Open Access Article
Open Access ArticleConstruction of stable porous organic cages: from the perspective of chemical bonds
Miao
Yang
ac,
Kongzhao
Su
 *ac and
Daqiang
Yuan
*ac and
Daqiang
Yuan
 *abc
*abc
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China. E-mail: skz@fjirsm.ac.cn; ydq@fjirsm.ac.cn
bFujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, Fujian, P. R. China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
First published on 28th August 2024
Abstract
Porous organic cages (POCs) are constructed from purely organic synthons by covalent linkages with intrinsic cavities and have shown potential applications in many areas. However, the majority of POC synthesis methods reported thus far have relied on dynamically reversible imine linkages, which can be metastable and unstable under humid or harsh chemical conditions. This instability significantly hampers their research prospects and practical applications. Consequently, strategies to enhance the chemical stability of POCs by modifying imine bonds and developing robust covalent linkages are imperative for realizing the full potential of these materials. In this review, we aim to highlight recent advancements in synthesizing chemical-stable POCs through these approaches and their associated applications. Additionally, we propose further strategies for creating stable POCs and discuss future opportunities for practical applications.
1. Introduction
Porous organic cages (POCs) and covalent organic frameworks (COFs) are both low-density crystalline materials constructed from organic building blocks through covalent bonds (Fig. 1).1–3 However, POC synthesis typically requires a convergent concave-shaped organic building block compared to COFs. Unlike extended COFs with framework structures linked by strong covalent bonds, POCs are distinct in their construction as they consist of discrete (zero-dimensional or 0D) covalent-bonded macromolecules with permanent intrinsic cavities, resulting in a 0D framework via weak intermolecular interactions.4–6 As a result, their porosity arises from the intrinsic cavities or extrinsic voids, resulting from inefficient molecule packing in the solid state. Moreover, this discrete nature makes POCs susceptible to structural changes in response to external stimulation,7–9 as well as endows POCs with advantages in solubility, processability, modification, and regeneration compared to COF materials.10–14The first organic cage compound can be traced back to the 1960s when Nobel Prize-winning chemist Jean-Marie Lehn introduced a crown ether-based cage molecule for cation binding.15 However, no permanent porosities of organic cages in the solid state have been explored since then, which may be attributed to the lack of appropriate gas adsorption instrumentation. Until 2009, Cooper et al. first confirmed that tetrahedral [4+4] imine-linked (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) POCs could serve as porous solids and exhibit permanent porosity with Brunauer–Emmett–Teller (BET) surface area up to 624 m2 g−1.16 Since then, POCs with different topologies, sizes, surface areas, and functions have increased substantially.17–24
N) POCs could serve as porous solids and exhibit permanent porosity with Brunauer–Emmett–Teller (BET) surface area up to 624 m2 g−1.16 Since then, POCs with different topologies, sizes, surface areas, and functions have increased substantially.17–24
The synthesized methods for POCs can be divided into two classes based on their bond formation during self-assembly:25 (1) irreversible linking chemistry, such as carbon–carbon bond formation,26 nucleophilic substitutions,27 Knoevenagel condensation;28 and (2) dynamic covalent chemistry (DCC),29–32 including imine condensation,33 boronic ester condensation,34 alkene/alkyne metathesis,35 amide bond formation,36 disulfide–sulfide bond formation,24 combination of imine and boronic ester condensations,37 combination of imine and alkene metathesis,38 and combination of imine and imide etc.39 The latter method typically yields higher results but exhibits lower stability, while the former demonstrates the opposite characteristics. To the best of our knowledge, most reported POCs are linked by the dynamically reversible imine condensation method, which have been extensively reviewed in recent publications.40–43 However, these POCs are susceptible to dissociation under acidic, basic, or even moist conditions due to dynamic imine linkages within their backbones. Consequently, they are not yet suitable for practical industrial applications such as catalysis, energy storage, pollutant removal, and gas storage and separation that involve exposure to reactive species and harsh physical conditions requiring materials with high chemical robustness. The stability (e.g., thermal, mechanical, hydrolytic, chemical, or photolytic stability) of materials is fundamental, and chemical stability is one of the most important characteristics of POCs which is deeply influenced by chemical bonds. Thus, there is a growing demand for methods in constructing chemically stable POCs to advance the development of porous materials across various fields.44 However, few reviews have been conducted on this topic.
Herein, we provide a comprehensive overview of recent advancements in the development and application of stable POCs. From the perspective of chemical bonds, we mainly present two strategies for preparing stable POCs: modifying imine linkages to increase the POCs' stability; using robust covalent linkages to prepare stable POCs. As for the former, to date, the proposed strategies for increasing the stability of imine linkage can be divided into three fundamentally different categories, that is, (1) constructing N-substituted imine derivatives, such as hydrazone-linked POCs;45–47 (2) transforming imine to amine linkages via keto–enol tautomerization48–50 and the direct reduction method;51–53 and (3) transforming imine into other chemically stable linkages, such as quinoline,54 carbamate,55 and amide bonds.56 As for the latter, stable POCs constructed from more robust linkages based on alkyne metathesis,57,58 imide formation,59 nucleophilic aromatic reactions,60,61 Knoevenagel reaction,28 and so on,62,63 have been recently achieved. Fig. 2 highlights selected significant reports on the efficient synthesis of stable POCs utilizing these strategies. Moreover, an outlook on future suggestions for efficient synthesis of stable POCs, along with the future opportunities that require attention, has also been presented.
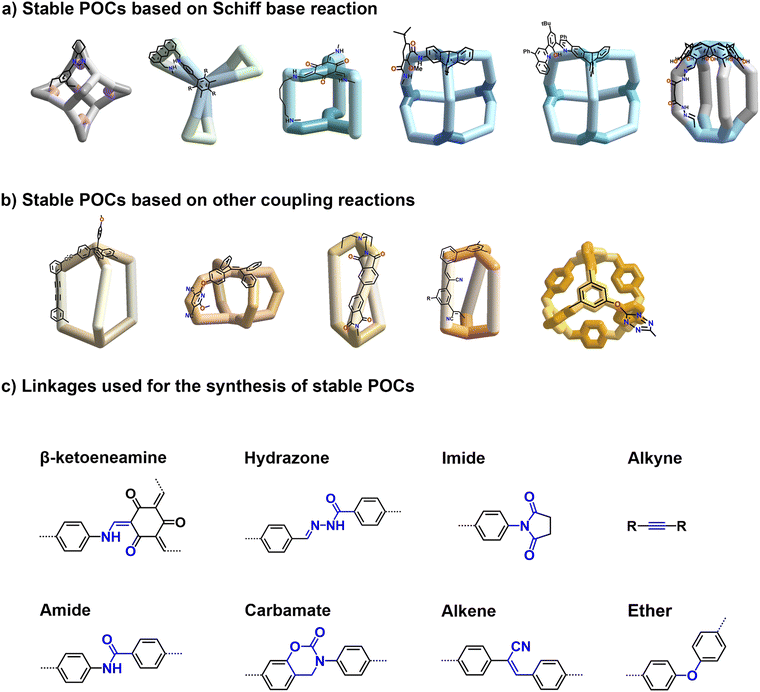 | ||
| Fig. 2 Selected stable POCs based on (a) modification of imine linkage, (b) usage of robust linkage strategies, and (c) common linkages for the synthesis of stable POCs. | ||
2. Stable POCs based on Schiff base reaction
Imine condensation is a widely adopted and straightforward synthetic method for fabricating porous organic materials, including porous organic polymers (POPs), COFs, and POCs. Since Cram reported the first utilization of imine bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) formation to prepare [2+4] lantern-shaped organic cage compound based on tetraformylcavitand and 1,3-diaminobenzene synthons in 1991,64 a large number of imine-linked organic cages with defined shapes and sizes, have significantly grown over the past three decades. However, the stability issues arising from imine linkage under harsh chemical conditions considerably restrict the practical utility of these imine-bonded POCs.
N) formation to prepare [2+4] lantern-shaped organic cage compound based on tetraformylcavitand and 1,3-diaminobenzene synthons in 1991,64 a large number of imine-linked organic cages with defined shapes and sizes, have significantly grown over the past three decades. However, the stability issues arising from imine linkage under harsh chemical conditions considerably restrict the practical utility of these imine-bonded POCs.
CC3, a robust organic cage formed via Schiff base reactions involving four molecules of 1,3,5-triformylbenzene and six molecules of 1,2-diaminocyclohexane,16 exhibits remarkable stability. The material maintains its structural integrity in pure water for up to four days and can withstand refluxing in water for four hours without decomposition.65 This impressive stability is attributed to the way POCs are constructed by stacking organic molecules that contain internal voids, held together by weak intermolecular forces. Moreover, in the case of CC3, the cage molecules are packed in a window-to-window arrangement to form diamond topology, which is the main reason for its enhanced stability. In the meanwhile, the material can adsorb up to 20.1 wt% water. Inspired by this resilience and utility, POCs hold promise as versatile materials with diverse applications. Hence, further exploration of stable imine-based POC materials is a significant area for development. This section reviews notable advancements in bolstering the stability of these compounds and their possible uses.
