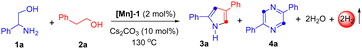Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Double dehydrogenative coupling of amino alcohols with primary alcohols under Mn(I) catalysis†
Ganesan
Sivakumar
 ,
Abhijith Karattil
Suresh
,
Smruti Rekha
Padhy
and
Ekambaram
Balaraman
,
Abhijith Karattil
Suresh
,
Smruti Rekha
Padhy
and
Ekambaram
Balaraman
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Tirupati, Tirupati – 517507, Andhra Pradesh, India. E-mail: eb.raman@iisertirupati.ac.in
First published on 23rd October 2024
Abstract
Herein, we unveil a method for synthesizing substituted pyrrole and pyrazine compounds via a double dehydrogenative coupling of amino alcohols with primary alcohols, facilitated by Mn(I)–PNP catalysis, which uniquely enables the simultaneous formation of C–C and C–N bonds.
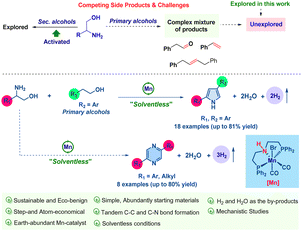
The essence of sustainable chemical synthesis lies in its commitment to reducing environmental impact and preserving resources. This approach involves devising synthetic pathways that leverage renewable resources, decrease energy demands, minimize waste, and avoid hazardous by-products.1 The sustainable synthesis of N-heterocyclic compounds is particularly noteworthy in contemporary science due to their extensive chemical and biological properties, essential in areas such as materials science, pharmaceuticals, and agrochemicals.2 Consequently, a plethora of methods for synthesizing N-heterocyclic compounds have been established.3 Of particular interest is the recent rise of acceptorless dehydrogenative coupling for producing aromatic N-heterocycles, a method that notably employs environmentally benign alcohols as starting materials. Extensive research has been conducted on various catalytic systems utilizing 3d and 4d-transition metals.4,5
In the realm of acceptorless dehydrogenative coupling (ADC), the spotlight has traditionally been on secondary alcohols, such as diols and activated benzylic alcohols, for crafting N-heterocyclic compounds, with both noble and base-metal catalysts playing a pivotal role.6 However, the synthesis techniques for N-heteroaromatics that employ unactivated primary alcohols, such as phenylethyl alcohol, remain underdeveloped despite the use of precious metal catalysts. Recently, we have demonstrated the synthesis of quinolines and pyridines by employing a variety of primary alcohols in a double dehydrogenative coupling process with amino alcohols facilitated by manganese-based catalysis.5k Following our research work, the research group of Banerjee also achieved a similar synthesis employing a nickel-catalysis system.7a This reflects an increased focus on primary alcohols in ADC, especially for the synthesis of the pyrrole derivatives. Expanding on this, we have honed our synthetic strategy to produce pyrrole via tandem double dehydrogenative coupling with primary and amino alcohols and to generate the pyrazine structure through dehydrogenative self-coupling of amino alcohols. The importance of pyrroles is underscored by their widespread presence in various natural products, pharmaceuticals, catalysts, and materials science.
Polypyrroles, known for their conductive properties, are utilized in the fabrication of batteries and solar cells.7b,c The conventional synthesis of pyrrole has been traditionally accomplished via well-known methodologies, including the Paal–Knorr, Knorr, and Hantzsch techniques.8 Recent advancements in research have introduced a variety of dehydrogenative coupling methods for the formation of pyrroles (see ESI†).4c,i,o,9 These methods predominantly utilize a variety of substituted secondary alcohols, ketones, and diols. However, the literature has not yet reported on the synthesis of pyrrole through dehydrogenative coupling involving primary alcohols. Our present work deals with the unprecedented synthesis of pyrrole directly from primary alcohols, employing a double dehydrogenative coupling with amino alcohol in a solvent-free environment (Scheme 1).
