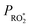Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
O3 chemistry of 2,5-dimethylfuran: mechanism development†
Niklas
Illmann
 * and
Vera
Rösgen
* and
Vera
Rösgen
University of Wuppertal, Institute for Atmospheric and Environmental Research, Gaußstraße 20, 42119 Wuppertal, Germany. E-mail: illmann@uni-wuppertal.de
First published on 19th August 2024
Abstract
Furans are emitted from biomass burning (BB) and contribute to the reactivity of BB plumes with a significant proportion. Consequently, the development of comprehensive furan oxidation schemes is one of the crucial elements towards a better understanding of BB plume chemistry. Nighttime oxidation is supposedly dominated by NO3 radicals and O3. The present study has chosen 2,5-dimethylfuran (25DMF) as a model compound for the development of an O3 oxidation mechanism for furans. Experiments were performed in the QUAREC atmospheric simulation chamber (QUAREC ASC) at 299 ± 2 K and a pressure of 980 ± 20 mbar under dry conditions (relative humidity < 0.1%) targeting the reaction kinetics, the OH formation and the oxidation mechanism. The reactions were monitored by long-path FTIR spectroscopy and a PTR-ToF-MS instrument. We determined a rate coefficient of (3.3 ± 1.0) × 10−16 cm3 molecule−1 s−1 for the target reaction using the relative-rate method. An OH yield of 25 ± 10% was obtained when using 1,3,5-trimethylbenzene as an OH tracer. Reaction products are formaldehyde, methyl glyoxal, ketene, glyoxal, methyl hydroperoxide, acetic anhydride, and acetic acid, respectively. The methyl glyoxal, glyoxal and formaldehyde yields were found to be sensitive to the overall peroxy radical level in the system. The PTR-MS data indicate further reaction products, which are tentatively assigned. A mechanism is postulated to account for the clearly identified reaction products. Overall, the obtained results indicate that O3 oxidation of furans might contribute to acidity in nighttime BB plumes.
Environmental significanceFuran derivatives have been shown to represent major components of biomass burning (BB) smoke. To date, research on furan oxidation mechanisms is dominated by daytime chemistry. In this study we look into the nighttime oxidation of 2,5-dimethylfuran by O3 to provide a basis for assessing the importance of O3 chemistry on nighttime BB plumes. We were able to show that such reactions may contribute to the reactivity and acidity inside plumes. The results can be used for the development of a comprehensive O3 oxidation scheme for furans to refine atmospheric models describing the ageing of nighttime BB plumes. |
Introduction
Furan derivatives (furans, furaldehydes, furan alcohols etc.) are versatile chemicals of use in industry for various applications and (easily) accessible from lignocellulosic biomass (see Jaswal et al.1 and references therein). 2,5-dimethylfuran (25DMF), in particular, raised interest as a biofuel candidate.2 Furan derivatives arise from pyrolysis of cellulose which explains their presence in biomass burning (BB) smoke, particularly from wildfires.3 For instance, furan derivatives accounted for up to 37% (by emission factor) of the smoke from different fuel types in laboratory studies.4 In light of their high reactivity towards atmospheric oxidants, such as OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–7 and NO3
5–7 and NO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 radicals, it is not surprising that furans contribute significantly to the total OH
8 radicals, it is not surprising that furans contribute significantly to the total OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and NO3
9 and NO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 reactivity of wildfires characteristic emissions.
10 reactivity of wildfires characteristic emissions.
The chemistry inside BB plumes has been one of the major topics in atmospheric science during the last decade, primarily due to intensifying occurrence and magnitude of wildfire events.11,12 A major scientific goal is the ability to predict ozone and particle formation (during plume ageing along the transport), which appears still challenging as a consequence of the multitude of possible chemical composition and reaction conditions inside plumes. Research on BB plume chemistry is still focused on the OH reactivity, since OH represents the predominant atmospheric oxidant on average and OH concentrations exceeding the typical tropospheric level by up to factor of 10 were found inside BB plumes for daytime conditions.3,13
More recently NO3 chemistry has started to receive increasing attention.10 However, O3 initiated oxidation of unsaturated non-methane hydrocarbons (NMHC) is, to the best of our knowledge, not extensively addressed in discussions on BB plume chemistry with only very few exceptions. Decker et al.10 found O3 reactions to account for up to 43% of the nighttime NMHC oxidation in agricultural BB plumes based on box-model assisted analysis of aircraft observations. Yet, they stated their reactivity calculations were limited mainly by a lack of kinetic and mechanistic data.
Furans are heterocyclic aromatic species, whose aromaticity is much less pronounced than that of benzene. They can be regarded as cyclic ethers exhibiting reactivities close to conventional dienes e.g. in organic synthesis. Consequently, they are susceptible to ozonolysis reactions. Yet, at present information on the reactivity of furans towards ozone in the gas-phase is limited. Atkinson et al.14 reported the first experimentally determined rate coefficient for the gas-phase ozonolysis of furan. Although the reactivity was found to be low and comparable to that ethene, this value did, however, confirm that furans, in general, are reactive towards ozone. Matsumoto15 showed that rate coefficients are significantly larger for higher substituted furans such as 2,5-dimethylfuran, which was subsequently supported by a theoretical study on several furan derivatives.16 Recently, the secondary organic aerosol formation from the 2,5-dimethylfuran ozonolysis was investigated and found to be negligible in the absence of SO2.17 Information on the ozonolysis mechanism of furans is, however, limited to one report on the OH production18 and a single theoretical calculation.16 No experimental data on reaction products were, to the best of our knowledge, ever reported in the literature.
Assessing the potential impact of O3 chemistry on the nighttime chemical evolution of wildfire emissions requires a detailed knowledge of the ozonolysis kinetics and mechanisms of major plume constituents such as furans. The present study thus intends to serve as a basis for the development of a comprehensive furan ozonolysis mechanism by investigating the O3 initiated oxidation of the symmetrical 2,5-dimethylfuran.
