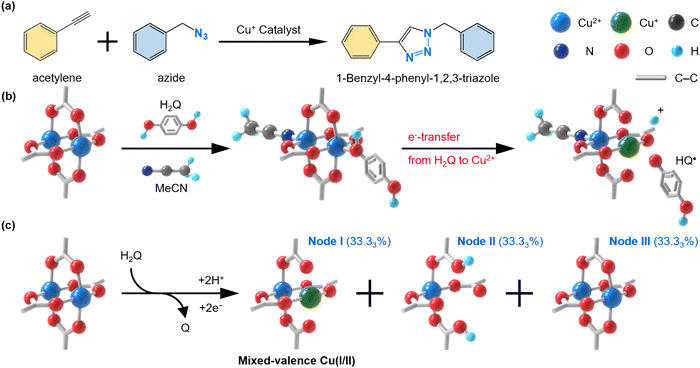
Hydroquinone-treated Cu3(BTC)2: a mixed-valence Cu(I/II) MOF catalyst for efficient cycloadditions†
Sun Ho
Park‡
 a,
Hye Mi
Kim‡
a,
Hye Mi
Kim‡
 a,
Mariana L.
Díaz-Ramírez‡
a,
Mariana L.
Díaz-Ramírez‡
 ab,
Sunggi
Lee
ab,
Sunggi
Lee
 *ab and
Nak Cheon
Jeong
*ab and
Nak Cheon
Jeong
 *ab
*ab
aDepartment of Physics & Chemistry, DGIST, Daegu 42988, Korea. E-mail: sunggi.lee@dgist.ac.kr; nc@dgist.ac.kr
bCenter for Basic Science, DGIST, Daegu 42988, Korea
First published on 4th November 2024
Abstract
We present mixed-valence Cu(I)1Cu(II)2(BTC)2 [henceforth Cu(I/II)-HKUST-1], post-synthetically prepared via the hydroquinone (H2Q) treatment of Cu(II)3(BTC)2 (also referred to as HKUST-1) and its subsequent catalytic activity. This Cu(I/II)-HKUST-1 exhibits exceptional structural integrity and superior catalytic performance in the copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction between phenylacetylene and benzyl azide. These findings highlight the potential of mixed-valence Cu-based MOFs as effective and sustainable heterogeneous catalysts for organic transformations, paving the way for future advancements in MOF-based catalysis.
Metal–organic frameworks (MOFs) are a highly versatile class of crystalline porous materials, composed of metal ions (or clusters) bridged by organic linkers, which exhibit extensive structural diversity. The pore size and chemical environment within MOFs can be precisely tuned by selecting appropriate metal ions and functionalized linkers, depending on the intended application.1 Notably, a wide variety of MOFs possess open metal sites (OMSs), which are vacant Lewis-acidic sites created by the removal of labile molecules, typically Lewis-basic solvents, from the primary coordination sphere of the metal center.2 Due to these distinctive characteristics, MOFs have garnered significant interest for applications, for example, in gas adsorption and separation,3,4 energy storage,5,6 and heterogeneous catalysis.7–9
Among the various types of MOFs, those with metal centers in multiple oxidation states (mixed-valence MOFs) have exhibited exceptional properties, such as charge transfer and charge delocalization, which contribute to high conductivity a desirable feature in redox chemistry and semiconductor development,10,11 and catalytic reactions.12,13 In these mixed-valence MOFs, the incorporation or removal of counter-ions, essential for maintaining the charge balance, not only alters the chemical environment of the pores but also induces structural changes, affecting pore size, window aperture, and available pore volume.14 However, the synthesis of intrinsically mixed-valence MOFs has been challenging because it requires metals and ligands to cooperate together precisely to enable redox isomerism.15 Specifically, direct synthetic methods often require modulators to control the formation of metal clusters in mixed oxidation states, but achieving precise control over the metal nodes in these mixed states remains difficult.16 Therefore, post-synthetic strategies have been developed to efficiently change the oxidation state of the metal centers while preserving the original MOF structure.17–20
