 Open Access Article
Open Access ArticleCluster helicates: promising functional materials
Sandra
Fernández-Fariña
 a,
Uxía
Barreiro-Sisto
b,
Miguel
Martínez-Calvo
a,
Uxía
Barreiro-Sisto
b,
Miguel
Martínez-Calvo
 a,
Rosa
Pedrido
a,
Rosa
Pedrido
 a and
Ana M.
González-Noya
a and
Ana M.
González-Noya
 *a
*a
aDepartamento de Química Inorgánica, Facultade de Química, Campus Vida, Universidade de Santiago de Compostela, Santiago de Compostela, Galicia E-15782, Spain. E-mail: ana.gonzalez.noya@usc.es
bDepartamento de Química Inorgánica, Facultade de Ciencias, Campus Terra, Universidade de Santiago de Compostela, E-27002, Lugo, Spain
First published on 10th October 2024
Abstract
Cluster helicates are a new type of supramolecular architecture that consists of a metal cluster wrapped around by ligands arranged in a helical mode. This class of compounds combines the unique properties of metal clusters with the sophisticated chiral arrangement found in helicates. Therefore, these architectures show promising applications in the development of advanced materials with specific electronic, magnetic, optical, catalytic and biological properties. This review delves into a few pioneering studies on cluster helicates and their applications as nanomagnetic, nanoelectric and catalyst materials. Furthermore, it emphasises the necessity for further exploration in this area and the fields related to the functional applications that cluster helicates can bring to the future of materials science.
Introduction
Supramolecular Chemistry is defined as “Chemistry beyond the molecule”. The interest in this discipline began in the late 20th century when the Nobel Prize in Chemistry was awarded to Lehn, Cram, and Pedersen for the development of Supramolecular Chemistry as a new field of study within Chemistry.1The main objective of Supramolecular Chemistry is the design and study of various systems formed by the spontaneous assembly of two or more components through non-covalent interactions, such as hydrogen bonds, van der Waals forces, or π–π interactions.2–4 These types of interactions are also present in biological systems such as proteins or DNA, being essential for the development of their main functions.5 In addition to non-covalent interactions, the coordinate bonds between organic strands and metal ions have enlarged the arsenal of supramolecular assemblies available for chemists.
The concept of helicity, a fundamental motif in nature that has fascinated scientists for centuries, continues to drive the pursuit of mimicking its intricate structures within the laboratory. This property provides valuable insights into the organization and function of complex molecules and materials. The intrinsic chirality associated with helicity can significantly influence the behavior and properties of these systems. Among the wide number of artificial helical architectures, helicates stand out as versatile metallosupramolecular platforms with applications in nanoscience, biomedicine, catalysis, and material sciences.
The term helicate was introduced by Jean-Marie Lehn in 1987 to describe a type of copper compounds that exhibit a helical architecture like the double helix structure of DNA.6 A helicate is composed of one or more organic ligands that coil helically around a series of metal ions, which define the axis of the helix.7
A significant advance in supramolecular helicates was the emergence of cluster helicates, a new type of metallohelicate complexes that were first described in 2005 by our research group (Fig. 1).8 This class of molecular architectures combines the distinct attributes of metal clusters with the sophisticated chiral arrangement found in metallohelicates.
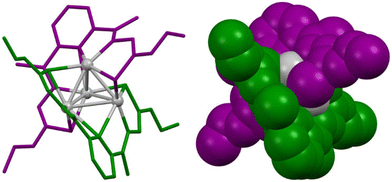 | ||
| Fig. 1 Ball-and-stick (left) and spacefill (right) representation of the first reported cluster helicate, a double-stranded silver(I) tetranuclear cluster helicate, with the two ligand strands colored in green and purple and the metal core shown in grey. Adapted from ref. 8. | ||
In this Frontier article we have included the limited examples of cluster helicates with a central metal core, but also the examples of compounds referred to in literature as cluster helicates, which show a cluster unit formed for a set of metals with co-ligands, where the organic ligands are coordinated in a helical arrangement.
Since their establishment, cluster helicates have been the subject of significant attention due to their remarkable properties and promising applications, with only a few examples reported nowadays in the literature. In this Frontier article, we explore the progress made in cluster helicates research, offering some insights into their promising applications as functional materials. The article is organized into different sections: we start by presenting examples emphasizing synthetic strategies for the development of cluster helicates. This is followed by an exploration of their magnetic and electrical properties, concluding with examples of cluster helicates exhibiting catalytic activity. This structured approach aims to provide an overview of the current state of the field, highlighting the potential of these systems for future applications.
Exploring routes to cluster helicates
As previously stated, the first examples of cluster helicates were reported in 2005 by our research group.8 In this pioneering work, a bisthiosemicarbazone ligand was employed together with an electrochemical synthesis and a Cu(I) salt precursor to obtain tetranuclear copper and silver neutral cluster helicates, respectively. The proposed route of obtaining neutral cluster helicates was tested again lately. This additional study confirmed that minor changes in the structure of the organic precursors do not prevent the formation of tetranuclear cluster helicates.9 Furthermore, it could be probed that electrochemical synthesis is also a convenient methodology to stabilize copper(I) and silver(I) cluster helicates.The first scientific articles focused on this topic found in the literature were mainly centred on the exploration of synthetic pathways for the obtaining of helicate clusters or derivatives. Thus, in 2006, Kaizaki et al. developed a new synthetic route for ionic cluster helicates by introducing a [FeII3O]4+ metal core into two mononuclear [FeIIL3]− units, giving rise to the first pentanuclear bis(triple-helical) iron cluster helicate, in which a [FeII3O]4+ core is wrapped by two terminal [FeIIL3]− units.10
In 2008, Li and colleagues synthesized nickel(II), zinc(II)11 and copper(I)12 ionic cluster helicates, and discovered that they could be employed as templates to obtain polymeric helices. This innovative approach enabled the transfer of helicity from the clusters to the polymeric strands. Consequently, they developed a helix-to-helix induction strategy for the design of coordination polymers from triple-stranded cluster helicates.
In 2013, Liu et al. reported the first example of a lanthanide-derived ionic cluster helicate using an anion-templated synthetic route. In this work, it was found that the size of the anion used in the complex synthesis has a significant impact on the resulting structure. Thus, when the anion is large, a circular helicate is formed. However, when the anion is smaller, a tetranuclear cluster helicate is isolated.13 Their findings demonstrated that the anion size plays an essential role in stabilising the cluster helicate architecture. This discovery highlights the importance of the selected anion in the formation and stability of complexes, which could have significant implications for the design of novel supramolecular materials.
The selective assembly of cluster helicates is a complex process, involving multiple factors. Among these, the nature of the metal ion and the ligand design are of particular importance. In the examples of cluster helicates found in the literature, transition, post-transition and lanthanide metal ions in different oxidation states have been employed. More specifically, the studies are focused on copper(I/II), silver(I), manganese(II/III), iron(II/III), nickel(II), zinc(II), cadmium(II), lanthanum(III), gadolinium(III), terbium(III), dysprosium(III) and holmium(III).
Consequently, the ligands chosen can contain soft, medium or hard donor atoms. Thus, all ligands, which have been employed so far include nitrogen donor atoms and either sulphur or oxygen atoms, depending on the nature of the metal ion (hard or soft Lewis acid) to be stabilized. It must be noted that most of the reported cluster helicates involve hard metal ions. The only examples stabilised with ligands containing sulphur donor atoms are those derived from bisthiosemicarbazone, prepared in our research group.8,9,22 Regarding ligand design, the great majority of the ligand strands conducting to cluster helicates contain a spacer and two binding domains to ensure the helical wrapping of the ligands around the set of metal ions. It is important to emphasize that most of the cluster helicate examples reported and discussed in this review feature co-ligands such as: μ-O, μ-OH, μ-OH2, μ-Cl, μ-Br, μ-I, acac, PhCOO−, NO3−, ClO4−, CN−, DMF and MeOH.
Table 1 summarizes the types of cluster helicates reported in the literature, including the parent ligands, the metal ions used, co-ligands if exist, the core nuclearity and the relevance commented along this work.
| Ligand | Metal ion | Co-ligands | Cluster core nuclearity | Relevance |
|---|---|---|---|---|
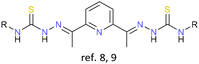
|
Cu+, Ag+ | None | {M4} cores | First examples and route to obtain cluster helicates |
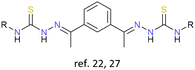
|
Cu+, Ag+ | None | {Cu4|Cu6} cores,22 {Ag4} core27 | Copper(I) cluster helicates as tyrosinase models;22 first example of desulfurization of a cluster helicate catalysed by metal ions27 |
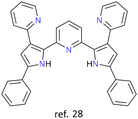
|
Ag+ | None | {Ag4} core | Hierarchical assembly of enantiomeric cluster helicates evocative of secondary structures of proteins |
|
Mn2+ | None | {Mn3} core | Ferromagnetic intramolecular magnetic interactions |
|
Mnn+ | μ-O | {Mn2[Mn3(μ3-O)]} core | Modulable oxidation states that may facilitate redox processes during catalytic oxidation of water |
|
Mnn+ | μ-O | {Mn2[Mn3(μ3-O)]} core | Modulable oxidation states that may facilitate redox processes during catalytic oxidation of water |
|
Mnn+ | μ-O | {Mn2[Mn3(μ3-O)]} core | Modulable oxidation states that may facilitate redox processes during catalytic oxidation of water |
|
Ga3+, Tb3+, Dy3+, Ho3+ | acac, μ-OH, μ-OH2 | {M2[M6(μ3-OH)4(μ4-OH)2(μ2-H2O)2]} octanuclear core | Ln8 cluster helicate reported for first time; magnetocaloric effect and slow magnetic relaxation properties |
|
Dy3+ | DMF, MeOH, PhCOO−, NO3− | {Dy4} core, {Dy6} core, {[Dy5Na(μ3-OH)2(NO3)3]2} core | Cluster helicates exhibit zero-field slow relaxation behavior expected for SMM features |
|
La3+ | MeOH, ClO4−, NO3− | {[La2(CH3OH)]3} core, {La4NO3} core | Anion templates processes conducting to cluster helicates |
|
Cu2+, Zn2+ | μ-OH | {M[M3(μ3-OH)]2} core | Selective catalysis in the peroxidative oxidation of C–H alkanes bonds to ketones |
|
Mn2+, Fe2+, Cd2+ | μ-O, μ-Cl, μ-Br, μ-I | {M2[M3(μ3-Cl)]2} core for cluster helicates, where M = Mn2+, Cd2+; {Fe2[Fe3(μ3-O)(μ-Cl)3]} cluster mesocate | Construction of novel triple-stranded cluster helicates/mesocates templated by a [MII3(μ3-O/X)]n+ triangle core |
|
Mnn+, Fe2+|3+, Ni2+, Cu2+, Zn2+ | μ-O | {FeII2[FeII3(μ3-O)]},10 {M2[M3(μ-OH)]} for M = Ni2+, Cu2+ and Zn2+,11,14 {MnII2[MnII2MnIII(μ-O)]}, {MnII2[MnIIMnIII2(μ-O)]},23 {FeII4[FeIII(μ3-O)]},25 cores | Synthetic route to ionic cluster helicates;10 ionic cluster helicates as template for polymeric helices;11 first time that a cluster helicate exhibits spin-frustration;14 interesting redox behavior for catalysis;23 pentanuclear iron complex that catalyses water oxidation25 |
|
Cu+ | CN− | {Cu5} core | Polymerization of cluster helicate cores |
|
Fe2+ | μ-O | {Fe2[Fe3(μ3-O)]} core | Spin-transition cluster systems that exhibit mixed-spin structures and synergy between spin transitions and magnetic interaction |
Cluster helicates as nanomagnetic or nanoelectric materials
From 2010 onwards, the magnetic properties of various cluster helicates started to be investigated due to their significant relevance arising from the interactions that can occur in metal clusters. For instance, Kawata and co-workers were the first to prepare a pentanuclear ionic copper(II) cluster helicate, which exhibited the rare and significant magnetic phenomenon of spin-frustration. This achievement set a new milestone in the field of molecular magnetism.14 Besides, Tong et al. used a pyridine-derived ligand to isolate four iron cluster helicates. Their work demonstrated that the spin states of the two apical [FeII(ligand)3]− units could be specifically modulated transitioning to the paramagnetic high spin state in the presence of a [Fe2OCl6]2− anion, whereas diamagnetic low spin states were observed with NCS−, ClO4−, and I− anions.15 These findings highlight the remarkable tunability of spin states and certain synergy between spin transitions and magnetic interaction by introducing different counteranions to the pentanuclear {Fe5} cluster helicate compounds.Following the line of cluster helicates with magnetic properties, in 2011, Rodríguez-Blanco et al. published a trinuclear ionic manganese(II) cluster helicate from a pyridine-derived ligand, exhibiting intramolecular ferromagnetic interactions.16 In 2013 Ruiz and co-workers reported the synthesis of manganese and cadmium triple-stranded cluster helicates templated by a [MII3(μ3-O/X)]n+ metal core that displays antiferromagnetic coupling. This finding revealed that the sizes in the triangle core and the cavity formed within the macromolecule determine if an octanuclear ionic cluster helicate is assembled instead of a pentanuclear mesocate depending on the anions allocated inside the cavity.17
The precise tuning of the spin state of metal ions represents a critical process that significantly contributes to the development of advanced materials with a wide range of applications, including molecular switches, sensors, and display devices. Tong et al. proceeded to deeply investigate this topic after their article was published in 2010.15 In 2016, they successfully introduced the spin-crossover (SCO) property into cluster helicates containing one apical [FeII(ligand)3]− unit that exhibits SCO behaviour (Fig. 2). This complex is composed by a [{Fe(μ-bpt)3}2Fe3(μ3-oxo)]2+ cation unit and one [{Fe2(μ-Cl)}(μ-bpt)(NCS)4(H2O)]2− anion. The pentanuclear cation contains a rare planar [FeII3(μ3-oxo)]4+ core. This cluster helicate displays six monoanionic bpt ligands wrapped around the pentanuclear cluster giving rise to two enantiomerically pure [Fe(μ-bpt)3]− units. This was the first example of a spin-crossover cluster helicate, a material that can switch between high-spin and low-spin, setting a new precedent in the field.18
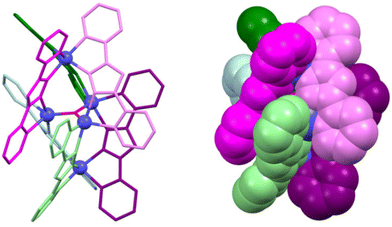 | ||
| Fig. 2 Ball-and-stick (left) and spacefill (right) representation of a cationic pentanuclear iron(II) cluster helicate with spin-crossover (SCO) behaviour. The anion is omitted for clarity. Adapted from ref. 18. | ||
The second example of SCO iron cluster helicates was reported in 2018 by Yan and co-workers,19 being the first occasion in which these compounds were explored as multifunctional materials. The same year, the first octanuclear cationic cluster helicates derived from gadolinium, terbium, dysprosium and holmium ions and Schiff base ligands were reported.20 The magnetic properties of these lanthanide-based cluster helicates were studied: the dysprosium cluster helicate exhibited slow magnetic relaxation, whereas the gadolinium cluster demonstrated a magnetocaloric effect, opening new horizons for advanced magnetic materials. Following this idea, Tang et al. synthetized a family of dysprosium-derived cluster helicates from a Schiff base ligand. They were able to establish a clear relationship between the synthetic conditions and the nuclearity of the cluster helicates. Besides, the magnetic properties of these helicates were studied, showing a zero-field slow relaxation behaviour, representative of single-molecule magnets SMM.21 These findings are set to transform the potential of cluster helicates as magnetic multifunctional materials.
Cluster helicates as catalyst materials
The exploration of cluster helicates as catalysts offer new potentialities for this type of architectures. Thus, in 2010 Bermejo and Casella designed and synthesized a double tetranuclear and a triple-stranded hexanuclear copper(I) cluster helicate from a thiosemicarbazone ligand. These cluster helicates can be considered as novel tyrosinase models, an enzyme that catalyzes the hydroxylation of aromatic compounds. This work demonstrated that these cluster helicates could efficiently hydroxylate the arene linker in the presence of O2. This process resulted in the formation of a dinuclear copper(II) complex, exhibiting a strong antiferromagnetic coupling between the two Cu(II) ions.22 The search for synthetic models for metalloenzymes has high scientific importance, not only due to their biological significance but also because of the potential industrial applicability of metal complexes in catalysing the transformation of organic substrates.In 2011, Collomb and collaborators reported the synthesis of a pentanuclear manganese cluster helicate, obtained through the previously described synthetic strategy of introducing a metal core into molecular units. This compound exhibits complex redox behaviour and remarkable stability in solution across four distinct oxidation states. This versatility suggests that cluster helicates may serve as promising candidates for catalysis involving oxidation processes.23
Subsequently, Cui and colleagues reported copper(II) and zinc(II) heptanuclear triple-stranded cluster helicates that contain a heptametallic dicubane core (Fig. 3). The structure of these compounds can be described as linear hexanuclear triple-stranded helicates encapsulating a single metal ion within its cavity. The metal ions are arranged into two M4O4 distorted cubane units, sharing one metal ion. This architecture of the dicubane cluster and hexadentate L ligands leads to the formation of a P-configured triple-stranded helicate. The helical arrangement of the hexanuclear complexes favours the formation of a hydrophobic pocket with a metal cluster containing a coordinatively unsaturated metal centre. Considering the structure of this cluster helicate, its catalytic activity was explored, demonstrating selectivity in the peroxidative oxidation of alkanes to alcohols and ketones.24 The authors also state that this type of compound could mimic the active site of metalloenzymes.
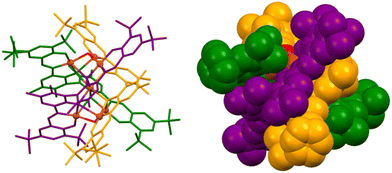 | ||
| Fig. 3 Ball-and-stick (left) and spacefill (right) representation of a triple-stranded heptanuclear copper(II) cluster helicate that performs selective oxidation of alkenes to ketones. Adapted from ref. 24. | ||
Furthermore, understanding the redox behaviour of cluster complexes is crucial to improve their electronic properties. In this context, Masaoka et al. published their work in Nature on a pentanuclear iron cluster helicate capable of catalysing the oxidation of water, although with a high overpotential.25 With the aim of improving the catalytic activity of this type of system, Krewald and Pantazis isolated the analogue pentanuclear manganese cluster helicate derived from a 3,5-bis(pyridin-2-yl)-pyrazol ligand. Their research focused on the influence of donor or acceptor groups within the ligand skeleton to achieve the redox potential required for water oxidation. The manganese system is compared with its pentanuclear iron analogue, which was reported to be catalytically active in oxygen evolution. While the iron complex demonstrates promising activity, manganese systems exhibit a broader range of oxidation states, presenting valuable opportunities for redox tuning in catalytic applications. They also concluded that introducing steric impediment is essential for changing the range of these potentials. The manganese complex derived from a negatively charged ligand reached higher oxidation states than those found in biological water oxidation.26 These works illustrate a key advantage of cluster helicates over non-cluster analogues, because their multiple metal centers could provide distinct and modulable oxidation states that may facilitate redox processes along the catalysis.
More recently, our research group reported the first example of a silver double-tetranuclear cluster helicate obtained from an electrochemical methodology.27 This cluster helicate, derived from a bisthiosemicarbazone ligand, undergoes a desulfurization process catalysed by metal ions that evolves into a cationic dinuclear helicate with a sulfate anion. This finding opens a new route for the interconversion between different helical structures.
The most recent article about cluster helicates found in the literature is from 2023. In this article, Luo et al. report tetranuclear silver cluster helicates (Fig. 4) that undergo hierarchical chiral assembly to generate a sheet structure that evocates the secondary structures of proteins.28 This particular architecture opens the pathway for increasing the nuclearity of coinage cluster helicates. By designing suitable ligands, we can create more protective environments, providing new possibilities for future research and applications.
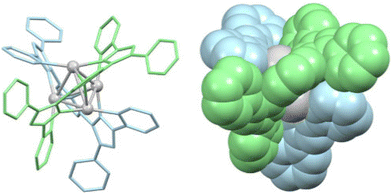 | ||
| Fig. 4 Ball-and-stick (left) and spacefill (right) represent a tetranuclear Ag(I) double-stranded cluster helicate that undergoes hierarchical assembly to generate a folded sheet. Adapted from ref. 28. | ||
The last two examples, along with Nitschke's supramolecular cages,7 demonstrate the potential of using silver ions to significantly enhance the diversity of cluster helicates. Besides, there is still ample opportunity to improve our understanding of the supramolecular evolution of cluster helicate architectures and the assemblies that could be formed.
Conclusions and outlook
In this Frontier article, we have deeply reviewed the recent progress in the development of cluster helicates. Considering the limited results found in the literature, we can state that this field remains significantly underexplored. The study of the unique properties of these architectures holds significant potential and needs further investigation.Despite the pathways reported to prepare cluster helicates, further investigation is required to better define the precise design factors to obtain them in a selective mode. One particularly intriguing area for future research is the exploration of their biological properties. In this sense, the architecture size and external surface of cluster helicates could be conveniently modulated to interact with biomolecules, such as proteins or different DNA supramolecular structures. Understanding how helicate clusters interact with biological systems could lead to new advances in drug delivery, bioimaging, and the development of novel metallodrugs. Furthermore, their potential applications as materials are vast and diverse. As demonstrated, cluster helicates could play a crucial role in the development of advanced materials with specific electronic, optical, magnetic, and catalytic properties. These materials could be utilized in nanotechnology, sensor technology, and sustainable energy solutions, among other areas. Overall, the potential for discovery in the field of helicate clusters is vast, and continued research could provide numerous advancements with multiple applications.
Author contributions
The manuscript was written, reviewed and edited through contributions of all authors that have given approval to the final version of the manuscript.Data availability
No primary research results, code or software have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the following FEDER co-funded grants: From Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia GRC GI-1584 (ED431C 2023/02). From Ministerio de Ciencia e Innovación, Project PID2021-127531NB-I00 (AEI/10.13039/501100011033/FEDER, UE) and RED2022-134091-T.References
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef
.
- M. Albrecht and M. Fiege, Coord. Chem. Rev., 2008, 252, 812–824 CrossRef CAS
.
- J. M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC
.
- G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483–3537 CrossRef CAS PubMed
.
- V. Vennelakanti, H. W. Qi, R. Mehmood and H. J. Kulik, Chem. Sci., 2021, 54–57 Search PubMed
.
- J. M. Lehn, A. Rigault, J. Siegel, J. Harrowfield, B. Chevrier and D. Moras, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 2565–2569 CrossRef CAS PubMed
.
- C. T. McTernan, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2021, 143, 664–670 CrossRef CAS PubMed
.
- M. R. Bermejo, A. M. González-Noya, R. M. Pedrido, M. J. Romero and M. Vázquez, Angew. Chem., 2005, 117, 4254–4259 CrossRef
.
- M. R. Bermejo, A. M. González-Noya, M. Martínez-Calvo, R. Pedrido, M. J. Romero and M. V. López, Eur. J. Inorg. Chem., 2008, 3852–3863 CrossRef CAS
.
- K. Yoneda, K. Adachi, K. Nishio, M. Yamasaki, A. Fuyuhiro, M. Katada, S. Kaizaki and S. Kawata, Angew. Chem., Int. Ed., 2006, 45, 5459–5461 CrossRef CAS PubMed
.
- J. Hou, M. Li, Z. Li, S. Zhan, X. Huang and D. Li, Angew. Chem., 2008, 120, 1735–1738 CrossRef
.
- S. Z. Zhan, M. Li, J. Z. Hou, J. Ni, D. Li and X. C. Huang, Chem. – Eur. J., 2008, 14, 8916–8921 CrossRef CAS PubMed
.
- B. Wang, Z. Zang, H. Wang, W. Dou, X. Tang, W. Liu, Y. Shao, J. Ma, Y. Li and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 3756–3759 CrossRef CAS PubMed
.
- R. Ishikawa, M. Nakano, A. Fuyuhiro, T. Takeuchi, S. Kimura, T. Kashiwagi, M. Hagiwara, K. Kindo, S. Kaizaki and S. Kawata, Chem. – Eur. J., 2010, 16, 11139–11144 CrossRef CAS PubMed
.
- X. Bao, J. D. Leng, Z. S. Meng, Z. Lin, M. L. Tong, M. Nihei and H. Oshio, Chem. – Eur. J., 2010, 16, 6169–6174 CrossRef CAS PubMed
.
- C. Rodríguez-Blanco, J. A. Rodríguez-Velamazán, J. Campo, B. Gillon, J. Sánchez Costa, P. Gamez and J. Luzón, J. Phys.: Conf. Ser., 2011, 325, 3–7 Search PubMed
.
- X. Bao, W. Liu, J. Liu, E. Ruiz and M. Tong, Inorg. Chem., 2013, 52, 1099–1107 CrossRef CAS PubMed
.
- Z. Yan, W. Liu, Y. Y. Peng, Y. C. Chen, Q. W. Li, Z. P. Ni and M. L. Tong, Inorg. Chem., 2016, 55, 4891–4896 CrossRef CAS PubMed
.
- K. H. Fan, Q. Huang, X. Y. Fang, L. W. Zhu and Z. Yan, Crystals, 2018, 8, 119 CrossRef
.
- M. X. Yao, L. Z. Cai, X. W. Deng, W. Zhang, S. J. Liu and X. Cai, New J. Chem., 2018, 42, 17652–17658 RSC
.
- J. Lu, X. L. Li, C. Jin, Y. Yu and J. Tang, New J. Chem., 2020, 44, 994–1000 RSC
.
- M. Martínez-Calvo, M. Vázquez López, R. Pedrido, A. M. González-Noya, M. R. Bermejo, E. Monzani, L. Casella and L. Sorace, Chem. – Eur. J., 2010, 16, 14175–14180 CrossRef PubMed
.
- S. Romain, J. Rich, C. Sens, T. Stoll, J. Benet-Buchholz, A. Llobet, M. Rodriguez, I. Romero, R. Clérac, C. Mathonière, C. Duboc, A. Deronzier and M.-N. Collomb, Inorg. Chem., 2011, 50, 8427–8436 CrossRef CAS PubMed
.
- Y. Fang, W. Gong, L. Liu, Y. Liu and Y. Cui, Inorg. Chem., 2016, 55, 10102–10105 CrossRef CAS PubMed
.
- M. Okamura, M. Kondo, R. Kuga, Y. Kurashige, T. Yanai, S. Hayami, V. K. K. Praneeth, M. Yoshida, K. Yoneda, S. Kawata and S. Masaoka, Nature, 2016, 530, 465–468 CrossRef CAS PubMed
.
- V. Krewald and D. A. Pantazis, Dalton Trans., 2016, 45, 18900–18908 RSC
.
- S. Fernández-Fariña, L. M. González-Barcia, M. J. Romero, J. García-Tojal, M. Maneiro, J. M. Seco, G. Zaragoza, M. Martínez-Calvo, A. M. González-Noya and R. Pedrido, Inorg. Chem. Front., 2022, 9, 531–536 RSC
.
- W.-C. Xiao, Q.-B. Nie and G.-G. Luo, Dalton Trans., 2023, 52, 6239–6243 RSC
.
| This journal is © The Royal Society of Chemistry 2024 |
