Intermetallic PdCu3 supported on nanodiamond–graphene for semi-hydrogenation of Phenylacetylene†
Received
16th September 2023
, Accepted 17th November 2023
First published on 17th November 2023
Abstract
The selective hydrogenation of phenylacetylene to styrene, instead of ethylbenzene, holds significant importance in the polymer industry. However, existing heterogeneous catalysts face the challenge of achieving exceptional activity while maintaining high selectivity. Herein, we introduce a novel intermetallic PdCu3 catalyst supported on defective nanodiamond–graphene (ND@G), showcasing not only high selectivity (95%) but also remarkable activity (turnover frequency: 2940 h−1, 6 times higher than that of the commercial Lindlar catalyst). Experimental results and DFT calculations reveal that the exceptional selectivity of the catalyst originates from the unique intermetallic structure of PdCu3 with abundant isolated Pd sites on its surface, which favors the rapid desorption of styrene. Simultaneously, the high activity is attributed to the electron transfer from ND@G to PdCu3, facilitating the reduction in the energy barrier of the rate-determining step for styrene formation.
1. Introduction
The selective hydrogenation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C to C
C to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is a vital industrial process used to purify hydrocarbon streams containing alkenes for the production of high-grade olefins in polymer manufacturing.1–7 A prominent application is seen in the production of polystyrene, where selective hydrogenation is essential to remove phenylacetylene impurities from styrene.8,9 Phenylacetylene impurities are commonly formed during the styrene synthesis process and can adversely affect polymerization catalysts, leading to their deactivation in polystyrene production units.10 Supported Pd catalysts exhibit outstanding efficiency in hydrogenation, attributed to their remarkably low activation energy for H2 dissociation.11–13 However, this impressive hydrogenation capability also renders Pd susceptible to excessive hydrogenation during alkyne hydrogenation, leading to the unwanted formation of alkane byproducts.
C is a vital industrial process used to purify hydrocarbon streams containing alkenes for the production of high-grade olefins in polymer manufacturing.1–7 A prominent application is seen in the production of polystyrene, where selective hydrogenation is essential to remove phenylacetylene impurities from styrene.8,9 Phenylacetylene impurities are commonly formed during the styrene synthesis process and can adversely affect polymerization catalysts, leading to their deactivation in polystyrene production units.10 Supported Pd catalysts exhibit outstanding efficiency in hydrogenation, attributed to their remarkably low activation energy for H2 dissociation.11–13 However, this impressive hydrogenation capability also renders Pd susceptible to excessive hydrogenation during alkyne hydrogenation, leading to the unwanted formation of alkane byproducts.
To this end, the industry has extensively embraced the Lindlar catalyst (Pd/CaCO3 modified by Pb salts), taking advantage of the poisoning effect of Pb on Pd to selectively convert alkynes to olefins.14,15 However, it is essential to note that this catalyst possesses notable drawbacks, such as the high toxicity of Pb and restricted olefins selectivity towards terminal alkynes. As a result, the pursuit of alternative and greener catalysts has become a major focus of development in this field. Notably, several Pb-free catalyst design strategies have been reported to date, demonstrating their effectiveness in achieving highly selective catalysts.16–21 One prominent approach is the site-isolation strategy, achieved through alloying Pd with inactive metals22–25 or creating single Pd atom catalysts.26,27 This strategy effectively inhibits the formation of β-H species and alters adsorption configurations, ultimately leading to enhanced selectivity.28–31 Additionally, catalyst support engineering provides another route for enhancing selectivity. For instance, using nitrogen-containing carbons or polymers as support effectively restrains the over-hydrogenation of monometallic Pd.32–35 Furthermore, mesoporous silica with hexagonal symmetric channels was reported to act as an effective support, leading to enhanced selectivity for monometallic Pd.36,37 It is believed that the confinement of Pd nanoparticles deep within the silica channels restricts reactant accessibility to Pd active sites, contributing to improved selectivity. However, these methods heavily rely on the passivation of Pd for selectivity enhancement, resulting in catalysts that often lack excellent activity and negatively impact the cost-effectiveness of using scarce Pd.38
Here, we report an intermetallic PdCu3 nanocatalyst immobilized on the defective nanodiamond–graphene support for the semi-hydrogenation of phenylacetylene. The resulting catalyst exhibits a remarkable selectivity (95%) at nearly complete conversion of phenylacetylene. This high selectivity arises from the structural benefit of intermetallic PdCu3, which contains an abundance of isolated Pd sites on its surface and thus favors the rapid desorption of the target product styrene. Moreover, our prepared catalyst demonstrates exceptional catalytic activity, with a high turnover frequency (TOF) of 2940 h−1, which is ∼6 times higher than the commercial Lindlar catalyst (490 h−1). Experimental characterizations and theoretical calculations indicate that the significantly enhanced catalytic activity comes from the strong electronic interaction between the nanodiamond–graphene support and intermetallic PdCu3 nanoparticles.
2. Experiments
2.1 Materials and chemicals
Copper dichloride (CuCl2, 99.7%), palladium chloride (PdCl2, 99%), ethanol (C2H5OH, 100%), Hydrochloric acid (HCl, 37%), phenylacetylene and its derivatives (99%) were purchased from Aladdin. Nano-diamond carbon (ND, 99.9%) with an average diameter of 30 nm was from Beijing Grish Hitech Co., and all the chemicals were used as received without further purification. Deionized water (H2O, 18.2 MΩ cm−1) used in all experiments was prepared by passing through an ultra-pure purification system.
2.2 Synthesis of ND@G and OLC supports
The ND powder (1.0 g) was first treated with a 1.0 M HCl at 80 °C for 20 hours to remove metallic contamination. Following thorough drying, the collected ND powder was annealed under an Ar atmosphere at 900 °C for 4 hours, with a temperature ramp of 5 °C, resulting in the formation of the ND@G support. The preparation process of OLC closely parallels that of ND@G, except for the thermal treatment conditions, which involved elevating the temperature to 1100 °C.
