 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorinated vs. non-fluorinated tetrahedral Tri4Tri4 porous organic cages for H2, CO2, and CH4 adsorption†
Tim
David
 a,
Robert
Oestreich
a,
Robert
Oestreich
 b,
Tobias
Pausch
b,
Tobias
Pausch
 a,
Yuki
Wada
a,
Yuki
Wada
 c,
Tom
Fleck-Kunde
c,
Tom
Fleck-Kunde
 a,
Masaki
Kawano
a,
Masaki
Kawano
 c,
Christoph
Janiak
c,
Christoph
Janiak
 b and
Bernd M.
Schmidt
b and
Bernd M.
Schmidt
 *a
*a
aInstitut für Organische Chemie und Makromolekulare Chemie, Heinrich-Heine-Universität Düsseldorf, Universitätsstraße 1, 40225 Düsseldorf, Germany. E-mail: bernd.schmidt@hhu.de
bInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, Universitätsstraße 1, 40225 Düsseldorf, Germany
cDepartment of Chemistry, School of Science, Institute of Science Tokyo, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan
First published on 6th November 2024
Abstract
We present the synthesis of two porous complementary tetrahedral Tri4Tri4 imine cages, exhibiting Brunauer–Emmett–Teller (BET) surface areas of 591 m2 g−1 and 753 m2 g−1, suitable for the adsorption of H2, CO2, and CH4. Comparisons in terms of crystallinity, thermal stability, porosity, and selectivity highlight the promising properties of fluorinated and non-fluorinated porous organic cages as functional materials.
Harnessing the power of organic synthesis in combination with subcomponent self-assembly of small and rigid building blocks into larger assemblies under thermodynamic control gives facile access to novel materials by molecular design.1 Besides metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), porous organic cages (POCs) are an emerging class of porous materials that are self-assembled in solution before precipitation as solid materials.2 These discrete, three-dimensional molecular assemblies differ from networks by enabling straightforward solution-phase processing and analysis while also allowing post-synthetic transformations that can modify the scaffolds with atomic precision.2,3 Dynamic covalent chemistry, particularly imine bond condensation4 and boronate ester formation,2a,b,e among others,2e can be employed to access POCs. In comparison to imine cages, the rigidity of boronate ester linkages is beneficial for the synthesis of large shape-persistent cages,2d–f as shown by Mastalerz and co-workers, who reported a giant boronate ester cage with cuboctahedral symmetry exhibiting an extraordinarily high surface area of SABET = 3758 m2 g−1 already in 2014, comparable to those observed for extended networks like MOFs and COFs.5 The group of Beuerle recently reported the first water-stable boronate ester cage,6 stable under ambient conditions with a well-defined microporous solid state structure (SABET = 2534 m2 g−1), paving the way for further applications of dynamic covalent boronate ester materials.7 In addition, the use of computational crystal structure prediction,8 along with computational design in supramolecular synthesis at both the molecular level and in the solid state,9 has been key to obtaining a complex, shape-persistent [4[2+3]+6] cage by reversible nucleophilic aromatic substitution.10 From the various accessible cage structures, the class of Tri4Tri4 cages surprisingly remains largely underrepresented. Despite their tetrahedral structure enabling the formation of three-dimensional pores, only a few porous cages have been reported to date.9,11a–c,12
Herein, we present the synthesis of two highly porous Tri4Tri4 imine cages, Et4H4 and Et4F4. When reacting the pre-organised 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene (Et) with the non-fluorinated trialdehyde (H) and the analogous highly fluorinated trialdehyde (F), Et4H4 and Et4F4 form, respectively, opening up the possibility to investigate the influence of fluorinated units in porous organic materials (Fig. 1a). Heating the building blocks in a chloroform/methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture at 60 °C without stirring results in the growth of cube-like crystals on the walls of the reaction vessel. Repeated solvent exchange against n-pentane and drying of the crystals in air gives Et4H4 in 84% and Et4F4 in 45% yield as colourless crystals (Fig. S9–S14, ESI†). The 1H NMR analysis of the redissolved crystals shows sharp signals, indicating