Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
211At on gold nanoparticles for targeted radionuclide therapy application
Jeffrey
Tanudji
 a,
Hideaki
Kasai
a,
Hideaki
Kasai
 *b,
Michio
Okada
*b,
Michio
Okada
 bc,
Tetsuo
Ogawa
bd,
Susan M.
Aspera
bc,
Tetsuo
Ogawa
bd,
Susan M.
Aspera
 e and
Hiroshi
Nakanishi
e and
Hiroshi
Nakanishi
 f
f
aDepartment of Applied Physics, The University of Osaka, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
bInstitute of Radiation Sciences, The University of Osaka, 1-1 Machikaneyama-cho, Toyonaka, Osaka 560-0043, Japan. E-mail: kasai@dyn.ap.eng.osaka-u.ac.jp
cDepartment of Chemistry, The University of Osaka, 1-1 Machikaneyama-cho, Toyonaka, Osaka 560-0043, Japan
dDepartment of Physics, The University of Osaka, 1-1 Machikaneyama-cho, Toyonaka, Osaka 560-0043, Japan
eResearch Initiative for Supra-Materials, Shinshu University, 4-17-1 Wakasato, Nagano, Nagano 380-8553, Japan
fNational Institute of Technology, Akashi College, 679-3 Nishioka, Uozumi-cho, Akashi, Hyogo 674-8501, Japan
First published on 3rd April 2024
Abstract
Targeted alpha therapy (TAT) is a methodology that is being developed as a promising cancer treatment using the α-particle decay of radionuclides. This technique involves the use of heavy radioactive elements being placed near the cancer target area to cause maximum damage to the cancer cells while minimizing the damage to healthy cells. Using gold nanoparticles (AuNPs) as carriers, a more effective therapy methodology may be realized. AuNPs can be good candidates for transporting these radionuclides to the vicinity of the cancer cells since they can be labeled not just with the radionuclides, but also a host of other proteins and ligands to target these cells and serve as additional treatment options. Research has shown that astatine and iodine are capable of adsorbing onto the surface of gold, creating a covalent bond that is quite stable for use in experiments. However, there are still many challenges that lie ahead in this area, whether they be theoretical, experimental, and even in real-life applications. This review will cover some of the major developments, as well as the current state of technology, and the problems that need to be tackled as this research topic moves along to maturity. The hope is that with more workers joining the field, we can make a positive impact on society, in addition to bringing improvement and more knowledge to science.
1. Introduction
1.1. Radiotherapy
Radiotherapy is a method of treating cancer by using radiation to kill cancer cells and other tumors.1 There are various types of radiotherapy but all of them generally fall into two categories: external treatment and internal treatment. External treatment involves utilizing an external radiation source (such as gamma-rays) to provide a concentrated dosage to the target areas. Internal treatment, also known as brachytherapy, involves placing the radioactive source near to the areas to be treated. This method allows for continuous therapy instead of in sessions which are prevalent in the external treatment variant.Regardless of the type of radiotherapy, all methods will use some sort of particle to provide treatment into the cancer cells. For external beam radiation therapy (EBRT), it uses either collimated X-ray or gamma-rays, electrons, or protons from outside sources to be fired into the affected areas.2 A recent iteration, known as heavy ion therapy, uses heavy ions in lieu of electrons or protons for treatment.3,4 The advantage of this method being that no operation is needed, which could complicate the health of the patients. Additionally, with the advancement of machinery, the target cells could be identified to great accuracy, allowing for a more accurate dosage to be delivered.
On the other hand, brachytherapy uses radioactive materials and implants them in a sealed container nearby the target cells. This procedure is useful for specific cancers, such as breast cancer or prostate cancer, where it is advantageous to apply a higher dosage to a concentrated area. The two treatments can be combined: by using the EBRT to target large cancer masses and brachytherapy to deliver doses to smaller cancer areas, it is possible to improve the efficacy of the overall treatments.
Another variant of the internal treatment is the radionuclide therapy or the unsealed source radiotherapy. It uses chemical and biological compounds to bind to the cancer cells or uses the tendency of the body to absorb it into the system, and thus is a form of targeted radiotherapy. An early example is the use of radioactive iodine (131I) to treat thyroid cancers.5 Since the thyroid will naturally absorb iodine for its own regulation, when 131I is ingested, the thyroid will absorb the radioactive iodine and the treatment will run its course.
1.2. Why use astatine-211/211At?
A similar treatment, targeted alpha therapy (TAT), is a new method of cancer treatment that is gaining traction in the medical world.6–10 It uses the radioactive decay of heavy elements as a source of α-radiation to treat cancer cells inside the body.11–13 Since α particles have short ranges but particularly large energies, this treatment shows promise for smaller cancers and metastatic cancers which are traditionally difficult to treat.13 Astatine-211 (211At) is a promising radionuclide candidate precisely due to its ability to release α particles during its decay.14 Its decay scheme is such that one 211At will result in one α particle, which is desirable due to the above-mentioned properties. There are several isotopes of astatine, but all of them are radioactive and have relatively short half-lives (<10 minutes), and only 211At is deemed feasible for use as an α-radiation source.15 Additionally, lower mass isotopes do not have the certainty of releasing α particles during their decay and larger mass isotopes mostly decay almost instantaneously.Due to the number of possible radionuclides that can be used for TAT, and the corresponding benefits and issues, this review will focus more on the use of 211At as a radionuclide, with other radionuclides being introduced whenever appropriate.
1.3. Gold nanoparticles as carriers in drug delivery systems
The current state of such treatment incorporates targeting compounds that will seek out and adsorb onto cancer-specific receptors. This means that if the radionuclide can be attached to the compound, it will be directed towards the cancer cells and be able to disrupt the growth of the cancer cells while keeping the healthy cells unharmed.The use of targeting compounds allows for the targeting radiotherapy to be used more in cancer treatments. Cancer cells have their own different receptors, meaning that a specific compound can be developed to bind to those receptors.11,16 An advantage of using a carrier is the increased amount of radionuclides that the carrier would be able to hold, thereby increasing the dosage to the targeted area. Fig. 1 shows a simple schematic of a comparison between two cases of targeted radionuclide therapy. The left side shows a traditional method with one radionuclide attaching to a targeting compound, while the right side shows the radionuclides being adsorbed on the AuNPs that have targeting compounds attached. From here, it is easy to see that the use of AuNPs would allow for a greater number of radionuclides to provide treatment to the cancer mass. Additionally, the carriers can also be appended with other drugs or imaging agents so that they would act in concert with the radionuclides to deliver an effective treatment. Finally, flexibility in synthesizing these nanoparticles (NPs) in terms of size and shape can be achieved, primarily from synthesis techniques covered in later sections. Smaller NPs have a high surface area to volume ratio, which allows for a higher number of particles for the same mass. Surfaces are crucial for any reaction since this is where all reaction processes take place. The atoms on the surface levels are also more reactive due to them having a lower coordination number.
