Recent advances in lanthanide-based POMs for photoluminescent applications
Kangting
Zheng
and
Pengtao
Ma
 *
*
Henan Key Laboratory of Polyoxometalate Chemistry, College of Chemistry and Molecular Sciences, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: mpt@henu.edu.cn; Fax: +86-371-23886876
First published on 19th January 2024
Abstract
Since the first formation of the famous “Peacock–Weakley” anions [Ln(W5O18)2]8/9−, a steady stream of breakthroughs have been made in the chemistry of multitalented lanthanide (Ln)-based polyoxometalates (POMs) for their potentially desirable properties. In particular, LnIII ions are generally recognised as the “vitamins of the modern industry” owing to their ability to cover a wide emission range, endowing Ln-based POMs with great potential for versatile and diverse luminescence-related applications. In this frontier, we discuss the synthesis strategies and intramolecular energy transfer in Ln-based POM derivatives. Then, the progressive improvements achieved with Ln-based POMs in photoluminescence applications are highlighted, focusing mainly on luminescent and fluorescent probes. Finally, the challenges for Ln-based POM materials for photoluminescence applications are discussed.
1. Introduction
As an exceptional subset of metal oxides, polyoxometalates (abbr. POMs) are a large exceptional family of assembled anionic compounds of polynuclear early transition metal oxides (MOx) (e.g. notably VV, MoVI, WVI, NbV, and TaV).1–6 POM materials with abundant structural units and chemical versatility are compellingly appealing7–10 and are widely recognized as the preferred choice in functional, magnetism, and catalysis materials.11–15 Under certain conditions, condensation reactions are triggered between the MOx components, in which the MOx octahedrons are structurally assembled in the form of typically symmetrical assemblies via edge-, corner- or face-sharing linkages. With the development of synthesis technology, a growing number of synthesis methods have been rapidly established, demonstrating that the composition of POMs can be customized by varying the constituent elements. Among them, introducing heteroatoms into the reaction system has been found to enable the synthesis of POM derivatives with more diverse structures. Moreover, the skeleton of POM readily dissociates from one or more MOx octahedra, producing lacunary POMs with unique functions to trap additional electrophilic components (lanthanide/transition metal, etc.) yielding mixed-addenda POMs by tuning the number and position of substituted sites. Therefore, lacunary POMs, known as “superligands” or “metalloligands”, are widely used as precursor units in the speciation of coordination polymers with nearly endless structural and compositional diversities. To summarize, in relation to the composition of POMs, several review articles include a more detailed Introduction.6,16–20Lanthanide (Ln)-based luminescent materials with the high color purity of the emitted light have attracted considerable attention in broad areas of sciences as potent candidates owing to their resistance to photobleaching, narrow f–f transitions, and long excited-state lifetimes.21–23 Trivalent LnIII ions with many complicated 4f energy levels, known as the “vitamins of modern industry”, feature fluorescence emission via intra-4f or 4f–5d transitions from visible (EuIII or TbIII) to near-infrared (NdIII or YbIII) light.22,24–29 This property can ensure the emergence of Ln ions with high color purity and Stokes shift, reducing excitation interference in the emission process.30–32 Consequently, in the field of luminescent materials, Ln-based materials could be employed as a crucial branch,33,34 generating considerable interest in applications as varied as bioimaging and biomedical analysis, lasers, chemical sensing, optical fibers, and amplifiers.35–38 Nevertheless, in principle, the direct excitation of 4f levels indicates a relatively inefficient process as a consequence of the Laporte forbidden f–f transitions, yielding extremely low molar absorption coefficients (ε < 10 L mol−1 cm−1).39–41 In this regard, the limitations of intramolecular excitation can be overcome by the formation of hybrid materials with the introduction of photosensitizers, also known as the Ln-sensitization or optical antenna effect.40,42–44 The essence of the antenna effect is that the dopants as luminescent sensitizers can harvest light, and then transfer efficient energy to the Ln centers as the energy of emitting levels is higher than that in the emitting levels for LnIII luminescence.45 In relation to the luminescence principles of Ln ions, several reviews have introduced a detailed summary and provided some insightful comments.21,46,47
The high coordination number is extremely advantageous for LnIII ions to be linkers and stabilizers to endow mixed-addenda POMs with a considerable increase in structural diversity.34,46,47 It is important that the synergistic combination between lacunary POMs and Ln components results in coordination compounds with characteristic functions and unique physicochemical properties.47–51 The thermal/chemical stability and acid/redox properties of POM moieties when judiciously combined with the highly peculiar properties of Ln ions (e.g. luminescence, magnetism) render Ln-based POMs as suitable candidates for implementation in different fields such as photoluminescence, magnetism, and photochromism. Dating back to the 1970s, a new field of Ln-based POM was opened up by a pioneering work, famous as “Peacock–Weakley” polyanions [Ln(W5O18)2]8/9−, reported by Weakley et al.52
Following the breakthrough of the “Peacock–Weakley” ions, research on Ln-based POM derivatives has witnessed dramatic developments, especially in the last few decades, judging from several review articles that have primarily introduced the synthesis and structure.53–55 Fundamental opinions on the principles of Ln-based POMs’ luminescence were proposed, based on extensive research by Yamase et al.5,21,56 In fact, the W–O charge transfer (CT) transitions in POM components will be executed under the excitation, leading to strong absorption in the ultraviolet range, manifesting a particularly pronounced action in energy transfer processes in POMs to act as photosensitizers. Typically, the 2p orbital electrons of the ligand oxygen will transfer to the d orbitals of the metal M (LMCT) upon excitation. At this point, the state of the POM components changes from the ground state 1A1g to the excited state 1T1u. The energy from the 1T1u → 3T1u states is then transferred to the excited levels of the LnIII ions, effectively enhancing the emission of the Ln centers. Among the luminescent Ln-based POMs, the Peacock–Weakley anion [Eu(W5O18)2]9− elucidates the long-lasting luminescent stability and the highest luminescent quantum yields.57
In this article, we would like to discuss the synthesis strategies from the view of energy transfer in Ln-based POM derivatives, and then further summarize the detailed study of photoluminescent applications such as those related to luminescence and fluorescence probes. In the final part, conclusions are discussed, together with further work that is urgently needed or challenges remaining in this field. We hope that this article will provide an overview of the recent novel functional developments of lanthanide-based POMs and provide inspiration for functional expansions.
2. Synthesis strategies and energy transfer in luminescent materials
Because the series usually have identical structures owing to the lanthanide contraction,46,58,59 it is feasible to introduce different LnIII ions into a system defined as a pre-functionalization strategy to emit a distinctive color. As could be expected, in the representative structure of this type, compound {[W2O5(OH)2(H2tart)2](H2tart){[W3O6Ln2(H2O)6][SeW9O33]2}2}14− will explicitly exhibit a different emission color under excitation, for Ln ions, i.e., Eu (red), Tb (green), Dy (blue), and Ho (orange).41Apart from that, the most popular solution to circumvent the limitation of low molar absorption coefficients of LnIII ions is the co-doping of a sensitizer in the Ln system, as previously proposed, the so-called “antenna effect”. Although the fact that the direct combination of POMs with LnIII ions has produced a plethora of types of architectures with hybrid frameworks, the incorporation of organic ligands will enrich the family of functionalized POM-based architectures with enhanced processability and robustness. Indeed, organic ligands (most with highly conjugated structures) could be envisaged as another “antenna” where the sensitization generally concerns the population of a triplet excited state of the conjugated organic ligand, which intrinsically exhibits a long lifetime, thus enabling energy to be efficiently transferred to the emitting LnIII center.