Cu-based mixed-valence MOFs, in particular, have attracted considerable attention due to the enhancements in properties such as conductivity,21 hydrolytic stability,20,22 adsorption,3,4 and catalytic activity7,8 that arise from changes in their electronic structure. Among these, Cu3(BTC)2 (BTC = benzene-1,3,5-tricarboxylate), also known as HKUST-1, serves as a benchmark material for the reduction of Cu(II) ions. For example, heating HKUST-1 under vacuum conditions partially reduces Cu(II) to Cu(I).3 Duke et al. demonstrated that a nearly complete reduction of Cu(II) in HKUST-1 to Cu(I) could be achieved at 225 °C under an argon atmosphere.21 Additionally, Szanyi and coworkers reported the effective reduction of Cu(II) centers in HKUST-1 using CO or H2 atmospheres, though the extent of reduction was influenced by the temperature rather than the nature of the reductive atmosphere, with the highest reduction occurring at 200 °C.23 However, a comprehensive structural characterization, including whether the reduction of Cu(II) occurs completely or partially to produce a mixed-valence MOF, is still lacking. Consequently, the precise mechanism underlying Cu(II) reduction and defect formation remains partially resolved. Recently, by contrast, our group introduced a novel approach, the coordinative reduction of Cu(II) in HKUST-1 using post-synthetic hydroquinone (H2Q) treatment, yielding a Cu(I)1/Cu(II)2 mixed-valence MOF [hereafter referred to as Cu(I/II)-HKUST-1] while maintaining structural integrity. In Cu(I/II)-HKUST-1, approximately 33% of Cu(II) ions were reduced to Cu(I), with half of these reduced ions remaining within the paddlewheel structure, thereby serving as potential catalytic active sites.20 By contrast, the remaining 67% of Cu(II) ions contribute to preserving the structural stability of Cu(I/II)-HKUST-1.
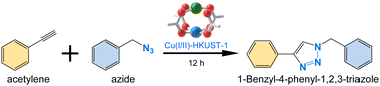
Meanwhile, it has been considered challenging to exploit a Cu(I)-MOF as catalyst in organic reactions, in particular, for the coupling of terminal alkynes and organic azides to form 1,4-disubstituted 1,2,3-triazoles (Fig. 1a),24 known as the copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction (also called as a type of click reaction). Although the reaction has been extensively studied using various molecular Cu(I) complexes generated by the reduction of Cu(II), oxidation of Cu(0), or through ligand-coupled reduction of Cu(II),25,26 the use of heterogeneous MOF catalysts without structural damage and significant loss of activity has rarely been successful, despite their preference for easier recovery, recyclability, and molecular diffusion all crucial for more efficient and sustainable processes. Therefore, mixed-valence Cu-based MOFs have recently been recognized as promising candidates for such catalytic applications.7,8,27,28
Herein, we demonstrate that post-synthetically H2Q-treated mixed-valence Cu(I/II)-HKUST-1 can be utilized as an efficient catalyst. This Cu(I/II)-HKUST-1 exhibits excellent catalytic activity in the CuAAC reaction between phenylacetylene and benzyl azide. Furthermore, Cu(I/II)-HKUST-1 demonstrates remarkable structural stability and recyclability, paving the way for further advancements in the design and synthesis of mixed-valence Cu-based MOFs.
Cu(I/II)-HKUST-1, containing 33% of Cu(I), was prepared according to our previously reported method.20 In brief, thermally activated HKUST-1 was treated with H2Q in anhydrous acetonitrile (MeCN) at 80 °C for 12 hours. During the H2Q treatment, 33% of Cu centers were reduced via a coordinative reduction mechanism, where H2Q, upon coordination with Cu(II), donates an electron to the Cu(II) center through the coordination bond, forming HQ• and Cu(I) (Fig. 1b). The subsequent change in the coordination number and geometry of Cu(I), which prefers two- or four-coordination, promotes the dissociation of HQ˙, enabling further reduction of remaining Cu(II) ions and the formation of p-benzoquinone (Q) (Fig. S1, ESI†). Consequently, three distinct types of nodes are formed in Cu(I/II)-HKUST-1: node I, formed by Cu(II)–Cu(I) paddlewheels; node II, consisting of Cu(II) Vacancy units; and node III, composed of Cu(II)–Cu(II) paddlewheels (Fig. 1c).