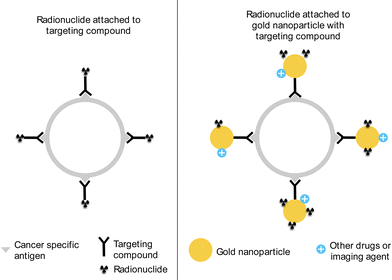 | ||
| Fig. 1 A simplified schematic of targeted radionuclide therapy with (left) radionuclide with targeting compound and (right) radionuclide on AuNP with targeting compound. | ||
Many studies have looked at gold being used in many biomedical applications due to its biocompatibility.17–22 This property makes AuNPs a good candidate to be used in vivo as carriers for TAT radionuclides. Additionally, there have been experimental studies performed that showed AuNPs being capable of acting as carriers for astatine.23–26
Aside from its biocompatibility, there are several advantages to using AuNPs for in vitro treatments: among those are surface plasmon resonance (SPR), causing biological radiosensitization to targeted cells, catalyzing the production of reactive oxygen species (ROS), as well as its ability to be labeled with other materials.10,18,27,28 SPR is a phenomenon that transfers light energy into electron movement, and thereby heat. This allows AuNPs to release concentrated amounts of heat into specified target areas, which damage the surrounding cancer cells. The ability of AuNPs to cause radiosensitization to cancer cells has also been documented.29–31 By having AuNPs nearby these cells, beam therapies such as X-ray can be tuned to a minimum to avoid damage to healthy cells while at the same time increasing the effect on the targeted areas. Finally, the production of ROS, such as singlet oxygen or superoxide ions, increases biological stress in the cancer cells.32,33 This oxidative stress can cause the cell's division mechanism to fail or damage the cell in such a way that the affected cell goes into a programmed cell death mode, killing the cell and stopping its proliferation.32,34–36 There are various works that look at generating or supplementing the production of ROS due to SPR, showing the multidimensional aspect of the utilization of AuNP.37–39 Additional works have also been included here to provide interested readers with the mechanism of ROS stressing biological systems, as well as the mechanism for dioxygen (O2) activation.40–43
2. Current state of radiotherapy
2.1. Production capability
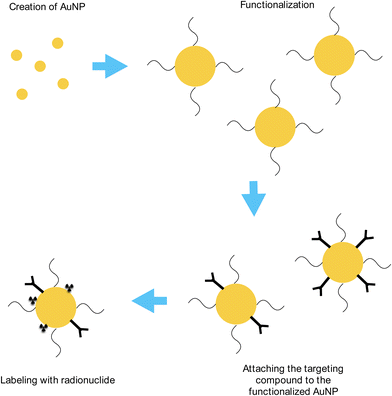
In order to use this type of treatment, both 211At and AuNPs would have to be made, and made consistently. A generalized and simplified diagram has been made to illustrate the process until it is ready to be used in situ, shown in Fig. 2. The current technology has to mature in order for trials, and eventually treatment, to be performed in patients reliably. One of the limiting factors in this treatment is the short half-life of 211At; at 7.2 hours, the production facility has to be close to the treatment centers (such as hospitals or specially designed radioactive wards) in order for the process to retain as much radioactive astatine as possible.13–15,23–26 As a result, other methods are being developed to extend the time ranges to take into consideration transportation issues as well as possibly short-term storage.| 209Bi(4He,2n)211At | (1) |
There are several facilities that can produce this, with the majority being located either in the United States or Japan. Trials involving human patients have been few and far between, and there is currently an effort in trying to determine the maximum tolerated dose of such treatment, as well as other side effects that could come about due to the application in patients.46
The University of Osaka's RCNP has cyclotrons capable of firing the beam at the aforementioned energy with up to 7 μA of beam current to produce a maximum of 500 MBq of 211At.47,48 Since α particles have a 2+ charge, the beam current is effectively a measure of how many charged particles are being fired into the target. The energy required for producing 211At varies due to the beam current being used, as well as the time that the production stays online. A rough calculation indicates the surface power density of the beam can be as high as 8 times the power density of an electric kettle (approximately 33 W cm−2vs. 4 W cm−2).
Following this, the product 211At has to be removed from the 209Bi slab via some treatment. There are two methods of separation: wet and dry.49–54 The wet separation method uses acid (i.e., HNO3 or HCl) to dissolve the solution and another compound to extract the 211At. The dry separation on the other hand heats the irradiated bismuth slab until the 211At boils off. The resulting gas is then cooled down before being added into a solution for use. Research is also underway on other methods, which have the potential to increase the yield of 211At.55,56
Another method to obtain 211At is by irradiating 209Bi with heavy-ions such as Li to create 211Rn, with the equations given as follows
| 209Bi(7Li,5n)211Rn | (2) |
| 209Bi(6Li,4n)211Rn | (3) |
The purpose of this alternative method is to both increase the production of 211At and offset the short lifetime of 211At for transportation since 211Rn has a longer half-life of 14.6 hours.14,57–59 It has been calculated that there is upwards of 80% 211At activity even after 24 hours due to this 211Rn/211At generator, providing a wider range of distribution of the radionuclide.
One of the most straightforward methods to synthesize AuNPs is by reducing chloroauric acid (HAuCl4) with reducing agents. The result is Au nanospheres of different sizes depending on the ratio of the acid and the reducing agent. The Turkevich–Frens method produces relatively uniform sized Au nanospheres around 10–20 nm in diameter by reducing HAuCl4 with sodium citrate solution, with bigger NPs resulting from a smaller citrate/acid ratio.18,66,67 The sodium citrate solution acts both as a reducing agent and a capping agent, which stops the growth of the Au nanospheres.67,68 A larger amount of citrate solution yields smaller NPs, and vice versa.
Another method to synthesize AuNPs is by using humic substances, such as fulvic acid and humic acid, as a reducer and stabilizer for the creation of AuNPs.69–71 The resulting AuNP sizes range from <10 nm to >100 nm in diameter and have been found to be stable. An additional property of this method is the ability to produce silver nanoparticles; the interested reader may want to look at these works, as well as the references contained therein.71–74
A third method is the Brust–Schriffin method, which uses sodium borohydride as a reducing agent and organic liquids (such as toluene) as anti-coagulants.75,76 In this method, the resulting Au nanospheres are <10 nm in diameter which are highly stable and thiol functionalized.
Functionalization is a process of binding some ligands or molecules to the NP, allowing it to have a broader application in the medical field.77–82 Standard NPs tend to agglomerate, which makes them useless at being created in the first place. By enveloping the surface of the NP with other compounds, it allows the AuNP to stay separated, as well as being able to bind to compounds such as targeting compounds, drugs, etc. to be used in the medical society. There are several methods for functionalization, including DNA, peptides, and polyethylene glycol (PEG).79–82
Since the body's own reticuloendothelial system (RES) will generally remove any injected NPs, a “covering” substance will be required for those nanoparticles in order to reach the target areas.83–86 One of these substances is PEG, an inert polymer that is widely used in medicine to allow for the NPs to bypass the RES recognition, and therefore stay in the bloodstream for a longer period.83–88 Additionally, PEG has been found to improve biocompatibility, as well as cellular uptake of the NPs that the PEG is attached to.
Following synthesis, verifications can be done in several ways, usually by Transmission Electron Microscopy (TEM) imaging or by absorption spectra.89–91 TEM imaging is easier to understand since the size and 2D shape is visible in the image. The absorption spectroscopy method measures the absorption characteristics of ultraviolet-visible light after passing through the sample. The amount of light at a specific wavelength that is absorbed corresponds to the size of the AuNP found in the sample. Calculations can also then be performed to provide information on the mass concentration of the AuNP as well as particle concentration in the solution.
For gold nanorods, one of the ways to produce them is by using the seed mediated growth method.92,93 Instead of an aggregation of gold atoms to form a sphere-like structure, this method utilizes an initial seed to grow the NP. The advantage of this method is the ability to control the size and shape of the AuNPs grown, partly depending on the initial size and shape of the seed.93 This allows for gold nanorods to be developed at high aspect ratios (i.e., high length to width ratio) which is otherwise difficult to obtain using the previous methods.
Gold nanoshells can be produced using several methods; but since they are effectively thin shelled gold nanospheres, the process first requires seedlings to start the growing process. The main types of gold nanoshell are core or hollow. Core gold nanoshells have solid cores in the middle, where the gold will attach and grow, while hollow gold nanoshells are just gold shells with nothing inside them. Several cores that can be used in creating gold nanoshells are silica, iron oxides, and polymers.94–96 Hollow gold nanoshells can be made by growing the gold in the acid solution on the silver nanoparticle, by replacing the silver atoms with gold ions.95–97
These different types of AuNPs can be used in various treatment or experiments depending on the goal of such applications. To provide even more choice, the AuNPs’ size and shapes can have an effect on their end uses. The reader is encouraged to look at review works, such as the ones listed in the reference, for a more comprehensive understanding.98–102
2.2. Biological trials
Since TAT is a promising concept, it is constantly being developed for various treatments, both experimentally and clinically.8,46,103–105 Experiments fall into two categories: in vitro and in vivo. In vitro experiments use cancer cells and expose them to treatment for a given amount of time to see the effect of the different variables, such as choice of targeting compound, concentration of 211At-labeled AuNP, size of AuNP, etc.24,26,105 These variables are sometimes permuted to obtain an optimized condition for such treatment.The setup for the experiment is similar for all the following trials: two sets of groups are prepared. One group is designated the control and is assumed to have 100% growth rate. The other group is divided into several treatment regimes, treated with different solutions containing the 211At–AuNP. Each treatment regime may have different variables being altered and is administered once to test their efficacies on the cellular material and the results are compared to the control group that received no treatment. The measurement used is the half maximal inhibition concentration or IC50. The data is plotted to provide a relationship between the concentration of 211At–AuNP versus the inhibition value. A smaller concentration value to achieve IC50 indicates the treatment is highly effective while a larger concentration value means the treatment is less or not as effective.
