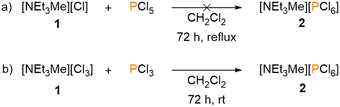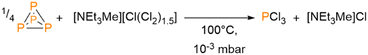Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A polychloride-enabled synthesis of [NEt3Me][PCl6] serving as a potential PCl3-storage and PCl5-reagent†
Moritz J.
Ernst
 ,
Merlin
Kleoff
,
Merlin
Kleoff
 ,
Patrick
Voßnacker
,
Patrick
Voßnacker
 ,
Günther
Thiele
,
Günther
Thiele
 ,
Christian
Müller
,
Christian
Müller
 * and
Sebastian
Riedel
* and
Sebastian
Riedel
 *
*
Freie Universität Berlin, Institute of Chemistry and Biochemistry, Fabeckstr. 34/36, Berlin 14195, Germany. E-mail: s.riedel@fu-berlin.de; c.mueller@fu-berlin.de
First published on 18th October 2024
Abstract
Reaction of the ionic liquid [NEt3Me][Cl3] with white phosphorus (P4) gives, quantitatively, hexachlorophosphate [NEt3Me][PCl6]. This compound shows similar reactivity as PCl5, as confirmed for the reaction with phenol, carboxylic acids and ammonium chloride. At elevated temperature, [NEt3Me][PCl6] releases PCl3 and can therefore be used as a potential PCl3-storage material.
Among all P1 building blocks, phosphorus(III) chloride (PCl3) is the most important, being produced on an industrial scale of approximately 350.000 t per a.1 This base chemical is widely employed for the synthesis of pharmaceuticals, agrochemicals, flame retardants and valuable organophosphorus compounds, such as PPh3.1,2 However, the use of PCl3 has been under debate,1,3–6 partly because it is a highly reactive and hazardous chemical that has caused severe accidents in the past.1 Moreover, the industrial synthesis of PCl3 is typically performed by the exothermic chlorination of molten white phosphorus (P4) with gaseous chorine at elevated temperatures.2 This procedure is “widely considered to be problematic” yet unavoidable as there is currently no useful alternative.3
Recently, Riedel and co-workers proposed trichlorides of the type [NEt3Me][Cl3] (1) as a reversible chlorine storage and chlorination agent that could pave the way to a new and improved chlorine technology.7 Using [NEt3Me][Cl3], new synthetic processes for base chemicals such as phosgene (COCl2) could be realized under mild conditions.8 In general, [NEt3Me][Cl3] shows an enhanced reactivity compared to elemental chlorine, while enabling the stabilization of anionic species.7 This unique reactivity was demonstrated for the synthesis of the ammonium salt [NEt3Me]2[SCl6], which contains the first hexachlorosulphate(IV) anion reported in the literature.9 Most importantly, [NEt3Me][Cl3] is a room-temperature ionic liquid with a low chlorine vapor pressure, serving as a safer and easier-to-handle alternative to elemental chlorine.10,11 Therefore, we investigated the use of [NEt3Me][Cl3] as a safe and simple agent for the preparation and handling of PCl3.
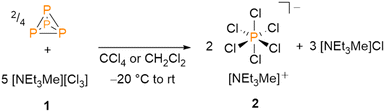
In contrast to the conversion of sulphur (S8) with [NEt3Me][Cl3] to [NEt3Me]2[SCl6], the reaction of red phosphorus (Pred) with the trichloride under ambient conditions turned out to be extremely exothermic and afforded no isolable products.9 Consequently, we attempted the reaction of P4 with [NEt3Me][Cl3] under milder and inert reaction conditions. Tetrachloromethane (CCl4) as a non-polar and rather inert reaction medium was chosen to prevent chlorination of the solvent. Thus, [NEt3Me][Cl3] was added dropwise to the suspension of P4 in CCl4 at T = −20 °C. While heating the reaction mixture slowly up to room temperature, the precipitation of a colourless powder was observed (Scheme 1).
