Open Access Article
Open Access ArticleA bridging bis-phosphanido-phosphinidene complex of lanthanum supported by a sterically encumbering PN ligand†
B.
Wittwer
,
F.
Heim
,
K.
Wurst
and
S.
Hohloch
 *
*
University of Innsbruck, Center for Chemistry and Biomedicine, Department of General, Inorganic and Theoretical Chemistry, Innrain 80–82, 6020 Innsbruck, Austria. E-mail: Stephan.Hohloch@uibk.ac.at
First published on 30th May 2024
Abstract
Synthesis of a bulky anilidophosphine ligand (short PNTerph) and its lanthanum complexes 1 and 3 is reported. When exposed to KPHMes, both complexes form the first example of a bis-phosphanido-phosphinidene complex 2. This complex undergoes Phospha-Wittig type reactions and its reactivity towards strong bases is further investigated.
Although metal–ligand multiple bonds (MLMB) are ubiquitous in (early) transition metal chemistry,1 their isolation in f-block chemistry is associated with severe challenges.2 Due to strong charge polarisation and postulated low covalency, resulting from the energy mismatch and low spatial overlap between the respective frontier orbitals,3 these species tend to be very reactive and hard to handle/isolate.
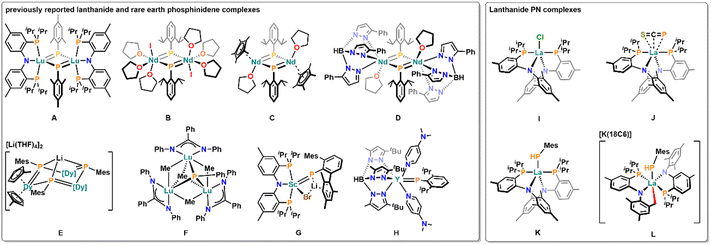
Nevertheless, in the last decade, a considerable number of examples with lanthanide and rare earth MLMB have emerged in the literature. These examples mostly focused on the use of group 15 and 16 elements (O, N). They included the facile isolation of various CeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4,5 and CeIV
O4,5 and CeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(Aryl)4,6 bonds as well as a nucleophilic CeIV carbene complex.7 While these “initial” systems mostly focus on the use of CeIV,8 which allows a higher covalent character of the MLMB, the Anwander group recently reported various strategies to isolate LnIII imido complexes (Ln = Ce, Nd, Sm, Dy, Ho, Lu).9–11 While their approaches eased the accessibility of imido complexes across the lanthanide series,9–11 for heavier group 15 elements, especially phosphorus, examples of MLMBs, especially terminal MLMBs, are still rare.12 Asides from a number of (multimetallic) ScIII phosphinidene complexes (e.g.Fig. 1 G)13 and two phosphinophosphinidene complexes,14 to date only six bridged phosphinidene complexes are known, which have been reported by Kiplinger (Fig. 1 A),15 Chen (Fig. 1 B–D),16,17 Layfield (Fig. 1 E)18 and Zhang (Fig. 1 F).19 In addition to the examples of ScIII phosphinidene complexes, the Anwander group recently reported the isolation of a terminal yttrium phosphinidene complex (Fig. 1 H).20
N(Aryl)4,6 bonds as well as a nucleophilic CeIV carbene complex.7 While these “initial” systems mostly focus on the use of CeIV,8 which allows a higher covalent character of the MLMB, the Anwander group recently reported various strategies to isolate LnIII imido complexes (Ln = Ce, Nd, Sm, Dy, Ho, Lu).9–11 While their approaches eased the accessibility of imido complexes across the lanthanide series,9–11 for heavier group 15 elements, especially phosphorus, examples of MLMBs, especially terminal MLMBs, are still rare.12 Asides from a number of (multimetallic) ScIII phosphinidene complexes (e.g.Fig. 1 G)13 and two phosphinophosphinidene complexes,14 to date only six bridged phosphinidene complexes are known, which have been reported by Kiplinger (Fig. 1 A),15 Chen (Fig. 1 B–D),16,17 Layfield (Fig. 1 E)18 and Zhang (Fig. 1 F).19 In addition to the examples of ScIII phosphinidene complexes, the Anwander group recently reported the isolation of a terminal yttrium phosphinidene complex (Fig. 1 H).20
We recently started to investigate the potential of anilidophosphine ligands to stabilize a variety of highly reactive lanthanide complexes starting from the halide complex I (Fig. 1).21 In this context, we found that the framework is capable of stabilizing an elusive η3 coordinated [SCP]− anion in complex J22 and a phosphanido complex K.21,23 Notably, deprotonation of the phosphanido ligand in K gave access to a C–H activated complex L, which was computationally and experimentally confirmed to form via a transient phosphinidene.24 Here, we aim to construct a novel anilidophosphine ligand to stabilize a putative terminal lanthanum phosphinidene complex by avoiding the presence of vulnerable C–H protons and offering severe steric protection, to tame the reactivity of such a species.