In vivo experiments require the use of live-animal testing, such as rats or mice that are implanted with cancer cells. Treatments are then administered based on the variables being tested. The goal is the same: to confirm their efficacy at curing cancers or at least controlling their growth.24,26,104,105 As can be seen in many works, a control group is set up and provided with a non-radiating solution (e.g. saline) and compared to the group that received treatments. These experiments are performed first to ensure 211At–AuNP's preliminary safety before testing them in human patients.
There are two main methods of delivery: direct injection of radionuclide labeled AuNP to the cancer masses as well as through intravenous injection.24,26 The first is a direct application to provide proof of concept that the AuNP can hold the radionuclides in place, as well as for the radionuclide to show its effect on the cancer cells. The second is much more interesting, since it will make real-life applications much easier to perform.
2.3. Experimental and theoretical works
There have been theoretical works that have embarked on understanding both the physics of the adsorption of radionuclides to AuNP as well as obtaining a more accurate model for further simulation use. Since astatine is a relatively newly discovered element, there are some works that look at the bonding characteristics of astatine, specifically at the halogen bonding of astatine and other halogens.106–109 Due to the importance of 211At as a future medical substance, more experiments are being carried out.23–26 One such result is obtained from experimental study by Huang et al., who found that even with a relatively small cumulative adsorption, the effect of targeted radiotherapy is controlling the growth of the tumor (see Fig. 3).26 This result is mirrored by another in vivo study that showed the efficacy of such methodology in rats, whereby the presence of 211At–AuNP of any size has a positive impact in reducing the growth of cancerous mass (see Fig. 4).25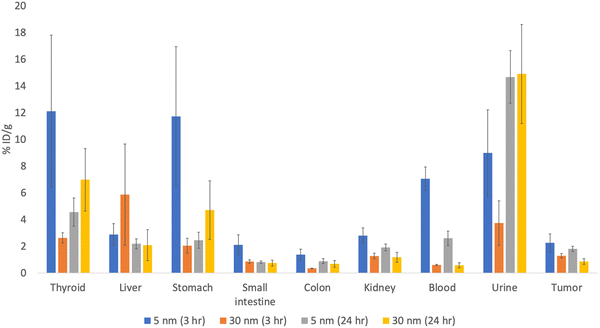 | ||
| Fig. 3 Percentage of 211At–AuNP adsorbed per gram of organ weight, adapted from data found in ref. 27. The blue and orange bars represent 211At-labeled 5 nm and 30 nm AuNPs, respectively, accumulated for 3 hours, whereas the gray and yellow bars represent 211At-labeled 5 nm and 30 nm AuNPs, respectively, accumulated for 24 hours. The %ID per g stands for the percentage of accumulated radioactive materials per weight of the organ/liquid; a higher value means more of the 211At–AuNPs are found in that system. Error bars are shown as black bounded vertical lines. | ||
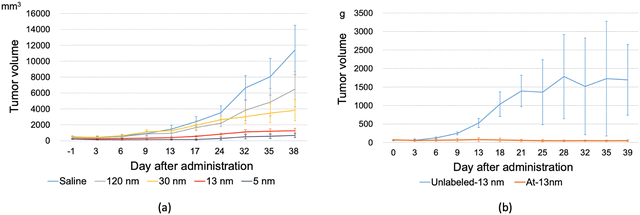 | ||
| Fig. 4 An abridged version of in vivo data for 211At-labeled AuNP of various diameters.26 It can be seen that the presence of 211At-labeled AuNP of any size contributes to limiting the growth of cancer cells. (a) The tumor size growth after administration of different sized 211At-labeled AuNP and (b) a comparison of the effect of 211At on the tumor size growth. | ||
With the promise of TAT treatments being developed in the medical field, the study turns into both finding more effective designs for its application, as well as elucidating the underlying physics that govern the process. The hope is for a better model to be developed before production begins. Due to the highly complex nature of the system, theoretical calculations can only cover a very small scope, such as the adsorption of the radionuclides to the gold surface.
Surface chemistry is crucial since any reaction between AuNPs and radionuclides occur at the surface level. The inside of the NP, termed as the bulk, do not take part in the adsorption process. Additionally, the lower coordination number of surface atoms make them more suited to reacting with other species. The issue comes when we decide how large the NP is. For calculation purposes, NPs, in particular nanospheres, of diameter circa 2.7 nm or above can be treated as a surface facet.110,111 While the majority of the NPs created have diameters above 5 nm, newer findings and control mechanism can produce NPs of 2.5 nm diameter, meaning that finite sized effects will become apparent when calculating systems based on these small sized NPs.
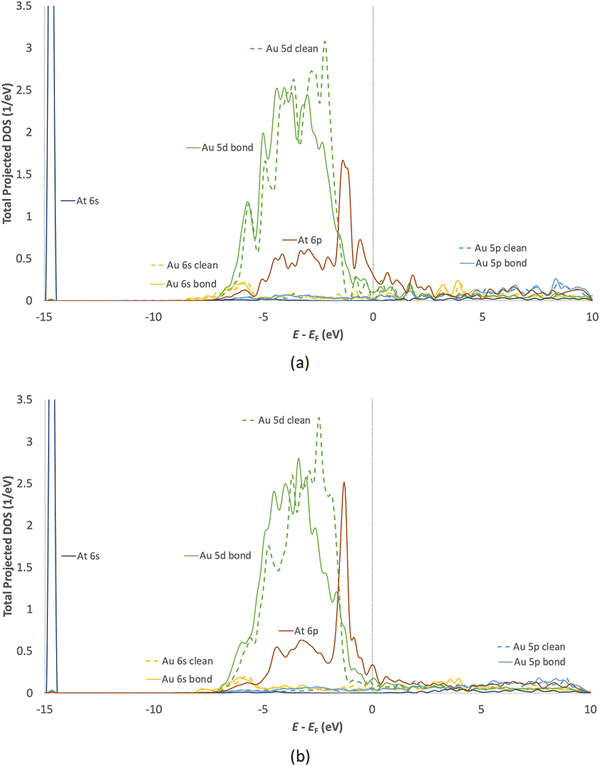
Owing to its nature as a halogen, a comparison between iodine adsorption and astatine adsorption seems fitting. Iodine is relatively well studied and is being used in its capacity as a radionuclide. It was found that covalent bonding is the primary method of adsorption of both iodine and astatine.113,114 Both iodine and astatine were found to adsorb on the fcc hollow site of the flat Au(111) surface and the edge-bridge site of Au(211) (adsorption of astatine on gold slab shown in Fig. 5), meaning that both adsorption profiles should be very similar. Additionally, the density of states (DOS) shows the 5d orbital of gold hybridizing with the 5p orbital and 6p orbital of iodine and astatine respectively, as well as the s orbitals of both adsorbates. Fig. 6 shows the local DOS of the adsorbed astatine on the flat Au(111) surface (top) as well as the stepped Au(211) surface (bottom). Comparison between the clean and bond DOS shows the effect of adsorption of At on the Au atom mainly on the large energy band of −4.5 eV to −2 eV. This is the region in which the majority of the 5d gold orbital is overlapping with the 6p orbital of the astatine, and hybridization is visible when looking at the similar peaks between the two DOS of the highlighted orbitals.
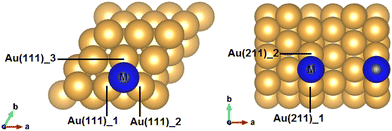 | ||
| Fig. 5 Most stable adsorption sites of a flat Au(111) and stepped Au(211).91 | ||
However, when charge transfers are analyzed, iodine is clearly an electron recipient while astatine is only weakly affected.113 This is an interesting finding since astatine falls into the halogen group in the periodic table, and halogens typically have strong electron affinity as they are one electron away from a complete shell. From the standpoint of electronegativity, astatine is less electronegative than Au, meaning that it is more likely for astatine to donate its electrons to Au during bonding.120,121 However, the amount of electrons transferred is not large since they bond covalently.
