Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cationic dinuclear complexes [M2(PCP)2μ-Cl][GaCl4] of the group 10 elements. metallophilic interactions and catalytic dehydrogenation of Me2NHBH3†
Fabio
Meyer
,
Pim
Puylaert
 ,
Daniel
Duvinage
,
Daniel
Duvinage
 ,
Emanuel
Hupf
,
Emanuel
Hupf
 * and
Jens
Beckmann
* and
Jens
Beckmann
 *
*
Institute für Anorganische Chemie und Kristallographie, Universität Bremen, Leobener Str. 7, 28359 Bremen, Germany. E-mail: hupf@uni-bremen.de; j.beckmann@uni-bremen.de
First published on 9th October 2024
Abstract
The facile synthesis of the cationic dinuclear group 10 complexes [M2(PCP)2μ-Cl]+ (M = Ni, Pd, Pt) by transmetallation from a simple Ga precursor is reported (PCP = 2,6-(Ph2P)C6H3). Their use for the catalysed dehydrogenation of Me2NHBH3 shows that Ni has a higher reactivity than Pt, whereas Pd is inactive.
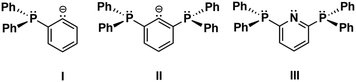
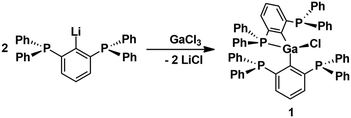
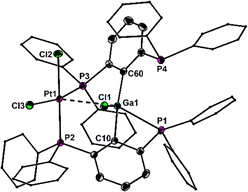
The stability of organometallic species relies essentially on a judicious choice of ligands and substituents, which provide kinetic and electronic stabilization to the metal atom. For this purpose, bidentate 2-diorganophosphinophenyl carbanions, [2-R2PC6H4]− (I, R = Ph, i-Pr) are frequently employed for a large number of main group and transition metal complexes (Fig. 1).1 Recently, we introduced the related bis(2,6-diphenylphosphino)phenyl carbanion, [2,6-(Ph2P)2C6H3]− (II),2 as a tridentate “PCP” ligand, which is also isoelectronic with the previously known neutral bis(2,6-diphenylphosphino)pyridine, 2,6-(Ph2P)2C5H2N (III), ligand (Fig. 1).3 So far, the PCP ligand was applied for the preparation of photoactive coinage metal complexes, [Cu4(PCP)3]+,4 and linear and cyclic [Au4(PCP)2(tht)2]2+, as well as a mixed-metal pincer complex, e.g. HgAu2(PCP)Cl3.2 In an effort to broaden the scope of the PCP ligand towards main group chemistry, we have now reacted the lithium reagent 2,6-(Ph2P)2C6H3]2Li5 with GaCl3 to give rise to the formation of [2,6-(Ph2P)2C6H3]2GaCl (1), which was isolated as colourless crystals in 95% yield (Scheme 1).
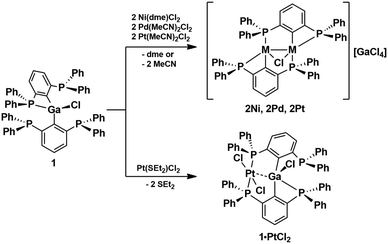
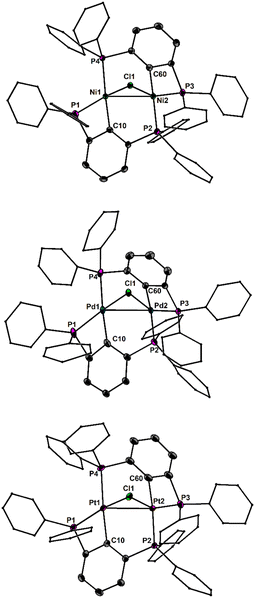
Notably, variations of the stoichiometric ratio did not give the mono- or trisubstituted Ga-species in significant amounts. The molecular structure of 1 shows that only one of the four P atoms is engaged in the first coordination sphere of the Ga atom (Fig. 2). The spatial arrangement may thus be described as distorted tetrahedral. The primary Ga–P bond length of 1 (2.567(1) Å) is substantially shorter than the remaining secondary Ga–P contacts (3.071(5), 3.382(5), 3.653(7) Å), which, however, are still within the van der Waals limit (4.2 Å). In solution, the P atoms are equivalent on the NMR time scale at room temperature, as the 31P NMR spectrum (CDCl3) of 1 