2.1 Transformation of imine to amine linkages
(1) To enhance the chemical stability of imine-linked POCs, a straightforward approach involves their reduction to amine-linked counterparts using hydride reagents. Beyond improving stability, these C–N linked POCs offer additional sites for post-synthetic functionalization and guest molecule binding.Zhang and coworkers successfully converted labile imine-linked POCs into robust C–N linked POCs through hydride reduction with reasonable yield (74%).66 The material exhibited high ideal CO2/N2 adsorption selectivity of 73 at STP, which was attributed to the unambiguous cage structure and the interaction between amine groups and CO2 molecules. Subsequently, numerous similar cages have been reported. Moreover, due to their stability and active amine sites, these POCs can undergo further functionalization as demonstrated by Cooper et al., who synthesized a [4+6] imine organic cage (CC1), constructed from four 1,3,5-triformylbenzene and six 1,2-diaminoethane, its reduction product (RCC1). By modifying RCC1, 12-arm dodecamine cages with different functional groups were regarded as promising supramolecular building blocks.67 Also reported by this group, RCC1 can be transformed into protonated cage salts to increase protonic conductivity.68 Su et al. reported two cases of metal-templated cages, which were subsequently reduced to pure covalent-organic cages for the meaningful incorporation of dithienylethene groups into organic cages.69 All the obtained cages have photochromic properties. In another notable development, Zhao et al. found that tetraphenylethylene-based POCs, derived from reduction and acidification processes, exhibit increased structural flexibility and hydrophilicity conducive to the creation of AIE-active POCs for biological applications.70
However, in many cases, the C–N linked POCs obtained by this method usually have a flexible skeleton, leading to their structural collapse and a dramatic decrease in porosity. Therefore, the main question that needs to be addressed is how to fix the collapsed skeleton. Cooper's group reported an effective method by using small carbonyl molecules such as formaldehyde to “tie” the flexible amine cages via post-synthetic modification to get shape-persistent cage compounds (Fig. 3).53 They demonstrated that this new protocol could improve the chemical robustness to acidic and basic conditions (pH 1.7–12.3) and tuned the internal cavity, pore environment, guest binding selectivity and so on, resulting in outstanding quantum sieves for hydrogen isotope separation.71 Additionally, they collaborated with Ibarra to conduct pioneering research on the capture of SO2 using CC3, RCC3, and a six-formaldehyde-tethered cage called 6FT-RCC3. Notably, among these materials, 6FT-RCC3 demonstrated an impressive capacity for capturing SO2 (13.78 mmol g−1) with excellent reversibility observed through over fifty adsorption–desorption cycles.72 More recently, Koh et al. reported that CC3 and 6FT-RCC3 have facilitated the rapid capture of Br2.73
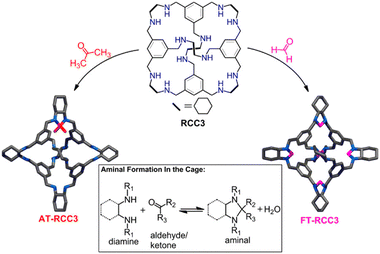 | ||
| Fig. 3 Synthesis of “tied” porous organic cages. Reproduced with permission: from ref. 53, Copyright 2014, The American Chemical Society. | ||
Benefiting from their exceptional stability, amine-linked POCs have found extensive application anchoring ultrafine metal nanoparticles within cavities as efficient supramolecular catalysts for numerous organic transformations under severe conditions.74,75 The size of the metal nanoparticles can be precisely controlled based on the host cavity's dimension. Zhang et al. initially utilized amine-linked [2+3] POC to provide well-regulated Au nanoparticle nucleation (particle diameter: 19 ± 4 Å) within a POC's cavity via gold–sulfur interactions.76 Since then, many groups have applied POCs with different desired shapes, sizes, and functionalities to stabilize various metal nanoparticles, such as Ru, Ag, Pd, and Pt nanoparticles.77–82 These nanocomposites are being examined for various organic transformations including photocatalytic nitroarene reduction, Suzuki–Miyaura cross-coupling reactions of haloarenes, alkoxycarbonylation of aryl iodides, and sequential conversions via aerobic hydroxylation and hydrogenation. Remarkably, these catalysts outperform conventional ones in stability, substrate scope, reactivity, and recyclability for the protection of POCs.
(2) Another easy method to make amine-linked POCs is based on a synthetic strategy that hinges on the Schiff base condensation reaction concept followed by keto–enol tautomerization.83 This method leads to the exceptional chemical stability of cages and facilitates their resistance to acids and bases.
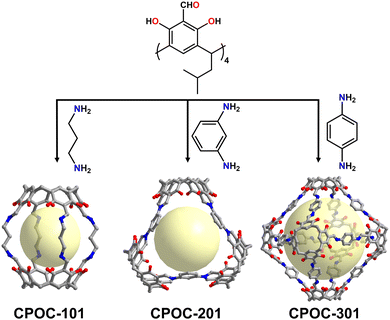
Banerjee et al. developed these chemical-stable amine-linked POCs employing trialdehyde resorcinol and aliphatic amine ligands, through imine condensation and keto–enol tautomerism reaction.48 Due to the different lengths of the diamines, the resulting organic cages showed an odd–even phenomenon. In addition, the authors also proved that all these organic cages can be stable in water, acids, and bases (Fig. 4). Inspired by this work, Yuan's Group successfully constructed three different types of POCs based on reticular chemistry by using C4RACHO with diamines by Schiff base condensation (Fig. 5). These include the [2+4] lantern-shaped organic cages with odd–even effect, [3+6] trigonal organic cages, [6+8] and [6+12] octahedral organic cages.49,84 Due to the presence of phenol hydroxyl group in the C4RACHO units, the resulting imine bonded POCs can also be spontaneously converted to amine bonded POCs through keto–enol tautomerization, which ensured the stability of the materials. In addition, the results show that the window diameters of these POCs can be continuously tuned from 3.8 to 11.6 Å. The cavity volume increased from 358 to 11243 Å3, and the BET surface area can be improved from 38 to 2803 m2 g−1. Subsequently, the octahedral CPOC-301 was used to purify ethylene (C2H4).85 Its excellent selectivity for ethane (C2H6) is due to the fact that C2H6 forms more C–H⋯π hydrogen bonds with calix[4]arene than C2H4, which leads to the preferential interaction of C2H6 over C2H4 in POCs. This work provides a new avenue for the isolation and purification of C2H4. Furthermore, CPOC-301 exhibits potential as a dual-functional photoelectrochemical energy storage cathode in a photo-assisted Li-organic battery when C60 is encapsulated within its cavity.86
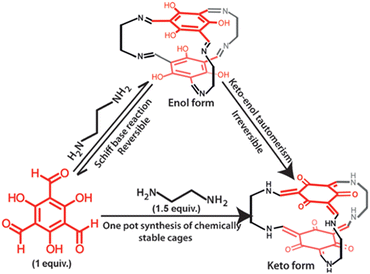 | ||
| Fig. 4 Synthesis of keto–enamine POCs. Reproduced with permission: from ref. 48, Copyright 2017, Wiley. | ||
In 2022, Wang et al. synthesized an ultra-stable chiral amine-linked octahedral organic cage (PAC 1) through the [4+6] condensation reaction of binaphthylenediamine with 2,4,6-triformylphloroglucinol.50 As expected, the crystallinity of PAC 1-S is kept in boiling water, as well as many harsh chemical conditions, such as high-concentrated acid and alkali, and 1 M H2SO4/MeOH/H2O solution. Moreover, stable PAC 1 can enantioselectively recognize axially chiral aromatic racemates. More recently, the introduction of Br and CH3 substituents has afforded two new pairs of axially chiral PAC enantiomers, namely R-PAC-2, S-PAC-2, and R-PAC-3, S-PAC-3.87 Among these, PAC-2 demonstrates successful encapsulation and selective binding of C70 over C60 in simulated carbon soot. Furthermore, C60-encapsulated PAC-2 can be utilized as an electrochemical chiral sensor on glassy carbon electrodes (GCE) for the detection of the chiral compound L-3,4-dihydroxyphenylalanine (DOPA), achieving a detection limit of 2.8 mM.88
Recently, Khashab et al. reported three cases of POCs with varying hydroxyl substitutions; notably, OC1, a [2+3] cage, synthesized from 1,3,5-triformylphloroglucinol and (1R,2R)-1,2-diaminocyclohexane, featuring a higher number of hydroxyl groups exhibited keto–enol tautomerism and demonstrated increased stability, biocompatibility, photostability, and cell permeability. Consequently, the organic cage (OC1) shows promise as an effective mitochondrial fluorescent probe.89
2.2 Transformation of imine into other chemically stable linkages
The dynamic nature of imine condensation allows for “self-error correction” within the reaction process to yield thermodynamically stable products at relatively high yields. Therefore, utilizing imine chemistry as a foundation and combining it with post-synthetic modification (PSM) undoubtedly represents a universal and efficient approach for obtaining high-yield, stable organic cage molecules.23 The PSM of imine bond provides an alternative approach to improve the backbones' chemical stability of porous organic materials, further providing tailored functionality for specific applications as demonstrated by COFs systems. Fortunately, recent studies have explored diverse transformations via imine bond PSM in POCs systems.Mastalerz et al. made significant contributions in this area. For instance, the imine-linked cage was synthesized following established procedures in literature; it underwent subsequent reduction treatment to yield an amine-bonded cage. This structure then reacted with N,N′-carbonylbisimidazole in DMF at ambient temperature to form the carbamate cage.55 Notably, this resulting cage exhibits resilience towards acid and base treatment, maintaining stability even in 1 M HCl at temperatures of up to 100 °C.