To validate whether the reduction arose, we examined the oxidation state of H2Q by analyzing the supernatant H2Q/MeCN solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of H2Q
2 ratio of H2Q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu) after the H2Q treatment. The 1H NMR spectrum of the supernatant solution shows proton peaks corresponding to H2Q (84%) and quinone (16%), indicating that approximately 32% of Cu(II) centers were reduced to Cu(I) (Fig. S2 and S3, ESI†). XPS analysis of Cu(I/II)-HKUST-1 further supports this finding, showing a band with a shoulder at approximately 933–935 and 952–954 eV, indicative of a mixture of Cu(II) and Cu(I), whereas MeCN coordinated HKUST-1 (MeCN-HKUST-1), which is not H2Q-treated, displays bands solely corresponding to Cu(II) (Fig. S4, ESI†). Integration of the fitted bands in the XPS spectrum of Cu(I/II)-HKUST-1 revealed that Cu(II) and Cu(I) are present at approximately 66% and 34%, respectively (Table S1, ESI†), corroborating the 1H NMR results.
Cu) after the H2Q treatment. The 1H NMR spectrum of the supernatant solution shows proton peaks corresponding to H2Q (84%) and quinone (16%), indicating that approximately 32% of Cu(II) centers were reduced to Cu(I) (Fig. S2 and S3, ESI†). XPS analysis of Cu(I/II)-HKUST-1 further supports this finding, showing a band with a shoulder at approximately 933–935 and 952–954 eV, indicative of a mixture of Cu(II) and Cu(I), whereas MeCN coordinated HKUST-1 (MeCN-HKUST-1), which is not H2Q-treated, displays bands solely corresponding to Cu(II) (Fig. S4, ESI†). Integration of the fitted bands in the XPS spectrum of Cu(I/II)-HKUST-1 revealed that Cu(II) and Cu(I) are present at approximately 66% and 34%, respectively (Table S1, ESI†), corroborating the 1H NMR results.
Half of the reduced Cu(I) centers likely dissociated from the node due to the protonation of BTC's carboxylates by a proton released from H2Q during the reaction, subsequently yielding a [Cu(MeCN)4]+ complex. The formation of the [Cu(MeCN)4]+ complex was confirmed by FT-IR spectroscopy, which showed bands at 2275 and 2303 cm−1 in the Cu(I/II)-HKUST-1 spectrum, corresponding to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibration modes that agreed well with those of the commercial [Cu(MeCN)4]BF4 complex (Fig. S5, ESI†).29 The dissociation of Cu(I) ions from the node subsequently to form [Cu(MeCN)4]+ ions did not alter the HKUST-1 crystal structure (Fig. S6, ESI†), however, it did created defects in the framework, as confirmed by the presence of mesopores in the N2 adsorption/desorption isotherm of Cu(I/II)-HKUST-1 performed at 77 K (Fig. S7, ESI†). Nevertheless, the surface area of Cu(I/II)-HKUST-1 (1196 m2 g−1), although reduced compared to MeCN-HKUST-1 (1965 m2 g−1) due to the presence of the [Cu(MeCN)4]+ complex and defect formation, was still comparable to typical HKUST-1 samples reported in other literature.20 The formation of mesopores with an average diameter of 38 Å, as indicated by the DFT pore size distribution analysis, also confirmed the presence of defects induced by the H2Q treatment (Fig. S8, ESI†). In addition, we observed that Cu(I/II)-HKUST-1 exhibits high thermal stability comparable to the pristine HKUST-1 (Fig. S9, ESI†).
N vibration modes that agreed well with those of the commercial [Cu(MeCN)4]BF4 complex (Fig. S5, ESI†).29 The dissociation of Cu(I) ions from the node subsequently to form [Cu(MeCN)4]+ ions did not alter the HKUST-1 crystal structure (Fig. S6, ESI†), however, it did created defects in the framework, as confirmed by the presence of mesopores in the N2 adsorption/desorption isotherm of Cu(I/II)-HKUST-1 performed at 77 K (Fig. S7, ESI†). Nevertheless, the surface area of Cu(I/II)-HKUST-1 (1196 m2 g−1), although reduced compared to MeCN-HKUST-1 (1965 m2 g−1) due to the presence of the [Cu(MeCN)4]+ complex and defect formation, was still comparable to typical HKUST-1 samples reported in other literature.20 The formation of mesopores with an average diameter of 38 Å, as indicated by the DFT pore size distribution analysis, also confirmed the presence of defects induced by the H2Q treatment (Fig. S8, ESI†). In addition, we observed that Cu(I/II)-HKUST-1 exhibits high thermal stability comparable to the pristine HKUST-1 (Fig. S9, ESI†).