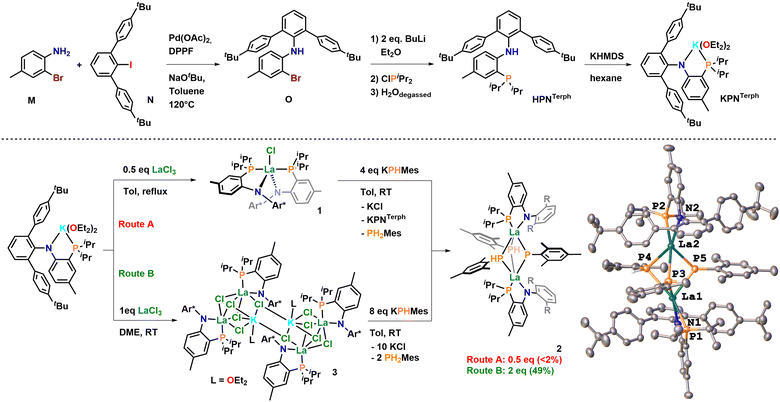
Synthesis of the new PN ligand HPNTerph is achieved by a modified synthetic route (Scheme 1, top), which includes α-bromination of o-toluidine to yield 2-bromo-toluidine M followed by Buchwald-Hartwig amination reaction using 4,4′′-di-tert-butyl-2′-iodo-1,1′:3′,1'′′-terphenyl N and subsequent bromide-lithium exchange on O followed by nucleophilic substitution on chlorodiisopropylphosphine. This procedure gives access to HPNTerph in 23% yield over all steps on a 5 g scale. Formation of the anilidophosphine was unambiguously determined by 31P NMR spectroscopy showing a singlet at −17.3 ppm (Fig. S9, ESI†) and by X-ray diffraction analysis of single crystals grown from concentrated diethyl ether solution (Fig. S77, ESI†). HPNTerph can be further converted to the corresponding lithium and potassium salts LiPNTerph and KPNTerph by deprotonation of the protonated ligand with BuLi or KHMDS. Formation of the corresponding salts is clearly indicated by the shift of the 31P{1H} NMR resonance from −17.3 ppm in HPNTerph to −10.3 ppm in LiPNTerph (Fig. S15, ESI†) and −12.2 ppm in KPNTerph (Fig. S24, ESI†). Additionally, the 7Li NMR of LiPNTerph shows a resonance at −0.63 ppm unambiguously proving the presence of a lithium ion (Fig. S18, ESI†). Unambiguous proof for the formation of KPNTerph was also given by X-ray diffraction analysis of single crystals grown form diethyl ether at room temperature (Fig. S78, ESI†). The structure reveals a monomeric potassium salt, in which the coordination sphere of the potassium ion K1 is completed by two additional ether ligands, forming a distorted tetrahedral environment around K1 (τ4′ = 0.72). The K1−P1 and K1−N1 are 3.3347(6) and 2.6923(13) Å and are comparable to previously reported potassium PN salts.21 Complexation reactions with lanthanum were initially conducted in boiling toluene using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
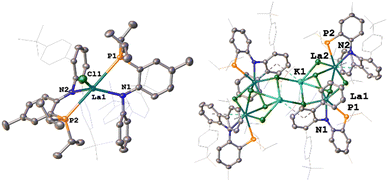
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry KPNTerphvs. LaCl3(THF). 1H and 31P{1H} NMR spectroscopy revealed the formation of a single species after reaction with a 31P{1H} NMR resonance at 0.36 ppm confirming successful salt metathesis reaction, yielding the anticipated bis-PNTerph complex 1. Structural characterisation of 1 reveals a pentacoordinate lanthanum center in a distorted square pyramidal coordination environment (τ5 = 0.17, Fig. 3). The La1−N and La1−P distances are 2.436(3) and 3.2117(11) Å on average and are comparable to the previously reported complex I.21 Analysis of the steric bulk of the co-ligands along the La−Cl bond reveals that the PNTerph ligand is sterically much more demanding compared to the PNMes ligand. Calculation of the %Vburied resulted in a value of 69.9% for 1 compared to 47.5% for I (Fig. 2 and Table S3, ESI†).25
1 stoichiometry KPNTerphvs. LaCl3(THF). 1H and 31P{1H} NMR spectroscopy revealed the formation of a single species after reaction with a 31P{1H} NMR resonance at 0.36 ppm confirming successful salt metathesis reaction, yielding the anticipated bis-PNTerph complex 1. Structural characterisation of 1 reveals a pentacoordinate lanthanum center in a distorted square pyramidal coordination environment (τ5 = 0.17, Fig. 3). The La1−N and La1−P distances are 2.436(3) and 3.2117(11) Å on average and are comparable to the previously reported complex I.21 Analysis of the steric bulk of the co-ligands along the La−Cl bond reveals that the PNTerph ligand is sterically much more demanding compared to the PNMes ligand. Calculation of the %Vburied resulted in a value of 69.9% for 1 compared to 47.5% for I (Fig. 2 and Table S3, ESI†).25