Then, in 2019, this group reported converting the imine-linked cage to amide-linked cage via Pinnick oxidation.56 To the best of our knowledge, due to the irreversible nature of the amide bonds, the yield of amide cage synthesised via the one-pot method is very low, and only a few examples of amide cages are reported with small cavities. Therefore, efficient synthesis of larger amide cages still needs to be explored. In this part, [4+6] cage containing twelve amide groups with durable shapes was prepared via PSM of salicylbisimine cage. The cage has superior chemical stability and thus can be further functionalized. This study obtained hydroxylated, nitrated, and brominated amide cages in 60%, 84%, and 83% yield, respectively. Among them, the nitrated cage could change the gas-sorption selectivity for CO2/CH4. Similarly, eight different [2+3] amide cages were obtained by this way and used as hosts for anion recognition.90 Also reported by this group, the Povarov reaction of [4+6] salicylimine cage with phenylacetylene led to a robust quinoline cage (Fig. 6).54 The key to success in this reaction was the reaction time. This reaction took them 20 hours and finally the product was obtained in 25% yield, which was resistant to harsh acidic conditions (pH – 1.9–15.2) without decomposition. The as-synthesized cage has a BET specific surface area of 698 m2 g−1. The cage also shows characteristic acidochromism and can be used as a thin film sensor. They also reported the transformation of imine cages into hydrocarbon cages through a three-step reaction.91 Although the yield of cages was up to 24% with this method, it is much higher than the previously reported one-step synthesis strategy.
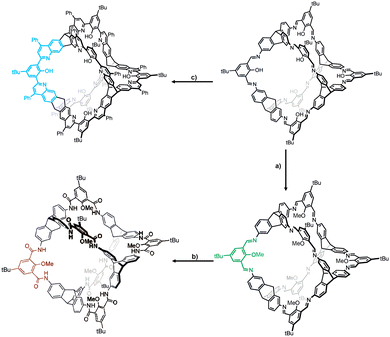 | ||
| Fig. 6 Synthesis of stable POCs. (a) CH3I, K2CO3, DMF, 80 °C, 16 h; (b) NaClO2, NaH2PO4, 2-methyl-2-butene, THF, H2O, rt, 16 h; (c) Sc(OTf)3, chloranil, phenylacetylene (neat), 100 °C, 20 h. | ||
Mukherjee et al. similarly used the Pinnick oxidation to convert [2+3] imine cage to amide cage in 48% yield.92 The specific cavity of this cage and the six amide groups allow it to bind F selectively. As a result, NMR titration experiments and computational studies demonstrated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex.
2 host–guest complex.
More recently, Schmidt and coworkers reported a novel, multi-step post-synthetic modification (PSM) of organic cages bearing fluorine substituents. This method generates stable benzylamine-linked organic cages with functional groups through azadefluorination cyclization (ADFC) reactions using readily available isocyanates.93 For instance, benzylamine-linked organic cages such as Et2A3-Br can be further reacted with various boronic acids via Suzuki–Miyaura coupling reactions to produce functionalized benzylamine-linked cages (Fig. 7). These reactions demonstrate the excellent chemical stability of the benzylamine-linked cages, as the cross-coupling reactions require stirring for up to 4 days under either basic or acidic conditions. Furthermore, this multi-step post-modification approach can also be employed to create benzylamine-linked organic macrocycles and cross-linked membranes from ditopic isocyanates.
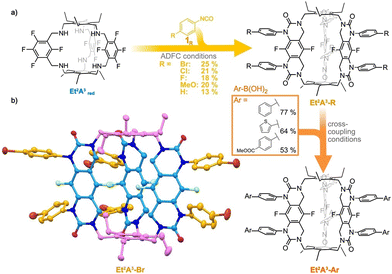 | ||
| Fig. 7 (a) Synthesis of benzylamine-linked POCs, and (b) the single-crystal structure of Et2A3-Br. Reproduced with permission: from ref. 93, Copyright 2024, Wiley. | ||
These examples demonstrate that chemically stable POCs can be obtained in reasonable or even high yields by transforming imine linkages into other linkages. This strategy appears to be an effective method for overcoming the stability drawbacks associated with imine-based POCs.
2.3 N-substituted imine linkages
Introducing N-alkyl/aryl groups onto the N atom of the imine bond yields the related N-substituted imine derivatives, namely hydrazone (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), a more robust counterpart of imine, which is stable enough against hydrolysis in water.94 Hydrazone stability arises from the adjacent N atom's lone pair electrons, which can delocalize into the C
N–N), a more robust counterpart of imine, which is stable enough against hydrolysis in water.94 Hydrazone stability arises from the adjacent N atom's lone pair electrons, which can delocalize into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, forming a –C−–N
N bond, forming a –C−–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+– charge-separated state, rendering the double bond less electrophilic and thereby more stable. As far as we know, several robust hydrazone-linked covalent organic frameworks (COFs) have been prepared and shown applications in water pollutant removal, proton conduction, energy production/storage, drug delivery, catalysis, etc.ref. 95–97, which have also driven the development of hydrazone-linked POCs.98–100
N+– charge-separated state, rendering the double bond less electrophilic and thereby more stable. As far as we know, several robust hydrazone-linked covalent organic frameworks (COFs) have been prepared and shown applications in water pollutant removal, proton conduction, energy production/storage, drug delivery, catalysis, etc.ref. 95–97, which have also driven the development of hydrazone-linked POCs.98–100
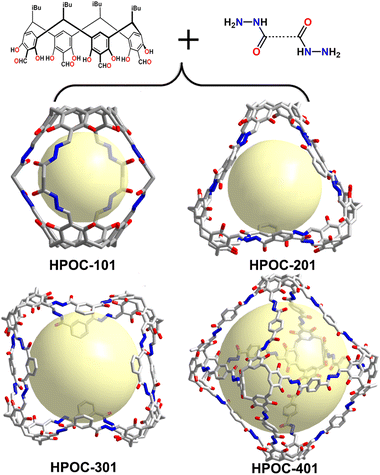
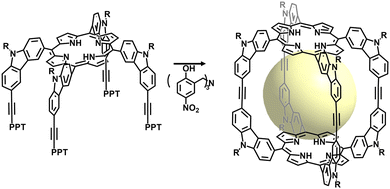
The synthesis of well-defined cage compounds via hydrazone bond formation was first reported in 2011 by Warmuth et al.,101 who prepared polyacylhydrazone nanocages from tetraformylcavitand and hydrazides. Although this work made a significant contribution, the resulting nanocapsules had cavity diameters limited to no larger than 25 Å and lacked characterization of their porosity, which is crucial for POCs applications. To address this issue, Yuan's group developed water-stable hydrazone-linked POCs (HPOCs) by coupling tetraformyl-functionalized calix[4]resorcinarene (C4RACHO) with various dihydrazides.45 By adjusting the length and angle of the hydrazide linkers, the size and crystal structure of the cage compounds could be simultaneously tuned, resulting in cavity volumes ranging from 580 to 6800 Å3 (Fig. 8). The experimental results demonstrated that C4RACHO could serve as an efficient building block in the formation of cage compounds due to its “convergent” structural properties. Additionally, HPOCs were found to effectively remove toxic pollutants such as radionuclide waste, heavy metal ions, and organic micropollutants from water. Their ability to chemically chelate metals makes them suitable for use in perovskite solar cells (PSC) to reconstitute buried interface,102 and can also act as catalysts after metal modification.103
Schneebeli et al. have recently synthesized a series of tetrahedral hydrazone cages with varying sizes through the reaction of hydrazides with different dialdehyde linkers.104 As the length of the linker increases, so does the size of the resulting molecular cages. The largest cage boasts a cavity volume of approximately 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3, making it one of the largest tetrahedral organic cages reported to date. Moreover, noteworthy improvements have been made in the synthesis route, reducing the solvent volume required by a factor of 6 and enabling gram-scale preparation of the molecular cage.
000 Å3, making it one of the largest tetrahedral organic cages reported to date. Moreover, noteworthy improvements have been made in the synthesis route, reducing the solvent volume required by a factor of 6 and enabling gram-scale preparation of the molecular cage.