So far, theoretical calculations have corroborated the adsorption strength of astatine on Au obtained from experiment, which is a good indication that the system being tested is of sufficient similarity.113,114,121 The simple equation for adsorption energies can be obtained by
| Eads = EM/Au(X) − (0.5 × EM2 + EAu(X)) | (4) |
Since one NP is much larger than an atom, it is fair to assume that there could be more than one radionuclide being adsorbed on its surface. A 2.5 nm diameter AuNP would consist of approximately 500 Au atoms, which could allow several radionuclides adsorbing on its surface. However, as will be explained in the next section, the actual application may not be close to the limit of adsorption due to many factors such as the binding of targeting compounds, functionalization, and the amount of radionuclides in the solution, etc.
3. Issues to be tackled
3.1. Increase in production of 211At
With the use of heavy elements for treatment, there are various issues that spring up, such as logistics, safety, ethical aspects in its use for patients, etc. One of the primary issues is the limited production of 211At. There are currently very few facilities in the world capable of producing 211At for use.46,47One problem that needs to be solved in order to increase production is the amount of beam current. As the minimum necessary energy to make 211At is 28 MeV, it falls to the beam current to increase its production. Since the beam current is proportional to the amount of α particles (He2+), the higher the beam current, the more α particles are being sent to the 209Bi target. A low beam current would yield a small amount of 211At, and, since 211At itself is radioactive, will result in an even smaller usable product.
Another problem is the saturation limit for each machine synthesizing the 211At. The saturation limit is a point whereby the rate of 211At being produced is equal to the rate of decay of 211At and is normally reached around 3 times the time for its half-life (i.e., 21 hours).48 This means that any production batch should not exceed the time limit lest energy is just being used to replenish the decaying radionuclide.
Additionally, engineering aspects must also be taken into consideration due to the heat that the process generates. As alluded to previously, the surface power density of the α beam is very high and causes overheating of the target easily. Currently, the beam is either oscillated on top of the bismuth target or being reduced by adjusting the slits to remove certain areas of the beam.47,48 More efficient and larger cooling systems must be developed so that as the beam current increases to accommodate more production capacity, such that the target bismuth does not overheat and melt in the process.
Another consideration is the chemical treatment to obtain the product from its stand/hold. The process takes time and causes a percentage of the 211At to undergo decay. Depending on the time elapsed, this treatment can yield 30% to 80% usable 211At, which necessitates an increase in production capability so that the required amount can be sent to testing. This is a classic “chicken-and-egg” problem that seems to be difficult to solve, unless both production capability and a more efficient and faster process can be found. Currently, developments are taking place on both fronts and hopefully, this issue can be remedied in the next few years.48,55,56
3.2. Use in human patients
Currently, there are ongoing clinical trials with 211At but none with AuNP as the carrier.46 There are several hurdles regarding this point, one of them being the supply of 211At. Another issue is the myriad of targeting compounds that can be used.A targeting compound, as mentioned previously, allows the 211At-labeled AuNPs to be directed towards the cancer cells and perform their treatment there. Unfortunately, different cancer cells can have different overexpressed genes (such as antigens), which makes the choice of a suitable targeting compound quite difficult.122 A specific targeting compound may also be targeting multiple different types of cancer cells, which may cause the treatment to go to a different cancer cell. While this may not be a bad thing if the patient's cancer is already at a high stadium level (meaning that the cancer has already spread to other parts of the body), but it may not be ideal if the treatment administered is focused on a particular target area. Preliminary gene tests would need to be done to determine which proteins or molecules can act as the targeting compound, and this further adds complexities to the procedure.
As one of the experiments found, there is an increase of the AuNP injected in the liver and stomach of the experimental subjects.26 This could be due to the liver breaking down red blood cells as they near their useful timespan. Even though these tests are done in mice, there are possibilities that the same result may arise when tested in humans, which raises the concern that the treatment may irradiate the healthy organs instead of solely going to the target tumor. While the tests show no damage of those organs by the treatment, it remains to be seen whether the human biology will display the same effect.
Another improvement is the small amount of radionuclides that was found in/near the tumor area. As can be seen in Fig. 3, roughly 2% of injected dose per gram of tumor was measured, compared to the large percentage measured in the liver.26 This is further exacerbated by the larger percentage of radionuclides that is expelled through urine. Given that this small amount of radiation is giving a positive outcome for controlling the cancer growth, the actual amount of radioactivity needed may be much smaller than what was injected into the test subject. This “inefficiency” can vastly increase the necessary amount of 211At needed for the treatment, creating unnecessary strain on both the patient and the production of the radionuclide. It is worth noting, however, that the experiment was done using passive targeting; other future works are looking at active targeting mechanisms to gauge their effectiveness as well as improve the efficiency of the 211At–AuNP treatment.105
Finally, there is the issue of cost. At the time of writing, while the cost of gold is relatively high, it is dwarfed by the cost of 211At production, which can be 50 times the cost of the gold being used.105 Part of this problem comes back to the supply, as well as the production being quite sensitive to the cost of electricity. For such costs to be born, patients will expect that the treatment be very effective and safe to them.
3.3. Gold nanoparticles as carriers
As we have covered before, the use of AuNP as carriers for these radionuclides also provide a source of issues that needs to be understood more clearly. One is the consideration of weight, as Au itself is a heavy element, meaning a small amount of Au atoms can end up having a large mass, which makes it unwieldy for intravenous transport. Another issue that was raised is that in experiments, AuNPs of around a few nanometers would exhibit a phenomenon of “drooping”, whereby the cancer receptors are unable to hold the AuNP straight but instead the AuNP would descend to the cancer cell due to its weight.123At a glance, the solution seems to be to use smaller NPs for testing and also eventually for treatment. However, making smaller NPs would require a higher degree of accuracy and control of the synthesis. In many of the experiments, there can still be a large deviation of sizes and imperfect shapes of AuNPs that are produced. While technology is improving, a consistent production capability must be achieved in order to solve these issues.
3.4. Radioactive decay products
One of the major issues of using α-particle treatment is the prevalence of heavy isotopes being the parent nuclide. This means that heavy elements such as lead, bismuth, etc. are being produced for every decay process. As we know, lead poses a significant danger to human health, as has been demonstrated by many real-life examples (lead in white paint, tetraethyl lead in leaded petrol, lead contamination of water supply, etc.). For TAT to gain acceptance by the greater public, these concerns must be addressed in such a way that is both scientifically rigorous and transparent so that the patients can form their own opinion whether to follow the TAT or seek other treatment methods.211At is known to decay into two possible daughter isotopes: 207Bi (41.7% probability) and 207Pb (58.2% probability) via different mechanisms.13,128 These two isotopes are relatively stable (207Bi will decay further to 207Pb, with a half-life of 31.22 years)124 and since they are in such small concentrations, experimental results are hard to come by. Bismuth has been found to be relatively nontoxic for living organisms, and the toxic threshold in humans is approximately 15 g versus a toxic threshold of approximately 1 mg for lead.125
Additionally, this decay produces energy, which is transformed mostly into kinetic energy to the two remaining products. The majority of the energy will go into the α-particle which is the crux of the treatment; however, a smaller amount will go to the daughter isotope that may cause the recoil of the said isotope from the AuNP. Depending on the direction of the separation, the daughter isotope may detach from its carrier, bury itself in (or even go through) the gold AuNP, or even be readsorbed on the surface of the AuNP after some event that drains the energy of the daughter isotope.
It is worth noting that since 211At releases only one α-particle during its decay, there are no problems relating to recoiling radioactive daughters as can be found in other chain decay radionuclides.125 Some radionuclides, e.g.225Ac, while capable of releasing multiple α-particles during their decay process, may be harder to control after their detachment from the carriers.126–128 This phenomenon could potentially cause unintended damage to nearby healthy organs or even healthy cells in the cancer mass’ vicinity.
