Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photochemical α-amination of carbonyl groups with iodinanes†
Suyuan
He
,
Boya
Feng
 ,
Yiben
Tang
,
Ruiping
Chen
,
Yujing
Guo
and
Rene M.
Koenigs
,
Yiben
Tang
,
Ruiping
Chen
,
Yujing
Guo
and
Rene M.
Koenigs
 *
*
RWTH Aachen University, Institute of Organic Chemistry, Landoltweg 1, 52074 Aachen, Germany. E-mail: rene.koenigs@rwth-aachen.de
First published on 15th August 2024
Abstract
We report on a photochemical reaction of silyl enol ethers with iminoiodinanes. This aza Rubottom reaction provides a direct access towards α-amino carbonyl compounds under catalyst free reaction conditions with light as the sole source of energy. Control experiments suggest the participation of triplet nitrene intermediates.
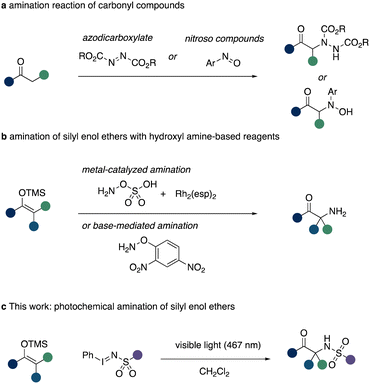
The introduction of an amino functional group in the α-position of a carbonyl group is of high relevance in the synthesis of α-amino acids or ketones, which themselves are fundamental building blocks in nature.1 A timely approach towards this endeavour can be realized via the direct α-amination of carbonyl compounds or their analogues, such as silyl enol ethers.1–4 The challenge in realizing such transformation, however, lies within the intrinsic nucleophilic properties of both the α-carbon atom of carbonyl groups and amines. A variety of different strategies based on Umpolung chemistry of either reagent have been realized.2 Among these, prominent examples being, e.g. the utilization of electrophilic amination reagents such as azodicarboxylates3 or nitroso compounds (Scheme 1a).4
An alternative approach to this synthetic challenge lies within the use of silyl enol ethers and a nitrene precursor.5 The latter can be utilized to in situ access an electrophilic nitrogen species that reacts with the nucleophilic silyl enol ether to initially form an aziridine ring. Subsequent desilylation and ring opening leads to the reformation of the carbonyl group with an amine group installed at the α-carbon atom. This reaction is resemblant of the classic Rubottom oxidation and this aza analogue recently attracted the attention of synthetic chemists to allow for direct α-amination of carbonyl groups.6–10 In this context, the Kürti group reported on HFIP mediated or rhodium catalysed amination reaction of silyl enol ethers using hydroxyl amine derived amination reagents (Scheme 1b),6 which themselves required a suitable protecting group strategy to access the reagent itself. Related approaches by the Chang group describe the utilization of carbamoyl azides in iridium catalysed nitrene transfer reactions to 1,3-dicarbonyl compounds.7 Notably, the use of specialized nitrene transfer reagents was reported by Takemoto requiring UV light conditions.8
Based on our interest in nitrene transfer reactions,11 we hypothesised that such Aza Rubottom12 oxidation reactions can be accessed under metal-free conditions using visible light to access the key nitrene species. Such approach would enable the access of important α-amino ketones in a straightforward fashion from simple and readily available reagents (Scheme 1c).
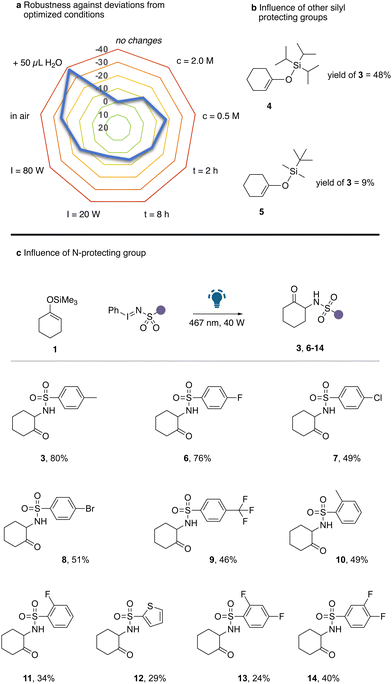
We commenced our studies by examining the reaction of cyclohexanone derived silyl enol ether 1 with iminoiodinane 2 under photochemical conditions. Only under irradiation with blue LEDs, the reaction proceeded equally well in dichloromethane (DCM) solvent with and without a photocatalyst (Table 1, entries 1–3). Further studies comprised the evaluation of different solvents. A broad spectrum of solvents, including ethers, chlorinated solvents, and polar solvents, proved compatible with the current reaction, yielding the desired α-amination product in moderate quantities (Table 1, entries 4–9). The highest yield of 3 was obtained using either acetonitrile or 1,2-dichloroethane (1,2-DCE). In subsequent steps, we examined reaction parameters (for details, see Tables S1–S5, ESI†) such as concentration, stoichiometry, reaction time, or the influence of air, moisture and light intensity, which quickly led to the optimized conditions (Table 1, entry 10). Only a small influence on the reaction outcome was observed upon deviations from optimum concentration, reaction time or light intensity. The critical reaction parameters for high reaction yield were identified as additional water or when the reaction was carried out in air (Scheme 2a). However, a significant influence of the silyl protecting group on the reaction outcome was observed (Scheme 2b). Increasing the steric demand of the silyl protecting group led to a significant reduction in product yield and both TBDMS protected silyl enol ether gave unsatisfactory reaction outcomes.