Experimental
Atmospheric simulation chamber (QUAREC ASC)
The experiments were performed in a cylindrical 1080 L quartz-glass atmospheric simulation chamber (QUAREC ASC) located at the University of Wuppertal. A white-type mirror system is installed inside the glass tube, which is coupled to a Fourier-transform infrared (FTIR) spectrometer and operated at an optical path length of 484.7 ± 0.8 m in total. The current set-up of the chamber is described in greater detail elsewhere.19Experimental approach
All experiments were carried out under dry conditions (r. h. ≪ 0.1%) at 980 ± 20 mbar of synthetic air (Messer, 99.9999%) and a temperature of 299 ± 2 K. A summary of the experimental conditions and initial concentrations is provided in Table S1 of the ESI† for all experiments. The ozonolysis reaction was initiated by adding O3 to the reaction mixture (according to the experiment type), which was generated by passing a stream of pure oxygen (Messer, 99.995%) through an electrical discharge in a homemade device. Aliquots of (0.6–1.3) × 1013 cm−3 O3 were added up to four times per experiment. The reaction mixtures were continuously stirred with all three fans mounted inside the chamber to keep the system as homogenous as possible. The mixtures were observed for about 7–30 min prior to adding O3 to determine wall losses of target species in each single experiment. Three types of experiments were performed targeting the OH formation, the determination of the ozonolysis rate coefficient and the product formation, respectively. 1,3,5-Trimethylbenzene (Sigma-Aldrich, 99%) was added as a tracer to estimate the OH production. Carbon monoxide (Air Liquide, 99.97%) was added in excess (≈2%) to scavenge OH radicals in the experiments investigating the product formation and the kinetics. In addition, the potential influence of stabilised Criegee intermediates (sCI) on the reaction system was examined by addition of (1.0–1.5) × 1014 cm−3 SO2 (Air Liquide, 99.9%) in one kinetic and two product study experiments. The rate coefficient was determined by the relative-rate method using E2-butene (Messer, 99%) and cyclohexene (Sigma-Aldrich, 99%) as reference compounds. Initial concentrations of 2,5-dimethylfuran (Sigma-Aldrich, 99%) were in the range (1.2–9.6) × 1013 cm−3.Instrumentation
The reaction mixtures were monitored using long-path FTIR spectroscopy and mass spectrometry. The FTIR spectrometer (Nicolet iS 50; Thermo Fisher Scientific) is operated with a liquid nitrogen cooled HgCdTe detector. The whole transfer optics housing, usually operated with dry air flushing, was purged with ultrapure nitrogen evaporated from a liquid nitrogen tank to allow monitoring of CO2. The reliability of the CO2 quantification was successfully proven in preceding work.19,20 FTIR spectra were recorded in the spectral range of 4000–700 cm−1 at a resolution of 1 cm−1. The time resolution of the FTIR data was varied in between the experiments by varying the number of scans co-added per spectrum between 20 and 50 resulting in averaging periods of about 32–81 s.A PTR-Tof 8000 (Ionicon Analytik GmbH, Innsbruck, Austria) was connected on-line to the reaction chamber and operated in H3O+ mode. The drift tube of the PTR-Tof-MS was kept at a temperature of 70 °C, 2.3 mbar pressure and 510 V drift voltage, which resulted in a reduced electric field strength of E/N ≈ 120 Td (1 Td = 1 × 10−17 V cm2). The sampling line of the instrument was operated at a temperature of 70 °C and coupled to a heated stainless steel line mounted on the end flange of the 1080 L chamber. The sample flow was about 200 mL min−1. Due to a lack of reliable calibration, the PTR-Tof-MS was used only for observing qualitatively the time profiles of the identified mass signals which were normalized by the m/z 21 (H318O+) signal.
Data analysis
The different substances were quantified via FTIR by manual subtraction with calibrated reference spectra from the internal laboratory database. For 2,5-dimethylfuran, acetic acid and acetic anhydride we used cross sections (base 10) of 3.8 × 10−18 cm molecule−1 (1060–880 cm−1), 2.6 × 10−19 cm2 molecule−1 (1177 cm−1), and 3.6 × 10−19 cm2 molecule−1 (1778 cm−1), respectively, which were determined in this study by injection of different known volumes into the chamber and applying the Beer–Lambert law. For ketene (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), we used a cross section of 7.7 × 10−19 cm2 molecule−1 (base 10) for the P-branch maximum at 2137 cm−1. The derivation of this value is outlined in Section D of the ESI.† The plots used for the calculation of the cross sections as well as a list of all literature values used for the quantification are given in the ESI.† Overall quantification errors in the experiments consist mainly of the uncertainty assigned to the absorption cross section and the subtraction procedure. The latter error was estimated for each compound by checking the reproducibility of the subtraction in selected spectra. Consequently, estimated relative uncertainties are about 11% for acetic acid, 7% for acetic anhydride, 6% for 2,5-dimethylfuran, 6% for formaldehyde, 11% for glyoxal, 21% for ketene, 3% for methyl glyoxal, and 12% for methyl hydroperoxide, respectively.
O), we used a cross section of 7.7 × 10−19 cm2 molecule−1 (base 10) for the P-branch maximum at 2137 cm−1. The derivation of this value is outlined in Section D of the ESI.† The plots used for the calculation of the cross sections as well as a list of all literature values used for the quantification are given in the ESI.† Overall quantification errors in the experiments consist mainly of the uncertainty assigned to the absorption cross section and the subtraction procedure. The latter error was estimated for each compound by checking the reproducibility of the subtraction in selected spectra. Consequently, estimated relative uncertainties are about 11% for acetic acid, 7% for acetic anhydride, 6% for 2,5-dimethylfuran, 6% for formaldehyde, 11% for glyoxal, 21% for ketene, 3% for methyl glyoxal, and 12% for methyl hydroperoxide, respectively.
The rate coefficient of the target reaction was determined using the relative-rate method which is based on relating the consumption of the target species to the consumption of a reference compound (ref) whose rate coefficient is accurately known. In the absence of significant wall losses, the rate coefficient can be determined by the following expression:
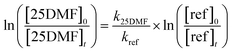 | (1) |
Since the relative-rate method has been described extensively in the literature,21 no further details on the derivation of the above equation will be given here. The recommended rate coefficients of 2.0 × 10−16 cm3 molecule−1 s−1 (E2-butene)22 and 7.8 × 10−17 cm3 molecule−1 s−1 (cyclohexene)23 were used for the calculation of k25DMF. The accuracy errors of these values are Δlog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = 0.1 for E2-butene22 and about 14% for cyclohexene.23
k = 0.1 for E2-butene22 and about 14% for cyclohexene.23
Product yields were calculated by relating the formation of the product to the consumption of the target species (Δ25DMF). The values reported within this work result from regression analysis over the linear range of each yield plot. In order to identify causes of non-linear behaviour, the time profiles were also simulated using a modelling approach introduced in preceding work.19 The profile of the initial reactant (25DMF), which is lost solely by reaction with O3, is approximated until the modelled time profile matches the experimental data. Both the yield (constant over the entire experimental run) and the wall loss of a reaction product are introduced as a variable parameter to be adjusted until a reasonable match between experimental and modelled profile is achieved if possible. A more detailed description of the procedure is outlined in Section F of the ESI.†
Results and discussion
A total of 21 experiments was performed investigating the rate coefficient (EXP1–EXP3, EXP21), the OH production (EXP4–EXP11) and the product formation (EXP12–EXP20) of the 2,5-dimethylfuran + O3 system. 25DMF did not exhibit a measurable wall loss in these experiments. The overall consumption of 25DMF was varied in the experiments and ranged from about 43% to 86%.Rate coefficient
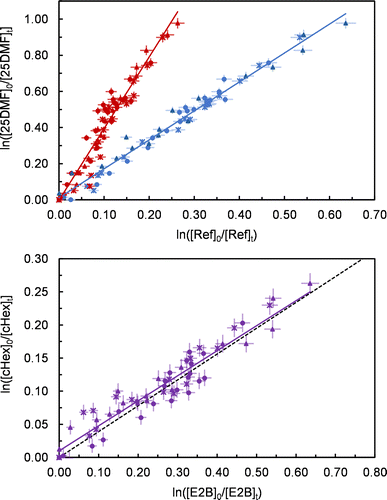
The use of two reference compounds and the resulting product formation during the reaction cause significant overlapping of absorption bands in the FTIR spectra, which hinders the spectral subtraction procedure. As a consequence, reliable subtraction was not possible in the case of cyclohexene and we only used the PTR-MS data for this compound. The relative-rate plots using the PTR-MS data are shown in Fig. 1. The rate coefficients obtained from these data agree within 13% (2σ).The experimentally observed rate coefficient ratio of cyclohexene and E2-butene using the PTR data matches the value based on the currently recommended rate coefficients. This suggests the amount of scavenger was high enough to suppress any influence of OH.