2.3 Synthesis of catalysts
In a typical synthesis, 30 mg of carbon support and transition metal salts were dispersed in 40 mL of deionized water. The mixture was vigorously stirred for 12 hours and then dried using rotary evaporation. Subsequently, the resulting powder was annealed under 5 vol% H2/Ar at 700 °C for 4 hours, with a heating rate of 5 °C min−1, followed by natural cooling to room temperature. In the preparation of the PdCu3/OLC and PdCu3/ND@G catalysts, the molar feed ratio of PdCl2 to CuCl2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1, and the Pd loading was kept constant at 2 wt% for all catalysts.
3.1, and the Pd loading was kept constant at 2 wt% for all catalysts.
2.4 Catalytic experiments
The selective hydrogenation of phenylacetylene was conducted in a stainless steel autoclave with a Teflon vessel, utilizing an H2 pressure of 0.4 MPa at 25 °C, and a stirring speed of 1000 rpm. For a typical procedure, 5.3 mg of catalyst, 1.0 mmol of phenylacetylene, and 2 mL of ethanol were placed in the vessel. The autoclave was purged with Ar five times before being filled with H2, and then the reaction mixture was magnetically stirred. The reaction products were monitored and analyzed using a GC-14C gas chromatograph equipped with both a flame ionization detector and a thermal conductivity detector. The selectivity hydrogenation test for phenylacetylene derivatives was performed under the same reaction conditions using the PdCu3/ND@G catalyst. The TOF calculation methods were provided in ESI.†
2.5 Characterizations
XRD analyses were performed using a Japan Rigaku DMax-γA rotation anode X-ray diffractometer equipped with graphite-monochromatized Cu-K radiation (λ = 1.54178 Å). The scan speed was set at 2° min−1, and the 2θ range was from 20 to 90°. For atomic-resolution HAADF-STEM and high-resolution bright-field STEM images, a JEM-ARM 200F atomic resolution analytical microscope operating at 200 kV was used. Low-magnification HAADF-STEM images and EDS elemental mapping images were acquired using the FEI Talos F200X microscope, which was equipped with a Super X-EDS system, also operating at 200 kV.
2.6 Theoretical and computational details
All spin-polarized density-functional theory (DFT) calculations were performed by utilizing the Vienna ab initio simulation package (VASP) codes.39–41 These calculations employed the Perdew–Burke–Ernzerhof exchange–correlation functional (PBE)42,43 and projector augmented wave (PAW) pseudopotentials44 with a kinetic energy cutoff of 500 eV. The convergence criteria for the electronic self-consistent iteration and force were set to 10−4 eV and 0.03 eV Å−1, respectively. To sample the Brillouin zone, a 2 × 2 × 1 Monkhorst–Pack k-point mesh was used. The DFT-D3 method was used for calculations to examine the effect of van der Waals interaction on reaction energetics.45,46
The computational hydrogen electrode model47 was used for the expression of the chemical potentials of protons and electrons at applied potential as in the semi-hydrogenation of phenylacetylene.
The elementary steps of the hydrogenation of phenylacetylene are as follows:48–52
| |  | (1) |
| |  | (2) |
| |  | (3) |
| |  | (4) |
| |  | (5) |
| |  | (6a) |
| | 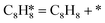 | (6b) |
| |  | (7) |
| | 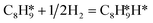 | (8) |
| |  | (9) |
| |  | (10) |
Thus, the Gibbs free energy change for steps 1 to 10 can be expressed as:
| |  | (11) |
| | 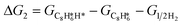 | (12) |
| | 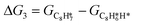 | (13) |
| | 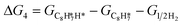 | (14) |
| |  | (15) |
| |  | (16a) |
| | 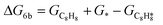 | (16b) |
| |  | (17) |
| | 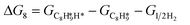 | (18) |
| | 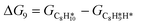 | (19) |
| | 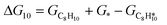 | (20) |
Here, * represents the surface vacancy; detail of correction of Gibbs free energy is expressed as:
| | | G = Edft + ZPE + CpdT − TdS | (21) |
Where
Edft is the reaction energy calculated by DFT, ZPE denotes the changes of zero-point energies, CpdT signifies the contribution of the change in heat capacity of a substance with temperature, and dS is the entropy during the reaction.
3. Results and discussion
The preparation of the ND@G support involves subjecting commercial nanodiamond powders to a high-temperature treatment at 900 °C in an argon atmosphere for 4 h. This annealing condition would induce the transformation of the outermost carbon layers of the nanodiamond into a sp2-hybridized graphene structure while preserving the pristine sp3-bonded texture of the inner core.53 High-resolution transmission electron microscopy (HRTEM) observations (Fig. S1a†) demonstrated the presence of the sp3 core/sp2 shell structure, wherein the shell consists of one or two graphene layers with a low degree of crystallinity. For comparison, another nanodiamond-derived support was prepared through annealing at 1100 °C for 12 h. HRTEM observations (Fig. S1b†) revealed an onion-like carbon morphology in this comparative sample (marked as OLC), indicating that the rigorous annealing treatment promoted the sp3-to-sp2 transition within the inner structure, transcending the limitations associated with surface transformations.12,54 Nitrogen sorption measurements (Fig. S1c and d†) demonstrated a notable increase in both specific surface area and pore volume for OLC (428 m2 g−1 and 0.65 cm3 g−1) compared to ND@G (359 m2 g−1 and 0.54 cm3 g−1), which could be attributed to the sp2-dominated architecture of OLC harboring a greater abundance of defective carbon structures.55
We employed ND@G and OLC as the carbon support to prepare the PdCu3 catalysts, respectively. The synthesis of PdCu3 catalysts involved wet impregnation of the carbon support with PdCl2 and CuCl2, followed by a thermal H2 reduction step. To overcome the kinetic energy barrier associated with alloying and atomic ordering required for the formation of intermetallic structure, the H2 reduction process was carried out at a temperature of 700 °C.56 The as-prepared catalysts were denoted as PdCu3/ND@G and PdCu3/OLC, respectively. XRD patterns revealed a strong match between the diffraction peaks of the prepared catalysts and the intermetallic PtCu3 standard card (Fig. 1a and b), which serves as a confirmation of the successful synthesis of the desired phase. The average particle size of PdCu3 for PdCu3/ND@G and PdCu3/OLC was estimated to be 9 and 13 nm, respectively, using the Debye–Scherrer equation based on the full-width at half-maximum peak at 49.2°.