| Entrya | Solvent | Other changes | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 0.2 mmol 2 (1 eq.), 1.0 mmol 1 (5 eq.) were taken in a reaction tube and 2 mL 1,2-DCE was added to the reaction mixture under argon atmosphere and irradiated with blue LEDs overnight. Further changes of the reaction conditions have been noted in the table. Isolated yield are reported. | |||
| 3 | DCM | 41 | |
| 1 | DCM | +Ru(bpy)3Cl2 (1mol%) | 41 |
| 2 | DCM | No light | Trace |
| 4 | EtOAc | 54 | |
| 5 | THF | 29 | |
| 6 | 1,4-Dioxane | 48 | |
| 7 | MeCN | 64 | |
| 8 | CHCl3 | 42 | |
| 9 | 1,2-DCE | 67 | |
| 10 | 1,2-DCE | 40 W LED (4 hours) | 80 |
Next, we examined the influence of the N-protecting group of the iodinane reagent in the reaction with silyl enol ether 1 under otherwise optimized conditions (Scheme 2c). Gratifyingly, a broad range of different aryl substituted sulfonyl protected iodinanes smoothly reacted to give the corresponding α-amino ketone in moderate to good yield. It is important to note that both ortho and para substituted aryl rings were well tolerated. Moreover, strongly electron-withdrawing substituents led to a decrease in reaction yield (9, 13, 14). Furthermore, the introduction of chloro (7) or bromo (9) substituents, ortho-substituents (10, 11) or a thienyl ring (12) led to a reduction in the product yield, which we assume to be related to potential interactions by (nucleophilic) heavy atoms with an electrophilic nitrene intermediate.
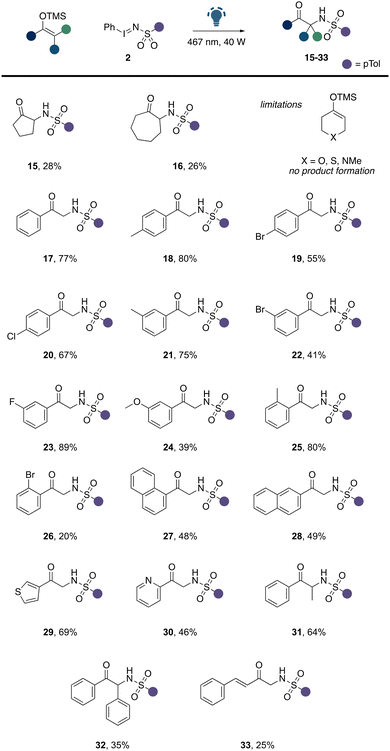
We then embarked on the evaluation of suitable silyl enol ether substrates in this α-amination reaction (Scheme 3). We commenced these studies by first evaluating the influence of the ring size of silyl enol ethers derived from cyclic ketones. However, a limitation with regards to the ring size of the ketone was observed and both 5- and 7-membered rings were significantly less tolerated under the reaction conditions (Scheme 3, 15, 16), which we assume to be related to challenges in purification and side reactions of the reaction product. Heteroatom-functionalized cyclohexanone derivatives were found incompatible, which we assume to competing reaction of the nucleophilic heteroatom with the electrophilic nitrene intermediate. Next, we explored acyclic ketones and studied the compatibility of acetophenone derived silyl enol ethers in this reaction. To our delight, a broad range of different acetophenone derivatives were well tolerated. Substituents on the aromatic ring of the acetophenone were tolerated in all positions. Electron-donating groups (18, 21, 24, 25), light halogen atoms (20, 23), as well as carbo- and heterocycles (27–30) were well found compatible with this transformation. Similarly, as previously observed, heavy halogen atoms such as bromine led to a significant reduction in product yield. We further explored β-substituted carbonyl compounds, which smoothly underwent α-amination (31, 32). Notably, the introduction of a sterically more demanding aromatic ring in the β position led to a slight reduction in the product yield (32). An example, of a vinyl substituted ketone further demonstrates the compatibility of suitable functional groups in this transformation (33).