shows only one signal at δ = −3.0 ppm. No qualitative change was observed at −30 °C. With the aim to prepare Ga-based Z-type complexes,6 we reacted 1 with two equivalents of Ni(dme)Cl2, Pd(MeCN)2Cl2 and Pt(MeCN)2Cl2, respectively. Surprisingly, in all cases rapid transmetallation occurred and the cationic dinuclear group 10 complexes7 [M2(PCP)2μ-Cl][GaCl4] (2M, M = Ni, Pd, Pt; PCP = 2,6-(Ph2P)2C6H3) formed, which were isolated as red, orange and red crystals in 92%, 72% and 64% yield, respectively (Scheme 2). The molecular structures of 2Ni, 2Pd and 2Pt reveal complete separation of the [M2(PCP)2μ-Cl]+ cations and the [GaCl4]− anions, which formed from the expelled Ga(iii) ions of 1 (Fig. 3). The spatial arrangement of the metal atoms is distorted square planar and defined by cis-P2CCl donor sets. The two metal atoms in the dinuclear motifs are bridged by the Cl atoms. The M–M distances of 1Ni (2.713(1) Å), 1Pd (2.807(1) Å) and 1Pt (2.818(1) Å) are indicative of metallophilic interactions8 and increase with the atomic number of the group 10 metals.
 | ||
| Fig. 2 Molecular structure of 1 showing 50% probability ellipsoids and the atomic numbering scheme. Selected bond lengths: Ga1–C10 1.975(2), Ga1–C60 1.994(2), Ga1–Cl1 2.250(1), Ga1–P1 2.567(1) Å. | ||
The outer P atoms of the structural motifs are situated in trans-position of the Cl atoms, whereas the inner P atoms are located in trans-position of the C atoms. The outer M–P bond lengths of 2Ni (2.141(1), 2.149(1) Å), 2Pd (2.236(2), 2.252(2) Å) and 2Pt (2.222(1), 2.227(1) Å) are generally shorter than the inner M–P bond lengths of 2Ni (2.230(1), 2.232(1) Å), 2Pd (2.354(2), 2.360(2) Å) and 2Pt (2.330(1), 2.334(1) Å), which is consistent with the larger trans-effect of the Pt–C bond compared to the Pt–Cl bond.9 The fact that the respective Pt–P bond lengths are shorter than the Pd–P bonds is attributed to the relativistic contraction of the 6s orbital of Pt.8 The 31P NMR spectra (CDCl3) of 2Ni, 2Pd, 2Pt show two distinctively different signals of equal intensity, which suggests that the molecular structures are retained in solution. The less shielded signals of 2Ni (δ = 9.2 ppm), 2Pd (δ = 8.2 ppm) and 2Pt (δ = 13.0 ppm) are assigned to the inner P atoms, whereas the more shielded signals of 2Ni (δ = −60.5 ppm), 2Pd (δ = −76.3 ppm) and 2Pt (δ = −76.1 ppm) belong to the outer P atoms. The assignment is based on the observed 1J(31P–123Pt) couplings of 1Pt of the inner (2073 Hz) and outer (4081 Hz) P atoms under the assumption that the magnitude inversely correlates with the bond lengths. The reaction of 1 with a different Pt-source, namely, Pt(Et2S)2Cl2, in an equimolar ratio, indeed afforded the Z-type complex 1·PtCl2, which was isolated as pale yellow crystals in 59% yield (Scheme 2). Attempts to prepare similar Ni and Pd complexes failed and gave only 2Ni and 2Pd as principal products.