Given the outstanding structural stability and large surface area of Cu(I/II)-HKUST-1, we explored its efficacy as a Cu(I)-based heterogeneous MOF catalyst for the CuAAC reaction between phenylacetylene and benzyl azide in ethanol (EtOH) media over 12 hours (Table 1). The conversion efficiency was monitored by 1H NMR spectroscopy, focusing on the characteristic signals of methylene groups in benzyl azide and 1-benzyl-4-phenyl-1,2,3-triazole (Fig. S10, ESI†). Initially, we optimized the reaction by controlling temperatures from 25 to 80 °C, using 0.007 mmol of Cu(I/II)-HKUST-1. While the conversion efficiency was modest at 25 °C (15%, entry 1 in Table 1), the efficiency was improved upon the temperature increase (66% at 50 °C, entry 2, Table 1). Eventually, the 100% conversion was achieved at 80 °C (entry 3, Table 1). We then optimized the reaction by verifying the catalyst loading amount. Subsequently, we found that 0.007 mmol yielded the highest conversion, while lower amounts resulted in significantly reduced conversions (66% and 64%, entries 4 and 5, Table 1). Additionally, we evaluated the reaction in different solvents, with MeCN resulting in the lowest conversion (4%, entry 6, Table 1), likely due to its strong coordination to the Cu(II) sites and its hindrance of the neighboring Cu(I) sites, thus, rendering them inactive.30 In contrast, toluene, a non-polar solvent, yielded a high conversion (95%, entry 7, Table 1), suggesting that non-polar and less-polar (EtOH) solvents are preferable for this reaction.
| Entry | Catalyst | Loading (mmol) | Temp. (°C) | Solvent | Conversion (%) |
|---|---|---|---|---|---|
| 1 | H2Q-HK | 0.007 | 25 | EtOH | 15 |
| 2 | H2Q-HK | 0.007 | 50 | EtOH | 66 |
| 3 | H2Q-HK | 0.007 | 80 | EtOH | 100 |
| 4 | H2Q-HK | 0.003 | 80 | EtOH | 64 |
| 5 | H2Q-HK | 0.005 | 80 | EtOH | 66 |
| 6 | H2Q-HK | 0.007 | 80 | MeCN | 4 |
| 7 | H2Q-HK | 0.007 | 80 | Toluene | 95 |
| 8 | Act-HK_150 °C | 0.007 | 80 | EtOH | 31 |
| 9 | Act-HK_200 °C | 0.007 | 80 | EtOH | 65 |
| 10 | None | 0 | 80 | EtOH | 0 |
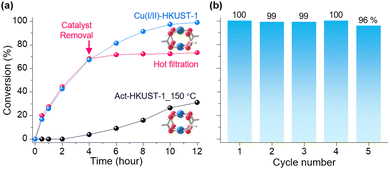
To assess the absence of Cu(I/II)-HKUST-1 and the impact of Cu's oxidation state on catalytic activity, we examined the reactions without any catalyst and with a thermally activated HKUST-1, which predominantly contains Cu(II). As expected, the reaction without any catalyst yielded no conversion (entry 10, Table 1), and the HKUST-1 samples activated at 150 °C and 200 °C (Act-HKUST-1_150 °C and Act-HKUST-1_200 °C, respectively) exhibited limited activities (31% and 65%, respectively, entries 8 and 9 in Table 1). The conversion profiles of Cu(I/II)-HKUST-1 and Act-HKUST-1_150 °C along the reaction time were further tested (Fig. 2a and Fig. S11, S12, ESI†). As a result, Cu(I/II)-HKUST-1 exhibited a steady increase in benzyl azide conversion, reaching 100% after 12 hours with no signs of catalyst deactivation, while Act-HKUST-1_150 °C showed only minimal catalytic activity, with the benzyl azide conversion reaching just 31% after 12 hours, likely due to the small presence of Cu(I) sites inevitably generated during thermal activation.3 To determine Cu ions leaching during the catalytic reaction, a hot filtration experiment and inductively coupled plasma (ICP) analysis were performed. The Cu(I/II)-HKUST-1 catalyst was removed after 4 hours of reaction, and the conversion remained unchanged over the subsequent 8 hours (Fig. 2a and Fig. S13, ESI†). ICP analysis of the supernatant revealed that 0.007% of the total Cu ions were detected, indicating negligible leaching occur (Table S2, ESI†). These results show that the reaction is heterogeneous, and the catalyst exhibits high stability during the reaction. Moreover, PXRD analysis confirmed that the structural integrity of Cu(I/II)-HKUST-1 remained intact after the reaction (Fig. S14, ESI†), underscoring its potential for efficient recyclability. Finally, we observed that the Cu(I/II)-HKUST-1 catalyst was easily recollected and recovered by washing with fresh EtOH and reactivated, with conversion above 96% over five cycles, confirming the excellent catalytic activity and recyclability of Cu(I/II)-HKUST-1 (Fig. 2b, Fig S15, and Table S3, ESI†). The framework of the Cu(I/II)-HKUST-1 remained intact during five cycles (Fig. S16, ESI†).