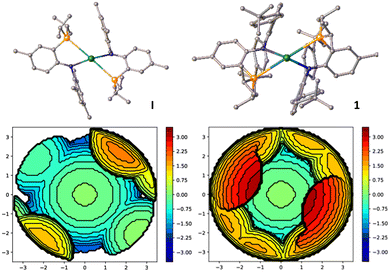 | ||
| Fig. 2 Comparison between the %Vburied along La-Cl bond between complex I (left) and complex 1 (right), showing the steric protection offered by the 1,3-terphenyl substituents. Further information can be found in the ESI† Table S3. | ||
Given this steric protection along with the absence of vulnerable protons, we assumed this system to be able to stabilize a desired terminal phosphinidene complex. Thus, we next attempted the salt metathesis reaction with one equivalent KPHMes targeting the putative phosphanido complex (PNTerph)2La(PHMes), analogous to complex K. However, analysis of the material after reaction revealed a very messy reaction with no clean species being formed. After numerous attempts of crystallisation, we found the formation of complex 2 in single crystalline quantity (>2%). X-ray diffraction analysis revealed two lanthanum centers, each supported by one PNTerph ligand (suggesting the loss of 1 eq., of KPNTerph during the reaction), being bridged by one phosphinidene and two phosphanido ligands (Scheme 1). The assignment of a phosphanido-phosphinidene complex is evident by X-ray diffraction analysis revealing four long La−P distances between 3.0268(11)–3.1130(10) Å for the bridging phosphanido ligands (P3 and P4), while the phosphinidene bridge (P5) displays La−P distances at 2.8471(11) and 2.8207(10) Å, clearly proving the phosphinidene character. The latter are substantially longer compared to Kiplinger's example using lutetium (2.5973(15)–2.6724(14) Å in A, Fig. 1)15 or to Chen's examples on neodymium (2.7314(15)/2.7769(16) Å in B;16 2.7827(10)/2.7456(11) Å in C,17 2.7808(16)/2.7911(15) Å in D,17Fig. 1), as well as to the terminal yttrium phosphinidene complex recently reported by Anwander and co-workers (2.4855(7) Å, complex H, Fig. 1),20 which is in line with the larger radius of La(III). However, since the formation of the phosphinidene complex 2 through the elimination of one equivalent of PNTerph is far from being atom efficient, we targeted a mono-PN ligated complex 3. While for PNMes, the synthesis of mono-PN complexes was not feasible by salt metathesis but only through protonolysis over 17 days using La(HMDS)3, with the novel KPNTerph ligand salt metathesis with LaCl3(THF)1.2 is easily achieved in DME and gives access to complex 3 in good yields of 76%. Formation of the complex is evident by 31P{1H} NMR spectroscopy showing a singlet at 8.96 ppm (compare complex 1 at 0.36 ppm, vide supra). Unambiguous proof for the formation of a mono-PN complex 3 was delivered again by X-ray diffraction analysis, which displayed the formation of an unexpected, tetranuclear -ate complex, in which two lanthanum ions are bridged by three chloride ligands, while the fourth chloride ligand is linking these units to a potassium ion, bridging the two dinuclear lanthanum units to give the tetranuclear scaffold. The bond distances between the PN ligands and the lanthanum ion are 3.1113(13) Å for La1−P1 and 2.409(4) Å for La1−N1 and are substantially shorter compared to the bis-PNTerph complex 1 but similar to the phosphinidene complex 2. This is in line with the enhanced steric pressure that is present in complex 1 with two bulky PNTerph ligands coordinated to the lanthanum ion. Indeed, and as anticipated, the reaction between complex 3 and eight equivalents of KPHMes gives clean access to the phosphanido-phosphinidene complex 2 in moderate yields of 49%. In line with the results from X-ray diffraction analysis, the 1H NMR spectrum shows six resonances in the CH3-mesityl region which corresponds to the presence of three