 |
| | Fig. 1 Structural and compositional characterization of the intermetallic PdCu3 catalysts. (a and b) XRD patterns of PdCu3/ND@G (a) and PdCu3/OLC (b). (c–e) HADDF-STEM images of PdCu3/ND@G; insets in c and e are the corresponding particle size distribution and the FFT pattern. (f) EDS elemental mapping of PdCu3/ND@G. | |
We conducted the high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) to confirm the formation of the intermetallic structure. As shown in Fig. 1c and S2a,† the PdCu3 nanoparticles are homogenously distributed throughout the ND@G and OLC support. Statistical analysis on more than 200 particles from the HAADF-STEM image, revealed an average particle size of 8.3 and 12.4 nm for PdCu3/ND@G and PdCu3/OLC, respectively. Note that these sizes are slightly smaller compared to the XRD result, as XRD tends to overestimate the geometric particle size due to its volume-weighted measurement nature.57 Aberration-corrected HAADF-STEM images revealed a periodic square array of Pd columns (bright dots) surrounded by Cu columns (dark dots) at the edges and corners of each unit cell along the [001] zone axis (Fig. 1d and e and S2b–d†), revealing the Pd atom was “isolated site” in the PdCu3 intermetallic structure. The corresponding fast Fourier transform (FFT) patterns further verified the presence of ordered intermetallic structures (Fig. 1e and S2d† insets). Energy-dispersive X-ray spectroscopy (EDS) elemental mapping confirmed the even dispersion of Pd and Cu within the individual PdCu3 intermetallic particle (Fig. 1f), implying the creation of phase-separation-free alloyed structures.
We conducted X-ray photoelectron spectroscopy (XPS) and temperature-programmed reduction (TPR) measurements to investigate the strong interaction between PdCu3 and ND@G. Analysis of high-resolution XPS spectra (Fig. 2a and b) revealed a significant downward shift in the binding energies of Pd 2d and Cu 2p for PdCu3/ND@G (334.2 eV and 931.3 eV, respectively) compared to PdCu3/OLC (336.1 eV and 933.0 eV), indicating the presence of an evident charge transfer behavior from ND@G to PdCu3 in the PdCu3/ND@G catalyst. This electron-donating capability of ND@G could be associated with the presence of excited electrons in its diamond core; and, the covalent bonds between the outer graphene shell and the diamond core can serve as tunnels for the transfer of excited electrons from the core to the shell, resulting in an increased electron density on the outer graphene shell.58–60 Compared to ND@G, the electron-donating capacity is lacking in the OLC support primarily due to its nearly exclusive composition of sp2-bonded graphene structures throughout the material. TPR profiles in Fig. 3c and d revealed the reduction temperature of monometallic Pd, Cu, and bimetallic Pd–Cu systems supported on ND@G and OLC. Typically, a higher reduction temperature signifies a stronger interaction between the metal species and the support.12,61,62 Comparative analysis showed a significant increase in the reduction temperatures for Pd and Pd–Cu samples supported by ND@G (198 °C and 208 °C, respectively), in comparison to their counterparts supported by OLC (187 °C and 185 °C). However, no discernible differences were observed between the Cu samples supported by OLC and ND@G, suggesting that the charge transfer behavior from ND@G to PdCu3 in the PdCu3/ND@G catalyst may originate from the strong interaction between Pd species and ND@G.63–66 Note that this strong interaction could be responsible for the better size control of PdCu3 nanoparticles achieved with ND@G compared to OLC during high-temperature preparation processes.
 |
| | Fig. 2 Strong electronic interactions. (a and b) High-resolution XPS Pd 3d spectra (a) and Cu 2p spectra (b) of PdCu3/ND@G and PdCu3/OLC. (c and d) TPR profiles of unreduced PdCu3, Pd, and Cu supported on ND@G (c) and OLC (d). | |
 |
| | Fig. 3 Catalytic performance of the catalysts for semi-hydrogenation of phenylacetylene. (a and b) Time-on-stream conversion and selectivity over PdCu3/ND@G (a) and PdCu3/OLC (b). (c) TOFs of PdCu3/ND@G and PdCu3/OLC. (d) Recycling experiments for PdCu3/ND@G. | |
We investigated the catalytic performance of the PdCu3/ND@G and PdCu3/OLC catalysts for the semi-hydrogenation of phenylacetylene to styrene in an autoclave reactor, employing mild conditions (25 °C and 0.4 MPa H2). For comparison, the commercial Lindlar catalyst and the ND@G-supported monometallic Pd catalyst (marked as Pd/ND@G) were evaluated under identical conditions. The time-on-stream conversion and selectivity were displayed in Fig. 3a and b and S3.† PdCu3/ND@G and PdCu3/OLC demonstrated a remarkable selectivity of approximately 95% and 92%, respectively, with the conversion of phenylacetylene nearing 100%; and, no noticeable decrease in selectivity was observed in either case when extending the reaction time. In contrast, the Pd/ND@G catalyst lost almost all its selectivity as the conversion approached 100% (Fig. S3a†). Additionally, we also tested the counterpart intermetallic PdCu/ND@G catalyst, which exhibited significantly lower selectivity compared with PdCu3 catalysts (Fig. S3b†). We attributed the remarkable selectivity of PdCu3/ND@G and PdCu3/OLC to the structural advantages offered by the intermetallic PdCu3 phase. In the case of PdCu3, the (111) surface plays a significant role in catalytic reactions as it is the most exposed surface. On this (111) surface, Pd atoms are individually surrounded by six nearest-neighboring Cu atoms (Fig. S4†). This isolated Pd site was believed to be favorable for adsorbing styrene through a weak π bonding, promoting its desorption, and thereby preventing further hydrogenation reactions.24
Note that PdCu3/OLC exhibited a considerably slower reaction rate compared to PdCu3/ND@G. The former required approximately fourteen times the reaction time (280 min) to achieve a near-complete conversion of phenylacetylene, whereas the latter achieved the same conversion in just 20 min. We quantitatively compared the catalytic activity of PdCu3/OLC and PdCu3/ND@G by calculating apparent turnover frequencies (TOFs) phenylacetylene conversions of 20–40% under kinetic control conditions. As revealed in Fig. 3c, the TOF of PdCu3/ND@G was found to be 2940 h−1, exhibiting nearly 13 times higher than PdCu3/OLC (235 h−1). Moreover, the selectivity and TOF of the commercial Lindlar catalyst were determined to be 79.5% and 490 h−1 (Fig. S3c†), respectively, which are much lower than PdCu3/ND@G. We conducted cyclic tests at a partial conversion level of approximately 50% to assess the stability of PdCu3/ND@G. The results, presented in Fig. 3d, consistently demonstrated that the phenylacetylene conversion remained within the range of 48–53% over four consecutive cycles. Additionally, the catalyst exhibited a nearly constant and remarkable selectivity towards styrene (>98%) throughout all cycles, showcasing its exceptional stability.