These issues must be taken into consideration as the technology matures enough for clinical trials to be held. Currently, clinical trials for 211At–Na are being performed in Osaka University Hospital for refractory thyroid cancer (i.e. cancer that does not respond to conventional treatment), which can lay the groundwork for future use of At-labeled AuNP in humans.129
3.5. Theoretical aspects
Modeling accurate systems remains an elusive problem that needs to be tackled. As shown here, the complexities of interdisciplinary issues prevent a full simulation from being carried out. Primarily, the various binding ligands, as well as new drugs being developed are too complex for density functional theory (DFT) based software to run. Molecular dynamics may be helpful in bridging some of the gaps, but there remains the issue of radiation of different types to consider.Additional physical and chemical theories can also be developed from this field. One such example is the filled 4f orbital that astatine has. Lanthanoid contraction, commonly found in the lanthanoid series has generated an interest in the community, due to interesting phenomena that do not occur in lower mass atoms. The presence of 4f electrons has been found to decrease the radius of the atoms with these electrons, making the higher mass atoms more compact than their preceding counterpart.130,131 Additionally, since the lanthanoid contraction affects heavier elements, relativistic effects are also at play, which alter the characteristics of those elements.
Pyykkö has written extensively about the characteristics of gold, including the effect of the relativistic treatment on orbital radial densities.132,133 It was found that around 10% of the lanthanoid contraction could be attributed to relativistic effects.132 Interestingly, while both relativistic effects and lanthanoid contraction would suggest a reduction of bond length for heavy element bonding, not all results point to that conclusion. Rossi et al. found that halogen bonding involving astatine, itself a heavier element than gold, has similar or increasing bond length after considering relativistic effects and spin–orbit coupling.108 Since we are heading into heavier elements, spin–orbit coupling effects will become more pronounced, and can affect the physics and chemistry of the orbitals. Considering these factors and other similar results further highlight the fact that more research needs to be done to verify theoretical results by experiments, which will in turn improve the accuracy of the theoretical model.
Furthermore, due to reasons alluded to earlier, the simulation of heavy elements would require corrections of a relativistic nature to be added into the system. Developments have been made to reduce the computational cost for fully relativistic DFT, and there are also works that have predicted the various characteristics of astatine as well as new compounds that can theoretically be obtained.109,117,130,134–136 However, as of now, it is still very difficult, due to the lack of supply, for some of these results to be verified experimentally. Interested readers may want to look at theoretical modeling developments in this area, including in superheavy elements (Z ≥ 104), to become more familiar with them.137–139
Since this problem is not purely of nuclear physics, consideration of the interaction of radioactive materials and surface physics are lacking. Studies on the adsorption coverage on the AuNPs are few, as well as the issue of combining two vastly different energy regimes between the adsorption process (on the order of eV) and the decay process (on the order of MeV).112–114,121,124 There is also the question of modeling multiple adsorptions on AuNPs. Experimental works have reported successful adsorption of 211At on AuNPs, but there are no data on how different concentrations of 211At affect the adsorption strength and stability of the radionuclides adsorbed on the AuNP. A similar theoretical study performed by Fernández and Balbá on multiple NO adsorptions on Au10− and Au9Zn− clusters found that after the first NO molecule is adsorbed, the subsequent adsorptions yield weaker adsorption energies.140 This result means that a trade-off needs to be found where the adsorption strength is optimized with the amount of adsorbates on the surface. Additionally, different sized AuNPs are made up of different facets, which may yield different adsorption strengths and numbers of possible adsorbates.141,142 Obtaining information on these can provide more knowledge on the science behind the adsorption characteristics of the radionuclides on AuNPs.
These issues are just some of the challenges that face this field in advancing the development of both technology and best-practices in the hopes of bringing this treatment to a wider scope of patients. It is crucial that both theoretical and experimental knowledge should work in concert so that the medical community can benefit from this cooperation. With the rise of more advanced techniques and fundamental understanding, we are hopeful that the state of development will mature quickly enough to bring a positive impact to many patients struggling with this illness.
3.6. Future plans for 211At on AuNP
As a developing field, the study of 211At on AuNP has potential to contribute to many fields. Aside from improving the medical knowledge and impact of such methodology, we can learn many things in both theoretical side and experimental best practices. Experimentally, we have learned that there are many areas that await both experimental verifications and optimization of best practices. The complex nature of experiments and clinical trials mean that there could be many correlations between variables that are yet unknown. As more 211At producing facilities come online, there would be more experiments and trials being performed, which can hopefully provide more knowledge on the subject matter.Theoretical advancements are also beneficial for the development of TAT; as we know more about the chemistry and physics of such elements and interactions, we can better model these systems with more confidence. The simulation of 211At-labeled AuNP would be very useful in bridging the gap between theory and experiment, as well as providing a base for future development. Additionally, a more complete theory can arise out of the collective knowledge of modeling and experimental verification which will give us a benchmark on the various variables that may affect the use of the 211At-labeled AuNP.
4. Summary
The development of TAT using 211At on AuNP has a promising future in the medical community. The present technology, along with future developments ensure that this research will continue to grow and make a big impact on the medical field for a long time to come. Interesting findings and challenges have also been brought up here, although these barely scratch the surface of this topic, along with the myriad of real-life problems that each of these discoveries will have to address before they can be utilized.As science becomes more complex, we should work together to develop this field in order to improve our quality of life as well as provide more understanding for our own fields. This is a great area of research to go into since the majority of the work done is of biological nature, and not much thought has gone into answering the fundamental questions that this topic offers. As physicists and chemists, we can contribute to the further development of this field via problems and issues that they face but do not usually consider. In exchange, we learn more about the science behind certain phenomena, as well as provide understanding for future problems that have yet to arise.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are indebted to Profs. W. A. Diño, K. Fukase, H. Kanda, and K. Washiyama, as well as Dr. X. Huang and Ms. E. Hilmayanti of Fukase laboratory in Osaka University for the interesting discussion and helpful suggestions for this review. Additionally, the authors would like to express their gratitude to Prof. H. Kato and Dr. X. Huang for their permissions for us to include their obtained results in this manuscript. Finally, the authors would also like to offer our condolences to one of the authors, T. Ogawa, who passed away during the preparation of this review. J. T. acknowledges the Matsuda Yosahichi Memorial Foundation for Foreign Students and The University of Osaka for the financial support.References
- National Institute of Health, Radiation Therapy to Treat Cancer, https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy (accessed August 2023) Search PubMed.
- W. Link, Principles of Cancer Treatment and Anticancer Drug Development, Springer International Publishing, Switzerland, 2019 Search PubMed.
- C. D. Schlaff, A. Krauze, A. Belard, J. J. O’Connell and K. A. Camphausen, Rad. Oncol., 2014, 9, 88 CrossRef PubMed.
- Y. Jin, J. Li, J. Li, N. Zhang, K. Guo, Q. Zhang, X. Wang and K. Yang, Front. Oncol., 2021, 11, 634913 CrossRef PubMed.
- S. C. Werner, E. H. Quimby and C. Schmidt, Am. J. Med., 1949, 7, 731–740 CrossRef CAS PubMed.
- D. A. Mulford, D. A. Scheinberg and J. G. Jurcic, J. Nucl. Med., 2005, 46, 199S–204S Search PubMed.
- J. Elqvist, S. Frost, J.-P. Pouget and P. Albertsson, Front. Oncol., 2014, 3, 324 Search PubMed.
- Y. Dekempeneer, M. Keyaerts, A. Krasniqi, J. Puttemans, S. Muyldermans, T. Lahoutte, M. D’huyvetter and N. Devoogt, Exp. Opin. Biol. Ther., 2016, 16, 1035–1047 CrossRef CAS PubMed.
- C. Kratochwil, F. Bruchertseifer, F. L. Giesel, M. Weis, F. A. Verburg, F. Mottaghy, K. Kopka, C. Apostolidis, U. Haberkorn and A. Morgenstern, J. Nucl. Med., 2016, 57, 1941–1944 CrossRef CAS PubMed.
- N. Daems, C. Michiels, S. Lucas, S. Baatout and A. Aerts, Nuc. Med. Bio., 2021, 100–101, 61–90 CrossRef CAS PubMed.
- National Research Council and Institute of Medicine Committee on State of the Science of Nuclear Medicine, Advancing Nuclear Medicine Through Innovation, The National Academic Press, Washington, DC, 2007 Search PubMed.
- International Atomic Energy Agency, Report of a Technical Meeting on “Alpha emitting radionuclides and radiopharmaceuticals for therapy, IAEA Headquarters, Vienna, Austria, 2013 Search PubMed.
- S. Poty, L. C. Francesconi, M. R. McDevitt, M. J. Morris and J. S. Lewis, J. Nucl. Med., 2018, 59, 878–884 CrossRef CAS PubMed.