the clean formation of both cages (Fig. 1b). Furthermore, 19F NMR analysis of Et4F4 reveals only one broad signal for the two independent aromatic fluorine atoms (Fig. S44, ESI†). Size determination using 1H DOSY experiments gives solvodynamic radii of rsolv = 1.04 nm (D = 3.98 × 10−10 m2 s−1 in CDCl3) for Et4H4 and rsolv = 1.24 nm (D = 3.35 × 10−10 m2 s−1 in CDCl3) for Et4F4, respectively (Fig. S1–S4, ESI†). Crystals suitable for single-crystal X-ray diffraction (SC-XRD) analysis can be obtained directly from the reaction mixture and reveal the cubic space groups F43c for Et4H4 and Fd3 for Et4F4. Et4F4 assembles in a face-to-face arrangement with centroid-to-centroid distances of 4.5 Å for the fluorinated tetraphenyl panels packing loosely and C–H⋯F contacts between the inner fluorine and the hydrogen of the phenyl core of a neighbouring cage's panel with a distance of 3.1 Å, resulting in interconnected windows and a three-dimensional pore network (Fig. S6, ESI†). Additional vertex-to-vertex arrangements of Et from four separate cages lead to isolated extrinsic pores that are inaccessible. In contrast, Et4H4 packs in a close window-to-window arrangement but also exhibits an extensively connected three-dimensional pore network. Powder X-ray diffraction (PXRD) analysis of dried cage crystals shows sharp diffraction for Et4F4, revealing a partly crystalline material before and after all gas sorption experiments (Fig. 2a). Whereas the PXRD analysis of Et4H4 shows broad diffraction, indicating the formation of a largely amorphous material upon activation (Fig. 2a). Additional thermogravimetric analysis shows a high thermal stability for both materials, with decomposition temperatures of 348 °C for Et4F4 and 352 °C for Et4H4, respectively (Fig. S21 and S22, ESI†). Et4F4's seemingly more robust networked cages and high thermal stability are most likely the result of several stabilising weak interactions between the fluorinated and (non-)fluorinated parts of the cages in the highly symmetric lattice.13 Jiang et al. outlined the improved crystallinity of fluorine-containing systems due to self-complementary electronic interactions between fluorinated and non-fluorinated counterparts.14,15 The pore sizes of Et4H4 range from 5.6 Å to 11.7 Å, derived from the SC-XRD data. Analogues are the diameters 3.4 Å and 6.4 Å for Et4F4, respectively. Non-local density functional theory (NLDFT) and grand canonical Monte Carlo (GCMC) calculations based on the N2 sorption isotherms at 77 K also show the smaller pore size of Et4F4 with a narrow pore size distribution around 5.8 Å (Fig. S29, ESI†). Contrarily, the pore size distribution of Et4H4 shows a broader maximum for micropores around 14 Å and some mesopores with pore diameters between 20 Å and 80 Å, which are larger than the cage compounds themselves (Fig. S26, ESI†), indicating cracks and a subsequent loss of crystallinity. This suggests that the solvent exchange and subsequent drying of the crystals obtained from the reaction mixture, in contrast to Et4F4, leads to a loss of crystallinity for Et4H4. The observed porosity of Et4H4, however, is suggested to be caused by the cage's large voids in the amorphous material. The pore widths of both cages and the kinetic diameters of H2 (2.89 Å), CO2 (3.30 Å), and CH4 (3.80 Å) indicate that both should be suitable for the adsorption of these gases.16 Therefore, the dried crystals were activated by heating under dynamic vacuum overnight, at 80 °C for Et4F4 and at 140 °C for Et4H4. Between the measurements, both samples were recycled by heating to 80 °C for two hours in vacuo. The obtained specific surface areas (SA) of 591 m2 g−1 and 753 m2 g−1 for Et4F4 and Et4H4, respectively, determined by the BET method, are comparable to the Tri4Di6 cage CC3 (SABET = 624 m2 g−1) and a Tri2Di3exo-functionalised salicylimine cage (SABET = 744 m2 g−1) of similar sizes.17,18 To the best of our knowledge, Et4F4 and Et4H4 exhibit among the largest specific surface areas reported for tetrahedral Tri4Tri4 cages. Et4F4 is additionally the largest fluorinated Tri4Tri4 imine cage, surpassing cage FC1 (SABET = 536 m2 g−1) previously published by our group.12 The pore volume of both cages was determined from the N2 sorption isotherms at 77 K by GCMC as well as NLDFT calculations, revealing