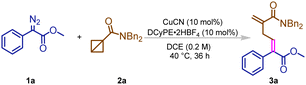Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed strain-enabled reaction of bicyclobutanes with diazo compounds to synthesize penta-1,4-dienes†
Peng
Yang‡
a,
Till
Brockmann‡
a and
Xiao-Feng
Wu
 *ab
*ab
aLeibnitz-Institut für Katalyse e.V, Albert-Einstein-Straße 29a, 18059 Rostock, Germany. E-mail: xiao-feng.wu@catalysis.de
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, Liaoning, China
First published on 16th October 2024
Abstract
Herein we report an interesting copper-catalyzed transformation of BCBs with diazo compounds. This reaction leads to the synthesis of substituted skipped penta-1,4-dienes in good to excellent yields with only one diastomer obtained, and the reaction can also be performed on a gram scale. The transformation is compatible with many different functional groups attached to the BCBs and the diazo compounds.
Bicyclo[1.1.0]butane (BCB), the smallest fused-ring carbocycle with the highest strain energy (64 kcal mol−1), caused by the unstable central C–C bond,1 was first synthesized by Wiberg and Ciula in 1959.2 Since then, BCBs have been indispensable synthetic building blocks because of their unusual bonding and the chemistry enabled by strain release, including carbene insertion,3 alkene4 and imine5 insertion, rearrangement reactions,6 functionalization7 and ring opening reactions with nucleophiles and electrophiles, to generate diverse molecular frameworks.8 Primarily utilizing p-character, the ring-opening of this high-energy bond has been accomplished with nucleophiles (including their use as bioconjugation agents), radicals, electrophiles, and transition metal catalysts.9 Beyond these reactions, the ring expansion of BCBs to bicyclo[n.1.1]alkanes has been achieved through formal addition of one, two, or three atoms.10 With the help of rearrangement reactions, Wipf and co-workers have even succeeded in producing cyclohexane derivatives from BCBs.11 This also shows that BCB compounds open up completely new possibilities.
The discovery of the chemistry of aliphatic diazo compounds was made a century ago. Theodor Curtius synthesized ethyl diazoacetate in 1883 for the first time, opening the wide field of diazo chemistry, which even still inspires new developments.12 The solution of the long controversial problem of constitution, the development of general and facile syntheses, and the application of diazo compounds in organic synthesis, facilitated by their high reactivity, have made this field a truly exciting one. Diazo compounds can be dediazonized to highly reactive free carbene intermediates under thermolytic or photolytic conditions.13 Transition metal-catalyzed reactions of diazo compounds generate metal carbene species that can undergo diverse conversions, such as X–H (X = C, Si, N, O, etc.) insertions, cyclopropanations, ylide formations and 1,2-migrations.14 Dirhodium catalysts usually exhibit superior catalytic activity for most reactions of diazo compounds.15 However, copper catalyzed reactions of diazo compounds have a longer history, which has dominated the literature before the advent of the dirhodium catalysis reactions.16 Copper catalysts have obvious advantages due to their lower cost, low toxicity and abundancy. Recently, copper catalyzed reactions of diazo compounds have been further developed with diazo compounds as versatile cross-coupling partners in various transformations.
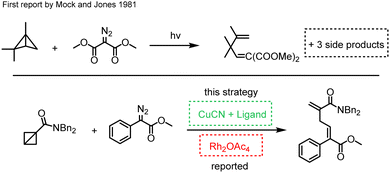
As early as in 1981, Mock and Jones reported a reaction on react free carbene with BCB to create penta-1,4-diene.17 They used C2N2 to synthesize skipped dienes, but with no stereoselectivity. Besides the main compound, they also obtained three side products. More recently, Hari and co-workers reported a new Rh2(OAc)4-catalyzed procedure for synthesizing pentadienes from bicyclobutanes and diazo compounds (Fig. 1).18 In their procedure, they used rhodium to produce a metal carbene from a diazo compound which then reacts with BCB to create a skipped diene. Under their conditions, the desired products were formed stereoselectively under mild conditions. It has been clearly noted that replacing rhodium with copper salt did not lead to the desired product under their conditions. Considering the advantages of copper catalysts and the attractiveness of the above discussed chemistry, herein we report our study on copper-catalyzed synthesis of penta-1,4-dienes from BCBs and diazo compounds. Many different diazo compounds and BCBs were tested and transformed successfully.