3. Stable POCs based on other coupling reactions
To expand the scope of stable COFs and POCs, it is crucial to develop new linkage chemistries that can be employed in one-pot or multi-step reactions, as demonstrated in organic synthesis. The implementation of these linkages has led to the creation of materials with novel properties (Table 1). Notably, certain stable linkages possess broader practical applications. These findings provide valuable insights for designing stable cages with new bonding modes. While previous studies have focused on cage structures formed through imine bond formations, this section will primarily discuss other coupling reactions, including imide linkage formation, base-catalyzed Knoevenagel condensation, alkyne metathesis, and ether linkage formation, which have successfully prepared stable POCs and COFs.105–108 Additionally, a brief introduction to stable POCs formed through other coupling reactions such as Click reaction, azo bond formation, Friedel–Crafts reaction will be presented.109–111 Although numerous studies exist, the number is limited due to the challenges in constructing POCs with less reversible or irreversible bonds, which often result in insoluble polymeric species with low product yields and oligomers. Despite these challenges, discovering novel POCs formed through these bonds remain essential for their practical industrial applications.| POC | Linkage | Chemical stability | Applications | Ref. |
|---|---|---|---|---|
| FT-RCC3 | Amine | pH 1.7–12.3 | Adsorption, separation | 53 and 71–73 |
| TpEDA/TpPDA | β-Ketoeneamine | Hot water | Mixed-matrix membrane | 48 |
| TpBDA/TpPNDA | 0.5 M HCl | |||
| 1 M NaOH | ||||
| PAC 1/2/3 | β-Ketoeneamine | Boiling water | Separation, molecular recognition | 50, 87 and 88 |
| 1 M HCl | ||||
| 1 M H2SO4 | ||||
| 12 M NaOH | ||||
| Amide cage | Amide | Conc. HNO3 | Adsorption | 56 |
| 2 M HCl | ||||
| 4 M H2SO4 | ||||
| 3 M NaOH | ||||
| Carbamate cage | Carbamate | Conc. HCl | Adsorption | 55 |
| 1 M NaOH | ||||
| NKPOC-1 | Imide | Water | Adsorption, separation, smart materials | 36, 59 and 112 |
| HPOCs | Hydrazone | Water | Adsorption, energy, catalysis | 45, 102 and 103 |
| Quinoline cage | Quinoline | 12 M HCl | Sensing | 54 |
| 36 M H2SO4 | ||||
| 15 M NaOH | ||||
| sp2C-POCs | Alkene | Boiling water | Adsorption, separation | 28 |
| Conc. HNO3 | ||||
| 12 M HCl | ||||
| 36 M H2SO4 | ||||
| 12 M NaOH | ||||
| Et2A3-Br | Benzylamine | Water | Adsorption | 93 |
| Conc. HCl |
3.1 sp2C linkages
The virtue of relatively low reversibility and in-plane p-electron delocalization makes sp2 carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) linkages based on various reactions suitable for constructing sp2 carbon-conjugated porous organic polymers and COFs with different pores and diverse applications. These include the Gilch reaction, Witting reaction, Mizoroki–Heck coupling reaction, Suzuki–Miyaura cross-coupling reaction, Yamamoto coupling reaction, and Knoevenagel condensation reaction.113,114
C) linkages based on various reactions suitable for constructing sp2 carbon-conjugated porous organic polymers and COFs with different pores and diverse applications. These include the Gilch reaction, Witting reaction, Mizoroki–Heck coupling reaction, Suzuki–Miyaura cross-coupling reaction, Yamamoto coupling reaction, and Knoevenagel condensation reaction.113,114
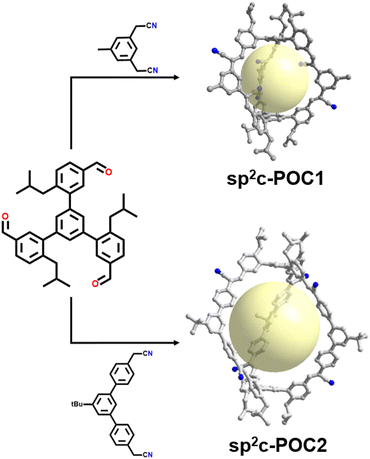
In contrast, the development of sp2 carbon-conjugated POCs has progressed more slowly than that of sp2 carbon-linked COFs. To our knowledge, only the Witting reaction and Knoevenagel condensation reaction have been achieved in the construction of sp2 carbon-linked POCs.115,116 The former was demonstrated by Wennerstrom et al. in 1982, who achieved a sp2 carbon-linked cage-shaped bicyclophane through sixfold Witting reactions between aromatic bisphosphonium salts and 1,3,5-benzenetricarbaldehyde, with a yield of only 2%.117 More recently, a series of novel sp2 carbon-linked POCs (sp2c-POCs) were synthesized by Yuan's team in 2023 through a one-step Knoevenagel reaction as illustrated in Fig. 9.28 These triangular prism-shaped sp2c-POCs are constructed from two bowel-shaped aromatic trialdehydes acting as planes and three V-shaped aromatic diacetonitriles molecules acting as the edges. They exhibit excellent stability, remaining intact even in concentrated HCl, concentrated HNO3 or saturated NaOH solutions. Furthermore, their porosity and related applications have been studied. For instance, sp2c-POC1 was found to effectively adsorb CO2, CH4 and C2 hydrocarbons, as well as separate CO2/CH4 and CO2/N2 gas mixtures.
3.2 Ethynylene linkages
Carbon–carbon triple bonds exhibit stability and reversibility, without Z/E isomer issues, which leads to multiple isomers that can be difficult to isolate and characterize.118 Their rigid and linear geometry makes alkyne bonds frequently employed in the synthesis of shape-persistent ethynylene-linked architectures over the past decades. Ethynylene-linked POCs are a type of molecular architecture consisting of interconnected organic units connected by ethynylene linkers.In 2013, Doonan et al. reported a [2+3] POC (Fig. 10), namely C1, formed by irreversible Eglinton homocoupling of two rigid, 4-[tris(4-ethynylphenyl)methyl]methoxybenzene.26 Following purification via chromatography, the yield was only 20%. The study demonstrated that two distinct crystalline polymorphs, C1α and C1β, could be obtained through kinetic control. Among them, C1α proved nonporous to N2, whereas C1β exhibited a high BET surface area (1153 m2 g−1) as determined by nitrogen adsorption at 77 K. This reaction has been used to construct cage-like organic compounds since 2007 when Chen et al. assembled lantern-shaped organic cages from terminal acetylene units with a yield of 58%.119 However, they focused on the crystal structure and packing of the molecular cages, without studying the porosity after removal of solvent guests. Nonetheless, these POCs were prepared through kinetically-controlled Eglinton homocoupling reaction, which lacks selectivity, high yields, and efficiency, thus limiting the broad applications of these rigid cage molecules.
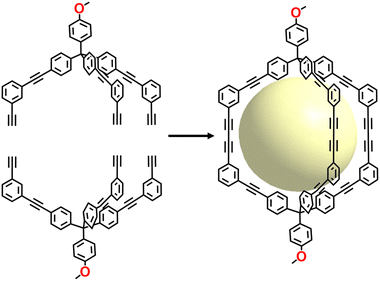
An alternative method for preparing ethynylene-linked POCs is one-step alkyne metathesis, a significant bond exchange reaction that facilitates the redistribution of alkyne groups via reversible cleavage and reformation of carbon–carbon triple bonds. A wide array of shape-persistent architectures have been synthesized through alkyne metathesis under thermodynamically controlled conditions due to its reversibility and self-correcting behavior.118
In 2011, Zhang et al. employed the alkyne metathesis reaction to synthesize a porphyrin-based POC (Fig. 11) through a one-step approach.57 This shape-persistent, three-dimensional cage architecture featuring confined cavities and rigid backbones enabled it to encapsulate C60 and C70 as host receptors. UV-vis titration experiments, NMR spectroscopy, and MALDI-MS confirmed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between the cage and the fullerenes. Notably, the association constant between the cage and C70 was 1000 times higher than that with C60, providing a promising platform for the efficient separation of C60 and C70. Subsequently, they constructed carbon–carbon bonded POCs using trialkynyl monomers via a similar synthetic route; however, the resulting product was a tetramer rather than the desired tetrahedral cage.58 This prompted them to conduct a detailed investigation of the reaction process using GPC traces and 1H NMR spectroscopy. The results showed that a critical dimeric cyclic compound was first formed in this reaction, followed by a “face-directed” formation of a tetramer with D2h symmetry. They also confirmed the strong binding interaction between the cage and C70. To understand the relationship between ethynylene-linked POC structures and organic synthons geometry, Zhang's group performed systematic experiments based on different organic synthons compositions. Based on their previous synthetic strategy, cubic and interlocked ethynylene-linked POCs have been obtained.35,120
1 complexes between the cage and the fullerenes. Notably, the association constant between the cage and C70 was 1000 times higher than that with C60, providing a promising platform for the efficient separation of C60 and C70. Subsequently, they constructed carbon–carbon bonded POCs using trialkynyl monomers via a similar synthetic route; however, the resulting product was a tetramer rather than the desired tetrahedral cage.58 This prompted them to conduct a detailed investigation of the reaction process using GPC traces and 1H NMR spectroscopy. The results showed that a critical dimeric cyclic compound was first formed in this reaction, followed by a “face-directed” formation of a tetramer with D2h symmetry. They also confirmed the strong binding interaction between the cage and C70. To understand the relationship between ethynylene-linked POC structures and organic synthons geometry, Zhang's group performed systematic experiments based on different organic synthons compositions. Based on their previous synthetic strategy, cubic and interlocked ethynylene-linked POCs have been obtained.35,120
In 2016, Moore and colleagues utilized alkyne metathesis to synthesize ethynylene-linked POCs (TdA). They employed a tripodal precursor, 1,3,5-tribenzyl-2,4,6-triethylbenzene derivative, featuring well-defined angles and alternating up–down conformation. This led to the successful synthesis of tetrahedral ethynylene-linked POCs with near-quantitative yields.121 Two years later, the researchers began examining the influence of the bite angle of tripodal precursors on cage formation.122 Their findings indicated that high yields in the synthesis of ethynylene-linked POCs can be achieved by designing and synthesizing precursors with bite angles slightly smaller than the theoretically optimal angle for the desired structure. In the same year, Moore collaborated with Gewirth and Nuzzo to develop solid–liquid lithium electrolyte nanocomposites using TdA as the base material. This nanocomposite displayed remarkable ionic conductivity, reaching values of approximately 1 × 10−3 S cm−1.123
3.3 Imide linkages
The imide linkage can be synthesized through the reaction between amine derivatives and acetic anhydride, which is less reversible and requires a high reaction temperature of up to 250 °C.124 An imide linkage represents a chemical bond between an amine (–NH2) group and a carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group. It is characterized by a nitrogen atom bonded to the carbonyl carbon. The resulting compound is referred to as an imide. Imide linkages are commonly found in various polymers, such as polyimides, which are known for their high thermal stability and mechanical strength.