Initially, we selected 2-phenylglycinol and 2-phenylethanol as representative substrates. A systematic investigation was conducted to assess diverse Mn-based catalysts, bases, and temperatures, aiming to determine the optimal reaction conditions for the selective synthesis of pyrrole derivatives (Table 1 and ESI†). Consequently, the reaction of 2-phenylglycinol (1a) with 2-phenylethanol (2a) in the presence of [Mn]-1 (2 mol%) and Cs2CO3 (10 mol%) under solvent-free conditions at 130 °C, emerged as the optimal protocol. The present catalytic system yielded product 3a with an isolated yield of 70%, while the self-dehydrogenated product 4a was produced in negligible quantities (Table 1, entry 1). A series of [Mn]-complexes were evaluated for their efficacy in synthesizing 2,4-disubstituted pyrrole (Table 1, entries 2–4, and ESI†). The [Mn]-1 complex emerged as particularly potent, significantly advancing the catalytic tandem transformation. Notably, in the absence of the Mn[I]-catalyst and base, the formation of product 3a was minimal (Table 1, entries 5 and 6). Exploring solvent influence with n-octane, 1,4-dioxane, m-xylene, THF, mesitylene, and toluene, we achieved a moderate yield of 3a under standard conditions (Table 1, entry 7). Notably, substituting Cs2CO3 with other bases such as NaOtBu, KOtBu, KOH, and KH led to a lower yield of 3a (Table 1, entry 8), indeed, with stronger bases favoring the by-product 4a. Additionally, lowering the reaction temperature was found to adversely affect the yield of 3a.
| Entry | Deviation from above | 3a Yieldb (%) | 4a Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: substrate 1a (0.5 mmol), 2a (0.6 mmol), [Mn]-1 (2 mol%), and Cs2CO3 (10 mol%) were heated at 130 °C (silicone oil-bath temperature) for 8 h under an argon atmosphere. b Isolated yield. | ||||
| 1 | No variation | 70 | <5 |
|
| 2 | [Mn]-2 as a catalyst | 63 | <10 | |
| 3 | [Mn]-3 as a catalyst | 60 | <10 | |
| 4 | Mn(CO)5Br/PhPNP-ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
<58 | <15 | |
| 5 | No [Mn]-catalyst | — | Trace | |
| 6 | No Cs2CO3 | — | Trace | |
| 7 | n-Octane, 1,4-dioxane, m-xylene, THF, mesitylene, toluene as a solvent | <50 | <10 | |
| 8 | NaOtBu, KOtBu, KOH, KH as a base | <52 | <25 | |
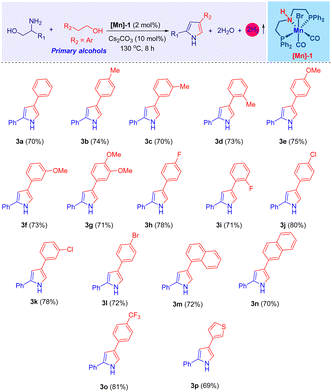
Upon establishing the optimal conditions for the tandem catalytic synthesis of pyrrole derivatives, we expanded our investigation to encompass the generality of the present [Mn]-catalysis, as detailed in Table 2. The compound 2-phenylglycinol (1a) was selected as a standard substrate. Thus, under optimal reaction conditions, 1a dehydrogenatively coupled with various phenethyl alcohols (2) and effectively led to the formation of the desired 2,4-disubstituted pyrroles (3), achieving high yields, as shown in Table 2. Indeed, the use of 2-phenylethanol bearing electron-donating methyl and methoxy groups on the aromatic ring led to the synthesis of 2,4-disubstituted pyrroles, achieving very good isolated yields (up to 75%; Table 2, products 3b–3g).
Notably, employing phenyl ethylalcohols with halogen substituents (–Br, –Cl, –F), such as compounds 2h–2l, resulted the corresponding pyrroles with excellent yields, ranging from 72% to 80% (Table 2, products 3h–3l). The significance of these halogenated derivatives lies in their potential to facilitate further functionalization reactions, thereby unlocking avenues for the synthesis of diverse molecular forms. Furthermore, the incorporation of extended π-conjugated systems, as seen with 2-(1-naphthyl)ethanol (2m) and 2-(2-naphthyl)ethanol (2n), culminated in the high yield of the expected compounds 3m–3n (up to 80% isolated yield). Under the optimal reaction conditions, 2-phenyl ethanol derivatives with electron-withdrawing functionalities, specifically 2-(4-(trifluoromethyl)phenyl)ethan-1-ol (2o), and heteroaromatic alcohols such as 2-(thiophene-2-yl)ethan-1-ol (2p), demonstrated excellent reactivity. This led to the successful synthesis of compounds 3o and 3p, achieving yields of 81% and 69%, respectively. However, it was found that aliphatic primary alcohols, modified 2-phenylglycinol derivatives, and aliphatic amino alcohols did not undergo the Mn-catalyzed reaction under the same conditions.