Ozone generators based on electrical discharge might produce also nitrogen oxides and eventually reactive species like NO3 radicals if trace amounts of N2 are present in the high purity grade oxygen used to generate ozone. While this is typically not relevant for most VOCs, it might, in principle, interfere in the present reaction system since 2,5-dimethylfuran is extremely reactive towards NO3.8 Yet, this interference were the largest during ozone addition and would slow down after the injection stops. As a consequence, significant non-linearity should be observed in the relative-rate plots, which is obviously not the case (Fig. 1). To ultimately rule out any influence of NO3 an excess of ozone was added to 1 atm of synthetic air, in the absence of reactive VOCs, and monitored for about 1 hour in the dark. After that, 2,5-dimethylfuran was added at atmospheric pressure and the mixture stirred for about 3 min for homogenization. After this period, 25DMF was almost entirely consumed without any hint for the formation of organic nitrate species.
The rate coefficient obtained from the experiment with SO2 added (EXP21) is indistinguishable from the values of experiments without SO2 injection (EXP1–EXP3). Consequently, any reaction between 2,5-dimethylfuran and stabilised Criegee intermediates appears not relevant in the experiments. Overall, these findings give confidence that the loss of 2,5-dimethylfuran after O3 addition is due to reaction with O3 solely.
The final rate coefficient of (3.3 ± 1.0) × 10−16 cm3 molecule−1 s−1 was obtained from the weighted average of all individual determinations. The quoted errors represent an expanded, conservative accuracy error of 30% (2σ) to cover all potential uncertainties.
The present value is slightly smaller than the sole experimentally determined rate coefficient of (4.2 ± 0.9) × 10−16 cm3 molecule−1 s−1 reported by Matsumoto,15 who followed the decay of ozone with a chemiluminescence detector for different initial 25DMF concentrations at 295 K. These values are roughly a factor of 2 smaller than suggested from theoretical calculations,16 which is still in reasonable agreement considering the fact that the theoretical study overestimates the experimentally determined rate coefficient for furan14 by a factor of ten.
OH production
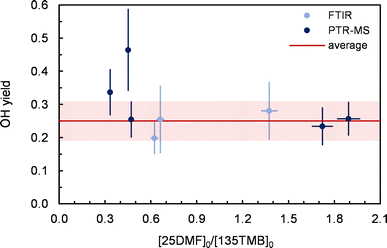
The formation of OH radicals was investigated in separate experiments using 1,3,5-trimethylbenzene (135TMB) as an OH tracer. In order to check for potential influence of the experimental conditions on the OH yield calculation, we varied the initial reactant concentration ratio (25DMF/135TMB) in the experiments. Two subsets of experiments were performed in which either FTIR (EXP4–EXP6, ESI Table S1†) or PTR-MS (EXP7–EXP11, ESI Table S1†) was used to observe the time profiles of the target species and the OH tracer. In the latter, the FTIR was used solely to determine the initial reactant concentrations.135TMB exhibits in these experiments a wall loss of up to 5 × 10−5 s−1. Yet, the decay observed for 135TMB is definitely larger when O3 is added to the mixture indicating the formation of OH in the reaction system. Plots of ln([135TMB]0/[135TMB]t) vs. time from data collected after one single O3 injection appear linear within statistical errors, which suggests a near steady-state OH level in each experiment. OH concentrations estimated from these plots were in the range from (2–6) × 106 cm−3, respectively.
In the initial phase of the reaction, production and loss rate of OH are dominated by 25DMF and 135TMB:
| 25DMF + O3 → αOH + products | (R1) |
| 25DMF + OH → products | (R2) |
| 135TMB + OH → products | (R3) |
Accordingly, by extrapolating to the start of the reaction, the OH yield α can be calculated as follows:
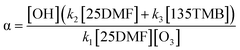 | (2) |
The detailed derivation of eqn (2) can be found in Section C of the ESI.†
As evident from Fig. 2, both the 25DMF/135TMB ratio and the analysis method did not influence the OH yield determination within the statistical uncertainties (2σ) but in two cases exceptionally high α-values were registered. Since consistently low OH yields were in most cases obtained from experiments performed after the chamber was conditioned with ozone, the most likely explanation is the OH yield determination was easily affected by OH production from the wall:
| O3 + wall → βOH | (R4) |
Combining the results of these experiments leads to an OH yield of 0.25 ± 0.06, depicted by the red range in Fig. 2. The error represents the statistical uncertainty (2σ). Yet, the overall accuracy error is certainly on the order of 40% when considering all uncertainties in the calculations. In addition, although the additional formation of OH, likely from the chamber wall, was largely minimized in these experiments, the OH yield should still be regarded as an upper limit since the reaction system itself might act as a secondary source of OH, e.g. through peroxy radical reactions:
| RO2 + HO2 → γOH + γRO + O2 | (R5) |
All this suggests that direct OH formation from the 2,5-dimethylfuran + O3 reaction is rather low.
Product determination
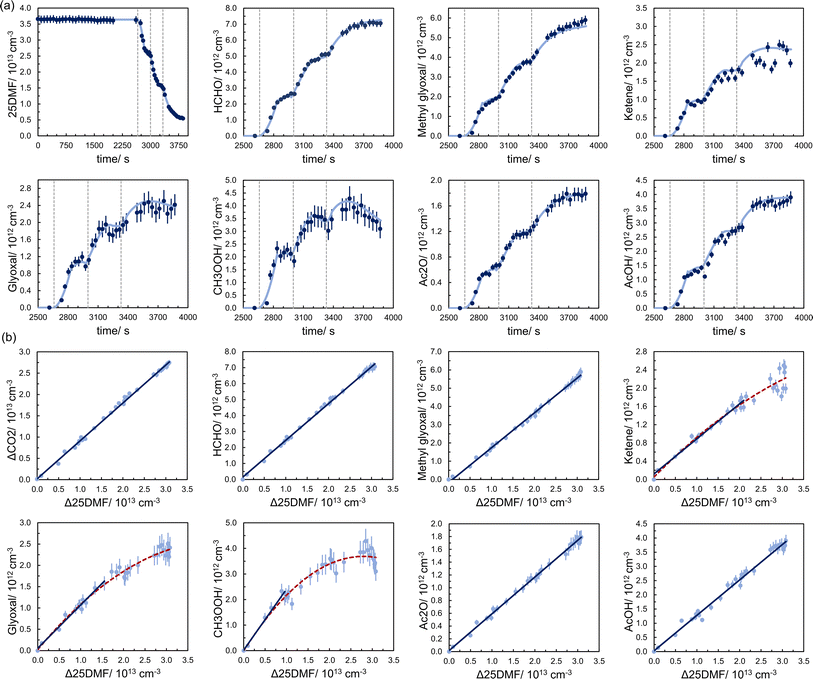
Fig. 3 shows, exemplary, time profiles and product formation (for species quantified by FTIR) for one experiment. The experimental data are adequately reproduced by the simulated time profiles when assuming a constant product yield and a unimolecular loss. The levels of glyoxal and methyl hydroperoxide, in particular, decrease once the reaction rate slows down significantly. Yet, this behaviour was reproducible for most of the experiments. The time profiles of methyl hydroperoxide, for example, were reproduced for the entire experimental duration when considering kwall in the range of (0.5–1.7) × 10−3 s−1 in the model calculation. Such large values can be explained by the constant stirring inside the chamber which might accelerate the loss rate for species exhibiting wall affinity, which is likely the case for methyl hydroperoxide (CH3OOH). Only in EXP19 and EXP20, which were performed at a later stage, the CH3OOH wall loss was almost zero.For methyl glyoxal, the model runs tend to either overestimate the experimental data in the beginning or underestimate the data in the end of the experiment like it is the case in EXP13 (Fig. 3). In the yield plots this is reflected in a minute curvature which might indicate a larger methyl glyoxal yield at higher 25DMF consumption levels. Yet, the deviation from linearity is extremely close to the precision error and cannot be confirmed unambiguously.