We utilized density functional theory (DFT) simulations to gain insights into the remarkable selectivity and activity of the PdCu3/ND@G catalyst. To investigate the potential influence of electronic interactions on the catalytic activity, we adopted distinct models for the PdCu3/OLC and PdCu3/ND@G catalysts. Specifically, we represented the former with free-standing PdCu3 and the latter with PdCu3 supported on a single graphene layer (Fig. S5a and b†). To validate the rationality of these models, we calculated the partial density of states of the d band (Fig. 4a), which showed a significant downshift of the d band center in the graphene-supported model (−3.99 eV) compared to the free-standing model (−1.74 eV). This shift indicates the occurrence of charge transfer from the graphene layer to PdCu3 in the graphene-supported model, which aligns with our XPS results. Moreover, we also constructed a free-standing monometallic Pd model as a reference to illustrate the effect of the intermetallic PdCu3 structure on selectivity (Fig. S4c†). We used the (111) surface to conduct the DFT calculations, as it is the most exposed surface for both monometallic Pd and intermetallic PdCu3, which is consistent with HAADF-STEM results.
 |
| | Fig. 4 DFT calculation results of monometallic Pd, PdCu3/OLC, and PdCu3/ND@G. (a) Partial density of states of d band, where Ed represents the d band center of Pd site. (b) The Gibbs free energy change of reaction pathway of phenylacetylene hydrogenation. (c) The Gibbs free energy variance between the rate-determining step barrier of C8H8 and C8H10. | |
The energy profile of the hydrogenation reaction pathway for phenylacetylene is depicted in Fig. 4b. The energy barriers and the structures of intermediates for each reaction step can be found in Table S1 and Fig. S5.† The corresponding results unveil the rate-determining step (RDS) in the formation of styrene (C8H8) and ethylbenzene (C8H10), which are associated with the desorption of C8H8 and the formation of 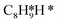 , respectively. The advantage of the desorption of C8H8 on PdCu3/ND@G surface is demonstrated by the adsorption energy (Fig. S6†). For comparing the C8H8 selectivity of the catalyst models, we utilized the energy variance (ΔG) between the rate-determining step (RDS) barrier of C8H8 (GRDS,C8H8) and C8H10 (GRDS,C8H10) as an estimation of selectivity. As depicted in Fig. 4c, both models corresponding to PdCu3/ND@G and PdCu3/OLC demonstrated a negative value of ΔG, while the monometallic Pd showed a positive value of ΔG, highlighting the selectivity advantage of the intermetallic PdCu3 over monometallic Pd. Moreover, the (GRDS,C8H8) of PdCu3/ND@G is 0.73 eV, significantly lower than that observed on PdCu3/OLC (1.06 eV, Fig. 4b), confirming that the PdCu3/ND@G catalyst with electron-riched properties favors the improvement of the activity.
, respectively. The advantage of the desorption of C8H8 on PdCu3/ND@G surface is demonstrated by the adsorption energy (Fig. S6†). For comparing the C8H8 selectivity of the catalyst models, we utilized the energy variance (ΔG) between the rate-determining step (RDS) barrier of C8H8 (GRDS,C8H8) and C8H10 (GRDS,C8H10) as an estimation of selectivity. As depicted in Fig. 4c, both models corresponding to PdCu3/ND@G and PdCu3/OLC demonstrated a negative value of ΔG, while the monometallic Pd showed a positive value of ΔG, highlighting the selectivity advantage of the intermetallic PdCu3 over monometallic Pd. Moreover, the (GRDS,C8H8) of PdCu3/ND@G is 0.73 eV, significantly lower than that observed on PdCu3/OLC (1.06 eV, Fig. 4b), confirming that the PdCu3/ND@G catalyst with electron-riched properties favors the improvement of the activity.
4. Conclusion
In summary, we successfully synthesized a highly efficient nanocatalyst for phenylacetylene semi-hydrogenation by using ND@G as a support to immobilize intermetallic PdCu3 nanoparticles. We confirmed that the high selectivity of the resulting catalyst originates from the structural advantage of intermetallic PdCu3 phases. More importantly, we demonstrated the presence of an electron transfer behavior from ND@G to PdCu3, which plays a pivotal role in enhancing the catalytic activity. Our work offers valuable insights into the development of highly efficient Pd-based semi-hydrogenation catalysts and proposes an efficient approach to enhance catalyst activity through the engineering of strong electronic interactions.