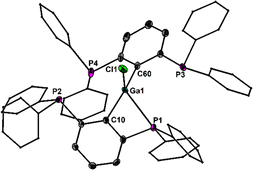
The molecular structure of 1·PtCl2 confirms the putative square planar arrangement of the Pt atoms, which adopts a cis-configuration of the two P atoms (Fig. 4). The Ga atom is situated perpendicular to Pt square plane. The Pt–Ga bond length (3.078(1) Å) suggests that the occupied dz2 orbital at the Pt atom tentatively donates electron density to the vacant orbital at the Ga atom suggesting Z-type coordination. The Ga–P bond length of 1·PtCl2 (2.543(1) Å) is shorter than in the parent 1 (2.567(1) Å). The 31P NMR spectrum (CDCl3, 243 K) of 1·PtCl2 shows four equally intense signals. The two more deshielded signals at δ = 30.8 and 24.7 showing 1J(31P–123Pt) couplings of 3430 Hz and 3016 Hz and are assigned to the P atoms involved in the coordination to the Pt atom. The two more shielded signals are doublets centred at δ = −1.7 and −10.1 ppm both showing the same J(31P–31P) couplings (102 Hz), which are indicative for through space interactions10 of the two remaining P atoms via their electron lone pairs. This is quite unexpected in light of the large P⋯P distance (3.731(9) Å).
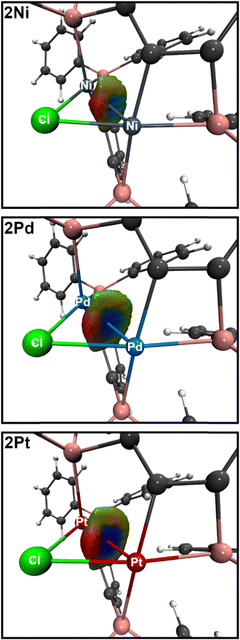
The most interesting structural feature of the cations 2M (M = Ni, Pd, Pt) are the metallophilic interactions, which were analyzed in more detail by using the electron density based bond descriptors AIM,11 NCI12 and IGMH.13 AIM analysis give rise to bond critical points between the metal–metal bond path for 2Pd and 2Pt, but is absent in case of the lighter 2Ni. However, the electron density minimum found along the direct Ni–Ni axis (0.22 e Å−3) is in line with the values found for the electron densities at the bcp for 2Pd (0.26 e Å−3) and 2Pt (0.32 e Å−3, Table S2, ESI†). These values are in the range found for other nickelophilic (0.18–0.36 e Å−3),14 palladophilic (0.21 e Å−3)15 and bimetallophilic interactions of the 6th period (0.28 e Å−3).16 The delocalization index δ(M|M) is also increasing from 2Ni (0.17) over 2Pd (0.26) to 2Pt (0.37), indicating metallophilic interactions in all three metal species, with increasing strengths from Ni to Pt. This is further corroborated by inspection of the IGMH partitioning. Each metal atom is defined as an individual fragment and the interaction between the two fragments are depicted in Fig. 5, showing attractive blue colored areas between the metals which are increasing from Ni to Pt. The NCI approach is also capable to unravel the weak non-covalent metallophilic interactions, however in the present case it is more difficult to distinguish the metallophilic interactions from other inter- and intramolecular interactions (Fig. S11–S13, ESI†)17 and therefore the IGMH method might be preferred in cases of weak interactions, being in the vicinity of other interactions.
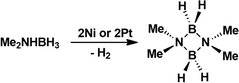
The hydrogen-rich dimethylamine borane, Me2NHBH3, undergoes thermally induced dehydrogenation at 130 °C to give quantitatively the aminoborane (Me2NBH2)2 comprising a four-membered ring structure.18 While no reaction occurs below 100 °C, Manners et al. have shown that the dehydrogenation can be effectively catalyzed by addition of 0.5–5 mol% of [M(1,5-cod)(μ-Cl)]2 (M = Rh, Ir; 1,5-cod = 1,5-cyclooctadiene) or RhCl3·3H2O within 24 h at 45 °C or even lower temperatures at longer reaction times.19,20 Bimetallic complexes offer interesting opportunities for catalytic reactions.21 The interplay of two metal atoms in close proximity to each other often leads to cooperative effects and higher activities compared to single site catalysts. In a first foray, we investigated the catalytic dehydrogenation of Me2NHBH3 at 70 °C.