- M. R. Zalutsky and M. Pruszynki, Curr. Radiopharm., 2011, 4, 177–185 CrossRef CAS PubMed.
- G. Audi, O. Bersillon, J. Blachot and A. H. Wapstra, Nucl. Phys. A, 2003, 729, 3–128 CrossRef.
- T. Shi, M. Wang, H. Li, M. Wang, X. Luo, Y. Huang, H.-H. Wang, Z. Nie and S. Yao, Sci. Rep., 2018, 8, 5551 CrossRef PubMed.
- R. Shukla, V. Bansal, M. Chaudhary, A. Basu, R. R. Bhonde and M. Sastry, Langmuir, 2005, 21, 10644–10654 CrossRef CAS PubMed.
- W. Cai, T. Gao, H. Hong and J. Sun, Nanotechnol., Sci. Appl., 2008, 1, 17–32 CrossRef CAS PubMed.
- N. Khlebstov and L. Dykman, Chem. Soc. Rev., 2011, 40, 1647–1671 RSC.
- S. A. Grant, C. S. Spradling, D. N. Grant, D. B. Fox, L. Jimenez, D. A. Grant and R. J. Rone, J. Biomed. Mater. Res., Part A, 2013, 102, 332–339 CrossRef PubMed.
- A. Orlando, M. Colombo, D. Prosperi, F. Corsi, A. Panariti, I. Rivolta, M. Masserini and E. Cazzaniga, J. Nanopart. Res., 2016, 18, 58 CrossRef.
- M. Kus-Liskiewicz, P. Fickers and I. Ben Tahar, Int. J. Mol. Sci., 2021, 22, 10952 CrossRef CAS PubMed.
- L. Dziawer, P. Koźmiński, S. Męczyńska-Wielgosz, M. Pruszyński, M. Łyczko, B. Was, G. Celichowski, J. Grobelny, J. Jastrzębski and A. Bilewicz, RSC Adv., 2017, 7, 41024–41032 RSC.
- H. Kato, X. Huang, Y. Kadonaga, D. Katayama, K. Ooe, A. Shimoyama, K. Kabayama, A. Toyoshima, A. Shinohara, J. Hatazawa and K. Fukase, J. Nanobiotechnol., 2021, 19, 223 CrossRef CAS PubMed.
- E. Sporer, C. B. M. Poulie, S. Lindegren, E. Anneheim, H. Jensen, T. Bäck, P. J. Kempen, A. Kjaer, M. M. Herth and A. I. Jensen, J. Nanotheranostics, 2021, 2, 196–207 CrossRef.
- X. Huang, K. Kaneda-Nakashima, Y. Kadonaga, K. Kabayama, A. Shimoyama, K. Ooe, H. Kato, A. Toyoshima, A. Shinohara, H. Haba, Y. Wang and K. Fukase, Pharmaceutics, 2022, 14, 2705 CrossRef CAS PubMed.
- M. Hu, J. Chen, Z.-Y. Li, L. Au, G. V. Hartland, X. Li, M. Marquez and Y. Xia, Chem. Soc. Rev., 2006, 35, 1084–1094 RSC.
- F. Silva, M. P. Cabral Campello and A. Paulo, Materials, 2021, 14, 4 CrossRef CAS PubMed.
- Y. Chen, J. Yang, S. Fu and J. Wu, Int. J. Nanomed., 2020, 15, 9407–9430 CrossRef CAS PubMed.
- S. Penninckx, A.-C. Heuskin, C. Michiels and S. Lucas, Cancers, 2020, 12, 2021 CrossRef CAS PubMed.
- C. Cunningham, M. de Kock, M. Engelbrecht, X. Miles, J. Slabbert and C. Vandevoorde, Front. Publ. Health, 2021, 9, 699822 CrossRef PubMed.
- T. Ozben, J. Pharm. Sci., 2007, 96, 2181–2196 CrossRef CAS PubMed.
- M. C. Maiuri, E. Zalckvar, A. Kimchi and G. Kroemer, Nat. Rev. Mol. Cell Biol., 2007, 8, 741–752 CrossRef CAS PubMed.
- M. Redza-Dutordoir and D. A. Averill-Bates, Biochim. Biophys. Acta, 2016, 1863, 2977–2992 CrossRef CAS PubMed.
- H. Nakamura and K. Takada, Cancer Sci., 2021, 112, 3945–3952 CrossRef CAS PubMed.
- B. Minaev, in Advances in Chemistry Research, ed. J. C. Taylor, Nova Science Publishers, Hauppauge, New York, 2022, vol. 75, ch. 8 Search PubMed.
- L. Gao, R. Liu, F. Gao, Y. Wang, X. Jiang and X. Gao, ACS Nano, 2014, 8, 7260–7271 CrossRef CAS PubMed.
- W. He, J. Cai, X. Jiang, J.-J. Yin and Q. Meng, Phys. Chem. Chem. Phys., 2018, 20, 16117–16125 RSC.
- F. Hong, C. Tang, Q. Xue, L. Zhao, H. Shi, B. Hu and X. Zhang, Langmuir, 2019, 35, 14833–14839 CrossRef CAS PubMed.
- B. F. Minaev, Russ. Chem. Rev., 2007, 76, 989–1011 CrossRef CAS.
- J. Chaca and J. M. Aran, J. Inflamm. Res., 2020, 13, 1057–1073 CrossRef PubMed.
- H. J. Shields, A. Traa and J. M. Van Raamsdonk, Front. Cell. Dev. Biol., 2021, 9, 628157 CrossRef PubMed.
- K. Murotomi, A. Umeno, M. Shichiri, M. Tanito and Y. Yoshida, Int. J. Mol. Sci., 2023, 24, 2739 CrossRef CAS PubMed.
- R. H. Larsen, B. W. Wieland and M. R. Zalutsky, Appl. Radiat. Isot., 1996, 47, 135–143 CrossRef CAS PubMed.
- K. Nagatsu, K. Minegishi, M. Fukada, H. Suzuki, S. Hasegawa and M.-R. Zhang, Appl. Radiat. Isot., 2014, 94, 363–371 CrossRef CAS PubMed.
- P. Albertsson, T. Bäck, K. Bergmark, A. Hallqvist, M. Johansson, E. Aneheim, S. Lindegren, C. Timperanza, K. Smerud and S. Palm, Front. Med., 2023, 9, 1076210 CrossRef PubMed.
- H. Kanda, M. H. Fukuda, Y. Kon, A. Shinohara, T. Suzuki, N. Takahashi, A. Toyoshima and T. Yorita, Presented in part at the Proceedings of the 40th Annual Conference of the Canadian Nuclear Society/the 45th Annual CNS/CAN Student Conference, Technical Session T2B – Medical Isotopes (Part 2), held virtually, June, 2021.
- H. Kanda, M. Fukuda, T. Yorita, Y. Yasuda, M. Nakao, K. Hatanaka, T. Saito, H. Tamura, S. Morinobu, K. Nagayama, H. Yoshida, S. Ano, D. Tomono, H. Kamano, N. Aoi, T. Shima, E. Ideguchi, S. Ota, N. Kobayashi, T. Furuno, S. Imajo, M. Murata, Y. Yamamoto, T. Suzuki, Y. Kon, Y. Morita, K. Takeda, T. Hara, T. H. Chong, H. Zhao, M. Kittaka, S. Matsui, T. Imura and K. Watanabe, Presented in part at the Proceedings of the 20th Annual Meeting of Particle Accelerator Society of Japan, Funabashi, August, 2023, (in Japanese).
- J. Koziorowski, O. Lebeda and R. Weinreich, Appl. Radiat. Isot., 1999, 50, 527–529 CrossRef CAS.
- S. Lidegren, T. Bäck and H. J. Jensen, Appl. Radiat. Isot., 2001, 55, 157–160 CrossRef PubMed.
- A. T. Yordanov, O. Pozzi, S. Carlin, G. Akabani, B. Wieland and M. R. Zalutsky, J. Radioanal. Nucl. Chem., 2004, 262, 593–599 CrossRef CAS.
- E. R. Balkin, D. K. Hamlin, K. Gagnon, M.-K. Chyan, S. Pal, S. Watanabe and D. S. Wilbur, Appl. Sci., 2013, 3, 636–655 CrossRef.