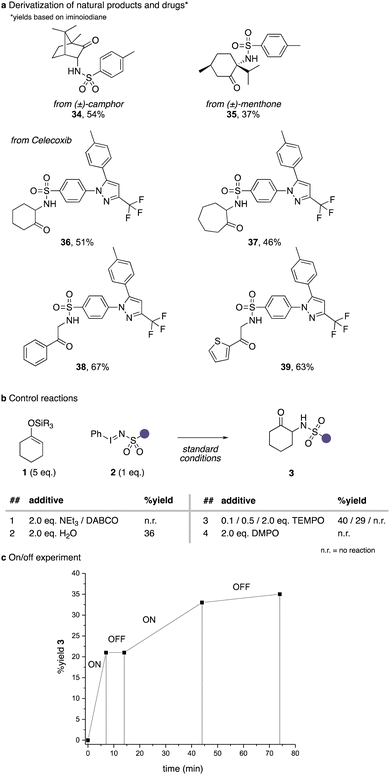
We next explored the applicability of this protocol in the α-amination of natural products and drug molecules (Scheme 4a). To our delight naturally occurring 6-membered ring ketones such as camphor or menthone underwent smooth and stereoselective amination reactions to yield 34 and 35 in moderate to good yield. We further examined an iodinane derived from the drug Celecoxib, which underwent smooth amination reactions of a range of different cyclic ketones and acetophenone derivatives (36–39).
In a last step, we examined different reaction additives to examine the reaction mechanism. In the presence of tertiary amines, the reaction was completely suppressed, which can be attributed to ylide formation reactions of a nitrene intermediate. The addition of 2 equivalents of water had a detrimental effect on the reaction yield. Finally, the addition of stoichiometric amounts of radical trapping reagents led to a complete inhibition of this reaction, which further underlines the participation of nitrene intermediates. The on/off experiment then shows that no background reaction is observed the moment the light source is switched off, which suggests that light is needed in this transformation and that no chain processes are occurring.
In summary, we herein describe a photochemical approach towards α-amination of carbonyl compounds. Control experiments suggest the participation of intermediates with unpaired electrons and the reaction stalls in the presence of radical trapping agents. We have demonstrated the application of this aza Rubottom in the amination reaction of silyl enol ethers derived from cyclic ketones and acetophenones that react only in the presence of light with readily available iminoiodinanes to the corresponding α-amino carbonyl compounds. Further applications in the derivatisation of natural products and drug molecules are shown.
R. M. K. thanks the Deutsche Forschungsgemeinschaft DFG, for grant KO5659/7-1. S. H., B. F. and Y. G. thank the China Scholarship Council for providing a scholarship.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- References alpha Amines: (a) Q. Yin, Y. Shi, J. Wang and X. Zhang, Chem. Soc. Rev., 2020, 49, 6141–6153 RSC; (b) Y. Zhang, J. Vanderghinste, J. Wang and S. Das, Nat. Commun., 2024, 15, 1474 CrossRef CAS PubMed; (c) C. Najera and J. M. Santano, Chem. Rev., 2007, 107, 4584–4671 CrossRef CAS PubMed; (d) L. A. T. Allen, R.-C. Raclea, P. Natheo and P. J. Parsons, Org. Biomol. Chem., 2021, 19, 498–513 RSC.
- (a) E. Erdik, Tetrahedron, 2004, 60, 8747–8782 CrossRef CAS; (b) A. Maity, P. Roychowdhury, R. G. Herrera and D. C. Powers, Org. Lett., 2022, 24, 2762–2766 CrossRef CAS PubMed.
- Selected references using azo dicarboxylates: (a) D. A. Evans and S. G. Nelson, J. Am. Chem. Soc., 1997, 119, 6452–6453 CrossRef CAS; (b) N. Kumaragurubaran, K. Juhl, W. Zhuang, A. Bøgevig and K. A. Jørgensen, J. Am. Chem. Soc., 2002, 124, 6254–6255 CrossRef CAS PubMed; (c) K. Juhl and K. A. Jørgensen, J. Am. Chem. Soc., 2002, 124, 2420–2421 CrossRef CAS PubMed; (d) X. Yang and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 3205–3208 CrossRef CAS; (e) Y.-S. Jiang, S.-S. Li, X.-L. Luo, L.-N. Chen, D.-N. Chen and P.-J. Xia, Org. Lett., 2023, 25, 6671–6676 CrossRef CAS.