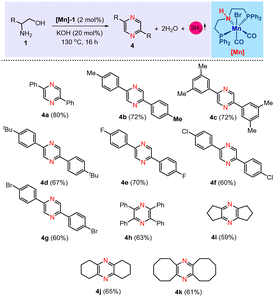
Furthermore, we have expanded the present Mn[I]-catalysis for the dehydrogenative self-coupling of β-amino alcohols, enabling the synthesis of pyrazine derivatives. The current methodology for self-coupling amino alcohols to produce pyrazines has been explored in the literature.4o,6d,10a Pioneering work by Milstein's group illustrated this process utilizing PNP–Ru and an acridine-derived PNP–Mn complex.6d,10a In continuation of our work on base metal catalysis, we have demonstrated a transformation akin to that of Milstein's group, utilizing a cobalt-based complex.6d In the current study, we have used a molecularly defined PNP–Mn catalyst, i.e., [Mn]-1, for the self-coupling of β-amino alcohols under acceptorless conditions, further expanding the utility of this catalytic system. After establishing the optimized conditions for the dehydrogenative self-coupling method in the synthesis of pyrazine derivatives (see ESI†), the reaction involving 2-phenylglycinol (1a), a catalytic amount of [Mn]-1 (2 mol%), and 20 mol% KOH under solvent-free conditions at 130 °C was identified as the optimal condition, affording the desired product 4a with an isolated yield of 80% (Table 3, product 4a). A variety of 2-phenylglycinol derivatives, including those with methyl (1b), dimethyl (1C), tert-butyl (1d) and halogen (–F, –Cl, and –Br) substituents (1e–1g), as well as aliphatic β-amino alcohols like 2-amino-1,2-diphenylethan-1-ol (1h), 2-aminocyclopentan-1-ol (1i), 2-aminocyclohexan-1-ol (1j) and 2-aminocyclooctan-1-ol (1k) were employed. These substrates successfully produced an array of substituted pyrazines, achieving yields as high as 80% (Table 3).
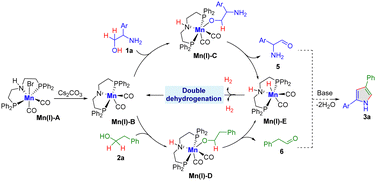
Control experiments were conducted to elucidate the underlying mechanism (Scheme 2). Initially, the generation of H2 gas was qualifiedly analyzed under optimal conditions (Scheme 2a). Further experiments showed that independent reactions of 2-phenylglycinol (1a) and 2-phenyl ethanol (2a) under the standard conditions led to the formation of 2-amino-2-phenylacetaldehyde (5) and phenylacetaldehyde (6), respectively. This was accompanied by the release of H2 gas, as outlined in Scheme 2b. These results suggest that the reaction follows the acceptorless dehydrogenative pathway. Moreover, the intermolecular coupling for C–N and C–C bond formation was substantiated by reacting in situ formed intermediate, such as aldehyde 6 with alcohol 1a, under standard conditions. This reaction yielded the anticipated 2,4-disubstituted pyrrole product in good yield (Scheme 2c). Notably, the presence of radical scavengers, such as TEMPO and BHT, led to a slight decrease in product yield. This observation suggests that a single electron transfer (SET) pathway cannot be entirely ruled out (Scheme 2d).
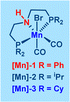
Based on the insights gained from control experiments and previous literature reports,5k,10b we propose a plausible reaction mechanism for the synthesis of pyrrole and pyrazine compounds via a double dehydrogenative coupling of amino alcohols with primary alcohols, facilitated by Mn(I)–PNP catalysis (Scheme 3).
Initially, the active Mn-catalyst Mn(I)-B is generated from the precatalyst Mn(I)-A in the presence of Cs2CO3. Subsequently, the intermediate complex Mn(I)-C is formed from the active catalyst Mn(I)-B by activating the O–H bond of 2-phenylglycinol through metal–ligand cooperation (MLC). Following this, the intermediate Mn(I)-C undergoes β-hydride elimination, resulting in the production of 2-amino-2-phenylacetaldehyde (5) and intermediate Mn(I)-E. Concurrently, 2-phenylacetaldehyde (6) is generated via intermediate Mn(I)-D, where 2-phenylethanol coordinates with the active Mn-catalyst Mn(I)-B, followed by β-hydride elimination to form intermediate Mn(I)-E. Subsequently, a base-mediated coupling takes place, bringing together 2-amino-2-phenylacetaldehyde (5) and 2-phenylacetaldehyde (6) to yield 2,4-disubstituted pyrrole 3a while eliminating two molecules of water. Finally, the active Mn catalyst Mn(I)-B is regenerated from intermediate Mn(I)-E, with the liberation of H2 gas through the MLC process.