To demonstrate the general applicability of the Cu(I/II)-HKUST-1 catalyst, we examined the reaction using various substrates with different functional groups. As a result, the catalyst showed high conversion for all substrates with efficiencies of 95% except for 2-fluorobenzyl azide (1i), which can be attributed to steric hindrance (Fig. 3 and Fig. S17, ESI†). Thus, these results demonstrate that Cu(I/II)-HKUST-1 is an effective heterogeneous catalyst for a wide range of substrates in the CuAAC reaction.
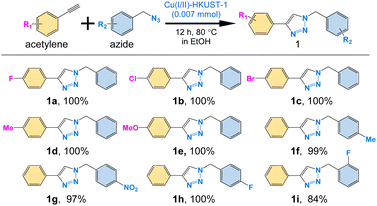 | ||
| Fig. 3 Various catalytic substrates and their conversion efficiencies for Cu(I/II)-HKUST-1 catalyzed azide–alkyne cycloaddition reaction. | ||
In this study, we successfully demonstrate the post-synthetic modification of HKUST-1 to produce mixed-valence Cu(I/II)-HKUST-1 via hydroquinone treatment, highlighting its strong potential for catalytic azide–alkyne cycloaddition applications. This mixed-valence Cu(I/II)-HKUST-1 not only maintained its structural integrity but also exhibited remarkable catalytic activity in the CuAAC reaction between phenylacetylene and benzyl azide. The Cu(I/II)-HKUST-1 catalyst showed superior performance in terms of conversion efficiency, structural stability, and recyclability, significantly outperforming the thermally activated HKUST-1, which primarily contains Cu(II) sites. These findings highlight the potential of mixed-valence Cu-based MOFs as effective and sustainable heterogeneous catalysts for the transformations of organic compounds. The ability of Cu(I/II)-HKUST-1 to retain high catalytic activity across multiple cycles further underscores its promise for future applications in catalysis and beyond.
This work was supported by the Ministry of Science and ICT (MSIT) of Korea under the auspices of the Basic Science Research Program sponsored by the National Research Foundation (NRF-2023R1A2C2004838) and by the DGIST R&D Program (24-HRHR+-01).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
-
Y. Belmabkhout and K. E. CordovaReticular Chemistry and Applications, De Gruyter, 2023 Search PubMed
.
- U. Kokcam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC
.
- P. S. Petkov, G. N. Vayssilov, J. Liu, O. Shekhah, Y. Wang, C. Woll and T. Heine, Chem. Phys. Chem., 2012, 13, 2025–2029 CrossRef PubMed
.
- H. Sun, X. Han, K. Liu, B. Shen, J. Liu, D. Wu and X. Shi, Ind. Eng. Chem. Res., 2017, 56, 9541–9550 CrossRef CAS
.
- X. Tang, C. Liu, H. Wang, L.-P. Lv, W. Sun and Y. Wang, Coord. Chem. Rev., 2023, 494, 215361 CrossRef CAS
.
- Z. Zhu, Y. Zeng, Z. Pei, D. Luan, X. Wang and X. W. D. Lou, Angew. Chem., Int. Ed., 2023, 62, e202305828 CrossRef PubMed
.
- Q. Fu, K. Xie, S. Tan, J. M. Ren, Q. Zhao, P. A. Webley and G. G. Qiao, Chem. Commun., 2016, 52, 12226–12229 RSC
.
- L. Xue, Y. Yang, S. Wu, Y. Huang, J. Li, Y. Xiang and G. Li, Anal. Chem., 2020, 92, 2972–2978 CrossRef CAS PubMed
.
- R. Yepez, S. García, P. Schachat, M. Sánchez-Sánchez, J. H. González-Estefan, E. González-Zamora, I. A. Ibarra and J. Aguilar-Pliego, New J. Chem., 2015, 39, 5112–5115 RSC
.
- J. G. Park, M. L. Aubrey, J. Oktawiec, K. Chakarawet, L. E. Darago, F. Grandjean, G. J. Long and J. R. Long, J. Am. Chem. Soc., 2018, 140, 8526–8534 CrossRef CAS PubMed
.