Initially, by using BCB 1a and diazo compound 2a in DCE, we checked different copper pre-catalysts. It was found that copper(I) salts yielded much better results than copper(II) salts (Table 1, entries 1–3). CuCN emerged as the best catalyst with 60% yield for this transformation, with the desired pentadiene 3a as the reaction product. Decreasing the loading of copper catalyst to 5% led to a decreased yield of 3a (Table 1, entry 4). Subsequently, we tested various nitrogen containing and phosphorus containing ligands (Table 1, entries 5–9). The tested nitrogen-based ligands exhibited no reaction while improved yields were obtained with phosphorus-based ligands. Ligands were proven to be crucial for the targeted reactions which may stabilize the copper intermediate (Table 1, entry 10). On the other hand, it was discovered that increasing the reaction time to 36 hours resulted in an increase in the yield. Solvents were screened without exception and DCE was found still the best solvent for this reaction (Table 1, entries 11–13). Afterwards we examined the influence of temperature on the reaction, and increasing the temperature proved to be ineffective (Table 1, entry 14), as it did further drive the conversion of substrates. Hence, we prolonged the reaction and the yield of 3a can be improved to 90% (Table 1, entries 15–16). Finally, we have been able to further improve the yield of the desired 3a by changing the ratio of the reactants (Table 1, entries 17–19).
| Entry | Catalyst | Ligand | Solvent | T (h) | Yieldb (%) |
|---|---|---|---|---|---|
a Reaction Conditions: 1a (0.4 mmol), 2a (0.2 mmol), CuCN (10 mol%), DCyPE·HBF4 (10 mol%), DCE (0.2 M), 40 °C, and 36 h.
b Isolated yield.
c CuCN (5 mol%).
d The reaction was carried out at 50 °C.
e 1a (1.0 equiv.).
f
1a (2.5 equiv.).
g
1a (3.0 equiv.). DPPP: 1,3-bis-(diphenylphosphino)-propane. DPPE: ethylene-bis(diphenylphosphine). bpy: 2,2′-bipyridine. dtppy: 4,4′-di-tert-butyl-2,2’-dipyridyl. DCyPE·2HBF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-bis(dicyclohexylphosphino)ethane bis(tetrafluorpbprate). 1,2-bis(dicyclohexylphosphino)ethane bis(tetrafluorpbprate).
|
|||||
| 1 | CuCN | DPPP | DCE | 14 | 60 |
| 2 | CuCl2 | DPPP | DCE | 14 | 18 |
| 3 | Cu(acac)2 | DPPP | DCE | 14 | 43 |
| 4c | CuCN | DPPP | DCE | 14 | 50 |
| 5 | CuCN | bpy | DCE | 14 | ND |
| 6 | CuCN | dtbpy | DCE | 14 | ND |
| 7 | CuCN | PPh3 | DCE | 14 | 62 |
| 8 | CuCN | DPPE | DCE | 14 | 55 |
| 9 | CuCN | DcyPE·2HBF4 | DCE | 14 | 77 |
| 10 | CuCN | — | DCE | 14 | ND |
| 11 | CuCN | DCyPE·2HBF4 | THF | 14 | 21 |
| 12 | CuCN | DcyPE·2HBF4 | Toluene | 14 | 15 |
| 13 | CuCN | DCyPE·2HBF4 | CH3CN | 14 | Trace |
| 14d | CuCN | DCyPE·2HBF4 | DCE | 14 | 74 |
| 15 | CuCN | DCyPE·2HBF4 | DCE | 24 | 88 |
| 16 | CuCN | DCyPE·2HBF4 | DCE | 36 | 90 |
| 17e | CuCN | DCyPE·2HBF4 | DCE | 36 | 30 |
| 18f | CuCN | DCyPE·2HBF4 | DCE | 36 | 95 |
| 19g | CuCN | DCyPE·2HBF4 | DCE | 136 | 95 |
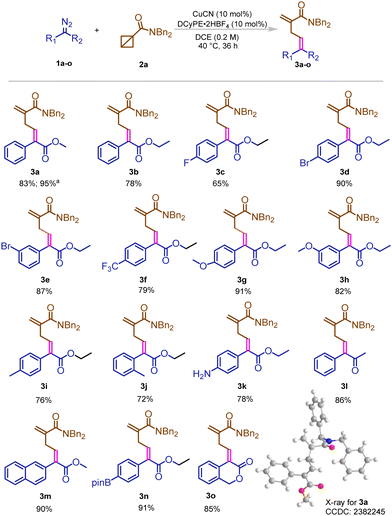