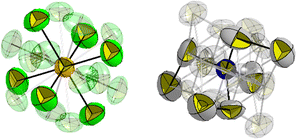
31P NMR spectra of the precipitate revealed a single resonance at δ(ppm) = −295.2. This signal is characteristic for the [PCl6]− anion in solution.12–16 The same reactivity is observed when performing the reaction in dichloromethane (CH2Cl2) instead of CCl4. After removing the solvent, the salt [NEt3Me][PCl6] (2) can be isolated quantitatively, based on the used amount of white phosphorus. Crystallization from both CH2Cl2 and acetonitrile (CH3CN) gave colourless crystals that were analysed by means of single crystal X-ray diffraction. The molecular structure of the [NEt3Me][PCl6] salt 2 is depicted in Fig. 1, selected bond lengths and angles are shown in the caption of the figure.
The crystallographic characterization of the product confirms the formation of [NEt3Me][PCl6], which crystallizes in the cubic space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The highly symmetric structure includes an octahedral [PCl6]− anion with Cl–P–Cl bond angles of 90° and P–Cl bond lengths of 2.11(2) Å, which is in the range of classical P–Cl-single bonds.‡ The cation [NEt3Me]+ is highly disordered, such that the methyl and ethyl groups are undistinguishable.§ The Raman spectrum of [NEt3Me][PCl6] also points to the octahedral symmetry in accordance with the reported spectra of the anion (Fig. S5, ESI†).12 The ESI-TOF mass spectrum of 2 shows a pattern that is similar to the one of the [PF6]− anion under ambient conditions, along with molecular ion peaks for oxidation products, such as [PO2Cl2]− (Fig. S7, ESI†).17
m. The highly symmetric structure includes an octahedral [PCl6]− anion with Cl–P–Cl bond angles of 90° and P–Cl bond lengths of 2.11(2) Å, which is in the range of classical P–Cl-single bonds.‡ The cation [NEt3Me]+ is highly disordered, such that the methyl and ethyl groups are undistinguishable.§ The Raman spectrum of [NEt3Me][PCl6] also points to the octahedral symmetry in accordance with the reported spectra of the anion (Fig. S5, ESI†).12 The ESI-TOF mass spectrum of 2 shows a pattern that is similar to the one of the [PF6]− anion under ambient conditions, along with molecular ion peaks for oxidation products, such as [PO2Cl2]− (Fig. S7, ESI†).17
The ammonium hexachlorophosphate reported here is one of very few examples of this compound class. In fact, with less than 20 crystallogaphically characterized structures containing the [PCl6]− anion, it is highly underrepresented in literature, particularly when compared to the [PF6]− anion, which is one of the most common weakly-coordinating anions, with over 25 thousand known structures. The synthesis described above is the first direct synthetic pathway to a [PCl6]− salt starting from white phosphorus. Other known synthetic routes towards [PCl6]− salts start either from phosphorus pentachloride (PCl5), or by using phosphorus chlorides or polyphosphorus compounds.13–16 Brockner and coworkers reported on the reactions of imidazolium chloride and pyridinium chloride salts with PCl5 yielding the respective [PCl6]− salts.12 In contrast, we found that the reaction of PCl5 with [NEt3Me][Cl] does not give [NEt3Me][PCl6] (Scheme 2a), while the reaction of [NEt3Me][Cl3] with PCl3 does result in the formation of [PCl6]− (Scheme 2b).
It should be noted that PCl5 exists as the ionic species [PCl4[PCl6]] in the solid.18,19 In solution, PCl5 exists primarily as PCl5, while in polar solvents a partial dissociation into [PCl4]+ and [PCl6]− has been observed.20 In contrast, for [NEt3Me][PCl6], only [PCl6]− species could be observed by 31P NMR spectroscopy.