Overall, the time profiles and yield plots suggest the formation of these species as first-generation products. The yields, obtained through regression analysis over the linear range of the plots, are in the range 23.0–24.4% for formaldehyde (HCHO), 15.1–20.0% for methyl glyoxal, 8–10% for ketene, 7–12% for glyoxal, 18–31% for CH3OOH, 4.8–6.2% for acetic anhydride, and 10–13% for acetic acid, respectively. Yield plots and time profiles of all product study experiments can be found in Section E of the ESI.†
The CO2 yields exhibit significant scatter and range from 81% to 107%. Since CO was used as an OH scavenger, production of CO2 arises from CO + OH. Consequently, the variation in the observed CO2 yield appears at least in part consistent with the observations from the OH tracer experiments. Yet, since the lower limit of the CO2 yield appears reproducible and is still more than a factor of 3 larger than the upper limit determined for the OH yield, a significant fraction of CO2 originates likely from 2,5-dimethylfuran + O3 itself.
The FTIR residual spectra (see Fig. S30 of the ESI†) do indicate additional unidentified reaction products. The absorption pattern in the range 1840–1600 cm−1 suggests the presence of different carbonyl groups. In addition, the absorption band centred at 1645 cm−1 might suggest the presence of unsaturated reaction products containing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. The most prominent absorption bands are centred at 1203 cm−1 and 1161 cm−1, which strongly suggest the formation of species containing C–O bonds. Absorption bands centred at 3586 cm−1 and 3499 cm−1 could correspond to a combination of O–H stretching vibrations and an overtone of C–O stretching vibrations.
C bond. The most prominent absorption bands are centred at 1203 cm−1 and 1161 cm−1, which strongly suggest the formation of species containing C–O bonds. Absorption bands centred at 3586 cm−1 and 3499 cm−1 could correspond to a combination of O–H stretching vibrations and an overtone of C–O stretching vibrations.
Table 1 summarizes mass signals whose build-up was observed during the reaction. The assignment remains, however, incomplete and in the majority of cases the m/z signals are not unique for a single species formed in the reaction system. For example, both HCHO and glyoxal contribute to m/z 31 (CH3O+) whereas fragments of methyl glyoxal (m/z 73, C3H5O2+) contribute to m/z 45. A fragment of a tricarbonyl likely contributes also to m/z 73. Tentative assignments, where possible, are discussed below in conjunction with a possible ozonolysis mechanism. The temporal evolution of the mass signals is provided in Section H of the ESI.†
| m/z | Formula | Assignment |
|---|---|---|
| a Assignment of species in italics remains uncertain and is tentative only, n.a. = not assigned, numbers in parentheses correspond to the numbering used in the mechanistic schemes. | ||
| 31.02 | CH3O+ | Formaldehyde, glyoxal fragment |
| 41.04 | C3H5+ | Propyne + fragmentsa |
| 43.02 | C2H3O+ | Ketene + fragments |
| 45.03 | C2H5O+ | Methyl glyoxal fragment |
| 59.01 | C2H3O2+ | Glyoxal |
| 59.05 | C3H7O+ | n.a. |
| 61.03 | C2H5O2+ | Acetic acid, acetic anhydride |
| 73.03 | C3H5O2+ | Methyl glyoxal, tricarbonyl (9) fragmenta |
| 75.04 | C3H7O2+ | Hydroxydicarbonyl (10) fragment |
| 85.03 | C4H5O2+ | n.a. |
| 87.04 | C4H7O2+ | n.a. |
| 103.04 | C4H7O3+ | Hydroxydicarbonyl (10) |
| 113.06 | C6H9O2+ | Epoxide |
| 117.05 | C5H9O3+ | n.a. |
| 145.05 | C6H9O4+ | n.a. |
Mechanism
The reaction products suggest the initial step of the 25DMF + O3 reaction proceeds dominantly through the concerted 1,3-cycloaddition resulting in a primary ozonide (POZ, Scheme 1). The m/z 113 signal (C6H9O2+) could technically correspond to a protonated epoxide. In contrast to simple alkenes epoxide formation accounts indeed for up to 5% of the reaction of acyclic conjugated dienes such as isoprene with O3.24 However, the assignment of m/z 113 remains uncertain and there is no clear evidence for epoxide formation in the FTIR spectra. Accordingly, we can neither rule out nor prove the existence of an epoxide pathway.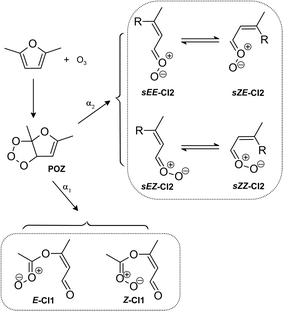 | ||
Scheme 1 Initial steps of the ozonolysis of 2,5-dimethylfuran and the (carbonyl oxides = Criegee Intermediates, CI) formed following decomposition of the primary ozonide (POZ). The E/Z-nomenclature follows the Cahn–Ingold–Prelog priority rules. For clarity and in the absence of ESI,† no differentiation between chemically activated and stabilized CI is drawn. R = –OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH3 for readability reasons. O)CH3 for readability reasons. | ||
The exothermicity of the 1,3-cycloaddition causes a prompt decomposition of the vibrationally excited POZ. Since there is no hint for products arising from a stepwise POZ dissociation route (O'Neil–Blumstein mechanism25), the POZ decomposition will likely proceed through two pathways and yield Criegee intermediates (CI) with a yield of unity. In both cases the carbonyl and the carbonyl oxide (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O+–O−) group remain within the same molecule, either adjacent to the remaining C
O+–O−) group remain within the same molecule, either adjacent to the remaining C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the former heterocyclic O atom (hereafter referred to as CI1) or vice versa (hereafter referred to as CI2), respectively (Scheme 1). Minimum two stereoisomers are possible for both Criegee Intermediates (CI) due to the orientation of the outer O atom of the carbonyl oxide (Scheme 1). The E/Z-nomenclature used here follows the Cahn–Ingold–Prelog priority rules.
C bond and the former heterocyclic O atom (hereafter referred to as CI1) or vice versa (hereafter referred to as CI2), respectively (Scheme 1). Minimum two stereoisomers are possible for both Criegee Intermediates (CI) due to the orientation of the outer O atom of the carbonyl oxide (Scheme 1). The E/Z-nomenclature used here follows the Cahn–Ingold–Prelog priority rules.
The carbonyl oxide group is part of a conjugated π-system in CI2. For these type of carbonyl oxides, theoretical calculations suggest internal rotation along the central single bond to be fast enough to reach an equilibrium between the conformers when the CI are stabilised.26 As a consequence, they are assumed to act as a single species.