a total pore volume of 0.39 cm3 g−1 and a micropore volume of 0.21 cm3 g−1 for Et4F4 and 0.59 cm3 g−1 and 0.24 cm3 g−1 for Et4H4, respectively. The Et4H4 cage adsorbs 14.5 mmol g−1 (28.9 wt%) of N2 at 77 K and 0.95p/p0 (Fig. 2c), as beyond this relative pressure, N2 condensation inside the pore network can be observed, which is often not considered. This is comparable to the adsorption performance of the substituted Tri4Di6 cages reported by Mastalerz et al., which also remain porous in their amorphous state while exhibiting specific surface areas ranging from 690 to 727 m2 g−1, with N2 uptake values between 17.4 and 21.4 mmol g−1 at 77 K and 0.95p/p0 bar.19 The
N2 sorption isotherm of Et4H4 can be classified as a combination of type-Ib (low p/p0 for the microporous region) and a type-II isotherm (higher p/p0, macroporous multi-layer region) with a wide H4 hysteresis loop.20Et4F4 exhibits a higher gas uptake of 10.1 mmol g−1 (22.0 wt%) N2 at 77 K and 0.95p/p0 (Fig. 2c), compared to the slightly larger CC3 (8.2 mmol g−1, 18.6 wt%, 1 bar).17 The N2 adsorption isotherm can again be described as a combination of a type-I and type-II isotherm with a H4 hysteresis loop. Noteworthy is the step in the H4 hysteresis loop at 0.5p/p0, more clearly seen in Et4F4 than in Et4H4, which we assign to two types of bottle-neck pores in combination with framework reconstruction.20 Exhibiting a hydrogen uptake of 6.1 mmol g−1 (1.2 wt%), Et4H4 adsorbs more H2 at 77 K and 1 bar than the almost twice as large Tri4Di6tert-butyl substituted adamantoid cage (SABET = 1377 m2 g−1, 5.6 mmol g−1) and is also comparable to the smaller sized CC2 (SABET = 533 m2 g−1, 5.9 mmol g−1) and CC3 (SABET = 624 m2 g−1, 5.0 mmol g−1) POCs reported.17,21 We further measured an adsorption of 3.0 mmol g−1 (11.8 wt%) for CO2 at 273 K and 1 bar. This value is again well comparable with the cages mentioned above showing CO2 uptakes of 2.7 mmol g−1 (11.8 wt%), 3.0 mmol g−1 (11.7 wt%), and 2.5 mmol g−1 (9.9 wt%), respectively.17,22 For CH4, we measured a low gas uptake at 273 K and 1 bar of 0.8 mmol g−1 (1.3 wt%) compared to CC2 (1.1 mmol g−1, 1.7 wt%) and CC3 (1.5 mmol g−1, 2.3 wt%), resulting in a higher selectivity of 10.3
1) mixture at 60 °C without stirring results in the growth of cube-like crystals on the walls of the reaction vessel. Repeated solvent exchange against n-pentane and drying of the crystals in air gives Et4H4 in 84% and Et4F4 in 45% yield as colourless crystals (Fig. S9–S14, ESI†). The 1H NMR analysis of the redissolved crystals shows sharp signals, indicating the clean formation of both cages (Fig. 1b). Furthermore, 19F NMR analysis of Et4F4 reveals only one broad signal for the two independent aromatic fluorine atoms (Fig. S44, ESI†). Size determination using 1H DOSY experiments gives solvodynamic radii of rsolv = 1.04 nm (D = 3.98 × 10−10 m2 s−1 in CDCl3) for Et4H4 and rsolv = 1.24 nm (D = 3.35 × 10−10 m2 s−1 in CDCl3) for Et4F4, respectively (Fig. S1–S4, ESI†). Crystals suitable for single-crystal X-ray diffraction (SC-XRD) analysis can be obtained directly from the reaction mixture and reveal the cubic space groups F43c for Et4H4 and Fd3 for Et4F4. Et4F4 assembles in a face-to-face arrangement with centroid-to-centroid distances of 4.5 Å for the fluorinated tetraphenyl panels packing loosely and C–H⋯F contacts between the inner fluorine and the hydrogen of the phenyl core of a neighbouring cage's panel with a distance of 3.1 Å, resulting in interconnected windows and a three-dimensional pore network (Fig. S6, ESI†). Additional vertex-to-vertex arrangements of Et from four separate cages lead to isolated extrinsic pores that are inaccessible. In contrast, Et4H4 packs in a close window-to-window arrangement but also exhibits an extensively connected three-dimensional pore network. Powder X-ray diffraction (PXRD) analysis of dried cage crystals shows sharp diffraction for Et4F4, revealing a partly crystalline material before and after all gas sorption experiments (Fig. 2a). Whereas the PXRD analysis of Et4H4 shows broad diffraction, indicating the formation of a largely amorphous material upon activation (Fig. 2a). Additional thermogravimetric analysis shows a high thermal stability for both materials, with decomposition temperatures of 348 °C for Et4F4 and 352 °C for