In this field, the feasible synthetic strategies can be primarily divided into three categories based on the energy transfer pathways: (1) between POM moieties and a single LnIII center; (2) between POM moieties, organic ligands (heterocyclic and aromatic carboxylates) and LnIII centers, (3) between POM moieties and doped LnIII components (double and triple LnIII centers). Moreover, the photostability could be effectively improved by preparing Ln-doped POM compounds.
In 2018, the Pr-based POMs presented that POM components can sensitize PrIII ions via energy transitions.60 There are a plethora of compounds of antenna effects caused by the transfer of energy from POM components to LnIII ions (SmIII,30,61 EuIII,30,61–63 TbIII,63 DyIII, and HoIII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64). Certain typical molecular structures are shown in Fig. 1, and the corresponding energy transfer mechanism is described in Fig. 2. It is worth mentioning the compound [Ln(H2O)6{H4(TaO2)6As4O24}]3− in which the {As4Ta6} segment can transfer the energy to the EuIII centers, facilitating EuIII emissions.65 In 2005, Zhang et al. developed a ternary system of EuIII-based POM derivatives, representing the first “organic group modified EuIII-based POMs”. In this ternary system, the organic group was able to absorb energy and sensitize the emission of EuIII ions through energy transfer.66
64). Certain typical molecular structures are shown in Fig. 1, and the corresponding energy transfer mechanism is described in Fig. 2. It is worth mentioning the compound [Ln(H2O)6{H4(TaO2)6As4O24}]3− in which the {As4Ta6} segment can transfer the energy to the EuIII centers, facilitating EuIII emissions.65 In 2005, Zhang et al. developed a ternary system of EuIII-based POM derivatives, representing the first “organic group modified EuIII-based POMs”. In this ternary system, the organic group was able to absorb energy and sensitize the emission of EuIII ions through energy transfer.66
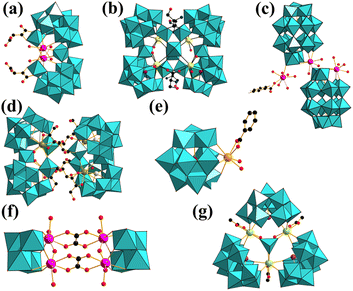 | ||
| Fig. 1 Some typical molecular structures for Ln-based POMs. (1a,601b,611c,301d,621e,671f,68 and 1g.69). | ||
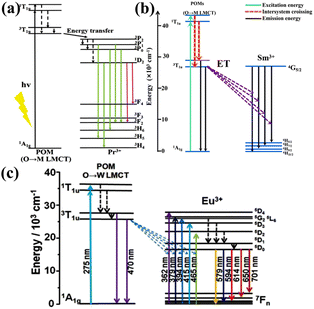 | ||
| Fig. 2 (a) Mechanisms for the sensitized emission of PrIII,60 (b) SmIII,61 (c) EuIII from POM components.62 | ||
In 2020, we confirmed that organic benzoic acid ligand in the compound [Sm(C7H5O2)(H2O)2(PW11O39)]5− can indeed sensitize Ln centers and improve the luminescence efficiency, corresponding to the π*–π transition of the benzoic acid group67 (Fig. 3). There are many examples of efficient sensitizers among organic ligands such as benzoate ligand → SmIII/EuIII,30 isonicotinic acid → SmIII,70 and 2-picolinic acid → EuIII/DyIII.71,72
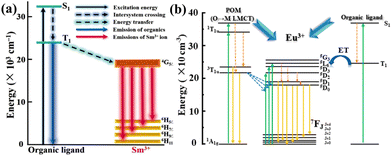 | ||
| Fig. 3 The mechanisms process for POMs, LnIII(a), and organic ligands (b).30,67 | ||
In comparison, the energy transfer process in co-doped Ln-based POMs containing two or more LnIII components is more complex. Initially, the ground state of Ln1 is converted under excitation, followed by the transfer of the f–f state photon energy to the ground state of Ln2. Finally, the conversion emission is successfully induced. Typically, for a known structured POM derivative with one type of LnIII ion, a series of isomorphic compounds could be purposefully obtained by introducing other LnIII ions or controlling different molar ratios of Ln ions with two or more centers.
Employing this strategy, the double-mixed DyIII/TmIII ion-based POM derivatives, [DyxTm1−x(C4H2O6)(PW11O39)]216−,73 verified the energy transfer TmIII → DyIII to efficiently facilitate emissions of the DyIII centers. In 2022, we offered a family of co-Eu/Tb-doped compounds based [{(As2W19O67(H2O))Ln(H2O)2}2(C2O4)]24−, probing the existence of the energy transfer (EuIII → TbIII) centers in the compound Eu0.1Tb0.99-POM.63 In addition, the energy transfer (DyIII → ErIII) was researched via the DyxEr(1−x)-POM (x = 0–1) derivatives based [Dy(C4H2O6)(PW11O39)]216−,75 where the derivative with the optimal doping ratio obtained was Dy0.8Er0.2-POM with the best photostability. Note that the triple-LnIII ion, due to the optimal doping ratio obtained in Dy0.8Er0.2-POM, showed the best photostability. Moreover, the triple-LnIII ion-doped POM derivatives, [EuxTbyTm1−x−y(C7H5O2)(H2O)2(PW11O39)]5−,76 representing a relatively high energy transfer efficiency between EuIII and TbIII ions.
Admittedly, Zhao's group achieved a lot of research progress in this field. In 2018, the HoIII/YbIII co-doped POM derivatives on the basis of [Ln2(OH)(TeW7O28)Sn2(CH3)4(W5O18)]214− demonstrated the maximum emission intensity in the Er0.40/Yb0.60 co-doped sample, primarily owing to the energy transfer between YbIII ions and ErIII.77 Furthermore, in 2020, they synthesized a series of HoIII/TmIII-co-doped POM derivatives based [SeO4Ln5(H2O)7(Se2W14O52)2]13−,74 confirming that TmIII ions can sensitize the emission of HoIII ions in the Ho0.5/Tm0.5 derivative (Fig. 4a). Furthermore, the red emitter EuIII ions and green emitter TbIII ions are co-doped in the [Gd2(C2O4)(H2O)4(OH)W4O16]210−. The compound Gd0.08Tb3.6Eu0.32W8 demonstrated the highest emission intensity, attributed to the energy transfer from TbIII to EuIII ions68 (Fig. 4b). Following a similar synthetic method, EuIII/TbIII/DyIII/GdIII-codoped species-based [(WO4){Tb(H2O)(Ac)(SbW9O31(OH)2)}3]17− demonstrated a two-step successive DyIII → TbIII → EuIII energy transfer process in the compound Dy1.2Tb3zEu0.03Gd1.77−3zW28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 (Fig. 4c).
69 (Fig. 4c).