O) group. It is characterized by a nitrogen atom bonded to the carbonyl carbon. The resulting compound is referred to as an imide. Imide linkages are commonly found in various polymers, such as polyimides, which are known for their high thermal stability and mechanical strength.
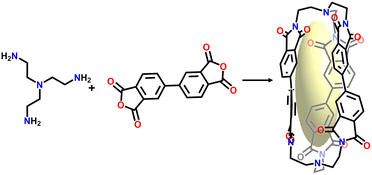
Zhang's group synthesized an imide-linked [2+3] organic cage (Fig. 12), obtaining soft porous crystals with intrinsic and extrinsic porosity through self-assembly.36 It was found that under the stimulation of different guest molecules, the crystal structure underwent a reversible phase transition (β phase ↔α phase ↔γ phase), and pores in this structure could selectively open or close to achieve the selective adsorption and separation of guest molecules.112 Furthermore, due to the dynamic nature of the POCs structure, the author also combined it with a polymer matrix to prepare a hybrid matrix membrane, which was used as a humidity-responsive actuator.59
3.4 Ether linkages
Nucleophilic substitutions are chemical reactions that involve the nucleophile attacking an electrophilic center, resulting in the displacement of a leaving group and the formation of stable covalent bonds. Several stable POCs have been prepared based on nucleophilic aromatic substitution reactions with ether bonds.125–129For example, Katz et al. reported early examples of ether-linked cage-like bicyclooxacalixarenes (Fig. 13a) with a yield of up to 95% by condensation of phloroglucinol with electron-poor pyridine and halogenated-substituted benzene synthons.130 Notably, these cage-like bicyclooxacalixarenes have been documented as good precursors for preparing cage-based framework materials for gas storage and separation applications.131–133
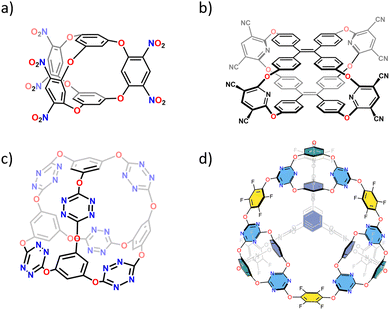 | ||
| Fig. 13 Synthesis of ether linked POCs (a) bicyclooxacalixarene, (b) tricyclooxacalixarene cage, (c) tetrazine-based cage, (d) [4[2+3]+6]cage. | ||
In subsequent years, various ether-bonded organic cages with diverse configurations and sizes, as well as assemblies featuring complex structures, were reported. Zhang et al. synthesized a tetragonal cage (Fig. 13b) using tetrahydroxytetraphenylethylene and 2,6-dichloropyridine-3,5-dicarbonitrile in the presence of cesium carbonate catalyst,60 with a yield of 21% and a BET surface area of 432 m2 g−1. It is noteworthy that the formation of the cage constrained the rotation of the tetraphenylethylene units, resulting in an aggregation-induced emission (AIE) effect. Carrillo et al. demonstrated the effectiveness of nucleophilic aromatic substitution of tetrazines with phenols and alkyl thiols for preparing macrocycle and cage compounds (Fig. 13c).61 Li et al. developed a new type of POCs featuring strong light absorption ability and photocatalytic activity by using porphyrins as building blocks.27 This was the first instance in which POCs supported single-atom catalysts to promote benzylamine coupling and dipeptide preparation under visible light irradiation. Apart from the [a+b] condensation-based POCs mentioned earlier, topologically complex ‘cage of cages’ structures can be achieved through hierarchical self-assembly strategy. The resulting [4[2+3]+6]cage (Fig. 13d) linked via ether bonds exhibits high BET surface area and exceptional stability in water, along with enhanced CO2 and SF6 uptakes.134
3.5 Other linkages
To further explore stable organic cage constructions, it is essential to consider additional types from an academic perspective. For instance, Flood's group utilized the widely recognized copper(I)-catalyzed azido–alkyne cycloaddition, also known as the click reaction, to synthesize a novel cryptand-like cage exhibiting strong binding affinity towards Cl− ions.135 Li et al. reported synthesizing a prism-shaped cage linked by six C–C bonds via the Friedel–Crafts reaction, which resulted in a π-electron-rich cavity capable of encapsulating various planar π-electron-poor guests.111 He et al. prepared an azo-linked organic cage through direct reaction between a triple-diazonium reagent and a triple-phenol molecule, demonstrating light-triggered fine-tuning of its cavity.110 Li's team efficiently synthesized shape-controlled organic cages ([2]Cage and [4]Cage) via a one-pot reaction involving 1,3,5-tris(2,4-dimethoxyphenyl)benzene and paraformaldehyde/isobutyraldehyde catalyzed by a Lewis acid. [2]Cage was successfully employed as a gas chromatographic stationary phase for the high-resolution separation of benzene/cyclohexane and toluene/methylcyclohexane.136 Furthermore, Wessjohann et al. utilized a one-pot, Ugi-type multiple multicomponent reaction to prepare organic cages containing multidimensional arrays of peptoid cores.137 It is important to note that the number of organic cages formed through these methods remains relatively small, offering significant scope for systematic studies on their assembly behavior, particularly those with cavities formed through more covalent bond formations. Additionally, exploring their potential applications is an essential direction for future research.4. Summary and outlook
In summary, numerous discrete POCs with diverse shapes and sizes have been developed utilizing various reversible and irreversible covalent condensation reactions. Despite this progress, these POCs frequently encounter stability concerns or low yields. As an emerging class of materials with promising practical applications, the need for efficient synthesis methods that produce stable POCs has become increasingly pressing. This review offers two guidelines for synthesizing stable POCs: (1) three strategies to stabilize imine bonds commonly used in POCs, such as incorporating hydrazone linkages, transforming imines into amines, and replacing imines with more chemically stable linkages; (2) four types of less reversible or irreversible bond formations, including imide formation, Knoevenagel condensation, alkyne metathesis, and nucleophilic aromatic reactions – for the efficient preparation of stable POCs. However, some strategies and covalent condensation reactions have been successfully applied to porous materials but not yet to POCs. Herein, we present three critical perspectives on synthesizing stable POCs and their advanced applications.1. The imine condensation method is a widely used and effective approach for synthesizing POCs, particularly those with large cavities that can accommodate more substantial guests. However, as the size of imine-linked POCs increases, their structural stability and porosity may be compromised due to the metastable nature of imine bonds. Consequently, improving the stability of imine linkages in these larger POCs is essential. Besides the transformation methods mentioned in Section 2.2, other strategies can enhance bond stability, as has been achieved in imine-linked COFs.63,138 These include cyclizations of imines into imidazole, oxazole, thiazole, indazole, as well as asymmetric addition, multicomponent reactions, and linkage exchange methods. Meanwhile, various methods for synthesizing highly chemically robust linkages, such as triazine,139 dioxin,140 arylamine,141 and C–C single bonds142 in stable COFs with large channels can be adopted to construct stable and functional POCs by selecting suitable organic synthons.
2. POCs are discrete, purely organic molecules characterized by their convergent structures, internal cavities, and open windows. When designing these molecules, careful consideration must be given to the shape of the organic synthons used in bond-forming reactions. It is noteworthy that most reported POCs are synthesized using two or three types of organic synthons. A concave-shaped synthon is generally required for efficient synthesis and to prevent the formation of infinite covalent organic framework (COF) structures. The optimal bite angle of these concave-shaped synthons can be calculated using computational tools,143–145 eliminating the need for repetitive trial-and-error optimization in experimental synthesis. Furthermore, building blocks such as calixarene,146 substituted 1,3,5-triphenylbenzene,147 substituted porphyrin,148 have been identified as excellent options for constructing POCs.
3. While the applications of well-developed COF materials are extensive, those of POCs remain in their infancy. To broaden their practical applications across energy, environment, sensors, catalysis, and other fields, functional groups like benzothiadiazole, phthalocyanine, porphyrin, azobenzene, viologen, triazine, and bipyridine can be incorporated into the stable skeletons of POCs. Their discrete and soluble nature offers the potential for more exposed sites compared to COFs in a solution state. Furthermore, POCs are promising candidates for 3D-printing, membrane, and porous liquid applications, given their advantageous characteristics such as good solubility and processability in solvents.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (22071244, 22275191), Youth Innovation Promotion Association CAS (2022305), and the Natural Science Foundation of Fujian Province of China (2022J01503, 2020J05087).Notes and references
- S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed.
- C. Liu, Y. Jin, Z. Yu, L. Gong, H. Wang, B. Yu, W. Zhang and J. Jiang, J. Am. Chem. Soc., 2022, 144, 12390–12399 CrossRef CAS PubMed.
- Z. Shan, X. Wu, B. Xu, Y.-L. Hong, M. Wu, Y. Wang, Y. Nishiyama, J. Zhu, S. Horike, S. Kitagawa and G. Zhang, J. Am. Chem. Soc., 2020, 142, 21279–21284 CrossRef CAS PubMed.
- M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed.
- G. Zhang and M. Mastalerz, Chem. Soc. Rev., 2014, 43, 1934–1947 RSC.
- T. Hasell and A. I. Cooper, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS.
- L. O. Alimi, F. Fang, B. Moosa, Y. Ding and N. M. Khashab, Angew. Chem., Int. Ed., 2022, 61, e202212596 CrossRef CAS.
- W. Wang, K. Su, E.-S. M. El-Sayed, M. Yang and D. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 24042–24050 CrossRef CAS PubMed.