Et4H4, respectively (Fig. S21 and S22, ESI†). Et4F4's seemingly more robust networked cages and high thermal stability are most likely the result of several stabilising weak interactions between the fluorinated and (non-)fluorinated parts of the cages in the highly symmetric lattice.13 Jiang et al. outlined the improved crystallinity of fluorine-containing systems due to self-complementary electronic interactions between fluorinated and non-fluorinated counterparts.14,15 The pore sizes of Et4H4 range from 5.6 Å to 11.7 Å, derived from the SC-XRD data. Analogues are the diameters 3.4 Å and 6.4 Å for Et4F4, respectively. Non-local density functional theory (NLDFT) and grand canonical Monte Carlo (GCMC) calculations based on the N2 sorption isotherms at 77 K also show the smaller pore size of Et4F4 with a narrow pore size distribution around 5.8 Å (Fig. S29, ESI†). Contrarily, the pore size distribution of Et4H4 shows a broader maximum for micropores around 14 Å and some mesopores with pore diameters between 20 Å and 80 Å, which are larger than the cage compounds themselves (Fig. S26, ESI†), indicating cracks and a subsequent loss of crystallinity. This suggests that the solvent exchange and subsequent drying of the crystals obtained from the reaction mixture, in contrast to Et4F4, leads to a loss of crystallinity for Et4H4. The observed porosity of Et4H4, however, is suggested to be caused by the cage's large voids in the amorphous material. The pore widths of both cages and the kinetic diameters of H2 (2.89 Å), CO2 (3.30 Å), and CH4 (3.80 Å) indicate that both should be suitable for the adsorption of these gases.16 Therefore, the dried crystals were activated by heating under dynamic vacuum overnight, at 80 °C for Et4F4 and at 140 °C for Et4H4. Between the measurements, both samples were recycled by heating to 80 °C for two hours in vacuo. The obtained specific surface areas (SA) of 591 m2 g−1 and 753 m2 g−1 for Et4F4 and Et4H4, respectively, determined by the BET method, are comparable to the Tri4Di6 cage CC3 (SABET = 624 m2 g−1) and a Tri2Di3exo-functionalised salicylimine cage (SABET = 744 m2 g−1) of similar sizes.17,18 To the best of our knowledge, Et4F4 and Et4H4 exhibit among the largest specific surface areas reported for tetrahedral Tri4Tri4 cages. Et4F4 is additionally the largest fluorinated Tri4Tri4 imine cage, surpassing cage FC1 (SABET = 536 m2 g−1) previously published by our group.12 The pore volume of both cages was determined from the N2 sorption isotherms at 77 K by GCMC as well as NLDFT calculations, revealing a total pore volume of 0.39 cm3 g−1 and a micropore volume of 0.21 cm3 g−1 for Et4F4 and 0.59 cm3 g−1 and 0.24 cm3 g−1 for Et4H4, respectively. The Et4H4 cage adsorbs 14.5 mmol g−1 (28.9 wt%) of N2 at 77 K and 0.95p/p0 (Fig. 2c), as beyond this relative pressure, N2 condensation inside the pore network can be observed, which is often not considered. This is comparable to the adsorption performance of the substituted Tri4Di6 cages reported by Mastalerz et al., which also remain porous in their amorphous state while exhibiting specific surface areas ranging from 690 to 727 m2 g−1, with N2 uptake values between 17.4 and 21.4 mmol g−1 at 77 K and 0.95p/p0 bar.19 The
N2 sorption isotherm of Et4H4 can be classified as a combination of type-Ib (low p/p0 for the microporous region) and a type-II isotherm (higher p/p0, macroporous multi-layer region) with a wide H4 hysteresis loop.20Et4F4 exhibits a higher gas uptake of 10.1 mmol g−1 (22.0 wt%) N2 at 77 K and 0.95p/p0 (Fig. 2c), compared to the slightly larger CC3 (8.2 mmol g−1, 18.6 wt%, 1 bar).17 The N2 adsorption isotherm can again be described as a combination of a type-I and type-II isotherm with a H4 hysteresis loop. Noteworthy is the step in the H4 hysteresis loop at 0.5p/p0, more clearly seen in Et4F4 than in Et4H4, which we assign to two types of bottle-neck pores in combination with framework reconstruction.20 Exhibiting a hydrogen uptake of 6.1 mmol g−1 (1.2 wt%), Et4H4 adsorbs more H2 at 77 K and 1 bar than the almost twice as large Tri4Di6tert-butyl substituted adamantoid cage (SABET = 1377 m2 g−1, 5.6 mmol g−1) and is also comparable to the smaller sized CC2 (SABET = 533 m2 g−1, 5.9 mmol g−1) and CC3 (SABET = 624 m2 g−1, 5.0 mmol g−1) POCs reported.17,21 We further measured an adsorption of 3.0 mmol g−1 (11.8 wt%) for CO2 at 273 K and 1 bar. This value is again well comparable with the cages mentioned above showing CO2 uptakes of 2.7 mmol g−1 (11.8 wt%), 3.0 mmol g−1 (11.7 wt%), and 2.5 mmol g−1 (9.9 wt%), respectively.17,22 For CH4, we measured a low gas uptake at 273 K and 1 bar of 0.8 mmol g−1 (1.3 wt%) compared to CC2 (1.1 mmol g−1, 1.7 wt%) and CC3 (1.5 mmol g−1, 2.3 wt%), resulting in a higher selectivity of 10.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) for CO2 over CH4, which is well comparable to the selectivity of 10