In summary, we have successfully demonstrated the direct synthesis of substituted pyrrole and pyrazine compounds through the double acceptorless dehydrogenative coupling of amino alcohols with primary alcohols under solvent-free conditions. This process results in the release of molecular hydrogen and water as by-products. The utilization of an earth-abundant manganese catalyst in combination with readily available starting materials enhances the atom efficiency of this approach, making it more environmentally friendly and sustainable for the synthesis of N-heterocycles.
This work is supported by MoE-STARS/STARS-2/2023-0232. E. B. acknowledges funding from Swarnajayanti Fellowship (SERB/F/5892/2020-2021). S. G. and S. R. P. thank IISER-Tirupati for fellowship, and A. K. S. acknowledges UGC for fellowship.
Data availability
The data supporting this article have been included in the main article and as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Notes and references
- (a) T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11, 190–195 CrossRef CAS PubMed; (b) S. A. Matlin, S. E. Cornell, A. Krief, H. Hopf and G. Mehta, Chem. Sci., 2022, 13, 11710–11720 RSC.
- A. Gomtsyan, Chem. Heterocycl. Compd., 2012, 48, 7–10 CrossRef CAS.
- (a) P. A. Keller, A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Comprehensive Heterocyclic Chemistry III, Elsevier, Oxford, U.K., 2008, vol. 7 Search PubMed; (b) I. Nakamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127–2198 CrossRef CAS PubMed.
- (a) R. Yamaguchi, K.-I. Fujita and M. Zhu, Heterocycles, 2010, 81, 1093 CrossRef CAS; (b) D. Srimani, Y. Ben-David and D. Milstein, Chem. Commun., 2013, 49, 6632 RSC; (c) S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed; (d) D. Forberg, J. Obenauf, M. Friedrich, S.-M. Hühne, W. Mader, G. Motz and R. Kempe, Catal. Sci. Technol., 2014, 4, 4188–4192 RSC; (e) A. Nandakumar, S. P. Midya, V. G. Landge and E. Balaraman, Angew. Chem., Int. Ed., 2015, 54, 11022–11034 CrossRef CAS PubMed; (f) N. Deibl, K. Ament and R. Kempe, J. Am. Chem. Soc., 2015, 137, 12804–12807 CrossRef CAS PubMed; (g) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed; (h) F. Li, L. Lu and P. Liu, Org. Lett., 2016, 18, 2580–2583 CrossRef CAS PubMed; (i) P. Daw, S. Chakraborty, J. A. Garg, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2016, 55, 14373–14377 CrossRef CAS PubMed; (j) R. H. Crabtree, Chem. Rev., 2017, 117, 9228–9246 CrossRef CAS PubMed; (k) B. Paul, M. Maji, K. Chakrabarti and S. Kundu, Org. Biomol. Chem., 2020, 18, 2193–2214 RSC; (l) M. Garbe, K. Junge and M. Beller, Eur. J. Org. Chem., 2017, 4344–4362 CrossRef CAS; (m) B. Maji and M. Barman, Synthesis, 2017, 3377–3393 CAS; (n) G. A. Filonenko, R. van Putten, E. J. M. Hensen and E. A. Pidko, Chem. Soc. Rev., 2018, 47, 1459–1483 RSC; (o) S. P. Midya, V. G. Landge, M. K. Sahoo, J. Rana and E. Balaraman, Chem. Commun., 2018, 54, 90–93 RSC; (p) A. Mukherjee and D. Milstein, ACS Catal., 2018, 8, 11435–11469 CrossRef CAS; (q) S. Shee, K. Ganguli, K. Jana and S. Kundu, Chem. Commun., 2018, 54, 6883–6886 RSC.
- (a) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46–60 CrossRef CAS PubMed; (b) S. Parua, R. Sikari, S. Sinha, G. Chakraborty, R. Mondal and N. D. Paul, J. Org. Chem., 2018, 83, 11154–11166 CrossRef CAS PubMed; (c) B. G. Reed-Berendt, K. Polidano and L. C. Morrill, Org. Biomol. Chem., 2019, 17, 1595–1607 RSC; (d) K. Das, A. Mondal, D. Pal and D. Srimani, Org. Lett., 2019, 21, 3223–3227 CrossRef CAS PubMed; (e) S. Waiba and B. Maji, ChemCatChem, 2020, 12, 1891–1902 CrossRef CAS; (f) N. Hofmann and K. C. Hultzsch, Eur. J. Org. Chem., 2021, 6206–6223 CrossRef CAS; (g) S. Shee, D. Panja and S. Kundu, J. Org. Chem., 2020, 85, 2775–2784 CrossRef CAS PubMed; (h) K. Bera and A. Mukherjee, Tetrahedron Lett., 2021, 81, 153326 CrossRef CAS; (i) M. Maji, D. Panja, I. Borthakur and S. Kundu, Org. Chem. Front., 2021, 8, 2673–2709 RSC; (j) I. Borthakur, A. Sau and S. Kundu, Coord. Chem. Rev., 2022, 451, 214257 CrossRef CAS; (k) G. Sivakumar, M. Subaramanian and E. Balaraman, ACS Sustainable Chem. Eng., 2022, 10, 7362–7373 CrossRef CAS.