- M. Ovalle, M. Kathan, R. Toyoda, C. N. Stindt, S. Crespi and B. L. Feringa, Angew. Chem., Int. Ed., 2023, 62, e202214495 CrossRef CAS PubMed.
- A. I. Cooper, ACS Cent. Sci., 2017, 3, 544–553 CrossRef CAS.
- T. Hasell, H. Zhang and A. I. Cooper, Adv. Mater., 2012, 24, 5732–5737 CrossRef CAS PubMed.
- Q. Song, S. Jiang, T. Hasell, M. Liu, S. Sun, A. K. Cheetham, E. Sivaniah and A. I. Cooper, Adv. Mater., 2016, 28, 2629–2637 CrossRef CAS.
- A. F. Bushell, P. M. Budd, M. P. Attfield, J. T. A. Jones, T. Hasell, A. I. Cooper, P. Bernardo, F. Bazzarelli, G. Clarizia and J. C. Jansen, Angew. Chem., Int. Ed., 2013, 52, 1253–1256 CrossRef CAS.
- A. He, Z. Jiang, Y. Wu, H. Hussain, J. Rawle, M. E. Briggs, M. A. Little, A. G. Livingston and A. I. Cooper, Nat. Mater., 2022, 21, 463–470 CrossRef CAS PubMed.
- B. Dietrich, J. Lehn and J. Sauvage, Tetrahedron Lett., 1969, 10, 2885–2888 CrossRef.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973–978 CrossRef CAS PubMed.
- X. Yang, Z. Ullah, J. F. Stoddart and C. T. Yavuz, Chem. Rev., 2023, 123, 4602–4634 CrossRef CAS PubMed.
- G. Montà-González, F. Sancenón, R. Martínez-Máñez and V. J. C. R. Martí-Centelles, Chem. Rev., 2022, 122, 13636–13708 CrossRef.
- V. Ramamurthy, Acc. Chem. Res., 2015, 48, 2904–2917 CrossRef CAS PubMed.
- W.-T. Dou, C.-Y. Yang, L.-R. Hu, B. Song, T. Jin, P.-P. Jia, X. Ji, F. Zheng, H.-B. Yang and L. Xu, ACS Mater. Lett., 2023, 5, 1061–1082 CrossRef CAS.
- A. Galan and P. Ballester, Chem. Soc. Rev., 2016, 45, 1720–1737 RSC.
- K. Kobayashi and M. Yamanaka, Chem. Soc. Rev., 2015, 44, 449–466 RSC.
- H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Chem. Soc. Rev., 2021, 50, 8874–8886 RSC.
- M. S. Collins, M. E. Carnes, B. P. Nell, L. N. Zakharov and D. W. Johnson, Nat. Commun., 2016, 7, 11052 CrossRef CAS PubMed.
- D. Chakraborty and P. S. Mukherjee, Chem. Commun., 2022, 58, 5558–5573 RSC.
- A. Avellaneda, P. Valente, A. Burgun, J. D. Evans, A. W. Markwell-Heys, D. Rankine, D. J. Nielsen, M. R. Hill, C. J. Sumby and C. J. Doonan, Angew. Chem., Int. Ed., 2013, 52, 3746–3749 CrossRef CAS PubMed.
- F. Zhang, J. Ma, Y. Tan, G. Yu, H. Qin, L. Zheng, H. Liu and R. Li, ACS Catal., 2022, 12, 5827–5833 CrossRef CAS.
- F. Qiu, X. Chen, W. Wang, K. Su and D. Yuan, CCS Chem., 2024, 6, 149–156 CrossRef CAS.
- M. Mastalerz, Angew. Chem., Int. Ed., 2010, 49, 5042–5053 CrossRef CAS PubMed.
- M. W. Schneider, I. M. Oppel, A. Griffin and M. Mastalerz, Angew. Chem., Int. Ed., 2013, 52, 3611–3615 CrossRef CAS PubMed.
- S. Bera, K. Dey, T. K. Pal, A. Halder, S. Tothadi, S. Karak, M. Addicoat and R. Banerjee, Angew. Chem., Int. Ed., 2019, 58, 4243–4247 CrossRef CAS PubMed.
- B. D. Egleston, K. V. Luzyanin, M. C. Brand, R. Clowes, M. E. Briggs, R. L. Greenaway and A. I. Cooper, Angew. Chem., Int. Ed., 2020, 59, 7362–7366 CrossRef CAS PubMed.
- P. Wagner, F. Rominger, W.-S. Zhang, J. H. Gross, S. M. Elbert, R. R. Schroeder and M. Mastalerz, Angew. Chem., Int. Ed., 2021, 60, 8896–8904 CrossRef CAS PubMed.
- S. Ivanova, E. Koester, J. J. Holstein, N. Keller, G. H. Clever, T. Bein and F. Beuerle, Angew. Chem., Int. Ed., 2021, 60, 17455–17463 CrossRef CAS PubMed.
- Q. Wang, C. Yu, C. Zhang, H. Long, S. Azarnoush, Y. Jin and W. Zhang, Chem. Sci., 2016, 7, 3370–3376 RSC.
- Z. Wang, N. Sikdar, S.-Q. Wang, X. Li, M. Yu, X.-H. Bu, Z. Chang, X. Zou, Y. Chen, P. Cheng, K. Yu, M. J. Zaworotko and Z. Zhang, J. Am. Chem. Soc., 2019, 141, 9408–9414 CrossRef CAS PubMed.
- N. Christinat, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2008, 47, 1848–1852 CrossRef CAS PubMed.
- Y. Jin, Q. Wang, P. Taynton and W. Zhang, Acc. Chem. Res., 2014, 47, 1575–1586 CrossRef CAS PubMed.
- H.-H. Huang, K. S. Song, A. Prescimone, A. Aster, G. Cohen, R. Mannancherry, E. Vauthey, A. Coskun and T. Solomek, Chem. Sci., 2021, 12, 5275–5285 RSC.
- K. Acharyya and P. S. Mukherjee, Angew. Chem., Int. Ed., 2019, 58, 8640–8653 CrossRef CAS PubMed.
- K. S. Gayen, T. Das and N. Chatterjee, Eur. J. Org. Chem., 2021, 861–876 CrossRef CAS.
- K. E. Jelfs, X. F. Wu, M. Schmidtmann, J. T. A. Jones, J. E. Warren, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 10653–10656 CrossRef CAS PubMed.
- P. Skowronek, B. Warzajtis, U. Rychlewska and J. Gawronski, Chem. Commun., 2013, 49, 2524–2526 RSC.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed.
- M. Yang, F. Qiu, E.-S. M. El-Sayed, W. Wang, S. Du, K. Su and D. Yuan, Chem. Sci., 2021, 12, 13307–13315 RSC.
- H. Li, H. Zhang, A. D. Lammer, M. Wang, X. Li, V. M. Lynch and J. L. Sessler, Nat. Chem., 2015, 7, 1003–1008 CrossRef CAS PubMed.
- T. Jiao, G. Wu, Y. Zhang, L. Shen, Y. Lei, C.-Y. Wang, A. C. Fahrenbach and H. Li, Angew. Chem., Int. Ed., 2020, 59, 18350–18367 CrossRef CAS PubMed.
- S. Bera, A. Basu, S. Tothadi, B. Garai, S. Banerjee, K. Vanka and R. Banerjee, Angew. Chem., Int. Ed., 2017, 56, 2123–2126 CrossRef CAS PubMed.
- K. Su, W. Wang, S. Du, C. Ji, M. Zhou and D. Yuan, J. Am. Chem. Soc., 2020, 142, 18060–18072 CrossRef CAS PubMed.
- D.-X. Cui, Y. Geng, J.-N. Kou, G.-G. Shan, C.-Y. Sun, K.-H. Zhang, X.-L. Wang and Z.-M. Su, Nat. Commun., 2022, 13, 4011 CrossRef CAS PubMed.
- M. Liu, L. Chen, S. Lewis, S. Y. Chong, M. A. Little, T. Hasell, I. M. Aldous, C. M. Brown, M. W. Smith, C. A. Morrison, L. J. Hardwick and A. I. Cooper, Nat. Commun., 2016, 7, 12750 CrossRef CAS PubMed.
- B. Mondal and P. S. Mukherjee, J. Am. Chem. Soc., 2018, 140, 12592–12601 CrossRef CAS PubMed.
- M. Liu, M. A. Little, K. E. Jelfs, J. T. A. Jones, M. Schmidtmann, S. Y. Chong, T. Hasell and A. I. Cooper, J. Am. Chem. Soc., 2014, 136, 7583–7586 CrossRef CAS PubMed.
- P.-E. Alexandre, W.-S. Zhang, F. Rominger, S. M. Elbert, R. R. Schroeder and M. Mastalerz, Angew. Chem., Int. Ed., 2020, 59, 19675–19679 CrossRef CAS PubMed.
- X. Y. Hu, W. S. Zhang, F. Rominger, I. Wacker, R. R. Schroder and M. Mastalerz, Chem. Commun., 2017, 53, 8616–8619 RSC.
- A. S. Bhat, S. M. Elbert, W.-S. Zhang, F. Rominger, M. Dieckmann, R. R. Schroeder and M. Mastalerz, Angew. Chem., Int. Ed., 2019, 58, 8819–8823 CrossRef CAS PubMed.
- C. Zhang, Q. Wang, H. Long and W. Zhang, J. Am. Chem. Soc., 2011, 133, 20995–21001 CrossRef CAS PubMed.