1 (w/w) for CO2 over CH4, which is well comparable to the selectivity of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) for the adamantoid cage by Mastalerz et al.17,21 The measured gas adsorptions of the highly fluorinated Et4F4 are much lower than for the non-fluorinated Et4H4. Et4F4 adsorbs at 1 bar 3.9 mmol g−1 (0.8 wt%) H2 (77 K), 1.5 mmol g−1 (6.3 wt%) CO2 (273 K), and 0.4 mmol g−1 (0.6 wt%) CH4 (273 K). The smaller, previously reported cage FC1 exhibits higher adsorption properties for H2 (7.5 mmol g−1 and 1.5 wt%) and CO2 (4.2 mmol g−1 and 19.0 wt%) than both here reported cages.12 To date, FC1 is the cage with the highest CO2 uptake ever reported for POCs.10 When calculating the gas uptake for a porous material in moles of gas per gramme material, the molecular weight and density of the material are not taken into account. This entails that smaller cages, such as FC1 (1309 g mol−1), frequently display higher gas uptakes since there are essentially more cage molecules with specific surface area per gramme of material available. To evaluate the quantitative uptake, we also calculated the gas uptake in moles of gas per mole of cage and volume of adsorbed gas per volume of the cage (Tables S3–S5, ESI†). For CO2, we calculated gas uptakes of 5.5 mol mol−1 for FC1 and 4.9 mol mol−1 together with 7.1 mol mol−1 for Et4F4 and Et4H4, respectively. In this regard, Et4H4 is the superior adsorbent material in direct comparison to FC1. With increasing pore sizes, the number of adsorbed gas molecules that directly interact with the surface area becomes less, and therefore higher relative pressure is needed for the pore filling through multilayer adsorption by adsorbate–adsorbate interactions to achieve a higher gas uptake. However, besides adsorption capacity, selectivity is arguably another critical property of a porous material for industrial applications such as gas purification.23 Natural gas, primarily composed of CH4, is a crucial energy source that is often contaminated with over 40% N2 and CO2, which must be removed before combustion.24 Therefore, we calculated the ideal adsorbed solution theory (IAST) selectivity for CO2 over CH4, based on the sorption isotherms at 273 K and 1 bar (Fig. 2d). For a 1
1 (w/w) for the adamantoid cage by Mastalerz et al.17,21 The measured gas adsorptions of the highly fluorinated Et4F4 are much lower than for the non-fluorinated Et4H4. Et4F4 adsorbs at 1 bar 3.9 mmol g−1 (0.8 wt%) H2 (77 K), 1.5 mmol g−1 (6.3 wt%) CO2 (273 K), and 0.4 mmol g−1 (0.6 wt%) CH4 (273 K). The smaller, previously reported cage FC1 exhibits higher adsorption properties for H2 (7.5 mmol g−1 and 1.5 wt%) and CO2 (4.2 mmol g−1 and 19.0 wt%) than both here reported cages.12 To date, FC1 is the cage with the highest CO2 uptake ever reported for POCs.10 When calculating the gas uptake for a porous material in moles of gas per gramme material, the molecular weight and density of the material are not taken into account. This entails that smaller cages, such as FC1 (1309 g mol−1), frequently display higher gas uptakes since there are essentially more cage molecules with specific surface area per gramme of material available. To evaluate the quantitative uptake, we also calculated the gas uptake in moles of gas per mole of cage and volume of adsorbed gas per volume of the cage (Tables S3–S5, ESI†). For CO2, we calculated gas uptakes of 5.5 mol mol−1 for FC1 and 4.9 mol mol−1 together with 7.1 mol mol−1 for Et4F4 and Et4H4, respectively. In this regard, Et4H4 is the superior adsorbent material in direct comparison to FC1. With increasing pore sizes, the number of adsorbed gas molecules that directly interact with the surface area becomes less, and therefore