inequivalent mesityl groups. Surprisingly no 1JPH coupling was detected in C6D6 at room temperature. Similarly, the 31P{1H} NMR spectrum in C6D6 at room temperature showed only very broad resonances for the mesityl phosphines between −50 to −65 ppm and a sharp signal at 13.7 ppm corresponding to the PN ligand (Fig. S38, ESI†). Strikingly a very broad resonance was visible at 333.8 ppm. Given the fact that Kiplinger's lutetium phosphinidene complex A (Fig. 1) showed a phosphinidene resonance at 186.8 ppm in its 31P{1H} NMR,15 the resonance at 333.8 ppm in 2 is strongly indicative of a phosphinidene complex. Cooling a sample of the product in toluene-d8 to 288 K, these signals in the 31P{1H} NMR become more defined (Fig. S40, ESI†) and the resonances from the mesitylphosphanido ligands appear at −50.3, −53.1 and −64.1 ppm. Further cooling of the sample to 233 K results in further splitting of these resonances, which we attribute to the formation of syn/anti isomers with respect to the PN orientation (Fig. S48, ESI†). Overall, three multiplets at −49.5 (1JPH = 256 Hz; 2JPP = 45 Hz, syn), −53.0 (1JPH = 230 Hz; 2JPP = 51 Hz, anti) and −69.5 ppm (1JPH = 260 Hz, anti) appear (Fig. S48, ESI†), revealing the expected 1JPH couplings for a phosphanido ligand. In addition, the phosphinidene resonance and the PN resonance split into two signals at 333.5/334.0 ppm (anti/syn) and 15.0/12.9 ppm (syn/anti). This interpretation is in line with the observation from the VT 1H NMR spectrum, which also reveals characteristic 1JPH couplings after cooling. Overall, the results from NMR spectroscopy clearly speak for the presence of the phosphanido-phosphinidene complex 2 in solution as well.
Given the fact, that Kiplinger's bis-μ-phosphinidene complex A only forms at 80 °C, we investigated the thermal stability of complex 2 to see if the formation of a similar bis-μ-phosphinidene complex would be feasible. While the complex is stable in solution for days at room temperature, at 333 K (60 °C) the formation of HPNTerph is observed after 24 h, which continues to form at temperatures up to 373 K (100 °C) (Fig. S50, ESI†). Additionally, all attempts to deprotonate the remaining phosphanido ligands using strong bases such as LiCH2TMS, KHMDS or KBn in the presence and absence of alkali metal scavengers (12C4, 18C6 or 222-Crypt) to make bis- or tris-μ-phosphinidene complexes have failed so far, giving only access to complicated reaction mixtures from which no defined material could be crystallized (Fig. S58, ESI†). However, complex 2 cleanly engages in Phospha-Wittig-type reactions, transferring the mesitylphosphinidene ligand to benzophenone giving access to the corresponding (diphenylmethylene)-(mesityl)phosphane (Fig. S60, ESI†). Considering the La complex, 1H and 31P{1H} NMR spectroscopy reveals the formation of multiple products in this reaction, and no crystals could be obtained to determine the fate of the lanthanum fragment.
In conclusion we have presented the synthesis of a novel, highly bulky PNTerph ligand and its coordination towards lanthanum(III) giving access to highly encumbered bis-PN complex 1 or hitherto inaccessible mono-PN complex 3via simple salt metathesis protocols. The reaction of 1 and 3 with four/eight equivalents of KPHMes gives access to an unprecedented phosphanido-phosphinidene complex 2, which is the first bridging phosphinidene complex of lanthanum and shows Phospha-Wittig type reactivity. The study gives important insights into stability and variability of PN ligands in lanthanide chemistry and delivers important results on the way to the first terminal phosphinidene complex of the (early) lanthanides.