The loss of SO2 in EXP 19 and EXP 20 is <2% and corresponds to a ΔSO2/Δ25DMF of <4%. Moreover, the product distribution was not measurably affected by the addition of SO2 suggesting that either the fraction of CI which is stabilised is very low or unimolecular reactions dominate over bimolecular processes even for the sCI. For simplicity and in the absence of additional information, we will not further differentiate between excited and stabilised CI in the following discussion.
The m/z 145 signal (C6H9O4+) suggests the formation of species retaining the carbon skeleton. In principle, the dioxirane route is accessible for most of the CI. Further isomerization will yield initially vibrationally excited acids and/or esters, which easily decompose. Yet, as observed for CI originating from some terpene ozonolysis,27,28 it appears possible that excited acids (or esters) from larger CI – like those cast from 25DMF + O3 – are stabilized by collision. Each of these species would contain two carbonyl groups with different chemical environments. Generally, this would be in accordance with the remaining carbonyl absorptions observed in the FTIR residual spectra.
The presence of both functionalities in the intermediate enables also an intramolecular reaction of the carbonyl oxide with the carbonyl group, in principle. If the internal energy distribution allows stabilisation, this might finally result in the formation of stabilised secondary ozonides (SOZ), which might contribute to the intense C–O absorption bands present in the FTIR residual spectra. The m/z 145 signal would correspond to protonated SOZ. Yet, it remains unclear if the SOZ structure remains intact after a proton transfer reaction.
Overall, the experimental clues towards formation of products retaining the C6 core structure are rather surprising. SOZ as well as acid formation (following the dioxirane route) was observed previously particularly for sesquiterpenes (C15).27,28 It appears that the excitation level of the present CI is rather low although all quantified reaction products confirm that CI fragmentation is still significant. Pathways accounting for those products are subsequently discussed below.
 | ||
| Scheme 2 Proposed mechanism for the fate of the carbonyl oxide Z-CI1 yielding in fragmentation of the C6 core structure. Species which were clearly identified as reaction products are marked in blue. | ||
Vinoxy-type radicals are typically considered as in equilibrium with the corresponding alkyl radical. Consequently, the most likely pathway is addition of oxygen yielding finally a thermalized carbonyl-substituted peroxy radical (DICARBO2, Scheme 2) at atmospheric pressure. Since the background level of NO is virtually zero after O3 addition, conventional bimolecular reactions of DICARBO2 are supposedly dominated by reaction with RO2 or HO2, respectively. Possible directly formed closed-shell reaction products are a multifunctional hydroperoxide (via DICARBO2 + HO2), a hydroxydicarbonyl and/or a tricarbonyl species (both through RO2 permutation reactions). In principle, these species might be present in the FTIR residual spectra as indicated by the presence of several different carbonyl absorption features. The PTR-MS data likely support the formation of the hydroxydicarbonyl (m/z 103, 75) and the tricarbonyl (m/z 73) compound.
For the alkoxy radical, formed from DICARBO2 by RO2 permutation reactions and possibly by HO2, decomposition is likely to be thermodynamically favoured due to the substitution pattern on both α-C atoms. The fragmentation yields either glyoxal + CHO or methyl glyoxal + an acetyl radical (CH3C(O)). Accordingly, the alkoxy radical of DICARBO2 provides one possible explanation for the formation of both dicarbonyls. The co-product of methyl glyoxal is immediately converted into an acetyl peroxy radical. Under the experimental conditions, the further chemistry would evolve into CH3O2 radicals (and the subsequent closed-shell reaction products), CO2, acetic acid and peracetic acid (PAA). However, the acetic acid yield is significantly larger than possible when considering only the acetyl peroxy radical reaction scheme (see e.g. Jenkin et al.30 and references therein) and strongly suggests another predominant source in the reaction system. In addition, there is no clear evidence for the formation of peracetic acid; we derive an upper limit of only 2% for the PAA yield based on the residual FTIR spectra.
DICARBO2 exhibits an aldehydic H atom close to the peroxy group which facilitates H-shift isomerisation reactions. These reactions are typically considered only at low radical concentrations close to atmospheric conditions since at higher peroxy radical levels bimolecular reactions predominate. However, both combined experimental and theoretical work on the methacrolein oxidation31 as well as SAR predictions32 indicate that H-shift isomerisation reactions are extremely rapid for peroxy radicals containing an aldehyde group. This pathway would provide an additional (small) source of OH and account for the formation of methyl glyoxal without producing acetyl (peroxy) radicals.
The fraction of DICARBO2 undergoing unimolecular isomerisation would be sensitive to the overall 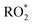 level, where
level, where 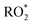 denotes the sum of individual RO2 species and HO2.
denotes the sum of individual RO2 species and HO2.
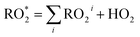 | (3) |
Although the exact concentration of 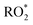 is not known, the steady-state level is expected to be roughly proportional to the production rate of peroxy radicals (
is not known, the steady-state level is expected to be roughly proportional to the production rate of peroxy radicals (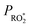 ). As such, the loss rate of 2,5-dimethylfuran can be used as a proxy for the overall peroxy radical level in the system:
). As such, the loss rate of 2,5-dimethylfuran can be used as a proxy for the overall peroxy radical level in the system:
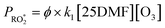 | (4) |
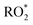 yield.
yield.
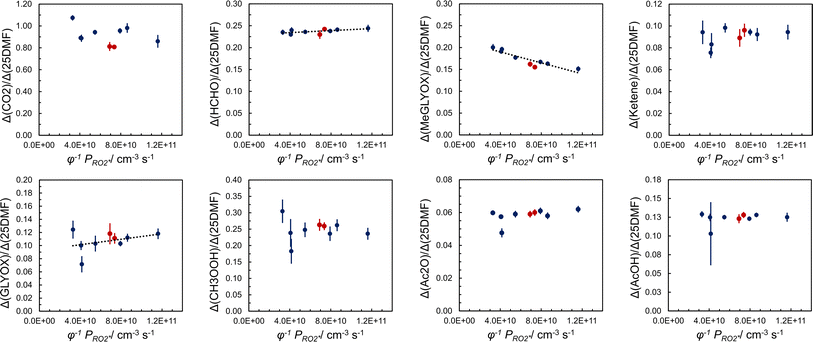
In order to assess whether product yields are sensitive to the 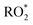 level in the system we tried to vary
level in the system we tried to vary 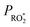 by changing both the initial concentration of 25DMF and the 25DMF loss rate in the different product study experiments. For each experiment, we determined an average
by changing both the initial concentration of 25DMF and the 25DMF loss rate in the different product study experiments. For each experiment, we determined an average 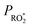 by considering only the time intervals, where significant consumption of 2,5-dimethylfuran is observed. The product yields in dependence of
by considering only the time intervals, where significant consumption of 2,5-dimethylfuran is observed. The product yields in dependence of 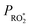 are shown in Fig. 4. It appears that a dependence is observed only for HCHO, glyoxal and methyl glyoxal. Both the HCHO and glyoxal yield increase with rising
are shown in Fig. 4. It appears that a dependence is observed only for HCHO, glyoxal and methyl glyoxal. Both the HCHO and glyoxal yield increase with rising 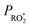 , whereas the methyl glyoxal yield decreases. In addition, the effect is much more pronounced in the case of methyl glyoxal, yielding a relative decrease of about 23% within the experimental limits compared to a relative increase of about 4% for HCHO.