- Q. Wang, C. Zhang, B. C. Noll, H. Long, Y. Jin and W. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10663–10667 CrossRef CAS PubMed.
- Z. Liu, Z. Wang, S.-Q. Wang, J. Li, Y. Chen, P. Cheng, M. J. Zaworotko and Z. Zhang, ACS Mater. Lett., 2023, 5, 2139–2147 CrossRef CAS.
- C. Zhang, Z. Wang, L. Tan, T.-L. Zhai, S. Wang, B. Tan, Y.-S. Zheng, X.-L. Yang and H.-B. Xu, Angew. Chem., Int. Ed., 2015, 54, 9244–9248 CrossRef CAS PubMed.
- T. Santos, D. S. Rivero, Y. Perez-Perez, E. Martin-Encinas, J. Pasan, A. Hernandez Daranas and R. Carrillo, Angew. Chem., Int. Ed., 2021, 60, 18783–18791 CrossRef CAS PubMed.
- L. Cusin, H. Peng, A. Ciesielski and P. Samori, Angew. Chem., Int. Ed., 2021, 60, 14236–14250 CrossRef CAS PubMed.
- A. Volkov, J. Mi, K. Lalit, P. Chatterjee, D. Jing, S. L. Carnahan, Y. Chen, S. Sun, A. J. Rossini, W. Huang and L. M. Stanley, J. Am. Chem. Soc., 2023, 145, 6230–6239 CrossRef CAS PubMed.
- M. L. Quan and D. J. Cram, J. Am. Chem. Soc., 1991, 113, 2754–2755 CrossRef CAS.
- T. Hasell, M. Schmidtmann, C. A. Stone, M. W. Smith and A. I. Cooper, Chem. Commun., 2012, 48, 4689–4691 RSC.
- Y. Jin, B. A. Voss, R. D. Noble and W. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6348–6351 CrossRef CAS PubMed.
- J. L. Culshaw, G. Cheng, M. Schmidtmann, T. Hasell, M. Liu, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2013, 135, 10007–10010 CrossRef CAS PubMed.
- M. Liu, L. J. Chen, S. Lewis, S. Y. Chong, M. A. Little, T. Hasell, I. M. Aldous, C. M. Brown, M. W. Smith, C. A. Morrison, L. J. Hardwick and A. I. Cooper, Nat. Commun., 2016, 7, 12750 CrossRef CAS PubMed.
- J.-H. Zhang, H.-P. Wang, L.-Y. Zhang, S.-C. Wei, Z.-W. Wei, M. Pan and C.-Y. Su, Chem. Sci., 2020, 11, 8885–8894 RSC.
- J. Dong, Y. Pan, K. Yang, Y. D. Yuan, V. Wee, S. Xu, Y. Wang, J. Jiang, B. Liu and D. Zhao, ACS Nano, 2022, 16, 2355–2368 CrossRef CAS PubMed.
- M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohtencatl, D. He, R. Clowes, S. Y. Chong, G. Schutz, L. Chen, M. Hirscher and A. I. Cooper, Science, 2019, 366, 613–620 CrossRef CAS PubMed.
- E. Martinez-Ahumada, D. He, V. Berryman, A. Lopez-Olvera, M. Hernandez, V. Jancik, V. Martis, M. A. Vera, E. Lima, D. J. Parker, A. I. Cooper, I. A. Ibarra and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 17556–17563 CrossRef CAS PubMed.
- S. Lee, I. Kevlishvili, H. J. Kulik, H.-T. Kim, Y. G. Chung and D.-Y. Koh, J. Mater. Chem. A, 2022, 10, 24802–24812 RSC.
- P. Bhandari and P. S. Mukherjee, ACS Catal., 2023, 13, 6126–6143 CrossRef CAS.
- V. Sharma and P. K. Bharadwaj, Dalton Trans., 2020, 49, 15574–15586 RSC.
- R. McCaffrey, H. Long, Y. Jin, A. Sanders, W. Park and W. Zhang, J. Am. Chem. Soc., 2014, 136, 1782–1785 CrossRef CAS PubMed.
- A. Singh, P. Verma, D. Samanta, A. Dey, J. Dey and T. K. Maji, J. Mater. Chem. A, 2021, 9, 5780–5786 RSC.
- X. Yang, J.-K. Sun, M. Kitta, H. Pang and Q. Xu, Nat. Catal., 2018, 1, 214–220 CrossRef CAS.
- J.-K. Sun, W.-W. Zhan, T. Akita and Q. Xu, J. Am. Chem. Soc., 2015, 137, 7063–7066 CrossRef CAS PubMed.
- L. Qiu, R. McCaffrey, Y. Jin, Y. Gong, Y. Hu, H. Sun, W. Park and W. Zhang, Chem. Sci., 2018, 9, 676–680 RSC.
- S.-Y. Zhang, Z. Kochovski, H.-C. Lee, Y. Lu, H. Zhang, J. Zhang, J.-K. Sun and J. Yuan, Chem. Sci., 2019, 10, 1450–1456 RSC.
- N. Sun, C. Wang, H. Wang, L. Yang, P. Jin, W. Zhang and J. Jiang, Angew. Chem., Int. Ed., 2019, 58, 18011–18016 CrossRef CAS PubMed.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed.
- L. Feng, Y.-X. Tan, E.-S. M. El-Sayed, F. Qiu, W. Wang, K. Su and D. Yuan, CCS Chem., 2024, 6, 149–156 CrossRef.
- K. Su, W. Wang, S. Du, C. Ji and D. Yuan, Nat. Commun., 2021, 12, 3703 CrossRef CAS PubMed.
- X. Zhang, K. Su, A. G. A. Mohamed, C. Liu, Q. Sun, D. Yuan, Y. Wang, W. Xue and Y. Wang, Energy Environ. Sci., 2022, 15, 780–785 RSC.
- J. Kou, Q. Wu, D. Cui, Y. Geng, K. Zhang, M. Zhang, H. Zang, X. Wang, Z. Su and C. Sun, Angew. Chem., Int. Ed., 2023, 62, e202312733 CrossRef CAS PubMed.
- J. Kou, Z. Zhu, J. Jiang, L. Chen, K. Zhang, G. Shan, X. Wang, Z. Su and C. Sun, Chem. Commun., 2024, 60, 6949–6952 RSC.
- D. Al Kelabi, A. Dey, L. O. Alimi, H. Piwonski, S. Habuchi and N. M. Khashab, Chem. Sci., 2022, 13, 7341–7346 RSC.
- J. C. Lauer, A. S. Bhat, C. Barwig, N. Fritz, T. Kirschbaum, F. Rominger and M. Mastalerz, Chem. – Eur. J., 2022, 28, e202201527 CrossRef CAS PubMed.
- T. H. G. Schick, J. C. Lauer, F. Rominger and M. Mastalerz, Angew. Chem., Int. Ed., 2019, 58, 1768–1773 CrossRef CAS PubMed.
- P. Bhandari and P. S. Mukherjee, Chem. – Eur. J., 2022, 28, e202201901 CrossRef CAS PubMed.
- T. Pausch, T. David, T. Fleck-Kunde, H. Pols, J. Gurke and B. M. Schmidt, Angew. Chem., Int. Ed., 2024, 63, e202318362 CrossRef CAS PubMed.
- D. K. Kolmel and E. T. Kool, Chem. Rev., 2017, 117, 10358–10376 CrossRef CAS PubMed.
- F. J. Uribe-Romo, C. J. Doonan, H. Furukawa, K. Oisaki and O. M. Yaghi, J. Am. Chem. Soc., 2011, 133, 11478–11481 CrossRef CAS PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC.
- C. Qian, W. Zhou, J. Qiao, D. Wang, X. Li, W. L. Teo, X. Shi, H. Wu, J. Di, H. Wang, G. Liu, L. Gu, J. Liu, L. Feng, Y. Liu, S. Y. Quek, K. P. Loh and Y. Zhao, J. Am. Chem. Soc., 2020, 142, 18138–18149 CrossRef CAS PubMed.
- Y.-Y. Xu, H.-K. Liu, Z.-K. Wang, B. Song, D.-W. Zhang, H. Wang, Z. Li, X. Li and Z.-T. Li, J. Org. Chem., 2021, 86, 3943–3951 CrossRef CAS PubMed.
- M. Sharafi, K. T. McKay, M. Ivancic, D. R. McCarthy, N. Dudkina, K. E. Murphy, S. C. Rajappan, J. P. Campbell, Y. Shen, A. R. Badireddy, J. Li and S. T. Schneebeli, Chem, 2020, 6, 1469–1494 CAS.
- H. Jedrzejewska, E. Wielgus, S. Kazmierski, H. Rogala, M. Wierzbicki, A. Wroblewska, T. Pawlak, M. J. Potrzebowski and A. Szumna, Chem. – Eur. J., 2020, 26, 1558–1566 CrossRef CAS PubMed.
- Z. Lin, T. J. Emge and R. Warmuth, Chem. – Eur. J., 2011, 17, 9395–9405 CrossRef CAS PubMed.
- F. Gao, C. Luo, X. Wang, C. Zhan, Y. Li, Y. Li, Q. Meng, M. Yang, K. Su, D. Yuan, R. Zhu and Q. Zhao, Adv. Funct. Mater., 2023, 33, 2211900 CrossRef CAS.
- M. Yang, X. Chen, Y. Xie, E.-S. M. El-Sayed, N. Xu, W. Wang, K. Su and D. Yuan, Sci. China: Chem., 2023, 66, 1763–1770 CrossRef CAS.