higher relative pressure is needed for the pore filling through multilayer adsorption by adsorbate–adsorbate interactions to achieve a higher gas uptake. However, besides adsorption capacity, selectivity is arguably another critical property of a porous material for industrial applications such as gas purification.23 Natural gas, primarily composed of CH4, is a crucial energy source that is often contaminated with over 40% N2 and CO2, which must be removed before combustion.24 Therefore, we calculated the ideal adsorbed solution theory (IAST) selectivity for CO2 over CH4, based on the sorption isotherms at 273 K and 1 bar (Fig. 2d). For a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2
1 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 composition, both cages exhibit nearly the same selectivity (8.80 and 8.71 for Et4H4 and Et4F4, respectively). With decreasing CO2 content, the selectivity of the non-fluorinated cage decreases to 6.6 at 95% CH4 in the gas composition, whereas the selectivity of the fluorinated cage increases to 9.7 for the identical composition (Fig. 2d). In addition to the recent studies of our group, Miljanić et al. reported the higher selectivity of fluorinated covalent triazine frameworks to CO2 in a CH4-enriched composition and the higher CO2-phillicity of fluorine-containing materials, resulting from attractive quadrupole interactions.12,13b The better selectivity of Et4F4, especially in CH4-enriched compositions (≥60%), shows the potential for the application of fluorinated materials in the purification of gases.
CH4 composition, both cages exhibit nearly the same selectivity (8.80 and 8.71 for Et4H4 and Et4F4, respectively). With decreasing CO2 content, the selectivity of the non-fluorinated cage decreases to 6.6 at 95% CH4 in the gas composition, whereas the selectivity of the fluorinated cage increases to 9.7 for the identical composition (Fig. 2d). In addition to the recent studies of our group, Miljanić et al. reported the higher selectivity of fluorinated covalent triazine frameworks to CO2 in a CH4-enriched composition and the higher CO2-phillicity of fluorine-containing materials, resulting from attractive quadrupole interactions.12,13b The better selectivity of Et4F4, especially in CH4-enriched compositions (≥60%), shows the potential for the application of fluorinated materials in the purification of gases.
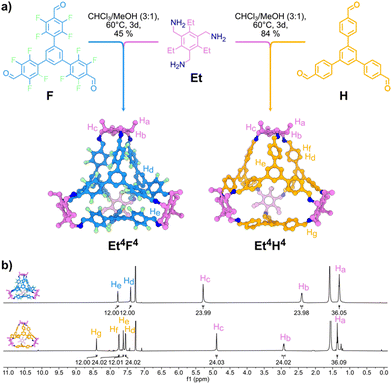 | ||
| Fig. 1 (a) Synthesis of crystalline Et4F4 and Et4H4 by combining H and F with 1.20 eq. Et, respectively; (b) 1H NMR spectra of Et4F4 and Et4H4 recorded in CDCl3 at 25 °C. | ||
In conclusion, we presented the successful synthesis and characterisation of two new porous Tri4Tri4 imine cages, Et4H4 and the highly fluorinated Et4F4. To the best of our knowledge, both cages are among the largest shape-persistent cages within this topology.11a–c,12 We further investigated the influence of the incorporation of highly fluorinated building blocks into porous materials in terms of retention of crystallinity, thermal stability, porosity, selectivity, and reusability. The fluorinated Et4F4 shows a much higher retention of the crystallinity, whereas Et4H4 was obtained as an amorphous material upon solvent removal. Both materials exhibit high thermal stability of approximately 350 °C and gas adsorption measurements further demonstrated that both cages are highly porous with BET surface areas of 591 m2 g−1 (Et4F4) and 753 m2 g−1 (Et4H4), which are accessible for the uptake of H2 and CO2, simultaneously showing a low affinity towards CH4. At 273 K and 1 bar, both cages nearly exhibit the same IAST selectivity for CO2 over CH4 for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition of the gases of 8.8 and 8.7 for Et4H4 and Et4F4, respectively. For compositions with decreasing amounts of CO2, the selectivity for CO2 of the non-fluorinated cage decreases, whereas the selectivity of the fluorinated cage increases, demonstrating the advantages of fluorine-containing building blocks in materials for gas purification. Unveiling significant relationships between the structural and electronic differences of fluorinated and non-fluorinated building blocks, offering new ways to tailor highly selective porous organic architectures for advanced functional materials.