, whereas the methyl glyoxal yield decreases. In addition, the effect is much more pronounced in the case of methyl glyoxal, yielding a relative decrease of about 23% within the experimental limits compared to a relative increase of about 4% for HCHO.
The increasing methyl glyoxal yield associated with a lower overall peroxy radical level supports the above hypothesis of a unimolecular pathway forming methyl glyoxal. Accordingly, the 1,4-H shift isomerisation of DICARBO2 appears competitive to bimolecular processes under the experimental conditions. The SAR approach32 yields kuni of about 7 × 10−2 s−1. Considering RO2 and HO2 levels inferred from previous work under similar experimental conditions33 a bimolecular loss rate in the order of 10−1 s−1 can be estimated when assuming average rate coefficients of 10−11 cm3 molecule−1 s−1 for the bimolecular processes, which is consistent with both the experimental observations as well as the SAR estimate. The opposite trend in the product yields, particularly for glyoxal, is consistent with the bimolecular loss of DICARBO2 and the decomposition of the subsequently formed alkoxy radical (11, Scheme 2).
The rovibronically excited carbonate (omitted for legibility reasons in Scheme 2) might theoretically be stabilised by collision and contribute to the m/z 145 signal. Though, fragmentation, particularly the release of CO2, appears likely. Scission may occur at different bonds in the carbonate molecule, in order that several fragmentation pathways appear possible, in general. Decomposition would yield, at least partly, the same species as fragmentation of the bis(oxy) biradical.
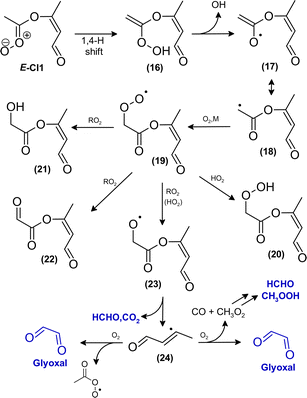 | ||
| Scheme 3 Proposed mechanism for the fate of the carbonyl oxide E-CI1 following 1,4-H isomerisation. Species which were clearly identified as reaction products are marked in blue. | ||
As discussed for DICARBO2, bimolecular reactions of the subsequently formed peroxy radical (19, Scheme 3) will be dominated by reaction with RO2 and HO2. However, the experimental data do not clearly support the consecutive formation of a multifunctional hydroperoxide, a hydroxydicarbonyl and/or tricarbonyl species. The corresponding alkoxy radical will likely decompose and contribute to the formation of HCHO and CO2.
The further fate of the remaining vinyl-type radical (24, Scheme 3) remains rather speculative. Such radicals are known from combustion chemistry and reactions with O2 were investigated particularly for low pressure conditions. Investigations, e.g. for the reaction of the vinyl radical with O2, suggest a mechanism involving a 3- or 4-membered transition state yielding predominantly HCHO + CHO or HCHO + H + CO, respectively.34–36 A detailed product analysis study on the C2H3 + O2 reaction combined with theoretical calculations suggest the mechanism to be valid even at room temperature and atmospheric pressure with the majority of CHO radicals promptly dissociating.37 Based on that, the most probable pathway is the reaction with O2 yielding glyoxal + CH3C(O) and/or glyoxal + CH3 + CO, respectively.
Kuwata et al.38 suggested the subsequent opening of the dioxolene intermediate resulting in a biradical (29, Scheme 4) that finally yields an epoxide species (30, Scheme 4). In fact, the formation of the epoxide remains unsettled, e.g. it might contribute to the m/z 145 signal (C6H9O4+). On the other hand, the FTIR residual spectra do not unambiguously show the presence of epoxides, as mentioned above.
Yet, in contrast to methyl vinyl ketone oxide, sZZ-CI2 possesses an α-hydrogen atom which might be subject of migration, either in the biradical species or the dioxolene intermediate itself (Scheme 4). In both cases, the products are acetic anhydride and ketene, which are found in the reaction system. However, considering the respective accuracy errors, the average ketene/acetic anhydride ratio of about 1.6 likely suggests an additional source of ketene in the system.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The bis(oxy) biradical will either decompose or further isomerize into a functionalized acid with excess internal energy (36, Scheme 5). The fragmentation of the biradical would also contribute to the formation of CO2. Yet, the further fate of the resulting unsaturated radical is rather unclear. The excited acid will either be stabilized by collision or fragment.
O bond. The bis(oxy) biradical will either decompose or further isomerize into a functionalized acid with excess internal energy (36, Scheme 5). The fragmentation of the biradical would also contribute to the formation of CO2. Yet, the further fate of the resulting unsaturated radical is rather unclear. The excited acid will either be stabilized by collision or fragment.
To date, there are only few studies on decomposition of vibrationally excited acids, mainly on formic and acetic acid (see e.g. Cox et al.22 and Newland et al.39 and references therein). Though, a common trait of chemically activated acids seems to be that fragmentation occurs via several pathways yielding either closed-shell molecules through rearrangement of chemical bonds or radical species initiated by bond scission. Recently, decarboxylation was also reported for excited trifluoroacetic acid resulting from a series of ozonolysis reactions.40 In the present system, isopropenyl acetate would be the co-product of such a decarboxylation channel, for which we find no evidence in neither the FTIR residual spectra nor PTR-MS data.
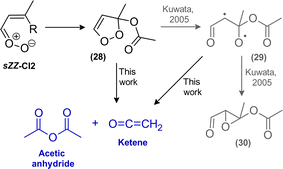
Given the molecular structure, E-CI2 appears the likely source of the non-RO2 acetic acid, which requires an H atom transfer in the chemically activated acid (36, Scheme 5), either from the OH or the CH3 group. In the latter case, an allene (R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) is formed as a co-product (37, Scheme 5). The shift from the OH group might proceed via a 6-membered transition state yielding either the co-products propyne + CO2 or an oxetene intermediate (38, Scheme 5), which might readily or even promptly rearrange to ketene.
CH2) is formed as a co-product (37, Scheme 5). The shift from the OH group might proceed via a 6-membered transition state yielding either the co-products propyne + CO2 or an oxetene intermediate (38, Scheme 5), which might readily or even promptly rearrange to ketene.
The accessibility of these pathways needs certainly to be examined by theoretical calculations. Both, propyne and the allene-type compound are highly endothermic species. There is no experimental evidence for the existence of an allene although it could technically correspond to the m/z 85 (C4H5O2+) signal. In the propyne channel the co-product CO2 acts as a strong thermodynamic sink. The formation of the alkyne might be argued by the presence of m/z 41 (C3H5+) although this ion signal is typically considered to be a fragment, e.g. known from isoprene.41 However, at present we do not find any other species in the proposed mechanism that could potentially yield C3H5+ fragments. In the FTIR residual spectra, a small absorption with a peak centred at 3334 cm−1 might correspond to propyne. Yet, this assignment remains uncertain. Based on available absorption cross sections42 the increase in this absorption feature would correspond to a yield of about 3% in the initial phase of the reaction.
The rearrangement of the oxetene intermediate (38, Scheme 5) is potentially the additional pathway necessary to explain the ketene yield observed, as discussed above. However, overall it seems that none of the proposed channels alone is able to account for the acetic acid yield suggesting a combination of these pathways and/or that additional unidentified reaction channels exist in the oxidation scheme.