Author contributions
The manuscript was written with the contributions of all authors. All authors have approved the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We acknowledge the funding support from the National Natural Science Foundation of China (Grants 22071225 and 22221003), the Fundamental Research Funds for the Central Universities (Grant WK2060190103), the Joint Funds from Hefei National Synchrotron Radiation Laboratory (Grant KY2060000175), Collaborative Innovation Program of Hefei Science Center of CAS (Grant 2021HSC-CIP015), USTC Research Funds of the Double First-Class Initiative, and the funding support from Shenzhen Science and Technology Program (Grants RCJC20200714114434086, JCYJ20200812160737002, and 20180921273B).
Notes and references
- M. Crespo-Quesada, F. Cárdenas-Lizana, A. L. Dessimoz and L. Kiwi-Minsker, Modern Trends in Catalyst and Process Design for Alkyne Hydrogenations, ACS Catal., 2012, 2, 1773–1786 CrossRef CAS.
- C. Li, Y. Chen, S. Zhang, J. Zhou, F. Wang, S. He, M. Wei, D. G. Evans and X. Duan, Nickel-Gallium Intermetallic Nanocrystal Catalysts in the Semihydrogenation of Phenylacetylene, ChemCatChem, 2014, 6, 824–831 CrossRef CAS.
- L. Zhao, X. Qin, X. Zhang, X. Cai, F. Huang, Z. Jia, J. Diao, D. Xiao, Z. Jiang, R. Lu, N. Wang, H. Liu and D. Ma, A Magnetically Separable Pd Single-Atom Catalyst for Efficient Selective Hydrogenation of Phenylacetylene, Adv. Mater., 2022, 34, 2110455 CrossRef CAS.
- M. Guo, S. Jayakumar, M. Luo, X. Kong, C. Li, H. Li, J. Chen and Q. Yang, The Promotion Effect of π-π Interactions in Pd NPs Catalysed Selective Hydrogenation, Nat. Commun., 2022, 13, 1770 CrossRef CAS PubMed.
- F. Huang, M. Peng, Y. Chen, X. Cai, X. Qin, N. Wang, D. Xiao, L. Jin, G. Wang, X. D. Wen, H. Liu and D. Ma, Low-Temperature Acetylene Semi-Hydrogenation over the Pd1-Cu1 Dual-Atom Catalyst, J. Am. Chem. Soc., 2022, 144, 18485–18493 CrossRef CAS PubMed.
- F. Huang, Y. Deng, Y. Chen, X. Cai, M. Peng, Z. Jia, P. Ren, D. Xiao, X. Wen, N. Wang, H. Liu and D. Ma, Atomically Dispersed Pd on Nanodiamond/Graphene Hybrid for Selective Hydrogenation of Acetylene, J. Am. Chem. Soc., 2018, 140, 13142–13146 CrossRef CAS.
- F. Huang, Y. Deng, Y. Chen, X. Cai, M. Peng, Z. Jia, J. Xie, D. Xiao, X. Wen, N. Wang, Z. Jiang, H. Liu and D. Ma, Anchoring Cu1 Species over Nanodiamond-graphene for Semi-Hydrogenation of Acetylene, Nat. Commun., 2019, 10, 4431 CrossRef PubMed.
- L. Li, W. Yang, Q. Yang, Q. Guan, J. Lu, S. H. Yu and H. L. Jiang, Accelerating Chemo- And Regioselective Hydrogenation of Alkynes over Bimetallic Nanoparticles in a Metal-Organic Framework, ACS Catal., 2020, 10, 7753–7762 CrossRef CAS.
- P. Guo, H. Liu and J. Zhao, Transforming Bulk Alkenes and Alkynes into Fine Chemicals Enabled by Single-Atom Site Catalysis, Nano Res., 2022, 15, 7840–7860 CrossRef CAS.
- V. R. Bakuru, K. Fazl-Ur-Rahman, G. Periyasamy, B. Velaga, N. R. Peela, M. E. DMello, K. S. Kanakikodi, S. P. Maradur, T. K. Maji and S. B. Kalidindi, Unraveling High Alkene Selectivity at Full Conversion in Alkyne Hydrogenation over Ni under Continuous Flow Conditions, Catal. Sci. Technol., 2022, 12, 5265–5273 RSC.
- R. Zhang, Z. Liu, S. Zheng, L. Wang, L. Zhang and Z. A. Qiao, Pyridinic Nitrogen Sites Dominated Coordinative Engineering of Subnanometric Pd Clusters for Efficient Alkynes' Semihydrogenation, Adv. Mater., 2023, 2209635 CrossRef CAS.
- F. Huang, Z. Jia, J. Diao, H. Yuan, D. Su and H. Liu, Palladium Nanoclusters Immobilized on Defective Nanodiamond-Graphene Core-Shell Supports for Semihydrogenation of Phenylacetylene, J. Energy Chem., 2019, 33, 31–36 CrossRef.
- X. Li, W. Zhang, Y. Liu and R. Li, Palladium Nanoparticles Immobilized on Magnetic Porous Carbon Derived from ZIF-67 as Efficient Catalysts for the Semihydrogenation of Phenylacetylene under Extremely Mild Conditions, ChemCatChem, 2016, 8, 1111–1118 CrossRef CAS.
- A. Swamy, K. S. Kanakikodi, V. R. Bakuru, B. B. Kulkarni, S. P. Maradur and S. B. Kalidindi, Continuous Flow Liquid-Phase Semihydrogenation of Phenylacetylene over Pd Nanoparticles Supported on UiO-66(Hf) Metal-Organic Framework, ChemistrySelect, 2023, 8, e202203926 CrossRef CAS.
- C. Shen, Y. Ji, P. Wang, S. Bai, M. Wang, Y. Li, X. Huang and Q. Shao, Interface Confinement in Metal Nanosheet for High-Efficiency Semi-Hydrogenation of Alkynes, ACS Catal., 2021, 11, 5231–5239 CrossRef CAS.
- K. Choe, F. Zheng, H. Wang, Y. Yuan, W. Zhao, G. Xue, X. Qiu, M. Ri, X. Shi, Y. Wang, G. Li and Z. Tang, Fast and Selective Semihydrogenation of Alkynes by Palladium Nanoparticles Sandwiched in Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2020, 59, 3650–3657 CrossRef CAS.