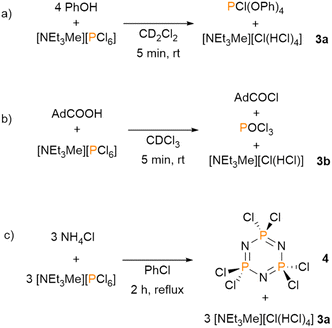
In 2009, Manners and coworkers showed that the [PCl6]− anion plays a key role in the PCl5-initiated living cationic polymerization of a phosphoranimine.21 Inspired by this work, we further investigated the reactivity of [NEt3Me][PCl6]. Reacting [NEt3Me][PCl6] with phenol in CD2Cl2 produces PCl(OPh)4, which shows a characteristic resonance at δ(ppm) = −23.7 in the 31P{1H} NMR spectrum (Scheme 3a and Fig. S13, ESI†). PCl(OPh)4 is the known product of the reaction of phenol and PCl5, while the reaction of phenol with PCl3 yields the phosphite P(OPh)3.22
Thus, this observation reveals a reactivity of [NEt3Me][PCl6] similar to the one of PCl5. Interestingly, HCl, which is liberated during the reaction of phenol with [NEt3Me][PCl6], adds to the resulting chloride salt giving the bichloride based ionic liquid [NEt3Me][Cl(HCl)n] (3, n = 1–4) as the only byproduct. Formation of the bichloride ionic liquid has recently been investigated by us.23 The bichloride can be detected by means of 1H NMR spectroscopy as a singlet resonance at δ(ppm) = 11.44. This shift slightly varies depending on the loading of the salt with HCl.21 Remarkably, the bichloride salt can be separated from the product mixture by extracting PCl(OPh)4 and can further be used in several classical reactions in which HCl is used. With this process in hand, a combined trichloride/bichloride process can be envisioned.23
Further studies confirmed the similar reactivity of [NEt3Me][PCl6] to PCl5 and the possibilities to use the combined process. Carboxylic acids were found to react with [NEt3Me][PCl6] in a similar fashion as PCl5, giving the respective acyl chlorides with POCl3 and, again, the bichloride [NEt3Me][Cl(HCl)n] (1H NMR (CD2Cl2) δ(ppm) = 12.63) as the only byproducts, as demonstrated with 1-adamantanecarboxylic acid (Scheme 3b).2
We subsequently turned our attention to a vast industrial process: the conversion of PCl5 with NH4Cl to hexachlorophosphazene. This compound is used, for example, for the synthesis of poly(dichlorophosphazene).24,25 It turned out that hexachlorophosphazene can also be easily obtained by reacting NH4Cl with [NEt3Me][PCl6] (Scheme 3c). The products can be separated by extracting hexachlorophosphazene with non-polar solvents. After extraction, the ionic liquid [NEt3Me][Cl(HCl)n] (1H NMR (CD2Cl2) δ(ppm) = 12.83) remains as a residue, which can be reconverted, e.g., by electrolysis, to [NEt3Me][Cl3] and H2, or used for further applications.23
During this work, we noticed that [NEt3Me][PCl6] releases PCl3 upon heating to temperatures above T = 100 °C. Thus, while [NEt3Me][PCl6] is stable at room temperature for a rather long period of time, it can be used as a PCl3 storage material as well. The release of PCl3 from 2 can be performed in CH2Cl2 with a conversion of approximately 50% based on the amount of [NEt3Me][PCl6] used. The reaction mixture noticeably turns orange during the release of PCl3. Raman spectroscopic analysis reveals that red phosphorus is formed as a byproduct during this process (Fig. S21, ESI†).¶ The release of PCl3 also proceeds in different chlorinated solvents, such as tetrachloromethane, tetrachloroethane, or dichloromethane. However, due to the formation of azeotropes, the separation of PCl3 from the solvents turned out to be rather challenging. As PCl3 is one of the most used phosphorus precursors in chemical industry, the direct synthesis of PCl3 without elemental chlorine could become industrially valuable (vide supra). To overcome the problem of the separation of PCl3 it is possible to react stochiometric amounts of [NEt3Me][Cl3] and white phosphorus in a closed system (Scheme 4).