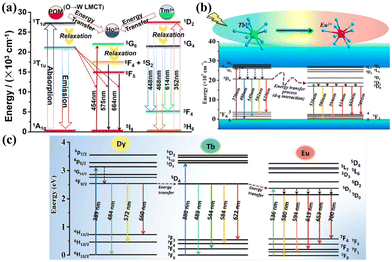 | ||
| Fig. 4 Energy transfer (ET) process of (a) POMs component, HoIII, and TmIII;74 (b) POMs component, EuIII, and TbIII;68 (c) POMs component, DyIII, TbIII, and EuIII.69 | ||
In addition to POM components and organic ligands, cationic surfactant (CS) usually acted as a stabilizer and a regulator in the Ln system.78–81 Note that there is a novel method for sensitization of LnIII ions in Ln-based POM composites with CS. Further exploration was conducted to study the influence of CS and Ln-based POMs with different mass ratios on the emission intensity. In 2017, Zhao et al. constructed a [{Sn(CH3)W2O4(IN)}{(TeW8O31)Sm(H2O)(Ac)}2]220−@CTAB composites with peanut-like and honeycombed morphologies (CTAB = cetyltrimethylammonium bromide).82 The energy transfer was performed from CTAB to SmIII centers within CTAB/POMs = 0.033/0.05 consolidation.
3. Photoluminescent applications
3.1 Multi-color emission
The ability to achieve multi-color emission by Ln-based POM materials is a potential application in many fields such as WLED, lighting, and displays, as unique features of Ln-based luminescent materials. A plethora of strategies, such as adjusting the excitation wavelength and the relative intensity of the emission peaks, have been used to achieve different color emissions from Ln-based POM materials.83–89 The CIE chromaticity diagram is often adopted to visually represent the luminescence information of materials with CIE chromaticity coordinates obtained from the emission spectra according to the International Commission on Illumination.Our group has performed several explorations in this field. In 2015, we synthesized a family of POM-ligated trinuclear Ln-clusters [Ln3(OH)(H2O)8(AsW9O33)(AsW10O35(mal))]222−.90 Different samples yielded different emission colors, from yellowish-green to green to reddish-orange by the introduction of different Ln ions. This method of changing the type of LnIII ions in POM compounds to afford different colors of emission is already well established, mainly because the different LnIII metals are typically isostructural.91 Furthermore, changing the excitation wavelength to coordinate the emission of Ln-based POMs is an obviously effective channel. In 2018, an Ln-based hybrid POM derivative, [{Pr(H2O)2}2{As2W19O68}{WO2(mal)}2]12−, showed excitation wavelength-dependent emission properties, achieving a reversible emission color switching simply by changing the excitation wavelength (380–470 nm)60 (Fig. 5a and b). In addition, we found that the novel reversible color-tunable photoluminescence Ln-based POM [Sm(C7H5O2)(H2O)2(PW11O39)]5− demonstrated emitting colors from blue to pink based on the excitation (260–350 nm)67 (Fig. 5c).
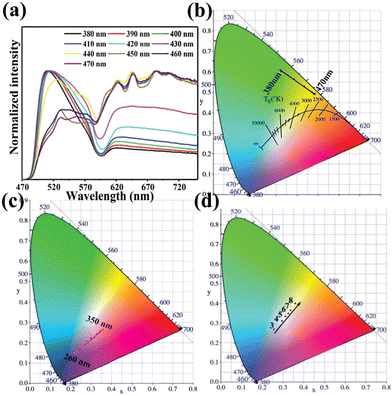 | ||
| Fig. 5 (a) PL emission spectra of compound [{Pr(H2O)2}2{As2W19O68}{WO2(mal)}2]12− under λex = 380–470 nm, and the CIE diagram (b).60 (c) The CIE 1931 diagram of Sm-based POMs on λex = 260–350 nm.67 (d) The CIE diagram of co-TmIII/DyIII-based POMs.73 | ||
In recent years, our group has devoted considerable efforts to co-doped LnIII-based POM materials for optical applications. The main reasons are as follows: (I) different ratios of two LnIII centres could produce multi-colour emission by changing the doping molar ratio of different LnIII ions; (II) intramolecular energy transfer occurs between different LnIII components with similar energy values, which enhances the luminescence intensity of another LnIII emission centre. There is an enormous variety of successful cases of achieving multi-colour luminescence by tuning the emission intensity of co-doped LnIII emission centres. In 2018, a family of co-TmIII/DyIII-based POMs, [DyxTm1−x(C4H2O6)(PW11O39)]216− was constructed, which displayed color reconcilable properties73 (Fig. 5d). The successful implementation is that different samples could provide adjustable emission colours from blue to yellow by adjusting the combination of TmIII/DyIII.
In 2022, co-doped compounds, [{(As2W19O67(H2O))Ln(H2O)2}2(C2O4)]24− {EuxTb1−x-POM} (x = 0.01, 0.04, 0.1, and 0.2), could potentially generate coordinated emission colours transitioning from green to red by modulating the ratio of EuIII/TbIII components.63 Moreover, we obtained another series of different ratios of doped-DyxEr(1−x) ions POM (x = 0–1) derivatives, which represent [Dy(C4H2O6)(PW11O39)]214− as the parent Dy-POM75 (Fig. 6). Among them, the compounds DyxEr(1−x)-POM (x = 1, 0.9, and 0.8) can emit cover the blue LED chip to emit white light in practical applications. As the doping amount of ErIII increases, the emission intensity of DyIII gradually decreases.
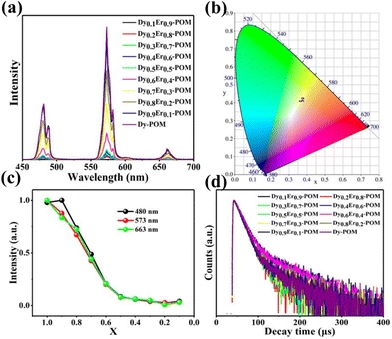 | ||
| Fig. 6 (a) PL emission spectra and (b) the CIE diagram of DyxEr(1−x)-POM; (c) corresponding emission intensity at 480, 573, and 663 nm; (d) PL decay time diagrams upon excitation at 367 nm and emission at 573 nm (x is 0.1–1) of DyxEr(1−x)-POM (λex = 367 nm).75 | ||
Zhao et al. demonstrated that multiple emission colors were achievable by doping TbIII ions into the polyanion [Eu2(C2O4)(H2O)4(OH)W4O16]210−. The samples emit green in the absence of TbIII, while the color can change to red emission by adding TbIII in different ratios.68 Recently, a family of co-Er/Yb-doped POM-based on the [Er(OH)(TeW7O28)LnSn2(CH3)4(W5O8)]214− polyanion with luminescence properties in the visible/NIR regions was synthesized.77 The emission intensity of ErIII ions reached the maximum with the ratio of Er/Yb of 0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60. Very recently, they probed the characteristic emission bands of co-HoIII/TmIII-existing in Ln-based POM-derivatives based on the polyanion [SeO4Ho5(H2O)7(Se2W14O52)2]13−. As the concentration of TmIII ions increases, the characteristic emission band of TmIII ions shifts slightly to red while the emission peak of HoIII ions shifts slightly to blue.74 When the concentration of HoIII and TmIII reaches a certain level (1
0.60. Very recently, they probed the characteristic emission bands of co-HoIII/TmIII-existing in Ln-based POM-derivatives based on the polyanion [SeO4Ho5(H2O)7(Se2W14O52)2]13−. As the concentration of TmIII ions increases, the characteristic emission band of TmIII ions shifts slightly to red while the emission peak of HoIII ions shifts slightly to blue.74 When the concentration of HoIII and TmIII reaches a certain level (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the strongest emission with the longest fluorescence lifetime can be generated by the energy transfer from the excited state TmIII to HoIII.