- Q. Zhu, X. Lu, S. Ji, H. Li, J. Wang and Z. Li, Fully Exposed Cobalt Nanoclusters Anchored on Nitrogen-Doped Carbon Synthesized by a Host-Guest Strategy for Semi-Hydrogenation of Phenylacetylene, J. Catal., 2022, 405, 499–507 CrossRef CAS.
- W. Zhang, W. Wu, Y. Long, J. Qin, F. Wang and J. Ma, Promoting Role of Iron Series Elements Modification on Palladium/Nitrogen Doped Carbon for the Semihydrogenation of Phenylacetylene, ChemCatChem, 2019, 11, 1510–1517 CrossRef CAS.
- S. Domínguez-Domínguez, Á. Berenguer-Murcia, D. Cazorla-Amorós and Á. Linares-Solano, Semihydrogenation of Phenylacetylene Catalyzed by Metallic Nanoparticles Containing Noble Metals, J. Catal., 2006, 243, 74–81 CrossRef.
- Z. Wang, L. Yang, R. Zhang, L. Li, Z. Cheng and Z. Zhou, Selective Hydrogenation of Phenylacetylene over Bimetallic Pd-Cu/Al2O3 and Pd-Zn/Al2O3 Catalysts, Catal. Today, 2016, 264, 37–43 CrossRef CAS.
- H. H. Wei, C. H. Yen, H. W. Lin and C. S. Tan, Synthesis of Bimetallic Pd-Ag Colloids in CO2-Expanded Hexane and Their Application in Partial Hydrogenation of Phenylacetylene, J. Supercrit. Fluids, 2013, 81, 1–6 CrossRef CAS.
- Z. S. Wang, C. L. Yang, S. L. Xu, H. Nan, S. C. Shen and H. W. Liang, Electronic Modulation of Pd-Based Bimetallic Catalysts with Sulfur-Doped Carbon Support for Phenylacetylene Semihydrogenation, Inorg. Chem., 2020, 59, 5694–5701 CrossRef CAS PubMed.
- Y. Shen, K. Yin, C. An and Z. Xiao, Design of a Difunctional Zn-Ti LDHs Supported PdAu Catalyst for Selective Hydrogenation of Phenylacetylene, Appl. Surf. Sci., 2018, 456, 1–6 CrossRef CAS.
- Z. Wang, H. Wang, Y. Shi, C. Liu and L. Wu, CuPd Alloy Decorated SnNb2O6 Nanosheets as a Multifunctional Photocatalyst for Semihydrogenation of Phenylacetylene under Visible Light, Chem. Eng. J., 2022, 429, 132018 CrossRef CAS.
- G. Wowsnick, D. Teschner, M. Armbrüster, I. Kasatkin, F. Girgsdies, Y. Grin, R. Schlögl and M. Behrens, Surface Dynamics of the Intermetallic Catalyst Pd2Ga, Part II - Reactivity and Stability in Liquid-Phase Hydrogenation of Phenylacetylene, J. Catal., 2014, 309, 221–230 CrossRef CAS.
- Z. Li, M. Hu, J. Liu, W. Wang, Y. Li, W. Fan, Y. Gong, J. Yao, P. Wang, M. He and Y. Li, Mesoporous Silica Stabilized MOF Nanoreactor for Highly Selective Semi-Hydrogenation of Phenylacetylene via Synergistic Effect of Pd and Ru Single Site, Nano Res., 2022, 15, 1983–1992 CrossRef CAS.
- F. Yang, S. Ding, H. Song and N. Yan, Single-Atom Pd Dispersed on Nanoscale Anatase TiO2 for the Selective Hydrogenation of Phenylacetylene, Sci. China Mater., 2020, 63, 982–992 CrossRef CAS.
- D. Teschner, J. Borsodi, A. Wootsch, Z. Révay, M. Hävecker, A. Knop-Gericke, S. D. Jackson and R. Schlögl, The Roles of Subsurface Carbon and Hydrogen in Palladium-Catalyzed Alkyne Hydrogenation, Science, 2008, 320, 86–89 CrossRef CAS.
- E. Guan, J. Ciston, S. R. Bare, R. C. Runnebaum, A. Katz, A. Kulkarni, C. X. Kronawitter and B. C. Gates, Supported Metal Pair-Site Catalysts, ACS Catal., 2020, 10, 9065–9085 CrossRef CAS.
- S. Zhang, Z. Xia, X. Chen, W. Gao and Y. Qu, Selective Semihydrogenation of Phenylacetylene to Styrene Catalyzed by Alloyed Palladium/Gold Catalysts Anchored on Cerium Oxide, ChemNanoMat, 2018, 4, 472–476 CrossRef CAS.
- D. Goud, A. Cherevotan, R. Maligal-Ganesh, B. Ray, S. D. Ramarao, J. Raj and S. C. Peter, Unraveling the Role of Site Isolation and Support for Semihydrogenation of Phenylacetylene, Chem. – Asian J., 2019, 14, 4819–4827 CrossRef CAS.
- D. Deng, Y. Yang, Y. Gong, Y. Li, X. Xu and Y. Wang, Palladium Nanoparticles Supported on Mpg-C3N4 as Active Catalyst for Semihydrogenation of Phenylacetylene under Mild Conditions, Green Chem., 2013, 15, 2525–2531 RSC.
- Y. Kuwahara, H. Kango and H. Yamashita, Pd Nanoparticles and Aminopolymers Confined in Hollow Silica Spheres as Efficient and Reusable Heterogeneous Catalysts for Semihydrogenation of Alkynes, ACS Catal., 2019, 9, 1993–2006 CrossRef CAS.
- X. Li, Y. Pan, H. Yi, J. Hu, D. Yang, F. Lv, W. Li, J. Zhou, X. Wu, A. Lei and L. Zhang, Mott-Schottky Effect Leads to Alkyne Semihydrogenation over Pd-Nanocube@N-Doped Carbon, ACS Catal., 2019, 9, 4632–4641 CrossRef CAS.