Under the optimized reaction conditions, diverse diazo compounds and BCBs were tested (Scheme 1). A variety of diazo esters and diazo ketones were able to react smoothly and gave the desired products in good to excellent yields (3a–3o). Different electron-withdrawing groups, such as bromo, fluoro and trifluormethyl groups (3c–3f), were tested and gave good to excellent yields of the corresponding products under the standard conditions. Electron donating groups also gave outstanding yields of the desired compounds. Substrates with a methyl group, methoxy group and amino group (3g–3k) were successfully converted to their predetermined products. Among these compounds, ketone was also successfully transformed and yielded good results (3l). Additionally, structurally interesting compounds were tested, a BPin group, which demonstrated a very good yield, as did a naphthalene group attached to the ester (3m–3o). The reaction was also performed on a large scale and 95% yield of 3a was produced without any problem and the structure was further confirmed by X-ray analysis (CCDC: 2382245†). However, it is also worth mentioning that the reactions failed when benzyl bicyclo[1.1.0]butane-1-carboxylate or (1-diazo-2,2,2-trifluoroethyl)benzene was tested in our model system.
Furthermore, the influence of different BCBs was also investigated (Scheme 2). Amides, with different substituents at the nitrogen atom, such as Bn groups and non-aromatic substituents were well tolerated in this transformation and excellent yields were obtained. In addition, BCBs with aromatic and aliphatic ketone moieties gave excellent yields in this reaction as well.
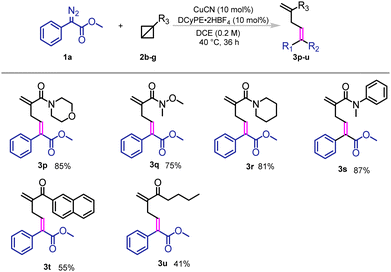 | ||
| Scheme 2 Substrate scope of BCBs. Reaction conditions: 1a (0.5 mmol), 2b–g (0.2 mmol), CuCN (10 mol%), DCyPE·2HBF4 (10 mol%), DCE (0.2 M), 40 °C, 36 h, yields of isolated products are shown. | ||
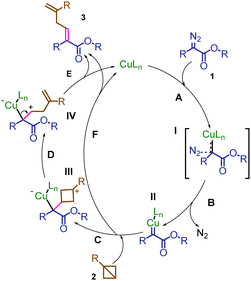
Based on our results and literature,13–18 a possible reaction pathway is proposed (Scheme 3). Initially, a copper carbene intermediate II was formed from the copper catalyst and diazo compound with the release of nitrogen. Then the BCB reacted with the copper carbene intermediate to give the final diene products.
In conclusion, we developed a new copper-catalyzed reaction between bicyclobutanes and diazo compounds to create penta-1,4-dienes (skipped dienes). Under mild conditions, we were able to achieve good to excellent yields of the desired products from a broad scope of diazo compounds and BCBs.
We acknowledge the financial support from the National Key R&D Program of China (2023YFA1507500) and the analytical department of Leibniz-Institute for Catalysis.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. Wiberg, G. Lampman, R. Ciula, D. Connor, P. Schertler and J. Lavanish, Tetrahedron, 1965, 21, 2749–2769 CrossRef; (b) M. D. Newton and J. M. Schulman, J. Am. Chem. Soc., 1972, 94, 767–773 CrossRef; (c) S. Hoz and D. Aurbach, Tetrahedron, 1979, 35, 881–883 CrossRef.