When the mixture is heated to T = 120 °C, PCl3 is produced and can be condensed under vacuum into a flask cooled with liquid nitrogen (Fig. S23, ESI†). This reaction can be scaled up to a multiple gram scale with yields of up to 74%. The reaction of [NEt3Me][Cl3] with white phosphorus can therefore be recommended as an alternative route to the preparation of PCl3, not only for laboratory use, but also from an industrial point of view, as the storage and handling of gaseous chlorine involved in the conventional synthesis of PCl3 can thus be avoided.
We have synthesized and characterized the novel ammonium hexachlorophosphate [NEt3Me][PCl6]. This salt is accessible directly from white phosphorus and the trichloride [NEt3Me][Cl3] and can be used efficiently as a synthon for PCl5. This was shown for the synthesis of PCl(OPh)4, acid chlorides and hexachlorophosphazene with the advantage of the direct accessibility from white phosphorus and without the use of gaseous chlorine. The byproduct of the reactions is the bichloride based ionic liquid [NEt3Me][Cl(HCl)n], which could also be further used for chemical conversions in which HCl is needed or be electrochemically recycled to [NEt3Me][Cl3]. Moreover, we demonstrated that [NEt3Me][PCl6] can be used as a PCl3 storage material, as the reaction of white phosphorus with [NEt3Me][Cl3] in a closed system gives PCl3, without the use of any gaseous starting materials.
We acknowledge financial support by the Werner Siemens-Stiftung and the Core Facility BioSupraMol supported by the DFG.
Data availability
The data supporting this article have been included as part of the ESI.† Furthermore, crystallographic data for [NEt3Me][PCl6] has been deposited under the CCDC number 2379260.Conflicts of interest
There are no conflicts to declare.Notes and references
- J.-L. Montchamp, Phosphorus, Sulfur Silicon Relat. Elem., 2013, 188, 66 CrossRef CAS.
- G. Bettermann, W. Krause, G. Riess and T. Hofmann, Phosporus Compounds, Inorganic in Ullmann‘s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012 Search PubMed.
- M. Donath, K. Schwedtmann, T. Schneider, F. Hennersdorf, A. Bauzá, A. Frontera and J. J. Weigand, Nat. Chem., 2022, 14, 384 CrossRef CAS PubMed.
- A. R. Jupp, S. Beijer, G. C. Narain, W. Schipper and J. C. Slootweg, Chem. Soc. Rev., 2021, 50, 87 RSC.
- B. M. Cossairt, N. A. Piro and C. C. Cummins, Chem. Rev., 2010, 110, 4164 CrossRef CAS PubMed.
- D. J. Scott, Angew. Chem., Int. Ed., 2022, 61, e202205019 CrossRef CAS PubMed.
- M. Kleoff, P. Voßnacker and S. Riedel, Angew. Chem., Int. Ed., 2023, 62, e202216586 CrossRef CAS PubMed.
- P. Voßnacker, A. Wüst, T. Keilhack, C. Müller, S. Steinhauer, H. Beckers, S. Yogendra, Y. Schiesser, R. Weber, M. Reimann, R. Müller, M. Kaupp and S. Riedel, Sci. Adv., 2021, 7, eabj5186 CrossRef PubMed.
- P. Voßnacker, A. Wüst, C. Müller, M. Kleoff and S. Riedel, Angew. Chem., Int. Ed., 2022, 61, e202209684 CrossRef PubMed.
- S. Rathmann, F. Braune, M. Kleoff, P. Voßnacker, B. Pölloth, C. Müller and S. Riedel, J. Chem. Educ., 2024, 101, 1394 CrossRef CAS.