- W. Long, N. A. Brunelli, S. A. Didas, E. W. Ping and C. W. Jones, Aminopolymer-Silica Composite-Supported Pd Catalysts for Selective Hydrogenation of Alkynes, ACS Catal., 2013, 3, 1700–1708 CrossRef CAS.
- N. Tiengchad, O. Mekasuwandumrong, C. Na-Chiangmai, P. Weerachawanasak and J. Panpranot, Geometrical Confinement Effect in the Liquid-Phase Semihydrogenation of Phenylacetylene over Mesostructured Silica Supported Pd Catalysts, Catal. Commun., 2011, 12, 910–916 CrossRef CAS.
- S. Domínguez-Domínguez, Á. Berenguer-Murcia, Á. Linares-Solano and D. Cazorla-Amorós, Inorganic Materials as Supports for Palladium Nanoparticles: Application in the Semi-Hydrogenation of Phenylacetylene, J. Catal., 2008, 257, 87–95 CrossRef.
- S. Song, K. Li, J. Pan, F. Wang, J. Li, J. Feng, S. Yao, X. Ge, X. Wang and H. Zhang, Achieving the Trade-Off between Selectivity and Activity in Semihydrogenation of Alkynes by Fabrication of (Asymmetrical Pd@Ag Core)@(CeO2 Shell) Nanocatalysts via Autoredox Reaction, Adv. Mater., 2017, 29, 1605332 CrossRef PubMed.
- C. H. Chu and C. W. Leung, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, A1133 CrossRef.
- G. Kresse and J. Furthmüller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- P. E. Blöchl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the Damping Function in Dispersion Corrected Density Functional Theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- J. Moellmann and S. Grimme, DFT-D3 Study of Some Molecular Crystals, J. Phys. Chem. C, 2014, 118, 7615–7621 CrossRef CAS.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- E. Skúlason, V. Tripkovic, M. E. Björketun, S. Gudmundsdóttir, G. Karlberg, J. Rossmeisl, T. Bligaard, H. Jónsson and J. K. Nørskov, Modeling the Electrochemical Hydrogen Oxidation and Evolution Reactions on the Basis of Density Functional Theory Calculations, J. Phys. Chem. C, 2010, 114, 18182–18197 CrossRef.
- Q. Feng, S. Zhao, Y. Wang, J. Dong, W. Chen, D. He, D. Wang, J. Yang, Y. Zhu, H. Zhu, L. Gu, Z. Li, Y. Liu, R. Yu, J. Li and Y. Li, Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes, J. Am. Chem. Soc., 2017, 139, 7294–7301 CrossRef CAS PubMed.
- J. K. Noerskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, Trends in the Exchange Current for Hydrogen Evolution, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- L. Xiang, H. Feng, M. Liu, X. Zhang, G. Fan and F. Li, Surface Defect-Induced Site-Specific Dispersion of Pd Nanoclusters on TiO2Nanoparticles for Semihydrogenation of Phenyl Acetylene, ACS Appl. Nano Mater., 2021, 4, 4688–4698 CrossRef CAS.
- V. Akhmedov, A. Aliyev, M. Bahmanov, V. Ahmadov and D. Tagiyev, Kinetics of Phenylacetylene Selective Hydrogenation to Styrene over Metal-free Polymeric Carbon Nitrides, Appl. Catal., A, 2018, 565, 13–19 CrossRef CAS.
- R. Wang, X. Sun, B. Zhang, X. Sun and D. Su, Hybrid Nanocarbon as a Catalyst for Direct Dehydrogenation of Propane: Formation of an Active and Selective Core-Shell Sp2/Sp3 Nanocomposite Structure, Chem. - Eur. J., 2014, 20, 6324–6331 CrossRef CAS.
- J. Liu, Y. Y. Yue, H. Y. Liu, Z. J. Da, C. C. Liu, A. Z. Ma, J. F. Rong, D. S. Su, X. J. Bao and H. D. Zheng, Origin of the Robust Catalytic Performance of Nanodiamond Graphene-Supported Pt Nanoparticles Used in the Propane Dehydrogenation Reaction, ACS Catal., 2017, 7, 3349–3355 CrossRef CAS.
- X. Duan, W. Tian, H. Zhang, H. Sun, Z. Ao, Z. Shao and S. Wang, Sp2/Sp3 Framework from Diamond Nanocrystals: A Key Bridge of Carbonaceous Structure to Carbocatalysis, ACS Catal., 2019, 9, 7494–7519 CrossRef CAS.
- W. J. Zeng, C. Wang, Q. Q. Yan, P. Yin, L. Tong and H. W. Liang, Phase Diagrams Guide Synthesis of Highly Ordered Intermetallic Electrocatalysts: Separating Alloying and Ordering Stages, Nat. Commun., 2022, 13, 7654 CrossRef CAS PubMed.
-
B. E. Warren, X-ray Diffraction, Courier Corporation, 1990 Search PubMed.
- Y. Lykhach, S. M. Kozlov, T. Skála, A. Tovt, V. Stetsovych, N. Tsud, F. Dvořák, V. Johánek, A. Neitzel, J. Mysliveček, S. Fabris, V. Matolín, K. M. Neyman and J. Libuda, Counting Electrons on Supported Nanoparticles, Nat. Mater., 2016, 15, 284–288 CrossRef CAS PubMed.
- X. Duan, Z. Ao, D. Li, H. Sun, L. Zhou, A. Suvorova, M. Saunders, G. Wang and S. Wang, Surface-Tailored Nanodiamonds as Excellent Metal-Free Catalysts for Organic Oxidation, Carbon, 2016, 103, 404–411 CrossRef CAS.
- L. Zhang, H. Liu, X. Huang, X. Sun, Z. Jiang, R. Schlögl and D. Su, Stabilization of Palladium Nanoparticles on Nanodiamond-Graphene Core-Shell Supports for CO Oxidation, Angew. Chem., Int. Ed., 2015, 54, 15823–15826 CrossRef CAS PubMed.