- O. Vestrheim, M. E. Schenkelberg, Q. Dai and S. T. Schneebeli, Org. Chem. Front., 2023, 10, 3965–3974 RSC.
- Y. Hu, C. Wu, Q. Pan, Y. Jin, R. Lyu, V. Martinez, S. Huang, J. Wu, L. J. Wayment, N. A. Clark, M. B. Raschke, Y. Zhao and W. Zhang, Nat. Synth., 2022, 1, 449–454 CrossRef.
- E. Jin, M. Asada, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. Chen and D. Jiang, Science, 2017, 357, 673–676 CrossRef CAS PubMed.
- Z. Lei, L. J. Wayment, J. R. Cahn, H. Chen, S. Huang, X. Wang, Y. Jin, S. Sharma and W. Zhang, J. Am. Chem. Soc., 2022, 144, 17737–17742 CrossRef CAS PubMed.
- Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS PubMed.
- C. Zhang, H. Wang, J. Zhong, Y. Lei, R. Du, Y. Zhang, L. Shen, T. Jiao, Y. Zhu, H. Zhu, H. Li and H. Li, Sci. Adv., 2019, 5, eaax6707 CrossRef CAS PubMed.
- L. He, C. Jiang, Z. Chen, D. Ma, L. Yi and Z. Xi, Org. Biomol. Chem., 2022, 20, 7577–7581 RSC.
- D. Zhu, B. Sun, L. Tong, Y. Wu, M. Cetin and H. Li, Org. Lett., 2022, 24, 8980–8985 CrossRef CAS PubMed.
- Z. Wang, Y. Zhang, J. Liu, T. Wang, J. Wang, K. Yu, Y. Chen, P. Cheng and Z. Zhang, ACS Mater. Lett., 2023, 5, 2754–2759 CrossRef CAS.
- J. Kang, S. Huang, K. Jiang, C. Lu, Z. Chen, J. Zhu, C. Yang, A. Ciesielski, F. Qiu and X. Zhuang, Adv. Funct. Mater., 2020, 30, 2000857 CrossRef CAS.
- X. Li, Mater. Chem. Front., 2021, 5, 2931–2949 RSC.
- Y. Liu, S. Fu, D. L. Pastoetter, A. H. Khan, Y. Zhang, A. Dianat, S. Xu, Z. Liao, M. Richter, M. Yu, M. Polozij, E. Brunner, G. Cuniberti, T. Heine, M. Bonn, H. I. Wang and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202209762 CrossRef CAS PubMed.
- S. Xu, G. Wang, B. P. Biswal, M. Addicoat, S. Paasch, W. Sheng, X. Zhuang, E. Brunner, T. Heine, R. Berger and X. Feng, Angew. Chem., Int. Ed., 2019, 58, 849–853 CrossRef CAS PubMed.
- H. E. Hogberg and O. Wennerstrom, Acta Chem. Scand., Ser. B, 1982, 36, 661–667 CrossRef.
- S. Huang, Z. Lei, Y. Jin and W. Zhang, Chem. Sci., 2021, 12, 9591–9606 RSC.
- C. Zhang and C.-F. Chen, J. Org. Chem., 2007, 72, 9339–9341 CrossRef CAS PubMed.
- Q. Wang, C. Yu, H. Long, Y. Du, Y. Jin and W. Zhang, Angew. Chem., Int. Ed., 2015, 54, 7550–7554 CrossRef CAS PubMed.
- S. Lee, A. Yang, T. P. Moneypenny, II and J. S. Moore, J. Am. Chem. Soc., 2016, 138, 2182–2185 CrossRef CAS PubMed.
- T. P. Moneypenny, II, A. Yang, N. P. Walter, T. J. Woods, D. L. Gray, Y. Zhang and J. S. Moore, J. Am. Chem. Soc., 2018, 140, 5825–5833 CrossRef PubMed.
- A. Petronico, T. P. Moneypenny, II, B. G. Nicolau, J. S. Moore, R. G. Nuzzo and A. A. Gewirth, J. Am. Chem. Soc., 2018, 140, 7504–7509 CrossRef CAS PubMed.
- Q. Fang, Z. Zhuang, S. Gu, R. B. Kaspar, J. Zheng, J. Wang, S. Qiu and Y. Yan, Nat. Commun., 2014, 5, 4503 CrossRef PubMed.
- M. M. Naseer, D.-X. Wang, L. Zhao and M.-X. Wang, Eur. J. Org. Chem., 2014, 7895–7905 CrossRef CAS.
- W.-J. Hu, L.-Q. Liu, M.-L. Ma, X.-L. Zhao, Y. A. Liu, X.-Q. Mi, B. Jiang and K. Wen, Inorg. Chem., 2013, 52, 9309–9319 CrossRef CAS PubMed.
- Y.-X. Li, M. Xue and Y. Yang, Org. Lett., 2021, 23, 6435–6438 CrossRef CAS PubMed.
- Z. Wang, Y. Luo, T.-L. Zhai, H. Ma, J.-J. Chen, Y. Shu and C. Zhang, Org. Lett., 2016, 18, 4574–4577 CrossRef CAS PubMed.
- L. Wang, L.-X. Zheng, M.-L. Ma, X.-L. Zhao, Y. A. Liu, X.-Q. Mi, B. Jiang and K. Wen, Supramol. Chem., 2013, 25, 409–415 CrossRef CAS.
- J. L. Katz, K. J. Selby and R. R. Conry, Org. Lett., 2005, 7, 3505–3507 CrossRef CAS PubMed.
- Q. Zhu, X. Wang, R. Clowes, P. Cui, L. Chen, M. A. Little and A. I. Cooper, J. Am. Chem. Soc., 2020, 142, 16842–16848 CrossRef CAS PubMed.
- C. Ji, K. Su, W. Wang, J. Chang, E.-S. M. El-Sayed, L. Zhang and D. Yuan, CCS Chem., 2022, 4, 3095–3105 CrossRef CAS.
- Q. Zhu, L. Wei, C. Zhao, H. Qu, B. Liu, T. Fellowes, S. Yang, A. Longcake, M. J. Hall, M. R. Probert, Y. Zhao, A. I. Cooper and M. A. Little, J. Am. Chem. Soc., 2023, 145, 23352–23360 CrossRef CAS PubMed.
- Q. Zhu, H. Qu, G. Avci, R. Hafizi, C. Zhao, G. M. Day, K. E. Jelfs, M. A. Little and A. I. Cooper, Nat. Synth., 2024, 3, 825–834 CrossRef.
- Y. Liu, W. Zhao, C.-H. Chen and A. H. Flood, Science, 2019, 365, 159–161 CrossRef CAS PubMed.
- X. Zhao, Y. Liu, Z.-Y. Zhang, Y. Wang, X. Jia and C. Li, Angew. Chem., Int. Ed., 2021, 60, 17904–17909 CrossRef CAS PubMed.
- D. G. Rivera and L. A. Wessjohann, J. Am. Chem. Soc., 2006, 128, 7122–7123 CrossRef CAS PubMed.
- Q. Guan, L.-L. Zhou and Y.-B. Dong, J. Am. Chem. Soc., 2023, 145, 1475–1496 CrossRef CAS PubMed.
- X. Zhu, C. Tian, S. M. Mahurin, S.-H. Chai, C. Wang, S. Brown, G. M. Veith, H. Luo, H. Liu and S. Dai, J. Am. Chem. Soc., 2012, 134, 10478–10484 CrossRef CAS PubMed.
- X. Guan, H. Li, Y. Ma, M. Xue, Q. Fang, Y. Yan, V. Valtchev and S. Qiu, Nat. Chem., 2019, 11, 587–594 CrossRef CAS PubMed.
- Z. Yang, J. Liu, Y. Li, G. Zhang, G. Xing and L. Chen, Angew. Chem., Int. Ed., 2021, 60, 20754–20759 CrossRef CAS PubMed.
- X.-N. Feng, Y. Yang, X. Cao, T. Wang, D.-M. Kong, X.-B. Yin, B. Li and X.-H. Bu, J. Am. Chem. Soc., 2023, 145, 21284–21292 CrossRef CAS PubMed.
- R. Greenaway, V. Santolini, M. J. Bennison, B. M. Alston, C. J. Pugh, M. A. Little, M. Miklitz, E. G. B. Eden-Rumps, R. Clowes, A. Shakil, H. J. Cuthbertson, H. Armstrong, M. E. Briggs, K. E. Jelfs and A. I. Cooper, Nat. Commun., 2018, 9, 2849 CrossRef CAS PubMed.
- L. Turcani, E. Berardo and K. E. Jelfs, J. Comput. Chem., 2018, 39, 1931–1942 CrossRef CAS PubMed.
- V. Santolini, M. Miklitz, E. Berardo and K. E. Jelfs, Nanoscale, 2017, 9, 5280–5298 RSC.
- M. Grajda, M. Wierzbicki, P. Cmoch and A. Szumna, J. Org. Chem., 2013, 78, 11597–11601 CrossRef CAS PubMed.
- C. Zhang, T. Jiao, L. Tong, H. Wang, Y. Pan and H. Li, Chem. Commun., 2020, 56, 3903–3906 RSC.
- D. A. Rothschild, W. P. Kopcha, A. Tran, J. Zhang and M. C. Lipke, Chem. Sci., 2022, 13, 5325–5332 RSC.
| This journal is © The Royal Society of Chemistry 2024 |