Conclusions
The present work confirms that furan derivatives are reactive towards ozone. Particularly substituted furans like 2,5-dimethylfuran exhibit a reactivity towards O3 similar to highly reactive monoterpenes like limonene or β-myrcene (see e.g. Cox et al.22 and references therein). Other higher substituted furans will likely possess similar reactivity towards ozone. The main atmospheric sink remains the reaction with OH under most daytime conditions. Yet, it appears that O3 chemistry of furan derivatives is not negligible, particularly under nighttime conditions. The ozonolysis of 2,5-dimethylfuran was shown to produce a range of small reactive (di)carbonyls, which are readily oxidized by OH and contribute to radical production by photolysis under typical daytime conditions. In addition, the reaction was shown to contribute to atmospheric acidity by forming acetic acid and acetic anhydride. This appears not substantially relevant for the atmosphere when considering a single compound. However, although the acetic acid pathways proposed here require confirmation, they are potentially valid for all furan derivatives possessing a CH3 group in position 2. As such, these channels might contribute to organic acid formation inside nighttime BB plumes, which appears indeed underpredicted by current global models43As said above, the present work intends to serve as a starting point for development of a comprehensive reaction scheme for the O3 chemistry of furans. Yet, there is clearly a need for further research. The carbon balance is still below 50% and although we were able to identify a unimolecular pathway of RO2, additional experiments should be performed under conditions closer to atmospheric radical levels. Further, there is certainly a need for theoretical calculations to assess the accessibility of reaction pathways proposed within this work.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
NI gratefully acknowledges Iulia Patroescu-Klotz and Peter Wiesen for support and encouragement to develop own research ideas. The laboratory infrastructure used in this study is supported by the German Federal Ministry for Education and Research (BMBF) under grant agreement 01LK2001H (ACTRIS-D).References
- A. Jaswal, P. P. Singh and T. Mondal, Furfural – a versatile, biomass-derived platform chemical for the production of renewable chemicals, Green Chem., 2022, 24, 510–551 RSC.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates, Nature, 2007, 447, 982–985 CrossRef PubMed.
- M. Müller, B. E. Anderson, A. J. Beyersdorf, J. H. Crawford, G. S. Diskin, P. Eichler, A. Fried, F. N. Keutsch, T. Mikoviny, K. L. Thornhill, J. G. Walega, A. J. Weinheimer, M. Yang, R. J. Yokelson and A. Wisthaler, In situ measurements and modeling of reactive trace gases in a small biomass burning plume, Atmos. Chem. Phys., 2016, 16, 3813–3824 CrossRef.
- L. E. Hatch, R. J. Yokelson, C. E. Stockwell, P. R. Veres, I. J. Simpson, D. R. Blake, J. J. Orlando and K. C. Barsanti, Multi-instrument comparison and compilation of non-methane organic gas emissions from biomass burning and implications for smoke-derived secondary organic aerosol precursors, Atmos. Chem. Phys., 2017, 17, 1471–1489 CrossRef CAS.
- A. Bierbach, I. Barnes and K. H. Becker, Rate coefficients for the gas-phase reactions of hydroxyl radicals with furan, 2-methylfuran, 2-ethylfuran and 2,5-dimethylfuran at 300 ± 2 K, Atmos. Environ., 1992, 26, 813–817 CrossRef.
- A. Bierbach, I. Barnes and K. H. Becker, Product and kinetic study of the OH-initiated gas-phase oxidation of furan, 2-methylfuran and furanaldehydes at ≈300 K, Atmos. Environ., 1995, 29, 2651–2660 CrossRef CAS.
- C. A. Whelan, J. Eble, Z. S. Mir, M. A. Blitz, P. W. Seakins, M. Olzmann and D. Stone, Kinetics of the Reactions of Hydroxyl Radicals with Furan and Its Alkylated Derivatives 2-Methyl Furan and 2,5-Dimethyl Furan, J. Phys. Chem. A, 2020, 124, 7416–7426 CrossRef CAS.
- M. J. Newland, Y. Ren, M. R. McGillen, L. Michelat, V. Daële and A. Mellouki, NO3 chemistry of wildfire emissions: a kinetic study of the gas-phase reactions of furans with the NO3 radical, Atmos. Chem. Phys., 2022, 22, 1761–1772 CrossRef CAS.
- M. M. Coggon, C. Y. Lim, A. R. Koss, K. Sekimoto, B. Yuan, J. B. Gilman, D. H. Hagan, V. Selimovic, K. J. Zarzana, S. S. Brown, J. M. Roberts, M. Müller, R. Yokelson, A. Wisthaler, J. E. Krechmer, J. L. Jimenez, C. Cappa, J. H. Kroll, J. de Gouw and C. Warneke, OH chemistry of non-methane organic gases (NMOGs) emitted from laboratory and ambient biomass burning smoke: evaluating the influence of furans and oxygenated aromatics on ozone and secondary NMOG formation, Atmos. Chem. Phys., 2019, 19, 14875–14899 CrossRef CAS.
- Z. C. J. Decker, K. J. Zarzana, M. Coggon, K.-E. Min, I. Pollack, T. B. Ryerson, J. Peischl, P. Edwards, W. P. Dubeì, M. Z. Markovic, J. M. Roberts, P. R. Veres, M. Graus, C. Warneke, J. de Gouw, L. E. Hatch, K. C. Barsanti and S. S. Brown, Nighttime Chemical Transformation in Biomass Burning Plumes: A Box Model Analysis Initialized with Aircraft Observations, Environ. Sci. Technol., 2019, 53, 2529–2538 CrossRef CAS PubMed.
- W. M. Jolly, M. A. Cochrane, P. H. Freeborn, Z. A. Holden, T. J. Brown, G. J. Williamson and D. M. J. S. Bowman, Climate-induced variations in global wildfire danger from 1979 to 2013, Nat. Commun., 2015, 6, 7537 CrossRef CAS PubMed.
- B. J. Harvey, Human-caused climate change is now a key driver of forest fire activity in the western United States, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11649–11650 CrossRef CAS PubMed.
- R. J. Yokelson, J. D. Crounse, P. F. DeCarlo, T. Karl, S. Urbanski, E. Atlas, T. Campos, Y. Shinozuka, V. Kapustin, A. D. Clarke, A. Weinheimer, D. J. Knapp, D. D. Montzka, J. Holloway, P. Weibring, F. Flocke, W. Zheng, D. Toohey, P. O. Wennberg, C. Wiedinmyer, L. Mauldin, A. Fried, D. Richter, J. Walega, J. L. Jimenez, K. Adachi, P. R. Buseck, S. R. Hall and R. Shetter, Emissions from biomass burning in the Yucatan, Atmos. Chem. Phys., 2009, 9, 5785–5812 CrossRef CAS.
- R. Atkinson, S. M. Aschmann and W. P. L. Carter, Kinetics of the reactions of O3 and OH radicals with furan and thiophene at 298 ± 2 K, Int. J. Chem. Kinet., 1983, 15, 1761–1772 Search PubMed.
- J. Matsumoto, Kinetics of the Reactions of Ozone with 2,5-Dimethylfuran and Its Atmospheric Implications, Chem. Lett., 2011, 40, 582–583 CrossRef CAS.
- M. Li, Y. Li and L. Wang, Gas-phase ozonolysis of furans, methylfurans, and dimethylfurans in the atmosphere, Phys. Chem. Chem. Phys., 2018, 20, 24735–24743 RSC.