- C. Ekberg, H. Jensen, S. P. Mezyk, B. J. Mincher and G. Skarnemark, J. Radioanal. Nucl. Chem., 2017, 314, 235–239 CrossRef CAS.
- A. Toyoshima and A. Shinohara, Radioisotopes, 2018, 67, 461 CrossRef CAS (in Japanese).
- D. H. Woen, C. Eiroa-Lledo, A. C. Akin, N. H. Anderson, K. T. Bennett, E. R. Birnbaum, A. V. Blake, M. Brugh, E. Dalodiere, E. F. Dorman, M. G. Ferrier, D. K. Hamlin, S. A. Kozimor, Y. Li, L. M. Lilley, V. Mocko, S. L. Thiemann, D. S. Wilbur and F. D. White, Inorg. Chem., 2020, 59, 6137–6146 CrossRef CAS PubMed.
- J. D. Burns, E. E. Tereshatov, G. Avila, K. J. Glennon, A. Hannaman, K. N. Lofton, L. A. McCann, M. A. McCarthy, L. A. McIntosh, S. J. Schultz, G. C. Tabacaru, A. L. Vonder Haar and S. J. Yennello, Sep. Purif. Technol., 2021, 256, 117794 CrossRef CAS.
- J. P. Greene, J. Nolen and S. Baker, J. Radioanal. Nucl. Chem., 2015, 305, 943–946 CrossRef CAS.
- J. R. Crawford, H. Yang, P. Kunz, D. S. Wilbur, P. Schaffer and T. J. Ruth, Nucl. Med. Biol., 2017, 48, 31–35 CrossRef CAS PubMed.
- Y. Feng and M. R. Zalutsky, Nucl. Med. Biol., 2021, 100–101, 12–23 CrossRef CAS PubMed.
- M. A. Hayat, Colloidal gold: principles, methods, and applications, Academic Press, San Diego, CA, 1989 Search PubMed.
- D. A. Giljohann, D. S. Seferos, W. L. Daniel, M. D. Massich, P. C. Patel and C. A. Mirkin, Angew. Chem., Int. Ed., 2010, 49, 3280–3294 CrossRef CAS PubMed.
- M. Das, K. H. Shim, S. S. A. An and D. K. Yi, Toxicol. Environ. Health Sci., 2012, 3, 193–205 CrossRef.
- J. Fan, Y. Cheng and M. Sun, Chem. Rec., 2020, 20, 1474–1504 CrossRef CAS PubMed.
- X. Hu, Y. Zhang, T. Ding, J. Liu and H. Zhao, Front. Bioeng. Biotechnol., 2020, 8, 990 CrossRef PubMed.
- I. Hammami, N. M. Alabdallah, A. Al Jomaa and M. Kamoun, J. King Saud Univ. Sci., 2021, 33, 101560 CrossRef.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- Z. Niu and Y. Li, Chem. Mater., 2014, 26, 72–83 CrossRef CAS.
- M. L. Machesky, W. O. Andrade and A. W. Rose, Chem. Geol., 1992, 102, 53–71 CrossRef CAS.
- D. S. dos Santos Jr., R. A. Alvarez-Puebla, O. N. Oliveira Jr. and R. F. Aroca, J. Mater. Chem., 2005, 15, 3045–3049 RSC.
- L. M. Ojwang, PhD thesis, Louisiana State University and Agricultural and Mechanical College, 2012.
- V. A. Litvin and B. F. Minaev, Mater. Chem. Phys., 2014, 144, 168–178 CrossRef CAS.
- R. Grillo, A. H. Rosa and L. F. Fraceto, Chemosphere, 2015, 119, 608–619 CrossRef CAS PubMed.
- V. A. Litvin and B. F. Minaev, Spectrochim. Acta, Part A, 2013, 108, 115–122 CrossRef CAS PubMed.
- M. Brust, M. Walker, D. Bethell, D. J. Schriffin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- S. R. K. Perala and S. Kumar, Langmuir, 2013, 29, 9863–9873 CrossRef CAS PubMed.
- S. Corra, U. Lewandowska, E. M. Benetti and H. Wennemers, Angew. Chem., Int. Ed., 2016, 55, 8542–8545 CrossRef CAS PubMed.
- R. Thiruppathi, S. Mishra, M. Ganapathy, P. Padmanabhan and B. Gulyás, Adv. Sci., 2017, 4, 1600279 CrossRef PubMed.
- G. Sanità, B. Carrese and A. Lamberti, Front. Mol. Biosci., 2020, 7, 587012 CrossRef PubMed.
- J. W. Lee, S.-R. Choi and J. H. Heo, ACS Appl. Mater. Interfaces, 2021, 13, 42311–42328 CrossRef CAS PubMed.
- F. Ahmad, M. M. Salem-Bekhit, F. Khan, S. Alshehri, A. Khan, M. M. Ghoneim, H.-F. Wu, E. I. Taha and I. Elbagory, Nanomaterials, 2022, 12, 1333 CrossRef CAS PubMed.
- H. Huang, R. Liu, J. Yang, J. Dai, S. Fan, P. Jiang, Y. Wei and X. Guo, Pharmaceutics, 2023, 15, 1868 CrossRef CAS PubMed.
- A. L. Klibanov, K. Maruyama, V. P. Torchilin and L. Huang, FEBS Lett., 1990, 268, 235–237 CrossRef CAS PubMed.
- R. Gref, Y. Minamitake, M. T. Peracchia, V. Trubetskoy, V. Torchilin and R. Langer, Science, 1994, 263, 1600–1603 CrossRef CAS PubMed.
- G. Zhang, Z. Yang, W. Lu, R. Zhang, Q. Huang, M. Tian, L. Li, D. Liang and C. Li, Biomaterials, 2009, 30, 1928–1936 CrossRef CAS PubMed.
- S. Chen, K. Yang, R. G. Tuguntaev, A. Mozhi, J. Zhang, P. C. Wang and X.-J. Liang, Nanomedicine, 2016, 12, 269–286 CrossRef CAS PubMed.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99A, 28–51 CrossRef PubMed.
- L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng and Q. Wang, Nanoscale, 2021, 13, 10748–10764 RSC.
- W. Shen, H. Zhou, T. Liu, P. Pei, J. Huang and X. Yi, Rad. Med. Protect., 2020, 1, 186–195 CrossRef.
- Q. Yao, X. Yuan, V. Fung, Y. Yu, D. T. Leong, D.-E. Jiang and J. Xie, Nat. Commun., 2017, 8, 297 CrossRef PubMed.
- J. B. Vines, J.-H. Yoon, N.-E. Ryu, D.-J. Lim and H. Park, Front. Chem., 2019, 7, 167 CrossRef CAS PubMed.
- M.-Z. Wei, T.-S. Deng, Q. Zhang, Z. Cheng and S. Li, ACS Omega, 2021, 6, 9188–9195 CrossRef CAS PubMed.
- N. U. Khan, J. Lin, M. R. Younas, X. Liu and L. Shen, Cancer Nanotechnol., 2021, 12, 20 CrossRef CAS.
- Y. Xia, K. D. Gilroy, H.-S. Peng and X. Xia, Angew. Chem., Int. Ed., 2017, 56, 60–95 CrossRef CAS PubMed.
- Y. Sun and Y. Xia, Anal. Chem., 2002, 74, 5297–5305 CrossRef CAS PubMed.
- Y.-C. Wang, E. Rhéume, F. Lesage and A. Kakkar, Molecules, 2018, 23, 2851 CrossRef PubMed.
- R. I. Ruvacalba Ontiveros, J. A. Duarte Moller, A. R. Carrasco Hernandez, H. Esperanza Esparza-Ponce, E. Orrantia Borunda, C. D. Gomez Esparza and J. M. Olivares Ramirez, in Current Topics in Biochemical Engineering, ed. N. Shiomi, Intechopen, London, UK, 2019, ch. 6 Search PubMed.
- A. Albanese, P. S. Tang and W. C. W. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- J. Yue, T. J. Feliciano, W. Li, A. Lee and T. W. Odom, Bioconjugate Chem., 2017, 28, 1791–1800 CrossRef CAS PubMed.
- C. Kinnear, T. L. Moore, L. Rodriguez-Lorenzo, B. Rothen-Rutishauser and A. Petri-Fink, Chem. Rev., 2017, 117, 11476–11521 CrossRef CAS PubMed.