- (a) M. Mastalir, M. Glatz, E. Pittenauer, G. Allmaier and K. Kirchner, J. Am. Chem. Soc., 2016, 138, 15543–15546 CrossRef CAS PubMed; (b) F. Kallmeier, B. Dudziec, T. Irrgang and R. Kempe, Angew. Chem., Int. Ed., 2017, 56, 7261–7265 CrossRef CAS PubMed; (c) N. Deibl and R. Kempe, Angew. Chem., Int. Ed., 2017, 56, 1663–1666 CrossRef CAS PubMed; (d) P. Daw, A. Kumar, N. A. Espinosa-Jalapa, Y. Diskin-Posner, Y. Ben-David and D. Milstein, ACS Catal., 2018, 8, 7734–7741 CrossRef CAS PubMed; (e) M. K. Barman, A. Jana and B. Maji, Adv. Synth. Catal., 2018, 360, 3233–3238 CrossRef CAS; (f) K. Das, A. Mondal and D. Srimani, J. Org. Chem., 2018, 83, 9553–9560 CrossRef CAS PubMed; (g) K. Das, A. Mondal and D. Srimani, Chem. Commun., 2018, 54, 10582–10585 RSC; (h) A. Mondal, M. K. Sahoo, M. Subaramanian and E. Balaraman, J. Org. Chem., 2020, 85, 7181–7191 CrossRef CAS PubMed.
- (a) M. Sk, A. Bera and D. Banerjee, ChemCatChem, 2023, 15(11), e202300412 CrossRef CAS; (b) H. Nishide and K. Oyaizu, Science, 2008, 319, 737–738 CrossRef CAS PubMed; (c) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- (a) T. A. Moss and T. Nowak, Tetrahedron Lett., 2012, 53, 3056–3060 CrossRef CAS; (b) Z. Wang, Knorr Pyrrole Synthesis, in Comprehensive Organic Name Reactions and Reagents, 2010, pp. 1634–1637.
- (a) S.-I. Murahashi, T. Shimamura and I. Moritani, J. Chem. Soc., Chem. Commun., 1974, 931 RSC; (b) Y. Tsuji, Y. Yokoyama, K.-T. Huh and Y. Watanabe, Bull. Chem. Soc. Jpn., 1987, 60, 3456–3458 CrossRef CAS; (c) S. J. Pridmore, P. A. Slatford, A. Daniel, M. K. Whittlesey and J. M. J. Williams, Tetrahedron Lett., 2007, 48, 5115–5120 CrossRef CAS; (d) N. D. Schley, G. E. Dobereiner and R. H. Crabtree, Organometallics, 2011, 30, 4174–4179 CrossRef CAS; (e) K. Iida, T. Miura, J. Ando and S. Saito, Org. Lett., 2013, 15, 1436–1439 CrossRef CAS PubMed; (f) T. Yan and K. Barta, ChemSusChem, 2016, 9, 2321–2325 CrossRef CAS PubMed; (g) B. Emayavaramban, M. Sen and B. Sundararaju, Org. Lett., 2017, 19, 6–9 CrossRef CAS PubMed; (h) K. Singh, L. M. Kabadwal, S. Bera, A. Alanthadka and D. Banerjee, J. Org. Chem., 2018, 83, 15406–15414 CrossRef CAS PubMed; (i) D. Srimani, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 4012–4015 CrossRef CAS PubMed; (j) M. Zhang, X. Fang, H. Neumann and M. Beller, J. Am. Chem. Soc., 2013, 135, 11384–11388 CrossRef CAS PubMed.
- (a) B. Gnanaprakasam, E. Balaraman, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 12240–12244 CrossRef CAS PubMed; (b) M. Peña-López, P. Piehl, S. Elangovan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 14967–14971 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedure, mechanistic insights, copy of NMR data. See DOI: https://doi.org/10.1039/d4cc03595j |
| This journal is © The Royal Society of Chemistry 2024 |