We are grateful to the University of Innsbruck for generous funding of this work.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Stradiotto and R. J. Lundgren, Ligand design in metal chemistry. Reactivity and catalysis, Wiley, Chichester, 2016 CrossRef; (b) C. C. Cummins, Angew. Chem., Int. Ed., 2006, 45, 862–870 CrossRef CAS PubMed.
- (a) M. A. Boreen, G. Rao, D. G. Villarreal, F. A. Watt, R. D. Britt, S. Hohloch and J. Arnold, Chem. Commun., 2020, 56, 4535–4538 RSC; (b) T. Chu, W. E. Piers, J. L. Dutton and M. Parvez, Organometallics, 2013, 32, 1159–1165 CrossRef CAS; (c) M. E. Garner, S. Hohloch, L. Maron and J. Arnold, Organometallics, 2016, 35, 2915–2922 CrossRef CAS; (d) G. R. Giesbrecht and J. C. Gordon, Dalton Trans., 2004, 2387–2393 RSC; (e) E. Lu, Y. Li and Y. Chen, Chem. Commun., 2010, 46, 4469–4471 RSC; (f) E. Lu, J. Chu, Y. Chen, M. V. Borzov and G. Li, Chem. Commun., 2011, 47, 743–745 RSC; (g) D. Schädle and R. Anwander, Chem. Soc. Rev., 2019, 48, 5752–5805 RSC; (h) C. Zhang, G. Hou, G. Zi, W. Ding and M. D. Walter, J. Am. Chem. Soc., 2018, 140, 14511–14525 CrossRef CAS PubMed.
- O. T. Summerscales and J. C. Gordon, RSC Adv., 2013, 3, 6682 RSC.
- L. A. Solola, A. V. Zabula, W. L. Dorfner, B. C. Manor, P. J. Carroll and E. J. Schelter, J. Am. Chem. Soc., 2017, 139, 2435–2442 CrossRef CAS PubMed.
- (a) M. P. Coles, P. B. Hitchcock, A. V. Khvostov, M. F. Lappert, Z. Li and A. V. Protchenko, Dalton Trans., 2010, 39, 6780–6788 RSC; (b) P. L. Damon, G. Wu, N. Kaltsoyannis and T. W. Hayton, J. Am. Chem. Soc., 2016, 138, 12743–12746 CrossRef CAS PubMed; (c) Y.-M. So, G.-C. Wang, Y. Li, H. H.-Y. Sung, I. D. Williams, Z. Lin and W.-H. Leung, Angew. Chem., Int. Ed., 2014, 126, 1652–1655 CrossRef; (d) M. K. Assefa, G. Wu and T. W. Hayton, Chem. Sci., 2017, 8, 7873–7878 RSC.
- (a) T. Cheisson, K. D. Kersey, N. Mahieu, A. McSkimming, M. R. Gau, P. J. Carroll and E. J. Schelter, J. Am. Chem. Soc., 2019, 141, 9185–9190 CrossRef CAS PubMed; (b) T. Cheisson, L. A. Solola, M. R. Gau, P. J. Carroll and E. J. Schelter, Organometallics, 2018, 37, 4332–4335 CrossRef CAS; (c) E. N. Lapsheva, T. Cheisson, C. Álvarez Lamsfus, P. J. Carroll, M. R. Gau, L. Maron and E. J. Schelter, Chem. Commun., 2020, 56, 4781–4784 RSC; (d) L. A. Solola, A. V. Zabula, W. L. Dorfner, B. C. Manor, P. J. Carroll and E. J. Schelter, J. Am. Chem. Soc., 2016, 138, 6928–6931 CrossRef CAS PubMed.
- (a) M. Gregson, E. Lu, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2013, 52, 13016–13019 CrossRef CAS PubMed; (b) M. Gregson, E. Lu, D. P. Mills, F. Tuna, E. J. L. McInnes, C. Hennig, A. C. Scheinost, J. McMaster, W. Lewis, A. J. Blake, A. Kerridge and S. T. Liddle, Nat. Commun., 2017, 8, 14137 CrossRef CAS PubMed; (c) M. Gregson, E. Lu, F. Tuna, E. J. L. McInnes, C. Hennig, A. C. Scheinost, J. McMaster, W. Lewis, A. J. Blake, A. Kerridge and S. T. Liddle, Chem. Sci., 2016, 7, 3286–3297 RSC.