1), the strongest emission with the longest fluorescence lifetime can be generated by the energy transfer from the excited state TmIII to HoIII.
Surprisingly, by changing the excitation wavelengths, the emission color of the EuIII/TbIII/DyIII/GdIII-codoped derivatives based on the polyanion {(WO4)[Ln(H2O)(Ac)(SbW9O31(OH)2)]3}17− can vary from blue to yellow in which a near-white-light emission case was achieved on λex = 378 nm.69 In 2019, Zhao et al. synthesized the nanosized CTA-encapsulated POM composites using the microwave method (cetyltrimethylammonium bromide = CTABr).92 The [SeO4Dy5(H2O)7(Se2W14O52)2]13−@CTA-5 min nanomaterial with the highly uniform small-sized nanoparticles demonstrated the highest emission among the nanomaterials at different stirring times.
Furthermore, as a hot trend in solid-state lighting, white light-emitting diodes (WLEDs) have attracted considerable attention for their high energy efficiency, high brightness, and low power consumption. As different LnIII ions can emit inherent CIE luminescence color coordinates, they are widely used in the production of WLED materials. More commonly, EuIII is widely used for red emission, DyIII or SmIII for yellow emission, TbIII for green emission, and TmIII for blue emission. Thus, EuIII, DyIII, SmIII, and Tb+ are used as common elements in the production of WLED materials. The white light point is referred to as the equal energy point (xee, yee), which is located near the center of the chromaticity diagram with the standard chromaticity coordinates (0.33, 0.33).93
At present, in the field of research on LnIII-based POM materials with white light emission, the synthesis of co-LnIII-doped POM derivative ions is primarily obtained by adjusting the concentration of each LnIII ion. As depicted in Fig. 7a, we report that the mixed DyIII/TmIII-based POM derivatives, [DyxTm1−x(C4H2O6)(PW11O39)]2, can emit a variety of colors from blue to white to yellow by adjusting the molar ratio of DyIII and TmIII.73 Similarly, for DyxEr(1−x)-POM based [Dy(C4H2O6)(PW11O39)]216−, the DyxEr(1−x)-based POM (x = 1, 0.9, and 0.8) samples emit macroscopic white light under blue irradiation, indicating that the samples can cover the blue LED chip to emit white light in practical applications75 (Fig. 7b). Zhao et al. confirmed that the co-doped LnIII-based POMs, Dy1.2Tb1.2Eu0.03Gd0.57W28, based [(WO4){Tb(H2O)(Ac)(SbW9O31(OH)2)}3]17− represent a near-white-light emission case upon excitation at 378 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 (Fig. 7c). Moreover, they illustrate that the co-doped Ln-based compound, Tb3.92Eu0.08W8-based [Gd2(C2O4)(H2O)4(OH)W4O16]210−, also displayed near white-light emission.68 The multicenter-Ln co-doped [EuxTbyTm1−x−y(C7H5O2)(H2O)2(PW11O39)]5−have been synthesized to study the white-light-emitting behavior with the molar ratio of EuIII/TbIII/TmIII = 0.06
69 (Fig. 7c). Moreover, they illustrate that the co-doped Ln-based compound, Tb3.92Eu0.08W8-based [Gd2(C2O4)(H2O)4(OH)W4O16]210−, also displayed near white-light emission.68 The multicenter-Ln co-doped [EuxTbyTm1−x−y(C7H5O2)(H2O)2(PW11O39)]5−have been synthesized to study the white-light-emitting behavior with the molar ratio of EuIII/TbIII/TmIII = 0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10
0.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.84.76
0.84.76
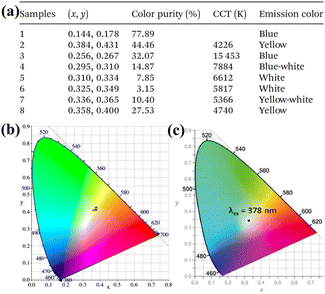 | ||
| Fig. 7 (a) CIE chromaticity coordinates, color purity, emitting color of co-DyIII/TmIII POMs derivatives.73 (b) CIE diagram of DyxEr(1−x)-POM.75 (c) CIE 1931 diagram of Dy1.2Tb1.2Eu0.03Gd0.57W28 upon excitation at 378 nm.69 | ||
3.2 Fluorescent probe
Fluorescent probe materials are often used as indicators and for the generation of excited fluorescence at a predetermined wavelength and are widely used in a variety of detection and labelling fields such as the determination of metal ions, pesticide residues, biomolecular content, traceability of biomolecules and cellular and subcellular structures. By detecting the changes in fluorescence intensity owing to the influence of the environment on the fluorescent probe, visual monitoring of the detected substance is achieved, providing the characteristics of the environment or specific information present in the environment. Based on the substance, there are many classifications of fluorescent probes, including metal ion fluorescent probes and biomolecular fluorescent probes.The detection of trace metal ions in drinking and irrigation water is important for a better understanding of their role in humans and animals. In this field, Eu-based POMs have a prominent position, as a hotspot of research, primarily because of the undisturbed high-intensity luminescence and ultrahigh sensitivity.95 In 2019, we found that the Eu-based POMs, [Eu(PHBA)(H2O)2(PW11O39)]5−, can serve as a luminescent probe for selective sensing of CrIII with a detection limit of 1.423 μM (quenching) and CaII with the detection limit of 0.676 mM (enhancing) in an aqueous solution showing photoluminescence intensity94 (Fig. 8). Furthermore, intense dynamic collision between EuIII ions and CrIII ions causes luminescence quenching, and the electrostatic interactions between hydroxyl groups and CaII ions lead to enhanced luminescence. In 2022, we fabricated a BaII-probe Ln-based arsenotungstate [{(As2W19O67(H2O))Eu(H2O)2}2(C2O4)]24− with the detection limit of 0.0105 mM for the selective detection of BaII ions in the presence of other metal ions. We suggested that the increase in fluorescence intensity could be caused by a possible interaction between BaII ions and oxygen sites in the POM backbone.63
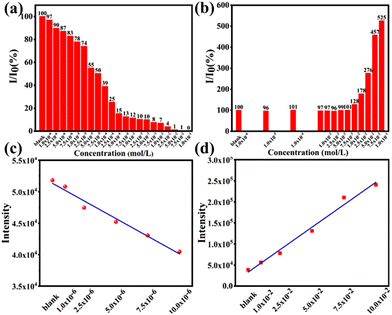 | ||
| Fig. 8 Changes of emission intensity with the addition of different concentrations of CrIII ion (a) and CaII ion (b). The linear relationship between emission intensities of CrIII (c) and CaII ions (d).94 | ||
In recent years, Zhao et al. synthesized a variety of Eu-based POM materials regarded as fluorescent probes. In 2020, a BaII-detector material, [{Eu6W14O40(H2O)18(Htpdc)2}{AsW9O33}6]34−, was successfully obtained with high sensitivity, good selectivity, and low detection limit (1.19 × 10−3 mM).96 Moreover, they constructed a ZnII/CuII-detector material, [Eu(H2O)6(pca)][Sn(CH3)2(H2O)]3[SbW9O33]−,70 with luminescent quenching for recognizing CuII and enhancement for ZnII in water (Fig. 9). As a continuation of earlier work, in 2020, they synthesized the Eu-based POMs material, [Eu4(H2O)4W6(H2glu)4O12(TeW9O33)4]14−, which can function as a “turn-off” luminescence probe to detect CuII ions in an aqueous solution with a limit of detection being 1.75 × 10−4 mM.62 Recently, they prepared an unprecedented Eu-POM aggregate as a bidirectional detection probe to continuously recognize both Mn2+ (on–off) and CO32− (off–on), succinctly summarized as an “on–off–on” mode.98
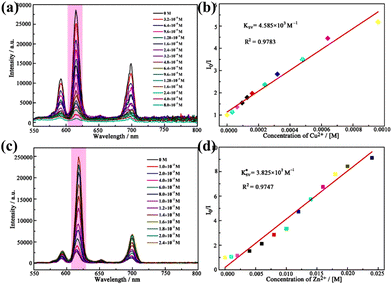 | ||
| Fig. 9 (a) Variation in the intensity of Eu–Sm-POMs dissolved in aqueous solution with enhancement of the CuII concentration. (b) Plot of the strength of the 613 nm peak and the CuII concentration and the linear fitting result. (c) Variation of the intensity dissolved in an aqueous solution with increasing ZnII concentration. (d) Plot of the intensity strength of the 613 nm peak and ZnII concentration.70 | ||
The fabrication of biological fluorescent probes as a possible research hotspot has been widely adopted in various fields such as medical imaging, bacterial detection, and biological warfare agents. Recently, Zhao et al. used the compound [Eu4(H2O)6(HPZDA)2(HFMA)2W8O21][SeW9O33]418− to engineer a fluorescent sensor to detect the main marker of anthrax spores (DPA) in the aqueous solution with a detection limit of 3.83 μM (enhancing)97 (Fig. 10). Moreover, the detection process could be followed through decay lifetime measurements of Eu-POMs with and without DPA.
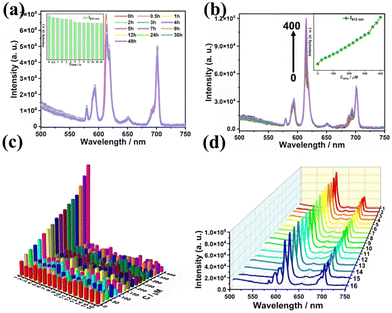 | ||
| Fig. 10 (a) Comparison of the emission spectra with the addition of different concentrations of DPA. (b) A linear relationship between the I/I0 ratio and the DPA concentration. (c) Comparison of the FL emission intensity with the addition of DPA (0–400 μM). (d) Comparison of the FL spectra in the presence of DPA and each different interferent.97 | ||
3.3 Fluorescent switch
As is known, photochromic POM-based materials with stability and reversibility are attractive and promising for applications in many fields.101 Among them, most strikingly, the fluorescence of Ln-based POM materials displays an effectively switchable behavior under irradiation. Recently, photochromic Ln-based POM materials have emerged with interesting potential in the application of fluorescent switches owing to their colour-reversible ability.Our group has made considerable progress in this field. In 2016, we isolated a photochromic mono-Dy-based POMs, [Dy(C4H2O6)(PW11O39)]216−, which displayed an effectively switchable behavior upon irradiation99 (Fig. 11a and b). Multinuclear Sm-implanted POMs [{(P2W17O61)Ln(H2O)3Sm(C6H5COO)(H2O)6}]{[(P2W17O61)Sm(H2O)3]}226− with switchable luminescence behavior induced by fast photochromism were synthesized.30 Moreover, we synthesized [{As4W44O137(OH)18(H2O)2(Ser)2}{Sm2(H2O)4(Ser)}2{Ln(H2O)7}2]2− in 2022, which possesses an effective luminescent switchable behavior, triggered by its fast-responsive photochromism effect61 (Fig. 11c and d). All these compounds demonstrated a gradual decrease in fluorescence intensity with the quenching phenomenon during the photochromism process, and reversible fluorescent switch behavior via alternating from irradiation (OFF) to in-air (ON) states, without major structural rearrangements.
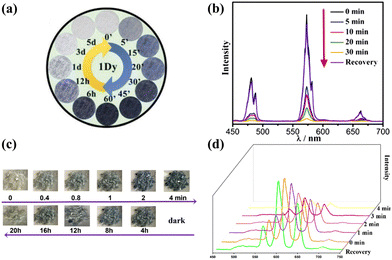 | ||
| Fig. 11 (a) The color evolution for Dy-POMs after irradiation; (b) emission spectral evolution after irradiation;99 (c) the color transformation for Sm-POMs on irradiation; (d) emission spectral evolution for Sm-POMs on irradiation.61 | ||
3.4 Drug activity
In recent decades, biological studies in medicine using Ln-based POM materials with broad-spectrum antiviral, antitumor, and antibacterial activities have become a burgeoning area of exploration.102–104Zhao et al. reported a series of Ln-bridged nanosized POM aggregates [Ln10W16(H2O)30O5(AsW9O33)8]46− [Ln = TbIII, TmIII] in 2016, and cytotoxicity tests indicated that TbIII and TmIII-based compounds can induce the apoptosis of human cervical cancer (HeLa) cells and human breast cancer (MCF-7) cells and kills cells, which was monitored from the evolution of fluorescence intensity.105 In 2018, they measured the drug activity of Ln-based POMs [{Eu(H2O)8}{K3Cu2WO(H2O)10}{AsW9O33}2]3−via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, inducing the apoptosis of HepG2 cells and HCT-116 cells through the activation of caspase-3 and the autophagy of HepG2 cells and HCT-116 cells through the involvement of lysosomes (Fig. 12).100
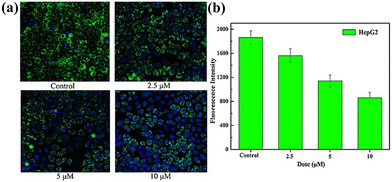 | ||
| Fig. 12 Compound [{Ln10W16(H2O)30O50}(AsW9O33)8]46− on the MMP in HepG2 cells. (a) Representative pictures from one of the three independent experiments with similar results. (b) The MMP (green) was detected with rhodamine 123/Hoechst staining through HCS of HepG2 cells in vitro.100 | ||
3.5 Other applications
In recent years, a few novel co-doped Ln-based POM materials have emerged as potential new optical markers in the domain of biomedical imaging. In 2018, PEG–NaGd(WO4)2:Eu, considered the X-ray luminescence nanoprobe in optical bioimaging, was reported by Yang et al.106 The maximum emission intensity was reached (λem = 615 nm) when the proportion of EuIII in the complex was 10%, stronger than that in all the other reported luminescent X-ray nanoprobes, thereby rendering it a prime candidate for exploitation as a biomarker in biological imaging (Fig. 13). In 2019, the nanoparticles Gd2(WO4)3:YbIII/HoIII@SiO2 nanoparticles (Gd2(WO4)3@SiO2–Pt–PEG) displayed yellow emission behavior under near-infrared excitation.107 After the invasion of the nanoparticles inside CT26 cells, fluorescence signals were detected at different invasion times. In addition, Gd2(WO4)3@SiO2–Pt–PEG demonstrated the magnetic resonance imaging property, which is positively correlated with the amount of GdIII.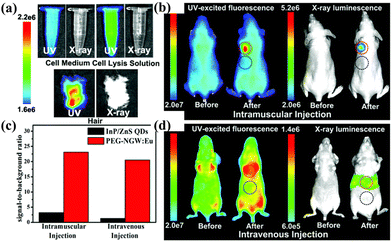 | ||
| Fig. 13 Luminescence imaging of (a) the cell medium, cell lysis, and mouse hair, (b) mice before and after intramuscular injection of InP/ZnS QDs (left) and PEG–NGW:Eu nanorods (right), and (c) mice before and after (30 min) intravenous injection of InP/ZnS QDs (left) and PEG–NGW:Eu nanorods (right) excitation. (d) Comparison of the intensity ratio in mice under excitation with InP/ZnS QDs or PEG–NGW:Eu nanorods.107 | ||
Furthermore, Ln-based POM materials have also shown a great deal of potential in the application of pH sensing and temperature sensing.108 In 2017, Ocaña et al. synthesized Eu-doped NaGd(WO4)2 nanophosphors, as a wide pH range (pH = 4–10) radiometric sensor: pH = 4 (blue), 7 (green), and 12 (red) (Fig. 14a and b).109 In 2018, Tu et al. designed a portable all-fiber thermometer using ErIII/YbIII-co-doped TWLN glasses, as a wide temperature range (293–569 K) radiometric sensor with the highest value of absolute temperature sensitivity (Sa) (86.7 × 10−4 K−1 at 553 K) (Fig. 14c and d).110
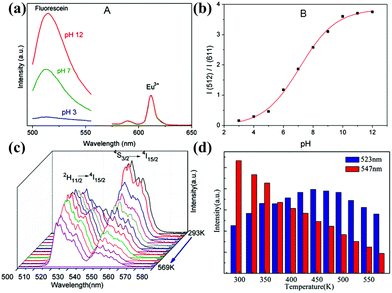 | ||
| Fig. 14 (a) Emission spectra of Eu:NaGd(WO4)2 NPs under pH-dependent; (b) intensity ratio of emissions at different pH values.109 (c) emission spectra of ErIII/YbIII-co-doped TWLN glass fiber with T/°C-dependent; (d) integrated up conversion intensities at 523 nm and 547 nm vs. T/°C.110 | ||
4. Conclusion
The ever-growing interest in Ln-based POMs is without doubt due to the compositional variability of these compounds, displaying unique chemical and physical properties because of the synergistic combination between POM coordinative structures and LnIII constituents. In this review, we briefly reviewed the preparation of POMs, Ln-based materials, and Ln-based POM derivatives, and then comprehensively summarized the photoluminescent applications of these Ln-based POMs such as in multicolor emission, fluorescent probes, drug activity, and fluorescent switches.However, as an important branch of photoluminescent materials, it is of great necessity to research how to improve the luminescence efficiency, which remains a challenging issue. Quantum yield and fluorescence intensity are essential parameters for the interpretation of luminescent materials. Firstly, LnIII ions with Laporte forbidden f–f transitions have low molar absorption coefficients. Secondly, in Ln-based POMs, although a double antenna effect occurs from the organic and POM ligands to the Ln centers, a low energy transfer efficiency may occur due to the weak emission of the POM fragment. It is necessary to investigate how to improve the luminescence efficiency of the target products. It is expected that the high quantum yield of high-nuclearity Ln-based POMs may be achieved. In addition, organic ligands with high energy absorption efficiency can be introduced by experimental syntheses such as spiropyran and viologen to improve the energy transfer efficiency between organic ligands and Ln centers. In addition, the inherent optical properties of Ln-based POM materials are expected to emerge with high potential for applications in light-emitting security printing, anti-counterfeiting, and encrypted information storage at different availability levels.
Although the design and manufacture of functional Ln-based POMs with unique properties are still very limited, these are expected to be overcome and run in more fields in tomorrow's world.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22071043).Notes and references
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag Berlin, 1983 Search PubMed.
- A. Blazevic and A. Rompel, Coord. Chem. Rev., 2016, 307, 42–64 CrossRef CAS.
- D. Li, P. Ma, J. Niu and J. Wang, Coord. Chem. Rev., 2019, 392, 49–80 CrossRef CAS.
- W. Du, Y. Liu, J. Sun, H. Wang, G. Yang and D. Zhang, Dalton Trans., 2022, 51, 9988–9993 RSC.
- T. Yamase, Chem. Rev., 1998, 307–326 CrossRef CAS PubMed.
- Z. Yang, J. Li, J. Niu and J. Wang, Dalton Trans., 2023, 52, 4632–4642 RSC.
- L. Sheng, L. Xiong and Z. Tian, Coord. Chem. Rev., 2023, 482, 215077 CrossRef.
- J. Liu, X. Zhang, Y. Li, S. Huang and G. Yang, Coord. Chem. Rev., 2020, 414, 213260 CrossRef CAS.
- N. Ogiwara, T. Iwano, T. Ito and S. Uchida, Coord. Chem. Rev., 2022, 462, 214524 CrossRef CAS.
- J. Zhang, Y. Huang, G. Li and Y. Wei, Coord. Chem. Rev., 2019, 378, 395–414 CrossRef CAS.
- A. Misra, K. Kozma, C. Streb and M. Nyman, Angew. Chem., Int. Ed., 2020, 59, 596–612 CrossRef CAS PubMed.
- V. Das, R. Kaushik and F. Hussain, Coord. Chem. Rev., 2020, 413, 213271 CrossRef CAS.
- K. Wassermann, M. H. Dickman and M. T. Pope, Angew. Chem., Int. Ed. Engl., 1997, 36, 1445–1448 CrossRef CAS.
- P. Ma, F. Hu, J. Wang and J. Niu, Coord. Chem. Rev., 2019, 378, 281–309 CrossRef CAS.
- H. J. Lun, S. Q. Dai, Y. X. Li, H. L. Guo, S. Andra, D. B. Dang and Y. Bai, Inorg. Chem., 2023, 62, 19749–19757 CrossRef CAS PubMed.
- D. Li, P. Ma, J. Niu and J. Wang, Coord. Chem. Rev., 2019, 392, 49–80 CrossRef CAS.
- X. Li, D. Zhao and S. Zheng, Coord. Chem. Rev., 2019, 397, 220–240 CrossRef CAS.
- C. Boskovic, Acc. Chem. Res., 2017, 50, 2205–2214 CrossRef CAS PubMed.
- J. Liu, X. Zhang, Y. Li, S. Huang and G. Yang, Coord. Chem. Rev., 2020, 414, 213260 CrossRef CAS.
- P. Mialane, C. Mellot-Draznieks, P. Gairola, M. Duguet, Y. Benseghir, O. Oms and A. Dolbecq, Chem. Soc. Rev., 2021, 50, 6152–6220 RSC.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- J. C. G. Bünzli, Acc. Chem. Res., 2006, 39, 53–61 CrossRef PubMed.
- J. Chen, T. Zhou and W. Sun, Dalton Trans., 2023, 52, 4643–4657 RSC.
- J. A. Mattocks, J. V. Ho and J. A. Cotruvo, J. Am. Chem. Soc., 2019, 141, 8172 CrossRef PubMed.
- S. V. Eliseeva and J. C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- T. Cheisson and E. J. Schelter, Science, 2019, 363, 489–493 CrossRef CAS PubMed.
- I. Olmez and G. E. Gordon, Science, 1985, 229, 966–968 CrossRef CAS PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- C. E. D. Cardoso, J. C. Almeida and C. B. Lopes, Nanomaterials, 2019, 9, 814 CrossRef CAS PubMed.
- B. Yan, R. Liang, K. Zheng, R. Li, P. Ma, J. Wang and J. Niu, Inorg. Chem., 2021, 60, 8164–8172 CrossRef CAS PubMed.
- Z. Ahmed, R. E. Aderne, J. Kai, H. I. P. Chavarria and M. Cremona, Thin Solid Films, 2016, 620, 34–42 CrossRef CAS.
- J. C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef PubMed.
- Q. Dong, X. Zhang, S. Liu, R. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu, J. Duan, R. Matsuda, W. Jin and B. Chen, Angew. Chem., Int. Ed., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- C. Boskovic, Acc. Chem. Res., 2017, 50, 2205–2214 CrossRef CAS PubMed.
- J. C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef PubMed.
- H. Dong, S. Du, X. Zheng, G. M. Lyu, L. Sun, L. Li, P. Zhang, C. Zhang and C. Yan, Chem. Rev., 2015, 115, 10725–10815 CrossRef CAS PubMed.
- P. Escribano, B. Julian-Lopez, J. Planelles-Arago, E. Cordoncillo, B. Viana and C. Sanchez, J. Mater. Chem., 2008, 18, 23–40 RSC.
- L. Liu, J. Jiang, G. Liu, X. Jia, J. Zhao, L. Chen and P. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 35997–36010 CrossRef CAS PubMed.
- A. M. Kaczmarek, K. V. Hecke and R. V. Deun, Inorg. Chem., 2017, 56, 3190–3200 CrossRef CAS PubMed.
- J. C. G. Bunzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- J. Liu, D. Wang, X. Xu, H. Li, J. Zhao and L. Chen, Inorg. Chem., 2021, 60, 2533–2541 CrossRef CAS PubMed.
- J. Chen, S. Wang, J. Lin and D. Chen, Nanoscale, 2019, 11, 22359–22368 RSC.
- J. Yang, Y. Liang, K. Li, G. Yang, Y. Zhu, S. Liu and W. Lei, Appl. Surf. Sci., 2018, 458, 769–780 CrossRef CAS.
- F. Kang, G. Sun, P. Boutinaud, H. Wu, F. X. Ma, J. Lu, J. Gan, H. Bian, F. Gao and S. Xiao, Chem. Eng. J., 2021, 403, 126099 CrossRef CAS.
- Z. Hou, Z. Cheng, G. Li, W. Wang, C. Peng, C. Li, P. Ma, D. Yang, X. Kang and J. Lin, Appl. Surf. Sci., 2018, 458, 769–780 CrossRef.
- B. Yan, H. Wu, P. Ma, J. Niu and J. Wang, Inorg. Chem. Front., 2021, 8, 4158–4176 RSC.
- C. M. Granadeiro, D. Julião, S. O. Ribeiro, L. Cunha-Silva and S. S. Balula, Coord. Chem. Rev., 2023, 476, 214914 CrossRef CAS.
- R. D. Peacock and T. J. R. Weakley, J. Chem. Soc. A, 1971, 1836–1839 RSC.
- G. Blasse, G. J. Dirksen and F. Zonnevijlle, J. Inorg. Nucl. Chem., 1981, 43, 2847–2853 CrossRef CAS.
- B. S. Bassil, U. Kortz and Z. Anorg, Allg. Chem., 2010, 636, 2222–2231 CrossRef.
- R. Wang, P. Y. Yu, J. Y. Tan, Y. Zhou and J. Zhang, Polyoxometalates, 2022, 1, 9140009 CrossRef.
- B. R. D. Peacock and T. J. R. Weakley, J. Chem. Soc. A, 1971, 1836–1839 RSC.
- M. T. Pope, Handbook on the Physics and Chemistry of Rare Earths, 2008, pp. 337–382 Search PubMed.
- B. S. Bassil and U. Kortz, CrystEngComm, 2015, 17, 8175–8179 RSC.
- C. Ritchie and C. Boskovic, Polyoxometalate Chemistry: Some Recent Trends, 2013, pp. 201–241 Search PubMed.
- T. Yamase and H. Naruke, Coord. Chem. Rev., 1991, 111, 83–90 CrossRef CAS.
- T. Yamase, Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 2009, pp. 297–356 Search PubMed.
- E. G. Moore, A. P. S. Samuel and K. N. Raymond, Acc. Chem. Res., 2009, 42, 542–552 CrossRef CAS PubMed.
- S. R. Li, W. D. Liu, L. S. Long, L. S. Zheng and X. J. Kong, Polyoxometalates, 2023, 2, 9140022 CrossRef.
- H. Wu, R. Wan, Y. Si, P. Ma, J. Wang and J. Niu, Dalton Trans., 2018, 47, 1958–1965 RSC.
- K. Zheng, Y. Ye, Y. Shi, Y. Xu, Z. Yang, P. Ma, J. Wang and J. Niu, Inorg. Chem., 2022, 61, 15871–15879 CrossRef CAS PubMed.
- Y. Zhang, D. Wang, B. Zeng, L. Chen, J. Zhao and G. Yang, Dalton Trans., 2020, 49, 8933–8948 RSC.
- H. Chen, K. Zheng, C. Chen, Y. Zhu, P. Ma, J. Wang and J. Niu, Inorg. Chem., 2022, 61, 3387–3395 CrossRef CAS PubMed.
- H. Li, X. Xu, Z. Tang, J. Zhao, L. Chen and G. Yang, Inorg. Chem. Front., 2021, 60, 18065–18074 CrossRef CAS PubMed.
- Z. Liang, H. Wu, V. Singh, Y. Qiao, M. Li, P. Ma, J. Niu and J. Wang, Inorg. Chem., 2019, 58, 13030–13036 CrossRef CAS PubMed.
- C. Zhang, R. C. Howell, D. McGregor, L. Bensaid, S. Rahyab, M. Nayshtut, S. Lekperic and L. C. Francesconi, C. R. Chim., 2005, 8, 1035–1044 CrossRef CAS.
- H. Wu, B. Yan, R. Liang, V. Singh, P. Ma, J. Wang and J. Niu, Dalton Trans., 2020, 49, 388–394 RSC.
- X. Xu, H. Li, S. Xie, L. Mei, R. Meng, L. Chen and J. Zhao, Inorg. Chem., 2020, 59, 648–660 CrossRef CAS PubMed.
- X. Xu, C. Lu, S. Xie, L. Chen and J. Zhao, Dalton Trans., 2020, 49, 12401–12410 RSC.
- H. Hu, J. Pang, P. Gong, L. Chen and J. Zhao, Inorg. Chem., 2020, 59, 11287–11297 CrossRef CAS PubMed.
- Y. Chen, L. Sun, S. Chang, L. Chen and J. Zhao, Inorg. Chem., 2018, 57, 15079–15092 CrossRef CAS PubMed.
- J. Jiang, Y. Chen, L. Liu, L. Chen and J. Zhao, Inorg. Chem., 2019, 58, 15853–15863 CrossRef CAS PubMed.
- H. Wu, M. Zhi, V. Singh, H. Li, P. Ma, J. Niu and J. Wang, Dalton Trans., 2018, 47, 13949–13956 RSC.
- Y. Zhang, Y. Liu, X. Xu, L. Chen and J. Zhao, Chem. – Asian J., 2020, 15, 1156–1166 CrossRef CAS PubMed.
- H. Wu, B. Yan, H. Li, V. Singh, P. Ma, J. Niu and J. Wang, Inorg. Chem., 2018, 57, 7665–7675 CrossRef CAS PubMed.
- H. Wu, M. Zhi, H. Chen, V. Singh, P. Ma, J. Wang and J. Niu, Spectrochim. Acta, Part A, 2019, 223, 117294 CrossRef CAS PubMed.
- J. Liu, M. Jin, L. Chen and J. Zhao, Inorg. Chem., 2018, 57, 12509–12520 CrossRef CAS PubMed.
- M. Liu and S. Guyot, J. Phys. Chem. B, 2005, 109, 22192–22200 CrossRef CAS PubMed.
- Q. Fu, G. Ran and W. Xu, Nano Res., 2019, 9, 3247–3256 CrossRef.
- Y. Jiang, Y. Zhao, X. Xu, K. Lin and D. Wang, RSC Adv., 2016, 6, 77481–71488 RSC.
- J. Zhao, P. Xu, Y. Li, J. Wu, J. Xue, Q. Zhu, X. Lu and W. Ni, Nanoscale, 2016, 8, 5417–5421 RSC.
- Q. Han, J. Liu, Y. Wen, L. Chen, J. Zhao and G. Yang, Inorg. Chem., 2017, 56, 7257–7269 CrossRef CAS PubMed.
- A. M. Kaczmarek, D. Ndagsi, I. V. Driessche, K. VanHecke and R. V. Deun, Dalton Trans., 2015, 44, 10237–10244 RSC.
- Y. Liu, G. Liu, J. Wang, X. Dong and W. Yu, Inorg. Chem., 2014, 53, 11457–11466 CrossRef CAS PubMed.
- J. Huang, W. You, G. Gong, G. Liu, P. Liu and B. Wang, Opt. Mater., 2019, 88, 534–539 CrossRef CAS.
- G. Wu, Y. Zhang, S. Kang, Z. Yu, X. Wang, D. Jin and L. Wang, Optik, 2021, 229, 166271 CrossRef CAS.
- Y. Zhou, B. Yan and X.-H. He, J. Mater. Chem. C, 2014, 2, 848–855 RSC.
- Y. Zhang, W. Gong, J. Yu, Z. Cheng and G. Ning, RSC Adv., 2016, 6, 30886–30894 RSC.
- Z. X. Yang, X. W. Liang, D. M. Lin, Q. J. Zheng and Y. Huo, Inorg. Chem., 2023, 62, 1466–1475 CrossRef CAS PubMed.
- P. Ma, R. Wan, Y. Si, F. Hu, Y. Wang, J. Niu and J. Wang, Dalton Trans., 2015, 44, 11514–11523 RSC.
- H. Li, Y. Liu, J. Liu, L. Chen, J. Zhao and G. Yang, Chem. – Eur. J., 2017, 23, 2673–2689 CrossRef CAS PubMed.
- Y. Zhang, Y. Li, J. Pang, Y. Liu, P. Li, L. Chen and J. Zhao, Inorg. Chem., 2019, 58, 7078–7090 CrossRef CAS PubMed.
- X. Yan, T. R. Cook, P. Wang, F. Huang and P. J. Stang, Nat. Chem., 2015, 7, 342–348 CrossRef CAS PubMed.
- H. Wu, H. Chen, M. Fu, R. Li, P. Ma, J. Wang and J. Niu, Dyes Pigm., 2019, 171, 107696–107706 CrossRef CAS.
- Y. C. Dai, S. Y. Zhang, X. X. Xiao, M. J. Li, J. C. Liu, L. J. Chen and J. W. Zhao, Polyoxometalates, 2023, 2, 9140041 CrossRef.
- D. Wang, Y. Li, Y. Zhang, X. Xu, Y. Liu, L. Chen and J. Zhao, Inorg. Chem., 2020, 59, 6839–6848 CrossRef CAS PubMed.
- T. Gong, S. Yang, Z. Wang, M. Li, S. Zhang, J. Liu, L. Chen and J. Zhao, Inorg. Chem. Front., 2023, 10, 2799–2810 RSC.
- S. Yang, T. Gong, Y. Dai, X. Xiao, J. Liu, L. Chen and J. Zhao, Inorg. Chem., 2023, 62, 17861–17869 CrossRef CAS PubMed.
- P. Ma, F. Hu, R. Wan, Y. Huo, D. Zhang, J. Niu and J. Wang, J. Mater. Chem. C, 2016, 4, 5424–5433 RSC.
- J. Luo, G. Jin, F. Zhang, Y. Liu, L. Chen, S. Xie and J. Zhao, Eur. J. Inorg. Chem., 2018, 143–152 CrossRef CAS.
- J. D. Compain, P. Deniard, R. Dessapt, A. Dolbecq, O. Oms and F. Secheresse, Chem. Commun., 2010, 46, 7733–7735 RSC.
- Z. Zhou, D. Zhang, L. Yang, P. Ma, Y. N. Si, U. Kortz, J. Niu and J. Wang, Chem. Commun., 2013, 49, 5189–5191 RSC.
- D. A. Judd, J. H. Nettles, N. Nevins, J. P. Snyder, D. C. Liotta, J. Tang, J. Ermolieff, R. F. Schinazi and C. L. Hill, J. Am. Chem. Soc., 2001, 123, 886–897 CrossRef CAS PubMed.
- J. D. Compain, P. Mialane, J. Marrot, F. Sécheresse, W. Zhu, E. Oldfield and A. Dolbecq, Chem. – Eur. J., 2010, 16, 13741–13748 CrossRef CAS PubMed.
- J. Zhao, H. Li, X. Ma, Z. Xie, L. Chen and Y. Zhu, Sci. Rep., 2016, 6, 26406 CrossRef CAS PubMed.
- T. Guo, Y. Lin, W. Zhang, J. Hong, R. Lin, X. Wu, J. Li, C. Lu and H. Yang, Nanoscale, 2018, 10, 1607–1612 RSC.
- G. Liu, Y. Chen, M. Jia, Z. Sun, B. Ding, S. Shao, F. Jiang, Z. Fu, P. Ma and J. Lin, Dalton Trans., 2019, 48, 10537–10546 RSC.
- C. Carrillo-Carrion, A. Escudero and W. J. Parak, Trends Anal. Chem., 2016, 84, 84–96 CrossRef CAS.
- M. Laguna, A. Escudero, N. O. Núñez, A. I. Becerro and M. Ocaña, Dalton Trans., 2017, 46, 11575–11583 RSC.
- Y. Tu, S. Zhao, D. He, T. Wu, H. Zhang, R. Lei, L. Huang and S. Xu, J. Mater. Chem. C, 2018, 6, 7063–7069 RSC.
| This journal is © The Royal Society of Chemistry 2024 |