- X. Sun, X. Wang, C. Wang, X. Sun, H. Liu, F. Wang, Y. Cao, S. Wang, X. Lu and C. Huang, Opt. Express, 2022, 30, 6051–6060 CrossRef CAS PubMed.
- A. Jang, A. T. Kendi, G. B. Johnson, T. R. Halfdanarson and O. Sartor, Int. J. Mol. Sci., 2023, 24, 11626 CrossRef CAS PubMed.
- H. Ma, F. Li, G. Shen, L. Pan, W. Liu, R. Liang, T. Lan, Y. Yang, J. Yang, J. Liao and N. Liu, Bioorg. Med. Chem., 2022, 55, 116600 CrossRef CAS PubMed.
- T. Kawabe, K. Kaneda-Nakashima, Y. Shirakami, Y. Kadonaga, K. Ooe, Y. Mang, H. Haba, A. Toyoshima, J. Cardinale, F. L. Giesel, N. Tomiyama and K. Fukase, Eur. J. Nucl. Med. Mol. Imaging, 2023, 50, 849–858 CrossRef PubMed.
- X. Huang and E. Hilmayanti, Private communications, Sep. 2023.
- N. Guo, R. Maurice, D. Teze, J. Graton, J. Champion, G. Montavon and N. Galland, Nat. Chem., 2018, 10, 428–434 CrossRef CAS PubMed.
- F. Zhou, Y. Liu, Z. Wang, T. Lu, Q. Yang, Y. Liu and B. Zheng, Phys. Chem. Chem. Phys., 2019, 21, 15310–15318 RSC.
- E. Rossi, M. De Santis, D. Sorbelli, L. Storchi, L. Belpassi and P. Belanzoni, Phys. Chem. Chem. Phys., 2020, 22, 1897–1910 RSC.
- L. Liu, S. Rahali, R. Maurice, C. Gomez Pech, G. Montavon, J.-Y. Le Questel, J. Graton, J. Champion and N. Galland, Chem. Sci., 2021, 12, 10855–10861 RSC.
- J. Kleis, J. Greeley, N. A. Romero, V. A. Morozov, H. Falsig, A. H. Larsen, J. Lu, J. J. Mortensen, M. Dulak, K. S. Thygesen, J. K. Nørskov and K. W. Jacobsen, Catal. Lett., 2011, 141, 1067–1071 CrossRef CAS.
- H. Kasai and M. C. S. Escaño, Physics of Surface, Interface and Cluster Catalysis, IOP Publishing, Bristol, UK, 2016 Search PubMed.
- Y. Demidov and A. Zaitsevskii, Chem. Phys. Lett., 2018, 691, 126–130 CrossRef CAS.
- J. Tanudji, S. M. Aspera and H. Kasai, e-J. Surf. Sci. Nanotechnol., 2023, 21, 318–323 CrossRef CAS.
- J. Tanudji, S. M. Aspera, H. Kasai, M. Okada, T. Ogawa and H. Nakanishi, e-J. Surf. Sci. Nanotechnol., 2023, 22, 38–45 CrossRef.
- K. Berei and L. Vasáros, in Halides, Pseudo-Halides and Azides: Supplement D: Part 1, ed. S. Patai and Z. Rappoport, John Wiley & Sons, UK, 1983, vol. 1, ch. 10, pp. 405–440 Search PubMed.
- K. Berei and L. Vasáros, in The Chemistry of Halides, Pseudo-Halides and Azides: Supplement D2, ed. S. Patai and Z. Rappoport, John Wiley & Sons, UK, 1995, ch. 14, pp. 787–819 Search PubMed.
- J. Champion, M. Seydou, A. Sabatié-Gogova, E. Renault, G. Montavon and N. Galland, Phys. Chem. Chem. Phys., 2011, 13, 14984–14992 RSC.
- G.-J. Meyer, J. Label. Compd. Radiopharm., 2018, 61, 154–164 CrossRef CAS PubMed.
- K. S. Kostecka, Substantia, 2020, 4, 63–70 Search PubMed.
- D. Leimbach, J. Karls, Y. Guo, R. Ahmed, J. Ballof, L. Bengtsson2, F. B. Pamies, A. Borschevsky, K. Chrysalidis, E. Eliav, D. Fedorov, V. Fedosseev, O. Forstner, N. Galland, R. F. Garcia Ruiz, C. Granados, R. Heinke, K. Johnston, A. Koszorus, U. Köster, M. K. Kristiansson, Y. Liu, B. Marsh, P. Molkanov, L. F. Pašteka, J. Pedro Ramos, E. Renault, M. Reponen, A. Ringvall-Moberg, R. E. Rossel, D. Studer, A. Vernon, J. Warbinek, J. Welander, K. Wendt, S. Wilkins, D. Hanstorp and S. Rothe, Nat. Commun., 2020, 11, 3824 CrossRef CAS PubMed.
- A. Serov, N. Aksenov, G. Bozhikov, R. Eichler, R. Dressler, V. Lebedev, O. Petrushkin, D. Piguet, S. Shishkin, E. Tereshatov, A. Türler, A. Vögele, D. Wittwer and H. W. Gäggeler, Radiochim. Acta, 2011, 99, 593–599 CrossRef CAS.
- J. B. Axelsen, J. Lotem, L. Sachs and E. Domany, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13122–13127 CrossRef PubMed.
- K. Washiyama, private communications, Aug. 2023.
- Table of Nuclides, Nuclear Data Center at KAERI, https://atom.kaeri.re.kr/nuchart/ (accessed October 2023).
- N. Yang and H. Sun, Encyclopedia of Environ. Health, 2011, pp. 414–420 Search PubMed.
- D. A. Scheinberg and M. R. McDevitt, Curr. Radiopharm., 2011, 4, 306–320 CrossRef CAS PubMed.
- M. Thoennessen, The Discovery of Isotopes: A Complete Compilation, Springer International Publishing, Switzerland, 2016 Search PubMed.
- E.-A. Salvanou, D. Stellas, C. Tsoukalas, B. Mavroidi, M. Paravatou-Petsotas, N. Kalogeropoulos, S. Xanthopoulos, F. Denat, G. Laurent, R. Bazzi, S. Roux and P. Bouziotis, Pharmaceutics, 2020, 12, 188 CrossRef CAS PubMed.
- T. Watabe, Gan to Kagaku Ryoho, 2022, 40, 12–16 Search PubMed (in Japanese).
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. Alaydrus, M. Sakaue and H. Kasai, Phys. Chem. Chem. Phys., 2016, 18, 12938–12946 RSC.
- P. Pyykkö, Chem. Rev., 1988, 88, 563–594 CrossRef.
- P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412–4456 CrossRef PubMed.
- L. Cheng, S. Stopkowicz and J. Gauss, Int. J. Quantum Chem., 2014, 114, 1108–1127 CrossRef CAS.
- N. Guo, D.-C. Sergentu, D. Teze, J. Champion, G. Montavon, N. Galland and R. Maurice, Angew. Chem., Int. Ed., 2016, 55, 15369–15372 CrossRef CAS PubMed.
- A. Petrone, D. B. Williams-Young, S. Sun, T. F. Stetina and X. Li, Eur. Phys. J. B, 2018, 91, 169 CrossRef.
- V. Pershina, Radiochim. Acta, 2011, 99, 459–476 CrossRef CAS.
- P. Schwerdtfeger, L. F. Pašteka, A. Punnett and P. O. Bowman, Nucl. Phys. A, 2015, 944, 551–557 CrossRef CAS.
- L. G. Machado de Macedo, C. A. Brito Negrão, R. Mendes de Oliveira, R. Ferreira de Menezes, F. Pirani and R. Gargano, Phys. Chem. Chem. Phys., 2023, 25, 633–645 RSC.
- E. M. Fernández and L. C. Balbás, Phys. Chem. Chem. Phys., 2023, 25, 17176–17185 RSC.
- T. Mori and T. Hegmann, J. Nanopart. Res., 2016, 18, 295 CrossRef PubMed.
- E. S. Varo, R. E. Tankard, J. Kryger-Baggesen, J. Jinschek, S. Helveg, I. Chorkendorff, C. D. Damsgaard and J. Kibsgaard, J. Am. Chem. Soc., 2024, 146, 2015–2023 CrossRef PubMed.
| This journal is © the Owner Societies 2024 |