- Y.-M. So and W.-H. Leung, Coord. Chem. Rev., 2017, 340, 172–197 CrossRef CAS.
- T. E. Rieser, D. Schädle, C. Maichle-Mössmer and R. Anwander, Chem. Sci., 2024, 15, 3562–3570 RSC.
- T. E. Rieser, R. Thim-Spöring, D. Schädle, P. Sirsch, R. Litlabø, K. W. Törnroos, C. Maichle-Mössmer and R. Anwander, J. Am. Chem. Soc., 2022, 144, 4102–4113 CrossRef CAS PubMed.
- D. Schädle, M. Meermann-Zimmermann, C. Schädle, C. Maichle-Mössmer and R. Anwander, Eur. J. Inorg. Chem., 2015, 1334–1339 CrossRef.
- (a) Q. Wen, B. Feng and Y. Chen, Acc. Chem. Res., 2023, 56, 3343–3357 CrossRef CAS PubMed; (b) J. Du, P. J. Cobb, J. Ding, D. P. Mills and S. T. Liddle, Chem. Sci., 2023, 15, 13–45 RSC.
- (a) Y. Lv, C. E. Kefalidis, J. Zhou, L. Maron, X. Leng and Y. Chen, J. Am. Chem. Soc., 2013, 135, 14784–14796 CrossRef CAS PubMed; (b) B. F. Wicker, J. Scott, J. G. Andino, X. Gao, H. Park, M. Pink and D. J. Mindiola, J. Am. Chem. Soc., 2010, 132, 3691–3693 CrossRef CAS PubMed.
- B. Feng, L. Xiang, K. N. McCabe, L. Maron, X. Leng and Y. Chen, Nat. Commun., 2020, 11, 2916 CrossRef CAS PubMed.
- J. D. Masuda, K. C. Jantunen, O. V. Ozerov, K. J. T. Noonan, D. P. Gates, B. L. Scott and J. L. Kiplinger, J. Am. Chem. Soc., 2008, 130, 2408–2409 CrossRef CAS PubMed.
- P. Cui, Y. Chen, X. Xu and J. Sun, Chem. Commun., 2008, 5547–5549 RSC.
- P. Cui, Y. Chen and M. V. Borzov, Dalton Trans., 2010, 39, 6886–6890 RSC.
- T. Pugh, F. Tuna, L. Ungur, D. Collison, E. J. L. McInnes, L. F. Chibotaru and R. A. Layfield, Nat. Commun., 2015, 6, 7492 CrossRef PubMed.
- K. Wang, G. Luo, J. Hong, X. Zhou, L. Weng, Y. Luo and L. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1053–1056 CrossRef CAS PubMed.
- T. E. Rieser, P. Wetzel, C. Maichle-Mössmer, P. Sirsch and R. Anwander, J. Am. Chem. Soc., 2023, 145, 17720–17733 CrossRef CAS PubMed.
- F. A. Watt, A. Krishna, G. Golovanov, H. Ott, R. Schoch, C. Wölper, A. G. Neuba and S. Hohloch, Inorg. Chem., 2020, 59, 2719–2732 CrossRef CAS PubMed.
- F. A. Watt, L. Burkhardt, R. Schoch, S. Mitzinger, M. Bauer, F. Weigend, J. M. Goicoechea, F. Tambornino and S. Hohloch, Angew. Chem., Int. Ed., 2021, 60, 9534–9539 CrossRef CAS PubMed.
- (a) F. A. Watt, N. Dickmann, R. Schoch and S. Hohloch, Inorg. Chem., 2020, 59, 13621–13631 CrossRef CAS PubMed; (b) B. Wittwer, K. N. McCabe, D. Leitner, M. Seidl, L. Maron and S. Hohloch, Inorg. Chem. Front., 2024, 11 10.1039/D4QI00868E.
- F. A. Watt, K. N. McCabe, R. Schoch, L. Maron and S. Hohloch, Chem. Commun., 2020, 56, 15410–15413 RSC.
- (a) L. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano and L. Cavallo, Organometallics, 2016, 35, 2286–2293 CrossRef CAS; (b) L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2341842–2341846 and 2341850. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc02244k |
| This journal is © The Royal Society of Chemistry 2024 |