Typically, NMR scale experiments with 4 mol% of 2Ni, 2Pd and 2Pt were carried out in 1,2-difluorobenzene and the progress was monitored by recording 11B NMR spectra. At the end of the reaction the spectrum showed almost exclusively a triplet centered at δ = 5.3 ppm with 1J(11B–1H) coupling of 112 Hz that was unambiguously assigned to the cyclic (Me2NBH2)2. The reaction proceeded three times faster upon using 2Ni (6 h) than 2Pt (18 h), whereas no reaction occurred in the presence of 2Pd (Scheme 3). Approximate turnover frequencies were determined as TOF = 4.16 h−1 (2Ni) and 1.38 h−1 (2Pt). The 31P NMR spectra of the reaction mixtures indicated that 2Ni (δ = 26 ppm) and 2Pt (δ = 29 ppm) changed into new yet unassigned species. In effort to further invest the kinetics of the dehydrogenation, the hydrogen gas evolution was monitored under the optimized reaction conditions for 2Ni and 2Pt (Fig. 6). The increase of the net pressure correlates well to the corresponding 11B NMR data.
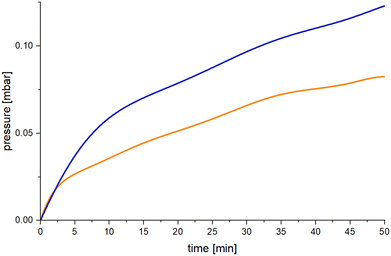 | ||
| Fig. 6 Net pressure generation of the 2Ni (blue) and 2Pt (orange) catalyzed dehydrogenation of Me2NHBH3 in 1,2-difluorobenzene at 70 °C. | ||
Again, 2Ni shows three times faster reaction times than 2Pt. After an initial sharp rise in pressure the reaction progressed approximately linear within the experiment. Notably, the use of 1·PtCl2 also drives the cyclization, but is very slow (96 h).
In summary, the reaction of [2,6-(Ph2P)2C6H3]2GaCl (1) with Ni(dme)Cl2, Pd(MeCN)2Cl2 and Pt(MeCN)2Cl2, respectively, surprisingly proceeded with transmetallation and gave rise to the formation of cationic dinuclear group 10 complexes [M2(PCP)2μ-Cl][GaCl4] (2M, M = Ni, Pd, Pt; PCP = 2,6-(Ph2P)2C6H3)] featuring a rare series of attractive metallophilic interactions between group 10 elements.22 The counterion [GaCl4]− was formed from the expelled Ga atom of 1. Only the reaction of 1 with Pt(Et2S)2Cl2, afforded an expected Z-type complex, namely 1·PtCl2. The dehydrogenation of Me2NHBH3 to give (Me2NBH2)2, is catalysed upon addition of 2Ni and 2Pt, whereas 2Pd is inactive. Efforts are underway to broaden the scope of applications in catalysis and bond activation.
We are grateful to the Deutsche Forschungsgemeinschaft (DFG) for financial support. This work is dedicated to the memory of Professor Ian Manners.
Data availability
Spectroscopic and crystallographic data including figures of NMR spectra as well as the results of DFT calculations are given in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. A. Bennett, S. K. Bhargava, N. Mirzadeh and S. H. Priver, Coord. Chem. Rev., 2018, 370, 69–128 CrossRef CAS.
- M. Olaru, J. F. Kögel, E. Lork, S. Mebs, M. Vogt and J. Beckmann, Chem. – Eur. J., 2020, 26, 275–284 CrossRef CAS.
- (a) A. L. Balch, Prog. Inorg. Chem., 1993, 41, 239–329 Search PubMed; (b) E. S. Smirnova, J. M. Muñoz Molina, A. Johnson, N. A. G. Bandeira, C. Bo and A. M. Echavarren, Angew. Chem., Int. Ed., 2016, 55, 7487–7491 CrossRef CAS.