- S. Y. Fu, C. H. Chang, A. S. Ivanov, I. Popovs, J. L. Chen, Y. F. Liao, H. K. Liu, S. Chirra, Y. W. Chiang, J. C. Lee, W. L. Liu, W. Kaveevivitchai and T. H. Chen, Angew. Chem., Int. Ed., 2023, 62, e202312494 CrossRef CAS PubMed
.
- H. Chen, Y. Liu, T. Cai, W. Dong, L. Tang, X. Xia, L. Wang and T. Li, ACS Appl. Mater. Interfaces, 2019, 11, 28791–28800 CrossRef CAS PubMed
.
- T. Yang, D. Yu, D. Wang, T. Yang, Z. Li, M. Wu, M. Petru and J. Crittenden, Appl. Catal., B, 2021, 286, 119859 CrossRef CAS
.
- D. M. D'Alessandro, Chem. Commun., 2016, 52, 8957–8971 RSC
.
- Y.-X. Li, J.-X. Shen, Z.-J. Diao, S.-C. Qi, X.-Q. Liu and L.-B. Sun, Fund. Res., 2022 DOI:10.1016/j.fmre.2022.08.012
.
- M. Ronda-Lloret, I. Pellicer-Carreño, A. Grau-Atienza, R. Boada, S. Diaz-Moreno, J. Narciso-Romero, J. C. Serrano-Ruiz, A. Sepúlveda-Escribano and E. V. Ramos-Fernandez, Adv. Funct. Mater., 2021, 31, 2102582 CrossRef CAS
.
- J. W. Yoon, Y.-K. Seo, Y. K. Hwang, J.-S. Chang, H. Leclerc, S. Wuttke, P. Bazin, A. Vimont, M. Daturi, E. Bloch, P. L. Llewellyn, C. Serre, P. Horcajada, J.-M. Grenèche, A. E. Rodrigues and G. Férey, Angew. Chem., Int. Ed., 2010, 49, 5949–5952 CrossRef CAS PubMed
.
- T. F. Liu, L. F. Zou, D. W. Feng, Y. P. Chen, S. Fordham, X. Wang, Y. Y. Liu and H. C. Zhou, J. Am. Chem. Soc., 2014, 136, 7813–7816 CrossRef CAS PubMed
.
- B. Tu, Q. Pang, H. Xu, X. Li, Y. Wang, Z. Ma, L. Weng and Q. Li, J. Am. Chem. Soc., 2017, 139, 7998–8007 CrossRef CAS PubMed
.
- D. Song, J. Bae, H. Ji, M.-B. Kim, Y.-S. Bae, K. S. Park, D. Moon and N. C. Jeong, J. Am. Chem. Soc., 2019, 141, 7853–7864 CrossRef CAS PubMed
.
- A. S. Duke, E. A. Dolgopolova, R. P. Galhenage, S. C. Ammal, A. Heyden, M. D. Smith, D. A. Chen and N. B. Shustova, J. Phys. Chem. C, 2015, 119, 27457–27466 CrossRef CAS
.
- M. Kanno, T. Kitao, T. Ito and K. Terashima, RSC Adv., 2021, 11, 22756–22760 RSC
.
- J. Szanyi, M. Daturi, G. Clet, D. R. Baer and C. H. F. Peden, Phys. Chem. Chem. Phys., 2012, 14, 4383–4390 RSC
.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed
.
- V. D. Bock, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2005, 51–68 Search PubMed
.
- M. S. Ziegler, K. V. Lakshmi and T. D. Tilley, J. Am. Chem. Soc., 2017, 139, 5378–5386 CrossRef CAS PubMed
.
- H. Noei, S. Amirjalayer, M. Müller, X. Zhang, R. Schmid, M. Muhler, R. A. Fischer and Y. Wang, ChemCatChem, 2012, 4, 755–759 CrossRef CAS
.
- W. Xue, J. Wang, H. Huang, Y. Cui and D. Mei, J. Phys. Chem. C, 2022, 126, 9652–9664 CrossRef CAS
.
- I. S. Kritchenkov, J. R. Shakirova and S. P. Tunik, RSC Adv., 2019, 9, 15531–15535 RSC
.
- S. H. Park, R. A. Peralta, D. Moon and N. C. Jeong, J. Mater. Chem. A, 2022, 10, 23499–23508 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04294h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |