Open Access Article
Open Access ArticleOrthogonal stimulation of structural transformations in photo-responsive MOF films through linker functionalization†
Sumea
Klokic
 ab,
Benedetta
Marmiroli
b,
Denys
Naumenko
b,
Giovanni
Birarda
ab,
Benedetta
Marmiroli
b,
Denys
Naumenko
b,
Giovanni
Birarda
 c,
Simone
Dal Zilio
c,
Simone
Dal Zilio
 d,
Miriam de J.
Velásquez-Hernández
d,
Miriam de J.
Velásquez-Hernández
 e,
Paolo
Falcaro
e,
Paolo
Falcaro
 e,
Lisa
Vaccari
e,
Lisa
Vaccari
 c and
Heinz
Amenitsch
c and
Heinz
Amenitsch
 *b
*b
aCERIC-ERIC, Elettra Sincrotrone Trieste – SAXS Beamline, S.S. 14, 163.5 km, Basovizza, Trieste 34149, Italy
bInstitute of Inorganic Chemistry, Graz University of Technology, 8010 Graz, Austria. E-mail: heinz.amentisch@tugraz.at
cElettra Sincrotrone Trieste – SISSI Bio Beamline, S.S. 14, 163.5 km, Basovizza, Trieste 34149, Italy
dIOM-CNR, Laboratorio TASC, S.S. 14, 163.5 km, Basovizza, Trieste 34149, Italy
eInstitute of Physical and Theoretical Chemistry, Graz University of Technology, 8010 Graz, Austria
First published on 22nd March 2024
Abstract
Controlling the magnitude of structural dynamics in flexible MOF films by an applied stimulus is largely desired for specific applications such as energy storage. Herein, by introducing chlorine substituents in an azobenzene-infiltrated Cl-DMOF-1 film structure, a significantly slowed structural response is obtained upon photo-switching of the system.
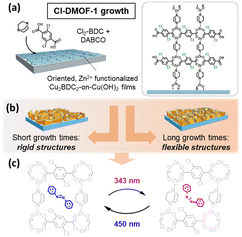
To date, the fabrication of structurally responsive MOF films1,2 is encouraged for the development of MOF-based devices, including sensors,3,4 cargo delivery systems5–7 and mechanical storage systems.8–10 Implementing a moiety within a film assembly that is responsive to an external stimulus allows for the remote control of MOF film properties.11 Here, light is a particularly attractive stimulus as it is readily available and renewable in the form of sunlight.12 So far, a large number of structurally flexible photo-active MOFs has been reported, such as DMOF-1 ([Zn2(bdc)2(dabco)], bdc = 1,4-benzenedicarboxylate and dabco = 1,4-diazabicyclo[2.2.2]octan),13 or [Zn(AzDC)(4,4′-BPE)0.5], AzDC = azobenzene-4,4′-dicarboxylic acid and 4,4′-BPE = trans-1,2-bis(4-pyridyl)ethylene.14 Most of these flexible structures are based on reticular design strategies, where molecular building blocks are replaced within the MOF system.15,16 These structures exhibit a tuned flexibility that provides an extra degree of freedom, which offers additional means to exploit dynamics in responsive MOF film systems alongside primary photon stimulation. This concept is introduced as orthogonal stimulation by Peh et al.15 and holds promise for tailoring responsiveness in flexible MOF systems. Following this approach, in this study, we demonstrate a chemical pathway to tune photo-induced structural transformations.15 Replacing terephthalic acid linkers with chloro-functionalized derivatives allows for the fabrication of Cl-DMOF-1 films that are isostructural to the DMOF-1 system (Fig. 1a, right)13,17 while exhibiting varied flexibility with changes in growth time (Fig. 1b). Although linker functionalization has been successfully shown for bulk DMOF-1 systems,18–21 structural photo-response and its duration for such modified structures has not been examined so far. We tackled this by incorporating azobenzene within Cl-DMOF-1 pores to obtain a photo-responsive film system, which was confirmed by infrared spectroscopic measurements. Time-resolved grazing incidence wide angle X-ray scattering measurements (GIWAXS) showed a significantly slowed structural photo-response compared to the DMOF-1 structure (Fig. 1c).22
To ensure sufficient light penetration accompanied by a controlled propagation of structural photo-response, Cl-DMOF-1 films were fabricated as heteroepitaxial film structures following a substrate-seeded approach.23,24 For this, in the first step, Cu(OH)2 nanobelts were deposited on glass substrates, which were then converted to Cu2bdc2 (see Scheme S1†).23
Subsequently, the Cu2bdc2-on-Cu(OH)2 films were treated in zinc acetate and placed at 60 °C in the methanolic linker solution containing dabco and a Cl2-bdc linker (Fig. 1a, see ESI† for experimental details). However, linker functionalization in MOFs can potentially vary the thermodynamic stability and, thus, the readiness of the structure to grow.25 To address this issue, in the first step, we altered the growth time of the Cl-DMOF-1 films from 10 to 270 min and studied the alignment of the structure along the Cu2bdc2-on-Cu(OH)2 film surface in a combinatory study by employing SEM (Fig. 2 and S2†) and GIWAXS measurements (Fig. S3 and S4†), which are discussed below.
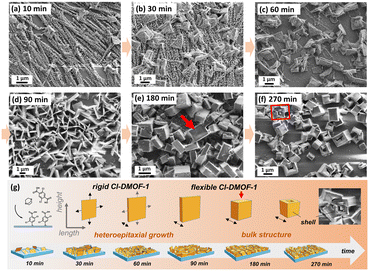
In Fig. 2, the top-view SEM images depict the time-dependent growth of the Cl-DMOF-1-on-Cu2bdc2-on-Cu(OH)2 film structures. Initially, at a growth time of 10 min, small crystallites appear (Fig. 2a), which were determined using GIWAXS measurements to correspond to the weak growth of the Cl-DMOF-110min structure (Fig. S3a†). According to the GIWAXS pattern in Fig. S3b,† sharp reflections appear for a growth of 30 min, which are attributed to the formation of the P4/mmm phase for the Cl-DMOF-130min (a = 10.93 Å, b = 10.93 Å, and c = 9.61 Å).17,26 Interestingly, (100) reflection shows a shift of Δq = 0.04 nm−1 between the out-of-plane (qOP = 5.82 nm−1) and the in-plane direction (qIP = 5.78 nm−1). On the contrary, (001)Cl-DMOF-1 and (101)Cl-DMOF-1 reflections remain unaltered, indicating that the (100) plane is distorted. This property is attributed to internal strain27 within the Cl-DMOF-1 structures, which becomes more evident at a growth time of 90 min, causing even splitting of (001)Cu2bdc2 reflection for the Cu2bdc2-on-Cu(OH)2 substructure (Fig. S4a,† out-of-plane pattern). Kitagawa and co-workers recently delineated that with a decreasing MOF film thickness, surface effects become more pronounced,28 in which heteroepitaxial structures can be a display of strain induced by lattice mismatch.24
Hence, to understand the impact of internal strain, we performed GIWAXS measurements while rotating the Cl-DMOF-190min film sample along the azimuthal angle φ (rotation around the surface normal, see Fig. S5a and b†). The behaviour of the (100)Cl-DMOF-1 reflection with respect to ϕ in the out-of-plane direction indicates the presence of two crystalline phases. At φ = 0°, the Cl-DMOF-190min structure was determined to grow in the P4/mmm phase (Fig. S5c and d†), as already encountered for structures grown at shorter times. By rotating the sample to larger φ-angles, a second phase appears that is shifted towards higher q, thus resembling the I4/mcm structure (Fig. S5c†). Consequently, the characteristic (001)Cu2bdc2 reflection of the epitaxial substructure shifts by Δq = 0.03 nm−1 towards smaller q in the out-of-plane direction (Fig. S5d†). It must be noted that the Cu2bdc2 substructure of the Cl-DMOF-190min film was confirmed to comprise a strong azimuthal angle dependence in the in-plane direction (Fig. S5e–g†), as was reported for the non-functionalized DMOF-1 film structure.22,23 The (001)Cl-DMOF-1 reflection located at qIP = 6.45 nm−1 shows no azimuthal angle dependence in the in-plane direction (Fig. S5b and d†). Based on these findings, we consider the growth of this second I4/mcm-like phase a result of relieving the internal strain within the Cl-DMOF-1 structures that arises mainly from the urge to match the Cl-DMOF-1 lattice with the lower Cu2bdc2-on-Cu(OH)2 structure. Thus, at growth times ≤90 min, strained but heteroepitaxially grown Cl-DMOF-1-on-Cu2bdc2-on-Cu(OH)2 film systems are obtained. However, a propagation of the growth time leads to non-epitaxially grown Cl-DMOF-1180min (Fig. S4b†) and Cl-DMOF-1270min structures (Fig. S4c†). Moreover, the absence of preferred alignment in both in-plane and out-of-plane directions is a characteristic of non-epitaxial and bulk structures.24
From SEM micrographs, the evolution of the Cl-DMOF-1 crystallite growth as a function of time demonstrates the formation of very thin crystallites after 30 min with a size of 1.4 μm in both length and height (Fig. 2b). The Cl-DMOF-160min crystallites continue to grow in their width (∼0.1 μm; Fig. 2c), which further increases for the Cl-DMOF-190min system (∼0.3 μm; Fig. 2d). However, prolonged growth of the Cl-DMOF-1180min system yields crystallites of significantly different shapes, where some crystallite surfaces are strongly bent inward (see the red arrow in Fig. 2e). After 270 min, the crystallite size resembles those of reported bulk crystallites18 with the difference that most larger crystallites comprise cracks (see Fig. 2f), which are referred to as the result of a long-range strain within the structure.29 Some of the Cl-DMOF-1270min crystallites display a hollow core resembling a shell-like structure, which is attributed to the ultimate product of internal stress release (Fig. 2g, see SEM image). The evolution of the Cl-DMOF-1 crystallite growth is schematically depicted in Fig. 2g, where we classify structures grown shorter than 90 min as heteroepitaxial, while subsequent growth yields bulk-like structures. Based on GIWAXS data, we propose for heteroepitaxial structures (growth time <90 min) that the (h00)Cl-DMOF-1 plane grows epitaxially onto the (00l)Cu2bdc2 plane with the a-axis of the Cl-DMOF-1 system aligning perpendicular to the substrate. For these structures, the (00l)Cl-DMOF-1 plane orients mainly in the out-of-plane direction, which results in the c-axis aligned parallel to the substrate (see Fig. S3a–c†). This alignment was already reported in our earlier study of the oriented DMOF-1 film structure,22 confirming the isoreticular growth approach for the presented functionalized heteroepitaxial film system. However, this is not the case for bulk-like Cl-DMOF-1 film structures (180–270 min), which show a sudden change in the alignment of their structure. This property is envisioned by the appearance of the (00l)Cl-DMOF-1 plane in the in-plane direction (≥90 min, Fig. S4a†). We presume that this inversion in alignment mainly arises from the strained ≥180 min structures, resulting in a complete loss of heteroepitaxy accompanied by the sudden change in the shape of the Cl-DMOF-1 crystallites yielding bulk-like structures (see SEM images in Fig. 2). The growth behaviour of the Cl-DMOF-1 films was additionally confirmed by infrared spectroscopic measurements. All pristine structures show strong vibrational bands located around 1388 cm−1 and 1587 cm−1 (Fig. 3 and S6–S8†), corresponding to the symmetric (υs) and asymmetric stretching (υas) of the carboxylic group bonded to the metal nodes, respectively.22
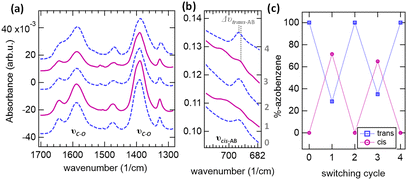
We further investigated the structural flexibility of the Cl-DMOF-1 films. For this, the photo-active guest molecule azobenzene was incorporated within the Cl-DMOF-1 pores following the vapour-assisted approach (<0.5 molecules per pore, Table S1†).22 The success of the infiltration process was confirmed by Fourier transform infrared (FT-IR) attenuated total reflectance (ATR) measurements of the respective Cl-DMOF-1 films. The results show the appearance of the characteristic trans-azobenzene vibrational band located around 691 cm−1 (see Fig. S6–S8†), which experiences a red shift for structures after prolonged growth (30–180 min, Table S2†). Similarly, a red shift in the υs vibrational band was determined, which is attributed to the carboxylate vibrations arising from the Cl-DMOF-1 structure (Table S2†). This behaviour indicates that the internal strain caused by the heteroepitaxial growth of the Cl-DMOF-1/AB structures is directly translated to the infiltrated azobenzene molecule within the pores. In the case of the weakly grown Cl-DMOF-1/AB10min structure (Fig. S6a†), where the main vibrational bands are attributed to the non-coordinated terephthalic acid linkers (see Fig. S6b†), azobenzene is expected to accumulate on the surface rather than being incorporated within the pores. Hence, this system can serve as a reference because the vibrational band of azobenzene corresponds to its non-strained, pristine trans-conformer located at υAB = 690 cm−1 (Fig. S6c†).30 In comparison, the Cl-DMOF-1/AB270min system contains azobenzene molecules in a relaxed state (υAB = 691 cm−1), which is attributed to the loss of epitaxy. Hence, these findings corroborate SEM and GIWAXS measurements; they show that at growth times exceeding 180 min, the internal stress is overcome, which leads to bulk-like, non-epitaxially grown structures.
In the next step, to test the photo-activity of the incorporated azobenzene molecules with respect to the duration of the structural transformation of the Cl-DMOF-1/AB systems, we performed ATR measurements combined with two light sources (343 nm and 450 nm; Scheme S2†). The results show that short growth times yield rigid Cl-DMOF-1/AB structures where no azobenzene isomerization was possible (Fig. S9a†), while the structures obtained after longer growth times exhibit sufficient flexibility to respond to the azobenzene isomerization process (≥60 min, Fig. S9b†). For the latter films, irradiation by 343 nm prompted the azobenzene within the Cl-DMOF-1/AB pores to enter its cis-state (υcis-AB = 698 cm−1, ON-state), which, after exposure to 450 nm, relaxed fully to its trans-conformer (υtrans-AB = 693 cm−1, OFF-state; Fig. 3b). The results for the DMOF-1/AB90min show that in the ON state, about 70% of the infiltrated azobenzene molecules are converted to the cis-conformer, while 30% of the trans-conformer remains (Fig. 3c). This significantly higher abundance of trans-azobenzene molecules within the Cl-DMOF-1/AB90min pores is attributed to the strong stabilization of this conformer owing to the chloro-functionalized MOF environment. The relaxed Cl-DMOF-1/AB90min structure was found to repeatedly allow the azobenzene to enter its trans-isomer, exhibiting a shift of Δυcis-AB = 1.05 cm−1 between the ON and OFF states (Fig. 3b).
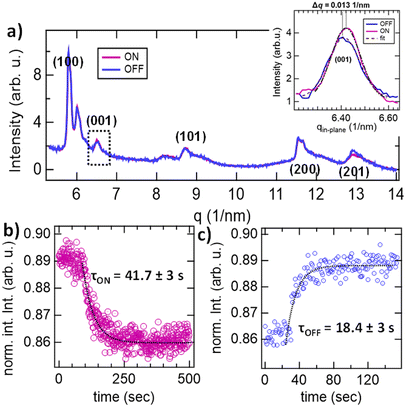
We further performed time-resolved GIWAXS measurements to investigate the structural transformations induced within the Cl-DMOF-1/AB90min structure upon photo-excitation. Here, only the Cl-DMOF-1/AB90min structure was tested to confirm the success of the isomerization process of azobenzene upon photo-stimulation (Fig. 4). This is because azobenzene remains non-responsive at shorter growth times (Cl-DMOF-1/AB30min; Fig. S9a†), and the bulk-like growth of Cl-DMOF-1/AB structures after 180 and 270 minutes produces inhomogeneous films that hinder light penetration, thus impeding their photo-physical characterization.1 Detailed analysis of the GIWAXS data for the Cl-DMOF-1/AB90min structure showed that 32% of the crystallites orient preferentially towards the out-of-plane direction (Fig. S10†). This result is comparable to the non-functionalized DMOF-1 film structure, which confirms that an oriented and heteroepitaxial Cl-DMOF-1 structure is grown, thus allowing the characterization of its photo-switch.22
Time-resolved GIWAXS measurements revealed that upon excitation with light of 343 nm, the (001) reflection commenced a shift by Δq = 0.013 nm−1, as determined from fitting results in the in-plane direction (Fig. 4a). This value is in good agreement with findings reported for the non-functionalized DMOF-1 film system.22 Hence, the Cl-DMOF-1/AB90min structure shrinks when azobenzene enters its cis-conformer, which is reversed by the light of 450 nm upon relaxation to its trans-conformer (Fig. 4b and c). This finding strongly supports the successful isoreticular design strategy as the structural response for the Cl-DMOF-1 is channelled similarly to the DMOF-1 structure.22 From the temporal evolution of the intensity for the (001) reflection under continuous illumination with the respective light sources, the switching constants were deduced for the ON state (343 nm, Fig. 4b) and the OFF state (450 nm, Fig. 4c). GIWAXS measurements showed that the structural response for the ON state was reached after τON = 41.7 ± 3 s (343 nm@130 mW cm−2), which was reversed within τOFF = 18.4 ± 3 s (Fig. 4b and c; 450 nm@17.2 mW cm−2). In comparison, we reported for the non-functionalized DMOF-1 film structure that the entire switch required 15 s (343 nm@95 mW cm−2, 450 nm@17.2 mW cm−2),22 therefore rendering the Cl-DMOF-1/AB90min film system to respond significantly slower at similar irradiances (see Table S3† for comparison of the two film systems). This finding is reasonable because chloro-functionalized switchable bulk structures are known to experience a certain loss in flexibility,16 as indicated by the lack of gate-opening behaviour in bulk Cl-DMOF-1 during N2 sorption experiments, while the DMOF-1 showed a subtle step in the adsorption branch P/P0 = 0.003 (Fig. S13†).
In conclusion, we herein prepared Zn-based MOF films using chlorinated terephthalic acid and dabco as ligands, yielding 3D-oriented MOF crystals infiltrated with photo-responsive azobenzene. Upon UV irradiation, we measured orthogonal stimulation. The chloro-functionality imparts rigidity to the oriented MOF film, leading to a strongly decelerated structural photo-response. Depending on the synthesis conditions, different photo-responsive behaviours were observed: films prepared with short reaction times (10–60 min) showed entirely rigid frameworks, while prolonged growth of the MOF crystals (90–270 min) yielded more flexible structures. Moreover, the azobenzene molecules in the MOF pores can be repeatedly isomerized when triggered by light opening possibilities for their application as mechanical storage systems.10,12,15,17
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Ing. Andrea Radeticchio and Dr Barbara Sartori for experimental and technical support. S. K. would like to acknowledge CERIC-ERIC for the postdoctoral position granted through its internal project ‘INCITE’. The authors also acknowledge the CERIC-ERIC Consortium for access to the Austrian SAXS beamline and SISSI beamline at ELETTRA through proposal No. 20212195 and 20222149.Notes and references
- R. Haldar, L. Heinke and C. Wöll, Adv. Mater., 2020, 32, e1905227 CrossRef PubMed.
- J. Liu and C. Wöll, Chem. Soc. Rev., 2017, 46, 5730–5770 RSC.
- A. H. Assen, O. Yassine, O. Shekhah, M. Eddaoudi and K. N. Salama, ACS Sens., 2017, 2, 1294–1301 CrossRef CAS PubMed.
- A. B. Kanj, J. Bürck, S. Grosjean, S. Bräse and L. Heinke, Chem. Commun., 2019, 55, 8776–8779 RSC.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef CAS PubMed.
- J. Gandara-Loe, B. E. Souza, A. Missyul, G. Giraldo, J.-C. Tan and J. Silvestre-Albero, ACS Appl. Mater. Interfaces, 2020, 12, 30189–30197 CrossRef CAS PubMed.
- A. Bui, S. G. Guillen, A. Sua, T. C. Nguyen, A. Ruiz, L. Carachure, M. D. R. Weber, A. Cortez and F. Tian, Colloids Surf., 2022, 650, 129611 CrossRef CAS PubMed.
- H. B. Wu and X. W. Lou, Sci. Adv., 2017, 3, eaap9252 CrossRef PubMed.
- M. Linares-Moreau, L. A. Brandner, M. de Velásquez-Hernández, J. Fonseca, Y. Benseghir, J. M. Chin, D. Maspoch, C. Doonan and P. Falcaro, Adv. Mater., 2023, 36, 2309645 CrossRef.
- J. Wieme, S. M. Rogge, P. G. Yot, L. Vanduyfhuys, S.-K. Lee, J.-S. Chang, M. Waroquier, G. Maurin and V. Van Speybroeck, J. Mater. Chem. A, 2019, 7, 22663–22674 RSC.
- Q. Huang and C. Wu, Mater. Today Sustain., 2022, 18, 100149 CrossRef.
- F. Bigdeli, C. T. Lollar, A. Morsali and H.-C. Zhou, Angew. Chem., Int. Ed., 2020, 59, 4652–4669 CrossRef CAS PubMed.
- N. Yanai, T. Uemura, M. Inoue, R. Matsuda, T. Fukushima, M. Tsujimoto, S. Isoda and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 4501–4504 CrossRef CAS PubMed.
- R. Lyndon, K. Konstas, B. P. Ladewig, P. D. Southon, C. J. Kepert and M. R. Hill, Angew. Chem., Int. Ed., 2013, 52, 3695–3698 CrossRef CAS PubMed.
- S. B. Peh, A. Karmakar and D. Zhao, Trends Chem., 2020, 2, 199–213 CrossRef CAS.
- T. Devic, P. Horcajada, C. Serre, F. Salles, G. Maurin, B. Moulin, D. Heurtaux, G. Clet, A. Vimont, J.-M. Grenèche, B. Le Ouay, F. Moreau, E. Magnier, Y. Filinchuk, J. Marrot, J.-C. Lavalley, M. Daturi and G. Férey, J. Am. Chem. Soc., 2010, 132, 1127–1136 CrossRef CAS PubMed.
- K. Griffiths, N. R. Halcovitch and J. M. Griffin, Chem. Mater., 2020, 32, 9925–9936 CrossRef CAS.
- M. Xie, N. Prasetya and B. P. Ladewig, Inorg. Chem. Commun., 2019, 108, 107512 CrossRef CAS.
- H. Hahm, K. Yoo, H. Ha and M. Kim, Inorg. Chem., 2016, 55, 7576–7581 CrossRef CAS PubMed.
- S. Henke, A. Schneemann, A. Wütscher and R. A. Fischer, J. Am. Chem. Soc., 2012, 134, 9464–9474 CrossRef CAS PubMed.
- N. C. Burtch and K. S. Walton, Acc. Chem. Res., 2015, 48, 2850–2857 CrossRef CAS PubMed.
- S. Klokic, D. Naumenko, B. Marmiroli, F. Carraro, M. Linares-Moreau, S. D. Zilio, G. Birarda, R. Kargl, P. Falcaro and H. Amenitsch, Chem. Sci., 2022, 13, 11869–11877 RSC.
- M. Linares-Moreau, L. A. Brandner, T. Kamencek, S. Klokic, F. Carraro, K. Okada, M. Takahashi, E. Zojer, C. J. Doonan and P. Falcaro, Adv. Mater. Interfaces, 2021, 8, 2101039 CrossRef CAS.
- P. Falcaro, K. Okada, T. Hara, K. Ikigaki, Y. Tokudome, A. W. Thornton, A. J. Hill, T. Williams, C. Doonan and M. Takahashi, Nat. Mater., 2017, 16, 342–348 CrossRef CAS PubMed.
- N. Novendra, J. M. Marrett, A. D. Katsenis, H. M. Titi, M. Arhangelskis, T. Friščić and A. Navrotsky, J. Am. Chem. Soc., 2020, 142, 21720–21729 CrossRef CAS PubMed.
- D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033–5036 CrossRef CAS PubMed.
- T. O. Erinosho, D. M. Collins, A. J. Wilkinson, R. I. Todd and F. Dunne, Int. J. Plast., 2016, 83, 1–18 CrossRef CAS.
- T. Haraguchi, K. Otsubo and H. Kitagawa, Eur. J. Inorg. Chem., 2018, 1697–1706 CrossRef CAS.
- H. Aggarwal, P. M. Bhatt, C. X. Bezuidenhout and L. J. Barbour, J. Am. Chem. Soc., 2014, 136, 3776–3779 CrossRef CAS PubMed.
- J. D. Webb, H. H. Neidlinger and J. S. Connolly, Polym. Chem., 1986, 7, 503–515 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ce00221k |
| This journal is © The Royal Society of Chemistry 2024 |