- (a) M. Olaru, E. Rychagova, S. Ketkov, Y. Shynkarenko, S. Yakunin, M. V. Kovalenkoc, A. Yablonsiky, B. Andreev, J. Beckmann and M. Vogt, J. Amer. Chem. Soc., 2020, 142, 373–381 CrossRef CAS; (b) G. Smolentsev, C. J. Milne, A. Guda, K. Haldrup, J. Szlachetko, N. Azzaroli, C. Cirelli, G. Knopp, R. Bohinc, S. Menzi, G. Pamfilidis, D. Gashi, M. Beck, A. Mozzanica, D. James, C. Bacellar, G. F. Mancini, A. Tereshchenko, V. Shapovalov, W. M. Kwiatek, J. Czapla-Masztafiak, A. Cannizzo, M. Gazzetto, M. Sander, M. Levantino, V. Kabanova, E. Rychagova, S. Ketkov, M. Olaru, J. Beckmann and M. Vogt, Nat. Commun., 2020, 11, 2131 CrossRef CAS.
- F. Meyer, T. Kuzmera, E. Lork, M. Vogt, J. Beckmann and Z. Allgem, Anorg. Chem, 2021, 647, 1890–1895 CrossRef CAS.
- S. Furan, M. Molkenthin, K. Winkels, E. Lork, S. Mebs, E. Hupf and J. Beckmann, Organometallics, 2021, 40, 3785–3796 CrossRef CAS.
- (a) P. Buchwalter, J. Rosé and P. Braunstein, Chem. Rev., 2015, 115, 28–126 CrossRef CAS; (b) E. Lu and S. T. Liddle, Group 10 Metal-Metal Bonds (Chapter 10) in Molecular Metal-Metal Bonds, Compounds, Synthesis, Properties. ed. S. T. Liddle, Wiley-VCH, Weinheim, Germany, 2015, 325–395 Search PubMed.
- (a) P. Pyykkö, Chem. Rev., 1997, 97, 597–636 CrossRef; (b) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931–1951 RSC; (c) M. J. Katz, K. Sakai and D. B. Leznoff, Chem. Soc. Rev., 2008, 37, 1884–1895 RSC; (d) S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741–2760 RSC; (e) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370–412 RSC; (f) H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS.
- M. D. Risi, W. Henderson and G. C. Saunders, Polyhedron, 2024, 255, 116992 CrossRef.
- J. C. Hierso, Chem. Rev., 2014, 114, 4838–4867 CrossRef CAS.
- R. W. F. Bader, Atoms molecules. a quantum theory, Cambridge University Press, Oxford UK, 1991 Search PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS.
- (a) C. Lefebvre, G. Rubez, H. Khartabil, J.-C. Boisson, J. Contreras-García and E. Hénon, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 RSC; (b) T. Lu, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS.
- A. S. Novikov, Inorg. Chim. Acta, 2018, 483, 21–25 CrossRef CAS.
- S. Furan, E. Lork, S. Mebs, E. Hupf and J. Beckmann, Z. Anorg. Allg. Chem., 2020, 646, 856–865 CrossRef CAS.
- R. Gericke, M. A. Bennett, S. H. Privér and S. K. Bhargava, Inorg. Chem., 2023, 62, 8846–8862 CrossRef CAS.
- S. Furan, M. Vogt, K. Winkels, E. Lork, S. Mebs, E. Hupf and J. Beckmann, Organometallics, 2021, 40, 1284–1295 CrossRef CAS.
- A. B. Burg and C. L. Randolph, J. Am. Chem. Soc., 1949, 71, 3451–3455 CrossRef CAS.
- C. A. Jaska, K. Temple, A. J. Lough and I. Manners, Chem. Commun., 2001, 962–963 RSC.
- C. A. Jaska, K. Temple, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2003, 125, 9424–9434 CrossRef CAS.
- (a) J. Campos, Nat. Rev. Chem., 2020, 4, 696–702 CrossRef CAS; (b) M. Navarro, J. J. Moreno, M. Peréz-Jiménez and J. Campos, Chem. Commun., 2022, 58, 11220–11235 RSC.
- O. Zheng, S. Borsley, G. S. Nichol, F. Duarte and S. L. Cockroft, Angew. Chem., Int. Ed., 2019, 131, 12747–12753 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: M = Ni, Pd, Pt. PCP = 2,6-bis(diphenylphosphino)phenyl. CCDC 2378087–2378091. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04296d |
| This journal is © The Royal Society of Chemistry 2024 |