- M. Tajuelo, D. Rodríguez, A. Rodríguez, A. Escalona, G. Viteri, A. Aranda and Y. Diaz-de-Mera, Secondary organic aerosol formation from the ozonolysis and oh-photooxidation of 2,5-dimethylfuran, Atmos. Environ., 2021, 245, 118041 CrossRef CAS.
- A. Alvarado, R. Atkinson and J. Arey, Kinetics of the gas-phase reactions of NO3 radicals and O3 with 3-methylfuran and the OH radical yield from the O3 reaction, Int. J. Chem. Kinet., 1996, 28, 905–909 CrossRef CAS.
- N. Illmann, R. G. Gibilisco, I. G. Bejan, I. Patroescu-Klotz and P. Wiesen, Atmospheric oxidation of α,β-unsaturated ketones: kinetics and mechanism of the OH radical reaction, Atmos. Chem. Phys., 2021, 21, 13667–13686 CrossRef CAS.
- N. Illmann, I. Patroescu-Klotz and P. Wiesen, Biomass burning plume chemistry: OH-radical-initiated oxidation of 3-penten-2-one and its main oxidation product 2-hydroxypropanal, Atmos. Chem. Phys., 2021, 21, 18557–18572 CrossRef CAS.
- R. Atkinson, Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions, Chem. Rev., 1986, 86, 69–201 CrossRef CAS.
- R. A. Cox, M. Ammann, J. N. Crowley, H. Herrmann, M. E. Jenkin, V. F. McNeill, A. Mellouki, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume VII – Criegee intermediates, Atmos. Chem. Phys., 2020, 20, 13497–13519 CrossRef CAS.
- D. J. Stewart, S. H. Almabrok, J. P. Lockhart, O. M. Mohamed, D. R. Nutt, C. Pfrang and G. Marston, Atmos. Environ., 2013, 70, 227–235 CrossRef CAS.
- R. Atkinson, J. Arey and E. C. Tuazon, Formation of O(3P) atoms and epoxides from the gas-phase reaction of O3 with isoprene, Res. Chem. Intermed., 1994, 20, 385–394 CrossRef CAS.
- H. E. O'Neal and C. Blumstein, A new mechanism for gas phase ozone–olefin reactions, Int. J. Chem. Kinet., 1973, 5, 397–413 CrossRef.
- L. Vereecken, A. Novelli and D. Taraborrelli, Unimolecular decay strongly limits the atmospheric impact of Criegee intermediates, Phys. Chem. Chem. Phys., 2017, 19, 31599–31612 RSC.
- R. Winterhalter, F. Herrmann, B. Kanawati, T. L. Nguyen, J. Peeters, L. Vereecken and G. K. Moortgat, The gas-phase ozonolysis of of β-caryophyllene (C15H24). Part I: an experimental study, Phys. Chem. Chem. Phys., 2009, 11, 4152–4172 RSC.
- T. L. Nguyen, R. Winterhalter, G. Moortgat, B. Kanawati, J. Peeters and L. Vereecken, The gas-phase ozonolysis of β-caryophyllene (C15H24). Part II: A theoretical study, Phys. Chem. Chem. Phys., 2009, 11, 4173–4183 RSC.
- T. L. Nguyen, J. Peeters and L. Vereecken, Theoretical study of the gas-phase ozonolysis of β-pinene (C10H16), Phys. Chem. Chem. Phys., 2009, 11, 5643–5656 RSC.
- M. E. Jenkin, M. D. Hurley and T. J. Wallington, Investigation of the radical product channel of the CH3C(O)O2 + HO2 reaction in the gas phase, Phys. Chem. Chem. Phys., 2007, 9, 3149–3162 RSC.
- J. D. Crounse, H. C. Knap, K. B. Ørnsø, S. Jørgensen, F. Paulot, H. G. Kjaergaard and P. O. Wennberg, Atmospheric Fate of Methacrolein. 1. Peroxy Radical Isomerization Following Addition of OH and O2, J. Phys. Chem. A, 2012, 116, 5756–5762 CrossRef CAS PubMed.
- L. Vereecken and B. Nozière, H migration in peroxy radicals under atmospheric conditions, Atmos. Chem. Phys., 2020, 20, 7429–7458 CrossRef CAS.
- N. Illmann, I. Patroescu-Klotz and P. Wiesen, Organic acid formation in the gas-phase ozonolysis of α,β-unsaturated ketones, Phys. Chem. Chem. Phys., 2023, 25, 106–116 RSC.
- R. R. Baldwin and R. W. Walker, Elementary reactions in the oxidation of alkenes, Symp. Combust. Proc., 1981, 18, 819–829 CrossRef.
- J. Y. Park, M. C. Heaven and D. Gutman, Kinetics and mechanism of the reaction of vinyl radicals with molecular oxygen, Chem. Phys. Lett., 1984, 104, 469–474 CrossRef CAS.
- B. K. Carpenter, Computational prediction of new mechanisms for the reactions of vinyl and phenyl radicals with molecular oxygen, J. Am. Chem. Soc., 1993, 115, 9806–9807 CrossRef CAS.
- A. Matsugi and A. Miyoshi, Yield of Formyl Radical from the Vinyl + O2 Reaction, Int. J. Chem. Kinet., 2014, 46, 260–274 CrossRef CAS.
- K. T. Kuwata, L. C. Valin and A. D. Converse, Quantum Chemical and Master Equation Studies of the Methyl Vinyl Carbonyl Oxides Formed in Isoprene Ozonolysis, J. Phys. Chem. A, 2005, 109, 10710–10725 CrossRef CAS PubMed.
- M. J. Newland, C. Mouchel-Vallon, R. Valorso, B. Aumont, L. Vereecken, M. E. Jenkin and A. R. Rickard, Estimation of mechanistic parameters in the gas-phase reactions of ozone with alkenes for use in automated mechanism construction, Atmos. Chem. Phys., 2022, 22, 6167–6195 CrossRef CAS.
- M. R. McGillen, Z. T. P. Fried, M. A. H. Khan, K. T. Kuwata, C. M. Martin, S. O'Doherty, F. Pecere, D. E. Shallcross, K. M. Stanley and K. Zhang, Ozonolysis can produce long-lived greenhouse gases from commercial refrigerants, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2312714120 CrossRef CAS.
- R. Taipale, T. M. Ruuskanen, J. Rinne, M. K. Kajos, H. Hakola, T. Pohja and M. Kulmala, Technical Note: Quantitative long-term measurements of VOC concentrations by PTR-MS – measurement, calibration, and volume mixing ratio calculation methods, Atmos. Chem. Phys., 2008, 8, 6681–6698 CrossRef CAS.
- E. Es-sebbar, A. Jolly, Y. Benilan and A. Farooq, Quantitative mid-infrared spectra of allene and propyne from room to high temperatures, J. Mol. Spectrosc., 2014, 305, 10–16 CrossRef CAS.
- M. A. H. Khan, K. Lyons, R. Chhantyal-Pun, M. R. McGillen, R. L. Caravan, C. A. Taatjes, A. J. Orr-Ewing, C. J. Percival and D. E. Shallcross, Investigating the Tropospheric Chemistry of Acetic Acid Using the Global 3-D Chemistry Transport Model, STOCHEM-CRI, J. Geophys. Res.: Atmos., 2018, 123, 6267–6281 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ea00045e |
| This journal is © The Royal Society of Chemistry 2024 |