1 composition of the gases of 8.8 and 8.7 for Et4H4 and Et4F4, respectively. For compositions with decreasing amounts of CO2, the selectivity for CO2 of the non-fluorinated cage decreases, whereas the selectivity of the fluorinated cage increases, demonstrating the advantages of fluorine-containing building blocks in materials for gas purification. Unveiling significant relationships between the structural and electronic differences of fluorinated and non-fluorinated building blocks, offering new ways to tailor highly selective porous organic architectures for advanced functional materials.
This work was supported by the Jürgen Manchot Foundation (PhD fellowship, T. D.) and by the Evanglisches Studienwerk Villigst (PhD fellowship, T. P.). B. M. S. acknowledges the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) SCHM 3101/6, B. M. S. and C. J. acknowledge funding for instrumentation through grant 440366605 (INST 208/793-1 FUGG) by the DFG. M. K. acknowledges the support from JSPS KAKENHI Grant (JP23H04878) in a Grant-in-Aid for Transformative Research Areas “Materials Science of Meso-Hierarchy”. We thank the crystallographic reviewer for comments regarding the refinements.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for has been deposited at the CCDC under 2388131 (Et4F4) and 2388132 (Et4H4) and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
There are no conflicts to declare.References
-
(a) F. B. L. Cougnon, A. R. Stefankiewicz and S. Ulrich, Chem. Sci., 2024, 15, 879 RSC
; (b) E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098 RSC
.
-
(a) S. Ivanova and F. Beuerle, Isr. J. Chem., 2024, 64, e202400025 CrossRef
; (b) T. Kunde, T. Pausch and B. M. Schmidt, Eur. J. Org. Chem., 2021, 5844 CrossRef CAS
; (c) X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406 RSC
; (d) M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411 CrossRef CAS PubMed
; (e) F. Beuerle and B. Gole, Angew. Chem., Int. Ed., 2018, 57, 4850 CrossRef CAS PubMed
; (f) T. Hasell and A. I. Cooper, Nat. Rev. Mater, 2016, 1, 16053 CrossRef CAS
.
-
(a) M. Yang, K. Su and D. Yuan, Chem. Commun., 2024, 60, 10476 RSC
; (b) T. Pausch, T. David, T. Fleck-Kunde, H. Pols, J. Gurke and B. M. Schmidt, Angew. Chem., Int. Ed., 2024, 63, e202318362 CrossRef CAS PubMed
; (c) H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Chem. Soc. Rev., 2021, 50, 8874 RSC
.
- K. Acharyya and P. S. Mukherjee, Angew. Chem., Int. Ed., 2019, 58, 8640 CrossRef CAS PubMed
.
- G. Zhang, O. Presly, F. White, I. M. Oppel and M. Mastalerz, Angew. Chem., Int. Ed., 2014, 53, 1516 CrossRef CAS PubMed
.
- P. H. Kirchner, L. Schramm, S. Ivanova, K. Shoyama, F. Würthner and F. Beuerle, J. Am. Chem. Soc., 2024, 146, 5305 CrossRef CAS PubMed
.
-
(a) S. Ivanova, E. Köster, J. J. Holstein, N. Keller, G. H. Clever, T. Bein and F. Beuerle, Angew. Chem., Int. Ed., 2021, 60, 17455 CrossRef CAS PubMed
; (b) S. Klotzbach, T. Scherpf and F. Beuerle, Chem. Commun., 2014, 50, 12454 RSC
.
- R. L. Greenaway, V. Santolini, A. Pulido, M. A. Little, B. M. Alston, M. E. Briggs, G. M. Day, A. I. Cooper and K. E. Jelfs, Angew. Chem., Int. Ed., 2019, 58, 16275 CrossRef CAS PubMed
.