- H. Armendáriz, A. Guzmán, J. A. Toledo, M. E. Llanos, A. Vázquez and G. Aguilar-Ríos, Isopentane Dehydrogenation on Pt-Sn Catalysts Supported on Al-Mg-O Mixed Oxides: Effect of Al/Mg Atomic Ratio, Appl. Catal., A, 2001, 211, 69–80 CrossRef.
- E. V. Golubina, E. S. Lokteva, A. V. Erokhin, A. A. Veligzhanin, Y. V. Zubavichus, V. A. Likholobov and V. V. Lunin, The Role of Metal–support Interaction in Catalytic Activity of Nanodiamond-supported Nickel in Selective Phenylacetylene Hydrogenation, J. Catal., 2016, 344, 90–99 CrossRef CAS.
- C. Yu, H. Xu, Q. Ge and W. Li, Properties of the Metallic Phase of Zinc-Doped Platinum Catalysts for Propane Dehydrogenation, J. Mol. Catal. A: Chem., 2007, 266, 80–87 CrossRef CAS.
- F. Jiang, L. Zeng, S. Li, G. Liu, S. Wang and J. Gong, Propane Dehydrogenation over Pt/TiO2−Al2O3 Catalysts, ACS Catal., 2015, 5, 438–447 CrossRef CAS.
- S. L. Xu, S. C. Shen, Z. Y. Wei, S. Zhao, L. J. Zuo, M. X. Chen, L. Wang, Y. W. Ding, P. Chen, S. Q. Chu, Y. Lin, K. Qian and H. W. Liang, A Library of Carbon-Supported Ultrasmall Bimetallic Nanoparticles, Nano Res., 2020, 13, 2735–2740 CrossRef CAS.
- P. Yin, Q.-Q. Yan and H.-W. Liang, Strong Metal-Support Interactions through Sulfur-Anchoring of Metal Catalysts on Carbon Supports, Angew. Chem., Int. Ed., 2023, e202302819 CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  *b,
Lei
Wang
*b,
Lei
Wang
 c,
Yuan
Kong
*b,
Chenliang
Su
c,
Yuan
Kong
*b,
Chenliang
Su
 a and
Hai-Wei
Liang
a and
Hai-Wei
Liang
 *b
*b
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C to C
C to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is a vital industrial process used to purify hydrocarbon streams containing alkenes for the production of high-grade olefins in polymer manufacturing.1–7 A prominent application is seen in the production of polystyrene, where selective hydrogenation is essential to remove phenylacetylene impurities from styrene.8,9 Phenylacetylene impurities are commonly formed during the styrene synthesis process and can adversely affect polymerization catalysts, leading to their deactivation in polystyrene production units.10 Supported Pd catalysts exhibit outstanding efficiency in hydrogenation, attributed to their remarkably low activation energy for H2 dissociation.11–13 However, this impressive hydrogenation capability also renders Pd susceptible to excessive hydrogenation during alkyne hydrogenation, leading to the unwanted formation of alkane byproducts.
C is a vital industrial process used to purify hydrocarbon streams containing alkenes for the production of high-grade olefins in polymer manufacturing.1–7 A prominent application is seen in the production of polystyrene, where selective hydrogenation is essential to remove phenylacetylene impurities from styrene.8,9 Phenylacetylene impurities are commonly formed during the styrene synthesis process and can adversely affect polymerization catalysts, leading to their deactivation in polystyrene production units.10 Supported Pd catalysts exhibit outstanding efficiency in hydrogenation, attributed to their remarkably low activation energy for H2 dissociation.11–13 However, this impressive hydrogenation capability also renders Pd susceptible to excessive hydrogenation during alkyne hydrogenation, leading to the unwanted formation of alkane byproducts.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1, and the Pd loading was kept constant at 2 wt% for all catalysts.
3.1, and the Pd loading was kept constant at 2 wt% for all catalysts.






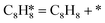

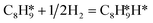



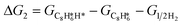
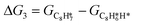
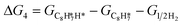


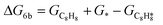

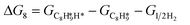
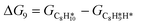
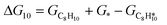
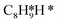 , respectively. The advantage of the desorption of C8H8 on PdCu3/ND@G surface is demonstrated by the adsorption energy (Fig. S6†). For comparing the C8H8 selectivity of the catalyst models, we utilized the energy variance (ΔG) between the rate-determining step (RDS) barrier of C8H8 (GRDS,C8H8) and C8H10 (GRDS,C8H10) as an estimation of selectivity. As depicted in Fig. 4c, both models corresponding to PdCu3/ND@G and PdCu3/OLC demonstrated a negative value of ΔG, while the monometallic Pd showed a positive value of ΔG, highlighting the selectivity advantage of the intermetallic PdCu3 over monometallic Pd. Moreover, the (GRDS,C8H8) of PdCu3/ND@G is 0.73 eV, significantly lower than that observed on PdCu3/OLC (1.06 eV, Fig. 4b), confirming that the PdCu3/ND@G catalyst with electron-riched properties favors the improvement of the activity.
, respectively. The advantage of the desorption of C8H8 on PdCu3/ND@G surface is demonstrated by the adsorption energy (Fig. S6†). For comparing the C8H8 selectivity of the catalyst models, we utilized the energy variance (ΔG) between the rate-determining step (RDS) barrier of C8H8 (GRDS,C8H8) and C8H10 (GRDS,C8H10) as an estimation of selectivity. As depicted in Fig. 4c, both models corresponding to PdCu3/ND@G and PdCu3/OLC demonstrated a negative value of ΔG, while the monometallic Pd showed a positive value of ΔG, highlighting the selectivity advantage of the intermetallic PdCu3 over monometallic Pd. Moreover, the (GRDS,C8H8) of PdCu3/ND@G is 0.73 eV, significantly lower than that observed on PdCu3/OLC (1.06 eV, Fig. 4b), confirming that the PdCu3/ND@G catalyst with electron-riched properties favors the improvement of the activity.



