. Auty, O. C. J. Andrén, F. Y. Hern, M. Malkoch and S. P. Rannard, Polym. Chem., 2015, 6, 573 RSC.
- F. L. Hatton, P. Chambon, T. O. McDonald, A. Owen and S. P. Rannard, Chem. Sci., 2014, 5, 1844 RSC.
- F. L. Hatton, L. M. Tatham, L. R. Tidbury, P. Chambon, T. He, A. Owen and S. Rannard, Chem. Sci., 2015, 6, 326 RSC.
- L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Macromolecules, 2007, 40, 7855 CrossRef CAS.
- A. D. Schlüter, A. Halperin, M. Kröger, D. Vlassopoulos, G. Wegner and B. Zhang, ACS Macro Lett., 2014, 3, 991 CrossRef PubMed.
- D. Wilms, F. Wurm, J. Nieberle, P. Böhm, U. Kemmer-Jonas and H. Frey, Macromolecules, 2009, 42, 3230 CrossRef CAS.
- C. Porsch, Y. Zhang, C. Ducani, F. Vilaplana, L. Nordstierna, A. M. Nyström and E. Malmström, Biomacromolecules, 2014, 15, 2235 CrossRef CAS PubMed.
- W. Li, X. Zhao and H. Liu, Polym. Chem., 2014, 5, 1905 RSC.
- R. M. England, J. I. Moss, A. Gunnarsson, J. S. Parker and M. B. Ashford, Biomacromolecules, 2020, 21(8), 3332 CrossRef CAS PubMed.
- J. M. J. Fréchet, M. Henmi, I. Gitsov, S. Aoshima, M. R. Leduc and R. B. Grubbs, Science, 1995, 269, 1080 CrossRef PubMed.
- R. W. Graff, X. Wang and H. Gao, Macromolecules, 2015, 48(7), 2118 CrossRef CAS.
- X. Wang and H. Gao, Polymers, 2017, 9, 188 CrossRef PubMed.
- J. Kim, C. Waldron, B. Cattoz and C. R. Becer, Polym. Chem., 2020, 11, 6847 RSC.
- S. E. Wright, A. Clarkson, J. M. Korns, E. Haljun, L. Lofving, M. Ourgessa and Y. D. Y. L. Getzler, Green Chem. Lett. Rev., 2022, 15, 683 CrossRef CAS.
- Y. Zheng, W. Turner, M. Zong, D. J. Irvine, S. M. Howdle and K. J. Thurecht, Macromolecules, 2011, 44, 1347 CrossRef CAS.
- S. R. Cassin, P. Chambon and S. P. Rannard, Polym. Chem., 2020, 11, 7637 RSC.
- S. R. Cassin, S. Flynn, P. Chambon and S. P. Rannard, RSC Adv., 2021, 11, 24374 RSC.
- O. B. Penrhyn-Lowe, S. Flynn, S. R. Cassin, S. Mckeating, S. Lomas, S. Wright, P. Chambon and S. P. Rannard, Polym. Chem., 2021, 12, 6472 RSC.
- O. B. Penrhyn-Lowe, S. R. Cassin, P. Chambon and S. P. Rannard, Nanoscale Adv., 2022, 4, 4051 RSC.
- S. Flynn, O. B. Penrhyn-Lowe, S. Mckeating, S. Wright, S. Lomas, S. R. Cassin, P. Chambon and S. P. Rannard, RSC Adv., 2022, 12, 31424 RSC.
- S. R. Cassin, S. Wright, S. Mckeating, O. B. Penrhyn-Lowe, S. Flynn, S. Lomas, P. Chambon and S. P. Rannard, Polym. Chem., 2023, 14, 1905 RSC.
- S. Flynn, B. Linthwaite, O. B. Penrhyn-Lowe, S. Mckeating, S. Wright, S. R. Cassin, P. Chambon and S. P. Rannard, Polym. Chem., 2023, 14, 5102 RSC.
- S. R. Cassin, S. Flynn., P. Chambon and S. P. Rannard, Polym. Chem., 2022, 13, 2295 RSC.
- G. Barany and R. B. Merrifield, J. Am. Chem. Soc., 1977, 99, 7363 CrossRef CAS PubMed.
- C.-H. Wong and S. C. Zimmerman, Chem. Commun., 2013, 49, 1679 RSC.
- O. Kanie, Y. Ito and T. Ogawa, J. Am. Chem. Soc., 1994, 116, 12073 CrossRef CAS.
- F. Zeng and S. C. Zimmerman, J. Am. Chem. Soc., 1996, 118, 5326 CrossRef CAS.
- A. Moreno, G. Lligadas, J. Adamson, D. S. Maurya and V. Percec, Polymers, 2023, 15, 1075 CrossRef CAS PubMed.
- M. Zhang, J. Wu, Z. Li, W. Hou, Y. Li, Y. Shi and Y. Chen, Polym. Chem., 2022, 13, 4895 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, full experimental details and characterisation. See DOI: https://doi.org/10.1039/d4cc02142h |
| This journal is © The Royal Society of Chemistry 2024 |