- Nitroso benzene: (a) N. Momiyama and H. Yamamoto, J. Am. Chem. Soc., 2004, 126, 5360–5361 CrossRef CAS PubMed; (b) N. Momiyama and H. Yamamoto, J. Am. Chem. Soc., 2005, 127, 1080–1081 CrossRef CAS PubMed; (c) D. J. Fisher, G. L. Burnett, R. Velasco and J. Read de Alaniz, J. Am. Chem. Soc., 2015, 137, 11614–11617 CrossRef CAS PubMed; (d) Q.-D. Wang, X. Liu, Y.-W. Zheng, Y.-S. Wu, X. Zhou, J.-M. Yang and Z.-L. Shen, Org. Lett., 2024, 26, 416–420 CrossRef CAS PubMed.
- Selected reviews on nitrene transfer reactions: (a) C. Empel and R. M. Koenigs, Chem Catal., 2022, 2, 2506–2514 CrossRef CAS; (b) H. J. Dequina, C. L. Jones and J. M. Schomaker, Chem, 2023, 9, 1658–1701 CrossRef CAS PubMed; (c) G. Dequirez, V. Pons and P. Dauban, Angew. Chem., Int. Ed., 2012, 51, 7384–7395 CrossRef CAS PubMed; (d) H. Hayashi and T. Uchida, Eur. J. Org. Chem., 2020, 909–916 CrossRef CAS; (e) Y. Luo, X. Zhang and Y. Xia, Chin. Chem. Lett., 2024, 35, 108778 CrossRef CAS.
- Z. Zhou, Q.-Q. Cheng and L. Kürti, J. Am. Chem. Soc., 2019, 141, 2242–2246 CrossRef CAS PubMed.
- M. Lee, H. Jung, D. Kim, J.-W. Park and S. Chang, J. Am. Chem. Soc., 2020, 142, 11999–12004 CrossRef CAS PubMed.
- For a report under UV light conditions with specialized iodinanes: Y. Kobayashi, S. Masakado and Y. Takemoto, Angew. Chem., Int. Ed., 2018, 57, 693–697 CrossRef CAS PubMed.
- (a) D. A. Evans, M. M. Faul and M. T. Bilodeau, J. Org. Chem., 1991, 56, 6744–6746 CrossRef CAS; (b) D. A. Evans, M. T. Bilodeau and M. M. Faul, J. Am. Chem. Soc., 1994, 116, 2742–2753 CrossRef CAS.
- (a) J. J. Bell-Tyrer, P. A. Hume, P. S. Grant, M. A. Brimble and D. P. Furkert, Chem. – Eur. J., 2023, 29, e202300261 CrossRef CAS PubMed; (b) M. Feng, A. J. Fernandes, A. Sirvent, E. Spinozzi, S. Shaaban and N. Maulide, Angew. Chem., Int. Ed., 2023, 62, e202304990 CrossRef CAS PubMed; (c) P. Mizar and T. Wirth, Angew. Chem., Int. Ed., 2014, 53, 5993–5997 CrossRef CAS PubMed.
- Selected articles: (a) F. Li, W. F. Zhou, C. Empel, O. Datsenko, A. Kumar, Y. Xu, J. H. M. Ehrler, I. Atodiresei, S. Knapp, P. M. Mykhailiuk, E. Proschak and R. M. Koenigs, Science, 2024, 383, 498–503 CrossRef CAS PubMed; (b) X. Zhao, Z. Tang, L. Shi, Y. Guo, R. M. Koenigs and X. Hao, Green Synth. Catal., 2024 DOI:10.1016/j.gresc.2024.05.001; (c) B.-G. Cai, C. Empel, W.-Z. Yao, R. M. Koenigs and J. Xuan, Angew. Chem., Int. Ed., 2023, 62, e202312031 CrossRef CAS PubMed; (d) Y. Guo, C. Pei and R. M. Koenigs, Nat. Commun., 2022, 13, 86 CrossRef CAS PubMed; (e) Y. Guo, C. Empel, C. Pei, H. Fang, S. Jana and R. M. Koenigs, Chem. Catal., 2022, 2, 2012–2023 CrossRef CAS; (f) Y. Guo, C. Pei, S. Jana and R. M. Koenigs, ACS Catal., 2021, 11, 337–342 CrossRef CAS.
- G. M. Rubottom, M. A. Vazquez and D. R. Pelegrina, Tetrahedron Lett., 1974, 15, 4319–4322 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03564j |
| This journal is © The Royal Society of Chemistry 2024 |