- P. Voßnacker, N. Schwarze, T. Keilhack, M. Kleoff, S. Steinhauer, Y. Schiesser, M. Paven, S. Yogendra, R. Weber and S. Riedel, ACS Sustainable Chem. Eng., 2022, 10, 9525 CrossRef.
- M. Gjikaj, J.-C. Leye, T. Xie and W. Brockner, CrystEngComm, 2010, 12, 1474 RSC.
- G. S. Harris and D. S. Payne, J. Chem. Soc., 1956, 3038 RSC.
- K. B. Dillon and A. W. G. Platt, Phosphorus, Sulfur Silicon Relat. Elem., 1984, 19, 299 CrossRef CAS.
- A. S. Muir, Polyhedron, 1991, 10, 2217 CrossRef CAS.
- B. Neumüller and K. Dehnicke, Z. Anorg. Allg. Chem., 2005, 631, 1471 CrossRef.
- Y. Lu, F. L. King and D. C. Duckworth, J. Am. Soc. Mass Spectrom., 2006, 17, 939 CrossRef PubMed.
- H. M. Powell, D. Clark and A. F. Wells, Nature, 1940, 145, 149 CrossRef CAS.
- D. Clark, H. M. Powell and A. F. Wells, J. Chem. Soc., 1942, 642 RSC.
- H. C. Suter, H. C. Knachel, V. P. Petro, J. H. Howatson and S. G. Shore, J. Am. Chem. Soc., 1973, 1474 CrossRef.
- V. Blackstone, A. J. Lough, M. Murray and I. Manners, J. Am. Chem. Soc., 2009, 131, 3658–3667 CrossRef CAS PubMed.
- L. W. Dennis, V. J. Bartuska and G. E. Maciel, J. Am. Chem. Soc., 1982, 104, 230 CrossRef CAS.
- G. H. Dreyhsig, P. Voßnacker, M. Kleoff, H. Baunis, N. Limberg, M. Lu, R. Schomäcker and S. Riedel, Sci. Adv., 2024, 10, eadn5353 CrossRef CAS PubMed.
- J. Emsley and P. B. Udy, J. Chem. Soc. A, 1970, 3025 RSC.
- M. Gorlov, N. Bredov, A. Esin, I. Sirotin, M. Soldatov, V. Oberemok and V. V. Kireev, Int. J. Mol. Sci., 2021, 22, 5958 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2379260. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04319g |
| ‡ The structure shows a [PCl6]− octahedron where the main position is 62.5% occupied. In addition, there are three further (rotational) disorders, each of them is occupied to 12.5%. In total there are six “main positions” of the chlorides with 0.75 occupation factor and 12 more with occupation factor 0.125. All four orientations of the [PCl6]− anion have an octahedral symmetry with only 90° angles and equal P–Cl bond lengths within the error tolerance. For details of the different orientations of the [PCl6]− anion see the ESI.† The comparably large size of the anion and the cation, combined with a highly delocalized charge of the anion, results in a pseudo-binary CsCl type packing that is caused by coulombic interactions with no preferential orientation of the anion. This is assumed to yield the observed rotational disorders. |
| § The same applies to the [NEt3Me] cation. The methyl and ethyl fragments are indistinguishable. Lower space group types show the same statistical occupation and even a triclinic solution with or without forced superstructure (no sign of superstructure in reciprocal space) does not provide any alignment. The central carbon atoms (bounded to N1) are disordered on 12 positions (in total 4 atoms, thus occupation factor 1/3), while the outer carbon atoms are disordered on six positions and thus, since statistically only 3 atoms, the occupation factor of these atoms is 0.5. A respective modelling of hydrogen atoms does not comprise any physically meaningful information and was thus omitted. The nitrogen and the phosphorus atom are the only fully occupied atoms. |
| ¶ It has once been reported that red phosphorus can be produced from white phosphorus in boiling PBr3 leading to the so called “light-red phosphorus”: R. Schenk, Z. Elektrochem., 1905, 8, 1. |
| This journal is © The Royal Society of Chemistry 2024 |