- K. B. Wiberg and R. P. Ciula, J. Am. Chem. Soc., 1959, 81, 5261–5262 CrossRef.
- X. Ma, D. L. Sloman, Y. Han and D. J. Bennett, Org. Lett., 2019, 21, 7199–7203 CrossRef PubMed.
- (a) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef; (b) Y. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef.
- B. Wölfl, N. Winter, J. Li, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2023, 62, e202217064 CrossRef.
- (a) R.-H. Dai, L. Han, Q. Wang and S.-K. Tian, Chem. Commun., 2021, 57, 8449–8451 RSC; (b) Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef; (c) M. Borgini, Q.-N. Huang, P.-P. Chen, S. J. Geib, K. N. Houk and P. Wipf, J. Am. Chem. Soc., 2024, 146, 14927–14934 CrossRef.
- R. E. McNamee, A. Dasgupta, K. E. Christensen and E. A. Anderson, Org. Lett., 2024, 26, 360–364 CrossRef PubMed.
- (a) M. A. A. Walczak and P. Wipf, J. Am. Chem. Soc., 2008, 130, 6924–6925 CrossRef PubMed; (b) P.-P. Chen, P. Wipf and K. N. Houk, Nat. Commun., 2022, 13, 7292 CrossRef PubMed; (c) T. Pinkert, M. Das, M. L. Schrader and F. Glorius, J. Am. Chem. Soc., 2021, 143, 7648–7654 CrossRef.
- (a) A. F. Becknell, J. A. Berson and R. Srinivasan, J. Am. Chem. Soc., 1985, 107, 1076–1078 CrossRef; (b) D. Bai, X. Guo, X. Wang, W. Xu, R. Cheng, D. Wei, Y. Lan and J. Chang, Nat. Commun., 2024, 15, 2833 CrossRef.
- (a) R. M. Bychek, V. Hutskalova, Y. P. Bas, O. A. Zaporozhets, S. Zozulya, V. V. Levterov and P. K. Mykhailiuk, J. Org. Chem., 2019, 84, 15106–15117 CrossRef; (b) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef PubMed; (c) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef PubMed; (d) Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef.
- M. J. Kerner and P. Wipf, Org. Lett., 2021, 23, 3615–3619 CrossRef.
- T. Curtius, Gesellschaft, 1883, 16, 2230–2231 Search PubMed.
- (a) M. P. Doyle, Chem. Rev., 1986, 86, 919–939 CrossRef; (b) T. Ye and M. A. McKervey, Chem. Rev., 1994, 94, 1091–1160 CrossRef CAS; (c) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911–936 CrossRef CAS; (d) Y. Zhang and J. Wang, Chem. Commun., 2009, 5350–5361 RSC; (e) Z. Zhang and V. Gevorgyan, Chem. Rev., 2024, 124, 7214–7261 CrossRef CAS PubMed.
- (a) X. Xu and M. P. Doyle, Acc. Chem. Res., 2014, 47, 1396–1405 CrossRef CAS; (b) H. M. Davies and R. E. Beckwith, Chem. Rev., 2003, 103, 2861–2904 CrossRef CAS PubMed; (c) H. M. Davies and J. R. Manning, Nature, 2008, 451, 417–424 CrossRef CAS PubMed; (d) H. M. Davies and D. Morton, Chem. Soc. Rev., 2011, 40, 1857–1869 RSC; (e) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef CAS; (f) D. Gillingham and N. Fei, Chem. Soc. Rev., 2013, 42, 4918–4931 RSC; (g) Y. Zhang and J. Wang, Coord. Chem. Rev., 2010, 254, 941–953 CrossRef CAS; (h) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS PubMed.
- M. P. Doyle, J. Org. Chem., 2006, 71, 9253–9260 CrossRef CAS PubMed.
- X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162–10173 RSC.
- G. B. Mock and M. JonesJr, Tetrahedron Lett., 1981, 22, 3819–3822 CrossRef CAS.
- G. A. Kadam, T. Singha, S. Rawat and D. P. Hari, ACS Catal., 2024, 14, 12225–12233 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General comments, general procedure, analytical data, and NMR spectra. CCDC 2382245. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04832f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |