Synthesis and structures of molecular beryllium Grignard analogues featuring terminal and bridging pseudohalides†
Corinna
Czernetzki
 ab,
Merle
Arrowsmith
ab,
Merle
Arrowsmith
 ab,
Malte
Jürgensen
ab,
Stephan
Hagspiel
ab,
Malte
Jürgensen
ab,
Stephan
Hagspiel
 ab and
Holger
Braunschweig
ab and
Holger
Braunschweig
 *ab
*ab
aInstitute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: h.braunschweig@uni-wuerzburg.de
bInstitute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
First published on 25th October 2024
Abstract
The carbene-stabilised beryllium Grignards [(CAAC)BeBrR] (R = CAACH 1a, Dur 1b; CAAC/H = 1-(2,6-diisopropylphenyl)-2,2,4,4-tetramethylpyrrolidin-2-yl/idene; Dur = 2,3,5,6-tetramethylphenyl) undergo salt metathesis with various pseudohalide salt precursors. Whereas with [NaNCS] the thiocyanato Grignards [(CAAC)Be(NCS)R] (R = CAACH 2a, Dur 2b) are obtained selectively, salt metatheses with [Na(OCP)(dioxane)2.3] and [K(OCN)] are fraught with side reactions, in particular scrambling of both neutral and anionic ligands, leading to complex product mixtures, from which the first examples of beryllium phosphaethynolate Grignards [(thf)2(CAACH)Be(OCP)] (3) and [(CAAC)Be(OCP)R] (R = CAACH 4a, Dur 4b), as well as the isocyanate-bridged hexamer [(CAAC)BrBe(1,3-μ-OCN)]6 (7) were determined as the main products. The complexity of possible side reactions is seen in complex 5, a byproduct of the salt metathesis of 1b with [Na(OCP)(dioxane)2.3], which hints at radical redox processes, OCP homocoupling, OCP coupling with CAAC, as well as OCP insertion into the Be–R bond. Finally, the unstable, tetrameric cyano-bridged beryllium Grignard [(thf)(CAACH)Be(1,2-μ-CN)] (8) was obtained by salt metathesis of 1a with [Na/KSeCN] alongside one equiv. CAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se. The new complexes were characterised by heteronuclear NMR and IR spectroscopy, as well X-ray crystallography.
Se. The new complexes were characterised by heteronuclear NMR and IR spectroscopy, as well X-ray crystallography.
Introduction
Named a century ago after their chemical resemblance with the halides,1 pseudohalides have since found applications ranging from organic cross-coupling or carboxylation reagents to ionic liquids and solar cell materials.2–7 Linear pseudohalogen anions, such as CN−, N3− or NCO−, are ambiphilic and thus capable of coordinating via both ends. As a result, the homoleptic alkaline earth pseudohalides, [AeY2], are usually oligomers featuring bridging pseudohalides.8Whereas the chemistry of classical and heavier Grignard reagents, [RAeX] (R = organyl, X = halide), is one of the mainstays of organic and inorganic chemistry,9–13 that of their pseudohalide derivatives is virtually unknown. The difficulties in isolating well-defined molecular group 2 Grignard analogues of the form [LnAeRY] (L = neutral donor), featuring a terminal unsymmetrical pseudohalide ligand (e.g. CN−, NCO−, NCS−, OCP−) are threefold: (1) the ambidentate nature of unsymmetrical pseudohalides, frequently resulting in the formation of mixtures of linkage isomers or oligomers,14 (2) the tendency of heteroleptic group 2 complexes to undergo Schlenk-type ligand scrambling,15 which results in the often irreversible formation of homoleptic compounds, and (3) the propensity of some unsymmetrical pseudohalides, like CN or NCO, to undergo unwanted nucleophilic addition reactions.16
In 1974, Klopsch and Dehnicke were the first to synthesise dianionic organomagnesium pseudohalides of the form [{Me2Mg}2(μ-Y)]2− and [{Me2Mg(η1-Y)}2]2− (Y = CN, N3, NCO), as determined by IR spectroscopy.17 In 1998 Böhland reported the first neutral thiocyanate and selenocyanate Grignards [(thf)nMg(NCE)R] (IR-E, R = Et, Ph, E = S, Se, Fig. 1A), which undergo Schlenk equilibration to the homoleptic species in solution.18 Much more recently, Gilliard isolated the first phosphaethynolate Grignard, II, stabilised against ligand redistribution by two bulky N-heterocyclic carbenes (NHCs).19 To date, complex II is the only structurally characterised pseudohalide Grignard analogue.
Long neglected due to the high toxicity of its compounds,‡ the last decade has seen a revival of organoberyllium chemistry,20–22 not least because of the landmark syntheses of the first Be(0) and Be(I) complexes.23–27 As the least polarisable and smallest of the group 2 dications, Be2+ forms largely covalent bonds with carbon. As a result, beryllium-based Grignard complexes tend to be more stable than their magnesium analogues towards Schlenk equilibration.28,29 Our group recently reported the first beryllium-based pseudohalide Grignards, the cyclic alkyl(amino)carbene (CAAC)-stabilised terminal azide complexes IIIR and the imine-stabilised azide-bridged dimer IV (Fig. 1B).30 Beyond this, only a handful of molecular beryllium pseudohalides have been reported, including tetrahedral homoleptic terminal cyanide, azide and thiocyanate complexes of the form [L2BeY2] (L = pyridine (V-Y), amine, imine, NHC (VI)),30–32 as well as a heteroleptic diamine-supported chloroberyllium azide (VII),33 and the tris(pyrazolyl)borate (Tp) complexes [(Tp)BeY] (VIII-Y, Y = CN, N3, NCS).34,35
In this study we report the synthesis, spectroscopic and structural characterisation of a range of monomeric and oligomeric Lewis-base-stabilised beryllium pseudohalide Grignards, including the first structurally characterised beryllium phosphaethynolates and isocyanates.
Results and discussion
Synthesis of beryllium pseudohalides
The dropwise addition of 1 equiv. [NaNCS] in THF to 1a26 and 1b36 in THF at −78 °C, followed by stirring for 3 h at room temperature yielded, after workup, the corresponding beryllium thiocyanates 2a and 2b as yellow (75%) and colourless (58%) solids, respectively (Scheme 1a). Their 9Be NMR spectra show a broad resonance at 15.5 (ω1/2 (full width at half maximum) ≈ 420 Hz) and 14.8 (ω1/2 ≈ 210 Hz) ppm, respectively, ca. 5 ppm upfield-shifted from that of their respective precursors (δ9Be = 20.0 (1a), 18.7 (1b) ppm). Their 13C NMR Ccarbene resonances at 249.6 and 240.1 ppm, respectively, are virtually identical to that of their respective precursors (δ13C = 249.7 (1a), 240.1 (1b) ppm). The 13C NMR resonance of the NCS moiety could not be detected, presumably owing to excessive broadening caused by strong coupling with the nearby quadrupolar 9Be and 14N nuclei. Complexes 1a and 1b represent the first examples of tricoordinate beryllium thiocyanates, previous examples being limited to tetracoordinate neutral35 or ionic complexes.37 The solid-state IR spectra of 2a and 2b show a single, very intense and relatively broad N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band at 2100 and 2089 cm−1, respectively, in the same range as other molecular and ionic terminal beryllium thiocyanates (ν(N
C stretching band at 2100 and 2089 cm−1, respectively, in the same range as other molecular and ionic terminal beryllium thiocyanates (ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) = 2065–2117 cm−1).35,37 Compounds 2a/b were stable in benzene up to 80 °C.
C) = 2065–2117 cm−1).35,37 Compounds 2a/b were stable in benzene up to 80 °C.
 | ||
| Scheme 1 Synthesis of beryllium isothiocyanates and phosphaethynolates. Isolated yields in parentheses. | ||
The 9Be and 31P NMR spectra of the reaction mixture of 1a with [Na(OCP)(dioxane)2.3] showed the formation of two main products in a 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. Workup revealed the major one of these to be the colourless tetracoordinate complex [(thf)2(CAACH)Be(OCP)], present as a mixture of two isomers, 3 and 3′, in an 88
4 ratio. Workup revealed the major one of these to be the colourless tetracoordinate complex [(thf)2(CAACH)Be(OCP)], present as a mixture of two isomers, 3 and 3′, in an 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ratio, respectively (δ9Be = 7.1 (s, ω1/2 ≈ 150 Hz, 3′), 4.7 (s, ω1/2 ≈ 145 Hz, 3) ppm; δ31P = −342.6 (s) ppm, Scheme 1b).§ Recrystallisation from pentane also yielded a few crystals of the expected tricoordinate CAAC-stabilised species 4a (δ31P = −339.4 ppm) as determined by X-ray crystallography (vide infra).¶ The 1H NMR spectrum of the major rotamer, 3, confirms the presence of two THF ligands, with 8H multiplets at 3.41 and 1.90 ppm, as well as the chiral CAACH ligand with its characteristic 1H singlet at 2.68 ppm for the BeCH resonance. The terminal nature and Be–O bonding of the OCP ligand is attested by a broad 13C{1H} NMR doublet at 156.6 (1JC–P = 13.3 Hz, OCP) and a 31P{1H} singlet at −342.6 (OCP) ppm. These are upfield- and downfield-shifted, respectively, from the range of 13C{1H} and 31P{1H} NMR OCP resonances observed for molecular terminal magnesium phospaethynolates (δ13C = 161–169 ppm, δ31P = −365–(−386) ppm).19,38,39 This may be rationalised by the much higher charge density of the Be2+ dication, which draws electron density away from the terminal phosphorus atom, thus favouring the O–C
12 ratio, respectively (δ9Be = 7.1 (s, ω1/2 ≈ 150 Hz, 3′), 4.7 (s, ω1/2 ≈ 145 Hz, 3) ppm; δ31P = −342.6 (s) ppm, Scheme 1b).§ Recrystallisation from pentane also yielded a few crystals of the expected tricoordinate CAAC-stabilised species 4a (δ31P = −339.4 ppm) as determined by X-ray crystallography (vide infra).¶ The 1H NMR spectrum of the major rotamer, 3, confirms the presence of two THF ligands, with 8H multiplets at 3.41 and 1.90 ppm, as well as the chiral CAACH ligand with its characteristic 1H singlet at 2.68 ppm for the BeCH resonance. The terminal nature and Be–O bonding of the OCP ligand is attested by a broad 13C{1H} NMR doublet at 156.6 (1JC–P = 13.3 Hz, OCP) and a 31P{1H} singlet at −342.6 (OCP) ppm. These are upfield- and downfield-shifted, respectively, from the range of 13C{1H} and 31P{1H} NMR OCP resonances observed for molecular terminal magnesium phospaethynolates (δ13C = 161–169 ppm, δ31P = −365–(−386) ppm).19,38,39 This may be rationalised by the much higher charge density of the Be2+ dication, which draws electron density away from the terminal phosphorus atom, thus favouring the O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P over the O+
P over the O+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P− resonance structure. Furthermore, magnesium phospaethynolates display significantly larger 13C–31P coupling constants (1JC–P = 25–60 Hz),19,38,39 the smallest one being observed for complex II (see Fig. 1A).19 Similarly small OCP 13C–31P coupling constants to that of 3 have been observed in iPr3Si(OCP),40 a β-diketiminate-stabilised scandium OCP complex (both 1JC–P = 10 Hz),41 and an OCP-substituted 1,3-dihydrodiazaborole (1JC–P = 17 Hz).42 Smaller 13C–31P coupling constants are associated with a higher degree of phosphaethynolate O–C
P− resonance structure. Furthermore, magnesium phospaethynolates display significantly larger 13C–31P coupling constants (1JC–P = 25–60 Hz),19,38,39 the smallest one being observed for complex II (see Fig. 1A).19 Similarly small OCP 13C–31P coupling constants to that of 3 have been observed in iPr3Si(OCP),40 a β-diketiminate-stabilised scandium OCP complex (both 1JC–P = 10 Hz),41 and an OCP-substituted 1,3-dihydrodiazaborole (1JC–P = 17 Hz).42 Smaller 13C–31P coupling constants are associated with a higher degree of phosphaethynolate O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P bonding, whereas larger ones are indicative of increased phosphaketene O
P bonding, whereas larger ones are indicative of increased phosphaketene O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P bonding,43 thereby confirming that the Be–O–C
P bonding,43 thereby confirming that the Be–O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P resonance structure is highly dominant in 3. This was also confirmed by IR spectroscopy of 3/3′, which shows an intense and relatively broad asymmetric OCP stretching band at 1728 cm−1, in the lower range of those observed for terminal magnesium phosphaethynolates (ν(OCP)asym = 1727–1770 cm−1)19,38,39 and similar to that of [(18-crown-6)K(OCP)] (ν(OCP)asym = 1730 cm−1).44 Compound 3/3′ was stable in benzene up to 60 °C but decomposed unselectively upon UV irradiation.
P resonance structure is highly dominant in 3. This was also confirmed by IR spectroscopy of 3/3′, which shows an intense and relatively broad asymmetric OCP stretching band at 1728 cm−1, in the lower range of those observed for terminal magnesium phosphaethynolates (ν(OCP)asym = 1727–1770 cm−1)19,38,39 and similar to that of [(18-crown-6)K(OCP)] (ν(OCP)asym = 1730 cm−1).44 Compound 3/3′ was stable in benzene up to 60 °C but decomposed unselectively upon UV irradiation.
The reaction of 1b with [Na(OCP)(dioxane)2.3] proved much less selective. The 9Be NMR spectrum of the crude reaction mixture after 30 min at room temperature evidenced the formation of a least two tricoordinate (δ9Be = 17.4 (br), 14.1 (br) ppm) and two tetracoordinate (δ9Be = 4.7 (s), 1.8 (s) ppm) beryllium complexes.45 The two main products observed by 31P NMR spectroscopy feature a sharp singlet at −328.6 (ca. 46%) ppm, which fits a phosphaethynolate complex, and a very broad resonance at −343.1 ppm (ω1/2 ≈ 320 Hz, ca. 36%), suggesting Be–P bonding, respectively, alongside several other minor unidentified products. Furthermore, the unexpected intense colouration of the reaction mixture (first dark purple, then dark brown), usually the hallmark of radical CAAC complexes,23,26,27,46 prompted us to measure a solution EPR spectrum. The latter indeed showed the presence of at least three radical species, none of which could be identified further, but which highlight that this seemingly simple salt metathesis is accompanied by complex redox processes.
Recrystallisation of the crude product mixture by diffusion of hexane into a saturated benzene solution yielded a few crystals of the tricoordinate beryllium phosphaethynolate 4b, which was identified as the major reaction product (δ9Be = 14.1 (br, ω1/2 ≈ 470 Hz) ppm; δ31P = −328.6 (s) ppm).||4b systematically cocrystallised with 30% of another tetracoordinate beryllium complex (6, δ9Be = 1.4 (br, ω1/2 ≈ 280 Hz) ppm; δ31P = −339.6 (s) ppm), containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Dur and terminal OCP substituents, as determined by 1H and 31P NMR spectroscopy. Unfortunately, the constitution of this complex could not be elucidated further. Further crystallisation yielded a few crystals suitable for X-ray diffraction analysis of an unexpected side-product, complex 5 (see Fig. S30 in the ESI†), which evidences the insertion of an OCP fragment into the Be–Dur bond, coupling of two PCO ligands via P–C bond formation, as well as CCAAC–CPCO and CCAACH–PPCO bond formation, the CAACH residue suggesting the involvement of a CAAC radical and hydrogen atom abstraction.
1 ratio of Dur and terminal OCP substituents, as determined by 1H and 31P NMR spectroscopy. Unfortunately, the constitution of this complex could not be elucidated further. Further crystallisation yielded a few crystals suitable for X-ray diffraction analysis of an unexpected side-product, complex 5 (see Fig. S30 in the ESI†), which evidences the insertion of an OCP fragment into the Be–Dur bond, coupling of two PCO ligands via P–C bond formation, as well as CCAAC–CPCO and CCAACH–PPCO bond formation, the CAACH residue suggesting the involvement of a CAAC radical and hydrogen atom abstraction.
The lack of selectivity of the reaction between 1b and [Na(OCP)(dioxane)2.3] reflects the multitude of possible side-reactions when attempting to synthesise main group phosphaethynolates, including ether cleavage of the dioxane ligands of [Na(OCP)(dioxane)2.3],19 OCP coupling,42,47 CAAC–PCO coupling,48,49 aryl transfer to the phosphorus atom from an s-block aryl reagent,42,50 photoinduced CO extrusion with radical P–P coupling,42,51,52 or photoinduced [PCO]˙ extrusion.51 Similar light-induced redox processes might be at play here and responsible for the radical species observed by EPR spectroscopy.
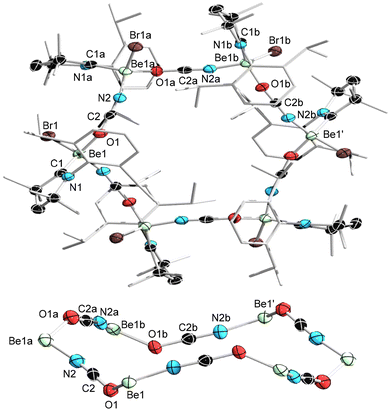
Attempts to obtain an isocyanate derivative of 1a by salt metathesis with [KOCN] at 60 °C yielded a mixture of four tetracoordinate beryllium complexes (δ9Be = 8.8, 7.0, 4.0, 2.0 ppm) in various ratios depending on the reaction time. Multiple attempts to improve the selectivity of the reaction by changing equivalents, solvent, temperature and reaction time failed. Recrystallisation of the crude product mixture from benzene yielded a few colourless single crystals of the hexameric complex 7, which is comprised of six [(CAAC)BeBr(OCN)] units, the OCN ligands bridging between two adjacent beryllium centres, thus forming a 24-membered (BeOCN)6 ring (Scheme 2a). It is noteworthy that 7 has lost the CAACH substituent but still contains an unreacted bromide substituent, suggesting the involvement of temperature- and/or sterics-induced Schlenk-type equilibria, which have been observed in beryllium Grignard analogues, albeit to a lesser degree than their magnesium counterparts.28,29 Attempts to obtain 7 more selectively by salt metathesis with (CAAC)BeBr2, however, proved unsuccessful. Taking into account potential ligand exchange processes and steric effects, the other products observed by 9Be NMR spectroscopy are likely to be either similarly OCN-bridged hexamers of the form [LBeY(1,3-μ-OCN)]6 (L = CAAC, Y = η1-OCN; L = THF, Y = CAACH), or dimeric [(THF)Be(OCN)]2, by analogy to Buchner's [(Me2S)Be(η1-OCN)(μ-OCN)]2 (δ9Be = 7.0 ppm),35 or mononuclear terminal beryllium isocyanates of the form [(THF)2BeY(η1-OCN)] (Y = CAACH, η1-OCN), akin to complex 3 and VIII-OCN (see Fig. 1B).35
Finally, the salt metathesis of 1a with the heavier isocyanate analogues [NaSeCN] and [KSeCN] proceeded selectively at room temperature in THF under elimination of CAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se,53 to yield a single beryllium-containing product with a broad 9Be NMR resonance at 3.4 ppm (ω1/2 ≈ 440 Hz). Recrystallisation from pentane at −30 °C yielded light brown crystals of the tetramer 8, which is comprised of four [(THF)Be(CAACH)(CN)] units, the CN ligands bridging between two adjacent beryllium centres, thus forming a 12-membered (BeCN)4 ring (Scheme 2b). The 9Be NMR shift of 8 is slightly downfield-shifted from those observed for the coordination isomers VIII-CN and VIII-NC at 1.9 and 1.6 ppm, respectively.35 Complex 8 proved extremely sensitive, even decomposing on the diffractometer at −173 °C. A full in-situ NMR-spectroscopic characterisation of the reaction mixture was marred by the fact that all four CN linkers of the (BeCN)4 ring are flip-disordered, yielding a complex mixture of isomers. Attempts to obtain a cyanoberyllium complex more directly by salt metathesis of 1a with [NaCN] or [KCN] required higher reaction temperatures (60–80 °C) and led to intractable product mixtures.
Se,53 to yield a single beryllium-containing product with a broad 9Be NMR resonance at 3.4 ppm (ω1/2 ≈ 440 Hz). Recrystallisation from pentane at −30 °C yielded light brown crystals of the tetramer 8, which is comprised of four [(THF)Be(CAACH)(CN)] units, the CN ligands bridging between two adjacent beryllium centres, thus forming a 12-membered (BeCN)4 ring (Scheme 2b). The 9Be NMR shift of 8 is slightly downfield-shifted from those observed for the coordination isomers VIII-CN and VIII-NC at 1.9 and 1.6 ppm, respectively.35 Complex 8 proved extremely sensitive, even decomposing on the diffractometer at −173 °C. A full in-situ NMR-spectroscopic characterisation of the reaction mixture was marred by the fact that all four CN linkers of the (BeCN)4 ring are flip-disordered, yielding a complex mixture of isomers. Attempts to obtain a cyanoberyllium complex more directly by salt metathesis of 1a with [NaCN] or [KCN] required higher reaction temperatures (60–80 °C) and led to intractable product mixtures.
X-ray crystallographic analyses
X-ray diffraction analyses provided the solid-state structures of 2a, 2b, 3, 4a, 4b, 5, 7 and 8. Due to excessive twinning, poor diffraction and rapid crystal decomposition the data for 8 is of insufficient quality to be discussed but provide conclusive proof of connectivity (Fig. S32 in the ESI†). Selected structural data for 2a, 2b, 3, 4a, 4b and 7 are provided in Table 1, together with relevant NMR- and IR-spectroscopic data.| 1a , | 1b , | 3 , , | 4a , , | 4b , | 7 , | |
|---|---|---|---|---|---|---|
| a YCE = NCS. b Z = N3. c Z = C4. d YCE = OCP. e Data for the only molecule of 3 in the asymmetric unit (out of 3), which does not display a disorder in the BeOCP moiety. f 4a cocrystallised with 4% of leftover starting material 1a, both molecules overlapping fully in the asymmetric unit, except for their Br/OCP ligands, which were freely refined, leading to relatively high esds. g YCE = OCN. h Structural data ranges provided for all three (CAAC)BBr(OCN) units present in the asymmetric unit. i n.d. = not determined. | ||||||
| δ 9Be | 15.5 | 14.8 | 4.7 | n.d.i | 17.0 | n.d. |
| ω 1/2 | 420 | 210 | 150 | n.d. | 340 | n.d. |
| νps | 2100 | 2089 | 1727 | n.d. | 1691 | n.d. |
| N1–C1 | 1.3011(15) | 1.2967(16) | — | 1.302(2) | 1.2998(18) | 1.292(4)–1.296(4) |
| N2–C3 | 1.4833(15) | — | 1.5151(17) | 1.486(2) | — | — |
| Be–C1 | 1.811(2) | 1.823(2) | — | 1.813(3) | 1.810(2) | 1.827(5)–1.836(5) |
| Be–C3 | 1.7655(19) | 1.743(2) | 1.776(2) | 1.763(3) | 1.745(2) | — |
| Be–Y | 1.6186(19) | 1.6197(19) | 1.626(2) | 1.534(3) | 1.557(2) | 1.665(4)–1.667(4) |
| Y–C2 | 1.1674(17) | 1.1740(17) | 1.224(2) | 1.273(5) | 1.2626(19) | 1.231(3)–1.234(3) |
| C2–E | 1.6080(14) | 1.6100(14) | 1.5841(17) | 1.531(5) | 1.5575(16) | 1.102(4)–1.138(4) |
| E–Be | — | — | — | — | — | 1.693(4)–1.712(5) |
| max. (YCE)⋯Beplane | ca. 0.28 | ca. 0.40 | — | ca. 0.38 | ca. 0.41 | |
| Be–Y–C2 | 170.42(12) | 169.84(12) | 144.73(13) | 159.8(4) | 132.99(13) | 130.1(2)–131.6(2) |
| Y–C2–E | 179.29(12) | 178.73(13) | 179.45(18) | 177.5(6) | 177.45(14) | 177.3(3)–178.0(3) |
| C2–E–Be | — | — | — | — | — | 167.1(3)–168.2(3) |
| Σ∠Be | 359.99(11) | 359.90(11) | — | 359.94(16) | 359.98(13) | — |
| |N1–C1–Be–Y| | 110.1(1) | 15.40(18) | — | 113.43(19) | 28.9(2) | 16.9(4)–20.1(4) |
| |Z–C3–Be–Y| | 63.8(2) | 78.2(2) | 51.3(2) | 62.1(2) | 114.3(2) | — |
| |N–C–Be–E| | — | — | — | — | — | 96.7(3)–99.8(3) |
Complexes 2a, 2b, 4a and 4b (Fig. 2) are trigonal-planar (Σ∠Be ca. 360°) and display a terminal pseudohalide ligand. The Be–C1 bond length to the neutral CAAC ligand (1.810(2)–1.823(2) Å) is similar to that in the precursors 1a and 1b (1.798(2), 1.829(2) Å).26,36 The CAAC and CAACH ligands in 2a and 4a adopt an anti conformation relative to each other so as to minimise steric repulsion. The duryl substituent in 2b is near-orthogonal to the beryllium plane (|C4–C3–Be1–N2| 78.2(2)°), but less so in 4b (|C4–C3–Be1–O1| 62.1(2)°), perhaps to minimise repulsion between the π systems of the duryl ring and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P moiety. The NCS and OCP moieties in 2a/b and 4a/b, respectively, approach linearity (N–C–S/O–C–P 177.45(14)–179.45(18)°) and are quasi-coplanar with the beryllium plane (S1/P1⋯Beplane < 0.41 Å).
P moiety. The NCS and OCP moieties in 2a/b and 4a/b, respectively, approach linearity (N–C–S/O–C–P 177.45(14)–179.45(18)°) and are quasi-coplanar with the beryllium plane (S1/P1⋯Beplane < 0.41 Å).
The steric constraints imposed by the anionic CAACH/Dur substituents have a significant impact on the orientation of the pseudohalide substituent relative to the CAAC ligand. In the duryl derivatives the NCS/OCP ligand tends towards coplanarity with the trigonal-planar CAAC carbene centre (2b |N1–C1–Be1–N2| 15.40(18); 4b |N1–C1–Be1–O1| 28.9(2)°) in order to maximise overlap between the pseudohalide and CAAC π systems. In the CAACH derivatives, however, the NCS/OCP ligand tends more towards orthogonality with the C1 plane as the much higher steric constraints override electronic effects (2a |N1–C1–Be1–N2| 110.1(1); 4a |N1–C1–Be1–O1| 113.43(19)°).
Complexes 2a/b are the first solid-state structures of tricoordinate beryllium thiocyanates. Accordingly, their Be1–N1 bonds (1.3011(15), 1.2967(16) Å) are necessarily shorter than those in the known tetracoordinate complexes (ca. 1.65–1.69 Å).35,37 The Be–N2–C2 angle in 2a/b deviates only slightly from linearity (ca. 170°), the NCS residue bending away from the CAAC and towards the CAACH/Dur ligand. The N2–C2 (1.1674(17), 1.1740(17) Å) and C2–S1 (1.6080(14), 1.6100(14) Å) bond lengths are typical for terminal beryllium thiocyanates (N–C ca. 1.14–1.17; C–S ca. 1.59–1.62 Å).35,37
Complexes 3, 4a and 4b are the first examples of beryllium phosphaethynolates. The Be1–O1 bond lengths range from 1.534(3) to 1.626(2) Å, being longer in the tetrahedral complex 3 than in the trigonal-planar complexes 4a/b. Furthermore, as Be1–O1 lengthens, O1–C2 shortens from 1.273(5) to 1.224(2) Å, while C2–P1 lengthens from 1.531(5) to 1.5841(17) Å, indicating small fluctuation in the degree of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P character in the predominantly O–C
P character in the predominantly O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P resonance form. In the only four structurally characterised terminal s-block phosphaethynolates the O–C bonds (1.196(3)–1.207(4) Å) are significantly shorter, while the C–P bonds (1.555(4)–1.582(3) Å) fall within the same range as those in 3 and 4a/b.37,54,55 Whereas in 4a/b the OCP substituent bends away from the CAAC ligand, the asymmetric unit of 3 contains two distinct topoisomers, one in which the OCP ligand points away from the CAAC ligand, the other towards. This is in agreement with the NMR data, which shows the presence of two rotamers, 3 and 3′, in solution. The Be–O1–C2 angle in 3 and 4a/b is much larger and varies more (132.99(13)–159.8(4)°) than the corresponding Be–N2–C2 angle in 2a/b. In the literature-known s-block phosphaethynolates the M–O–C angle varies even more, from highly bent in [Na(OCP)(dibenzo-18-crown-6)] (138.1(2)°)37 to nearly linear in [Na(OCP)(dme)2] (107.7(3)°, dme = dimethyoxyethane),55 but without apparent correlation with the O–C and C–P bond lengths and thereby the degree of O–C
P resonance form. In the only four structurally characterised terminal s-block phosphaethynolates the O–C bonds (1.196(3)–1.207(4) Å) are significantly shorter, while the C–P bonds (1.555(4)–1.582(3) Å) fall within the same range as those in 3 and 4a/b.37,54,55 Whereas in 4a/b the OCP substituent bends away from the CAAC ligand, the asymmetric unit of 3 contains two distinct topoisomers, one in which the OCP ligand points away from the CAAC ligand, the other towards. This is in agreement with the NMR data, which shows the presence of two rotamers, 3 and 3′, in solution. The Be–O1–C2 angle in 3 and 4a/b is much larger and varies more (132.99(13)–159.8(4)°) than the corresponding Be–N2–C2 angle in 2a/b. In the literature-known s-block phosphaethynolates the M–O–C angle varies even more, from highly bent in [Na(OCP)(dibenzo-18-crown-6)] (138.1(2)°)37 to nearly linear in [Na(OCP)(dme)2] (107.7(3)°, dme = dimethyoxyethane),55 but without apparent correlation with the O–C and C–P bond lengths and thereby the degree of O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P character.
P character.
The centrosymmetric hexameric complex 7 crystallises in the chiral monoclinic space group I2/a (Fig. 3). The OCN ligands bridge between the six beryllium centres, forming a central 24-membered (BeOCN)6 ring, within which the beryllium stereocentres alternate between the R and S configurations. Complex 7 is the first structurally characterised beryllium isocyanate and, to our knowledge, the largest known molecular (MOCN)n ring structure. The magnesium aluminate trimer [Me2Al{μ-O(SiMe3)}2Mg(μ-OCN)]3 presents a central 12-membered (MgOCN)3 ring,56** while [(CpiPr)U(μ-OCN)]4 features a 16-membered (UOCN)4 ring.57 The Be–O (ca. 1.67 Å) and Be–N (ca. 1.70 Å) bond lengths are slightly longer than those in the tetrahedral phosphaethynolate 3 (1.626(2) Å) and known terminal beryllium thiocyanates (1.65–1.69 Å),31–35 respectively. Each O–C–N–Be fragment is nearly linear (O–C–N ca. 178, C–N–Be ca. 168°), whereas the Be–O–C moieties are strongly bent (ca. 131°). The O–C (ca. 1.23 Å) and C–N (ca. 1.12 Å) bond lengths indicate a predominance of the O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N resonance form. The only other structurally characterised s-block complex with an OCN unit bridging between two metal centres is an anionic magnesium porphyrin complex featuring an Mg–O–C–N–K linkage to the K+ countercation, which shows significantly more pronounced O
N resonance form. The only other structurally characterised s-block complex with an OCN unit bridging between two metal centres is an anionic magnesium porphyrin complex featuring an Mg–O–C–N–K linkage to the K+ countercation, which shows significantly more pronounced O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N character (O–C 1.175(5), C–N 1.195(5) Å), resulting in much larger Mg–O–C (157.2(3)°) and much smaller C–N–K (123.1(3)°) angles.58
N character (O–C 1.175(5), C–N 1.195(5) Å), resulting in much larger Mg–O–C (157.2(3)°) and much smaller C–N–K (123.1(3)°) angles.58
Finally, the tetrameric complex 8 also crystallises in the chiral monoclinic space group I2/a (Fig. S32 in the ESI†). The CN ligands bridge between the four beryllium centres, forming a central 16-membered (BeCN)4 ring. This structural motif is relatively common, in particular in group 13 cyanide complexes,59–62 but has never been reported for s-block complexes. Due to poor diffraction data quality and extensive structural disorders, structural parameters may not be discussed, but the data show that all four bridging cyano ligands are flip-disordered, as was already apparent from the solution NMR data (vide supra). This coordinative CN/NC isomerism is also observed in other structurally characterised cyanoberyllium complexes.32,35
Conclusions
The synthesis of well-defined beryllium-based pseudohalide Grignards from the CAAC-stabilised beryllium Grignards [(CAAC)(CAACH)BeBr] (1a) and [(CAAC)DurBeBr] (1b) has proven less straightforward than expected. With the exception of the first tricoordinate thiocyanate derivatives, [(CAAC)(CAACH)Be(η1-NCS)] (2a) and [(CAAC)DurBe(η1-NCS)] (2b), which are readily accessible by salt metathesis with [NaNCS], the syntheses of phosphaethynolate, isocyanate and cyanide derivatives are complicated by Schlenk-type ligand exchange and other undesirable side reactions. The syntheses of the OCP derivatives, in particular, are fraught with numerous side reactions, including scrambling of both the neutral and anionic ligands, OCP homocoupling, OCP coupling with the CAAC ligand, insertion of OCP into the Be–organyl bond, and ill-defined radical-generating redox processes. Nonetheless, the first examples of beryllium phosphaethynolates, tetracoordinate [(thf)2(CAACH)Be(η1-OCP)] (3) and tricoordinate [(CAAC)RBe(η1-OCP)] (R = CAACH 4a, Dur 4b) were obtained and characterised by NMR and IR spectroscopy, as well as single-crystal X-ray crystallography. While attempts to obtain OCN derivatives also proved very unselective, fractional crystallisation yielded the first solid-state structure of a beryllium isocyanate, a hexameric complex featuring a unique (BeOCN)6 ring, [(CAAC)BrBe(1,3-μ-OCN)]6. Finally, attempts to synthesise a beryllium selenocyanate Grignard by salt metathesis with [Na/KSeCN] resulted instead in selenium abstraction by the CAAC ligand and the relatively selective generation of the tetrameric, self-stabilising mixed cyano/isocyanoberyllium Grignard [(thf)(CAACH)Be(1,3-μ-CN/NC)]4. Overall, this study shows that CAAC ligands, which have enabled the isolation of numerous, otherwise inaccessible low-valent main group complexes,46,63,64 are ill suited to the stabilisation of pseudohaloberyllium Grignards as they are easily displaced by THF, which is often required to solubilise the pseudohalide salt precursor, and may undergo side reactions with the pseudohalide substituents.Data availability
Electronic Supplementary Information (ESI) Available: Synthetic procedures, NMR spectra and X-ray crystallographic details.Crystallographic data have been deposited with the Cambridge Crystallographic Data Center as supplementary publication no. 2379134 (5), 2379135 (2a), 2379136 (3), 2379137 (7), 2379138 (2b), 2379139 (8), 2379140 (4b), 2379141 (4a).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the Julius-Maximilians-Universität Würzburg and Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; project number 466754611).References
- L. Birckenbach and K. Kellermann, Ber. Dtsch. Chem. Ges. B, 1925, 58, 786 CrossRef.
- F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef CAS PubMed.
- M. R. Netherton and G. C. Fu, Adv. Synth. Catal., 2004, 346, 1525 CrossRef CAS.
- Y.-G. Chen, X.-T. Xu, K. Zhang, Y.-Q. Li, L.-P. Zhang, P. Fang and T.-S. Mei, Synthesis, 2018, 50, 35 CrossRef CAS.
- S. Arlt, K. Bläsing, J. Harloff, K. C. Laatz, D. Michalik, S. Nier, A. Schulz, P. Stoer, A. Stoffers and A. Villinger, ChemistryOpen, 2021, 10, 62 CrossRef CAS PubMed.
- P.-Y. Lin, A. Loganathan, I. Raifuku, M.-H. Li, Y.-Y. Chiu, S.-T. Chang, A. Fakharuddin, C.-F. Lin, T.-F. Guo, L. Schmidt-Mende and P. Chen, Adv. Energy Mater., 2021, 11, 2100818 CrossRef CAS.
- F. Cheng, J. Zhang and T. Pauporté, ChemSusChem, 2021, 14, 3665 CrossRef CAS PubMed.
- C. Glidewell, Inorg. Chim. Acta, 1974, 11, 257 CrossRef CAS.
- S. Harder and J. Langer, Nat. Rev. Chem., 2023, 7, 843 CrossRef PubMed.
- A. Guérinot and J. Cossy, Acc. Chem. Res., 2020, 53, 1351 CrossRef PubMed.
- Y. Nassar, F. Rodier, V. Ferey and J. Cossy, ACS Catal., 2021, 11, 5736 CrossRef CAS.
- C. E. I. Knappkea and A. Jacobi von Wangelin, Chem. Soc. Rev., 2011, 40, 4948 RSC.
- S. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard and B. L. Feringa, Chem. Rev., 2008, 108, 2824 CrossRef CAS PubMed.
- J. L. Burmeister, Coord. Chem. Rev., 1990, 105, 77 CrossRef CAS.
- A. Tuulmets, M. Mikk and D. Panov, Main Group Met. Chem., 1997, 10, 1 Search PubMed.
- M. Hvastijová, J. Kohout, J. W. Buchler, R. Boča, J. Kožíšek and L. Jäger, Coord. Chem. Rev., 1998, 175, 17 CrossRef.
- A. Klopsch and K. Dehnicke, Chem. Ber., 1975, 108, 420 CrossRef CAS.
- H. Böhland, F. R. Hofmann, W. Hanay and H. J. Berner, Z. Anorg. Allg. Chem., 1989, 577, 53 CrossRef.
- A. D. Obi, H. R. Machost, D. A. Dickie and R. J. Gilliard, Jr., Inorg. Chem., 2021, 60, 12481 CrossRef CAS PubMed.
- D. Parveen, R. K. Yadav and D. K. Roy, Chem. Commun., 2024, 60, 1663 RSC.
- M. R. Buchner, Chem. – Eur. J., 2019, 25, 12018 CrossRef CAS PubMed.
- K. J. Iversen, S. A. Couchman, D. J. D. Wilson and J. L. Dutton, Coord. Chem. Rev., 2015, 297–298, 40 CrossRef CAS.
- M. Arrowsmith, H. Braunschweig, M. A. Celik, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, T. Kramer, I. Krummenacher, J. Mies, K. Radacki and J. K. Schuster, Nat. Chem., 2016, 8, 890 CrossRef CAS PubMed.
- J. T. Boronski, A. E. Crumpton, L. L. Wales and S. Aldridge, Science, 2023, 380, 1147 CrossRef CAS PubMed.
- J. T. Boronski, A. E. Crumpton, A. F. Roper and S. Aldridge, Nat. Chem., 2024, 16, 1295 CrossRef CAS PubMed.
- C. Czernetzki, M. Arrowsmith, F. Fantuzzi, A. Gärtner, T. Tröster, I. Krummenacher, F. Schorr and H. Braunschweig, Angew. Chem., Int. Ed., 2021, 60, 20776 CrossRef CAS PubMed.
- G. Wang, J. Walley, D. Dickie, A. Molino, D. Wilson and R. J. Gilliard, J. Am. Chem. Soc., 2020, 142, 4560 CrossRef CAS PubMed.
- M. R. Buchner, L. R. Thomas-Hargreaves, C. Berthold, D. F. Bekiş and S. I. Ivlev, Chem. – Eur. J., 2023, 29, e202302495 CrossRef CAS PubMed.
- C. Helling and C. Jones, Chem. – Eur. J., 2023, 29, e202302222 CrossRef CAS PubMed.
- C. Czernetzki, T. Kunz, S. Huynh, A. Lamprecht, J. Sprenger, M. Finze, M. Arrowsmith and H. Braunschweig, Angew. Chem., Int. Ed., 2024, 63, e202401279 CrossRef CAS PubMed.
- H. Böhland, W. Hanay, M. Noltemeyer, A. Meller and H. G. Schmidt, Fresenius’ J. Anal. Chem., 1998, 361, 725 CrossRef.
- A. V. G. Chizmeshya, C. J. Ritter, T. L. Groy, J. B. Tice and J. Kouvetakis, Chem. Mater., 2007, 19, 5890 CrossRef CAS.
- B. Neumüller and K. Dehnicke, Z. Anorg. Allg. Chem., 2007, 633, 2262 CrossRef.
- D. Naglav, D. Bläser, C. Wölper and S. Schulz, Inorg. Chem., 2014, 53, 1241 CrossRef CAS PubMed.
- C. Berthold, M. Müller, S. I. Ivlev, D. M. Andrada and M. R. Buchner, Dalton Trans., 2023, 52, 13547 RSC.
- C. Czernetzki, M. Arrowsmith, L. Endres, I. Krummenacher and H. Braunschweig, Inorg. Chem., 2024, 63, 2670 CrossRef CAS PubMed.
- B. Neumüller and K. Dehnicke, Z. Anorg. Allg. Chem., 2010, 636, 1206 CrossRef.
- R. J. Gilliard, Jr., D. Heift, J. M. Keiser, Z. Benko, A. L. Rheingold, H. Grützmacher and J. D. Protasiewicz, Dalton Trans., 2018, 47, 666 RSC.
- Z. Li, X. Chen, M. Bergeler, M. Reiher, C.-Y. Su and H. Grützmacher, Dalton Trans., 2015, 44, 6431 RSC.
- D. Heift, Z. Benko and H. Grützmacher, Dalton Trans., 2014, 43, 5920 RSC.
- L. N. Grant, B. Pinter, B. C. Manor, H. Grützmacher and D. J. Mindiola, Angew. Chem., Int. Ed., 2018, 57, 1049 CrossRef CAS PubMed.
- D. W. N. Wilson, A. Hinz and J. M. Goicoechea, Angew. Chem., Int. Ed., 2018, 57, 2188 CrossRef CAS PubMed.
- J. M. Goicoechea and H. Grützmacher, Angew. Chem., Int. Ed., 2018, 57, 16968 CrossRef CAS PubMed.
- A. R. Jupp and J. M. Goicoechea, Angew. Chem., Int. Ed., 2013, 52, 10064 CrossRef CAS PubMed.
- J. K. Buchanan and P. G. Plieger, Z. Naturforsch., B: J. Chem. Sci., 2020, 75, 459 CrossRef CAS.
- S. Kundu, S. Sinhababu, V. Chandrasekhar and H. W. Roesky, Chem. Sci., 2019, 10, 4727 RSC.
- G. Becker, G. Heckmann, K. Hubler and W. Schwarz, Z. Anorg. Allg. Chem., 1995, 621, 34 CrossRef CAS.
- W. Yang, K. E. Krantz, D. A. Dickie, A. Molino, D. J. D. Wilson and R. J. Gilliard, Jr., Angew. Chem., Int. Ed., 2020, 59, 3971 CrossRef CAS PubMed.
- B. Li, C. Wölper, H. M. Weinert, H. Siera, G. Haberhauer and S. Schulz, Eur. J. Inorg. Chem., 2024, 27, e202300622 CrossRef CAS.
- S. J. Urwin and J. M. Goicoechea, Chem. – Eur. J., 2023, 29, e202203081 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, T. Szilvási, E. Ballestero-Martínez, H. Grützmacher and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 4333 CrossRef CAS PubMed.
- D. W. N. Wilson, W. K. Myers and J. M. Goicoechea, Dalton Trans., 2020, 49, 15249 RSC.
- M. Tretiakov, Y. G. Shermolovich, A. P. Singh, A. P. Samuel, H. W. Roesky, B. Niepötter, A. Visschera and D. Stalke, Dalton Trans., 2013, 42, 12940 RSC.
- M. Westerhausen, S. Schneiderbauer, H. Piotrowski, M. Suter and H. Nöth, J. Organomet. Chem., 2002, 189, 643 Search PubMed.
- G. Becker, W. Schwarz, N. Seidler and M. Westerhausen, Z. Anorg. Allg. Chem., 1992, 612, 72 CrossRef CAS.
- H. Phull, D. Alberti, I. Korobkov, S. Gambarotta and P. H. M. Budzelaar, Angew. Chem., Int. Ed., 2006, 45, 5331 CrossRef CAS PubMed.
- M. A. Boreen, K. N. McCabe, T. D. Lohrey, F. A. Watt, L. Maron, S. Hohloch and J. Arnold, Inorg. Chem., 2020, 59, 8580 CrossRef CAS PubMed.
- K. Ezzayani, A. Ben Khelifa, E. Saint-Aman, F. Loiseau and H. Nasri, Polyhedron, 2016, 117, 817 CrossRef CAS.
- M. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, G. Bringmann, M. A. Celik, R. D. Dewhurst, M. Finze, M. Grüne, M. Hailmann, T. Hertle and I. Krummenacher, Angew. Chem., Int. Ed., 2016, 55, 14464 CrossRef CAS PubMed.
- M. Molon, K. Dilchert, C. Gemel, R. W. Seidel, J. Schaumann and R. A. Fischer, Inorg. Chem., 2013, 52, 14275 CrossRef CAS PubMed.
- W. Uhl, T. Abel, E. Hagemeier, A. Hepp, M. Layh, B. Rezaeirad and H. Luftmann, Inorg. Chem., 2011, 50, 325 CrossRef CAS PubMed.
- W. Uhl and M. Matar, Z. Naturforsch., B: J. Chem. Sci., 2004, 59, 1214 CrossRef CAS.
- S. K. Kushvaha, A. Mishra, H. W. Roesky and K. Chandra Mondal, Chem. – Asian J., 2022, 17, e202101301 CrossRef PubMed.
- M. Melaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046 CrossRef CAS PubMed.
- D. Naglav, M. R. Buchner, G. Bendt, F. Kraus and S. Schulz, Angew. Chem., Int. Ed., 2016, 55, 10562 CrossRef CAS PubMed.
- M. R. Buchner and M. Müller, ACS Chem. Health Saf., 2023, 30, 36 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, and X-ray crystallographic details. CCDC 2379134–2379141. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02457e |
| ‡ Caution! Beryllium and its compounds are regarded as toxic and carcinogenic. Because the biochemical mechanisms that cause beryllium-associated diseases are still unknown, special (safety) precautions are strongly advised.65,66 |
| § The same reaction carried out in benzene instead of THF to avoid CAAC-THF ligand scrambling proved much less selective. The low isolated yield of 3 of 33% is due to the difficulty in separating 3 from the CAAC ligand released during the reaction, as both have very similar solubility in pentane. |
| ¶ The calculated 9BeNMR shift of 4a at the B3LYP-D3(BJ)-def2-SVP level of theory using 2b (δ9Be = 14.8 ppm) as a reference is 14.6 ppm, therefore 4a is unlikely to be the minor product observed at δ9Be = 7.1 ppm. |
| || Compound 4b slowly decomposed within the reaction mixture upon exposure to light. |
| ** The structural data for [Me2Al{μ-O(SiMe3)}2Mg(μ-OCN)]3 are of insufficient quality to allow comparison with 7. |
| This journal is © The Royal Society of Chemistry 2024 |