-
(a) A. R. Basford, S. K. Bennett, M. Xiao, L. Turcani, J. Allen, K. E. Jelfs and R. L. Greenaway, Chem. Sci., 2024, 15, 6331 RSC
; (b) A. Tarzia, E. H. Wolpert, K. E. Jelfs and G. M. Pavan, Chem. Sci., 2023, 14, 12506 RSC
; (c) V. Santolini, M. Miklitz, E. Berardo and K. E. Jelfs, Nanoscale, 2017, 9, 5280 RSC
.
- Q. Zhu, H. Qu, G. Avci, R. Hafizi, C. Zhao, G. M. Day, K. E. Jelfs, M. A. Little and A. I. Cooper, Nat. Synth., 2024, 3, 825 CrossRef
.
-
(a) J. C. Lauer, W.-S. Zhang, F. Rominger, R. R. Schröder and M. Mastalerz, Chem. – Eur. J., 2018, 24, 1816 CrossRef CAS PubMed
; (b) K. Tian, X. Wang, M. P. Schuldt, S. M. Elbert, F. Rominger and M. Mastalerz, Org. Mater., 2023, 5, 91 CrossRef CAS
; (c) S. M. Elbert, F. Rominger and M. Mastalerz, Chem. Eur. J., 2014, 20, 16707 CrossRef CAS PubMed
.
- T. Kunde, E. Nieland, H. V. Schröder, C. A. Schalley and B. M. Schmidt, Chem. Commun., 2020, 56, 4761 RSC
.
-
(a) Z. Zhang and O. Š. Miljanić, Org. Mater., 2019, 1, 19 CrossRef CAS
; (b) Z. Yang, S. Wang, Z. Zhang, W. Guo, K. Jie, M. I. Hashim, O. Š. Miljanić, D.-E. Jiang, I. Popovs and S. Dai, J. Mat. Chem. A, 2019, 7, 17277 RSC
; (c) T.-H. Chen, I. Popov, W. Kaveevivitchai, Y.-C. Chuang, Y.-S. Chen, A. J. Jacobson and O. Š. Miljanić, Angew. Chem., Int. Ed., 2015, 54, 13902 CrossRef CAS PubMed
; (d) T.-H. Chen, I. Popov, W. Kaveevivitchai, Y.-C. Chuang, Y.-S. Chen, O. Daugulis, A. J. Jacobson and O. Š. Miljanić, Nat. Commun., 2014, 5, 5131 CrossRef CAS PubMed
.
- X. Chen, M. Addicoat, S. Irle, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2013, 135, 546 CrossRef CAS PubMed
.
- B. M. Schmidt, A. K. Meyer and D. Lentz, CrystEngComm, 2017, 19, 1328 RSC
.
- N. Mehio, S. Dai and D.-E. Jiang, J. Phys. Chem. A, 2014, 118, 1150 CrossRef CAS PubMed
.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973 CrossRef CAS PubMed
.
- M. W. Schneider, I. M. Oppel and M. Mastalerz, Chem. – Eur. J., 2012, 18, 4156 CrossRef CAS PubMed
.
- M. W. Schneider, I. M. Oppel, H. Ott, L. G. Lechner, H.-J. S. Hauswald, R. Stoll and M. Mastalerz, Chem. – Eur. J., 2012, 18, 836 CrossRef CAS PubMed
.
- K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K. S. W. Sing and M. Thommes, Pure Appl. Chem., 2015, 87, 1051 CrossRef
.
- M. W. Schneider, I. M. Oppel, O. Presly and M. Mastalerz, Angew. Chem., Int. Ed., 2011, 50, 1046 CrossRef PubMed
.
- M. W. Schneider, I. M. Oppel, A. Griffin and M. Mastalerz, Angew. Chem., Int. Ed., 2013, 52, 3611 CrossRef CAS PubMed
.
-
(a) L. Yu, L. Hao, C. Zhang, L. Qiao, J. Pang, H. Wang, H. Chang, W. Fan, R. Wang, D. Sun, L. Fan and Z. Kang, J. Membr. Sci., 2024, 711, 123231 CrossRef CAS
; (b) G. Wu, J.-R. Wu, Y. Wang and Y.-W. Yang, Chem., 2009, 9, 2918 CrossRef
.
- J. R. Long, D. M. D'Alessandro and B. Smit, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectroscopic data, gas sorption data, crystallographic data. CCDC 2388131 and 2388132. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05277c |
| This journal is © The Royal Society of Chemistry 2024 |

