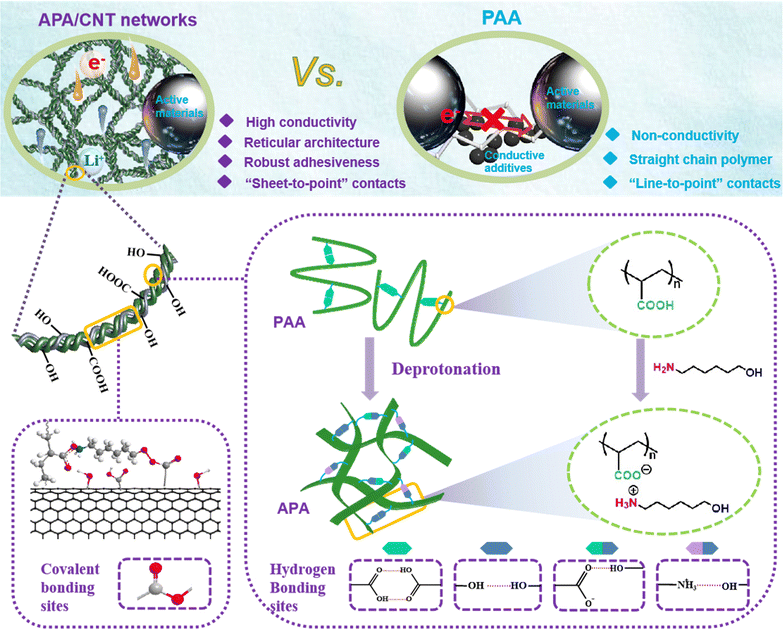
A new universal aqueous conductive binder via esterification reinforced electrostatic/H-bonded self-assembly for high areal capacity and stable lithium-ion batteries†
Farong
Zhang‡
a,
Hongyu
Xia‡
a,
Tongye
Wei
 *b,
Huaming
Li
ac,
Mei
Yang
*b,
Huaming
Li
ac,
Mei
Yang
 *ac and
An-Min
Cao
*ac and
An-Min
Cao
 *de
*de
aCollege of Chemistry, Xiangtan University, Xiangtan 411105, Hunan Province, P. R. China. E-mail: yangmei@xtu.edu.cn
bHunan Institute of Advanced Sensing and Information Technology, Xiangtan University, Xiangtan 411105, Hunan Province, P. R. China
cKey Laboratory of Polymeric Materials & Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymeric Materials of College of Hunan Province, and Key Lab of Environment-Friendly Chemistry and Application in Ministry of Education, Xiangtan University, Xiangtan 411105, Hunan Province, P. R. China
dCAS Key Laboratory of Molecular Nanostructure and Nanotechnology and Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing 100190, P. R. China
eUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 14th November 2023
Abstract
Because of insufficient adhesivity and mechanical properties, conventional polymeric binders fail to accommodate immense volume changes and maintain interparticle connections upon the repeated charge/discharge processes, especially in high active material loading and thick electrodes. Here, a new water-soluble conductive binder (APA/CNT) composed of carboxylic carbon nanotubes interwoven in flexible membranes of the neutralising product of 6-amino-1-hexanol and commercial poly(acrylic acid) (PAA) is developed. The APA/CNT is expected to form robust conductive and elastic network by means of the esterification reinforced electrostatic/H-bonded self-assembly, in which the continuous conductive skeletons can segregate and grasp active nanoparticles by an efficient and robust “sheet-to-point” bonding mode, which endows the as-fabricated anode and cathode with extraordinary structural and interfacial stability, and enhanced electronic conductivity. The as-constructed Si/C anode exhibits superior cycling stability and a high rate performance even under a high mass loading of 15 mg cm−2, and achieves a high areal capacity of 7.79 mA h cm−2, far exceeding those of other binder-based Si/C anodes. Moreover, the as-fabricated full cell also displays enhanced electrochemical behaviour and cycling durability. This simple and easy strategy should give a valuable new way to design a cost-effective, universal yet environmentally friendly binder with dependable adhesiveness for high-performance devices.
Broader contextThe rapid development of emerging markets such as electric vehicles has new requirements for power devices with a higher energy density, the core of which relies on the highly efficient construction of thick electrodes with a large areal capacity. However, the traditional polymeric binder and carbon additive mixture cannot successfully accommodate the huge volume change and maintain good conductivity when cycling. This issue is more serious for high mass loaded cathodes, which are preferentially used as a simple approach to enable high energy density Li-ion batteries. In this work, a new water-soluble conductive binder (APA/CNT) composed of carboxylic carbon nanotubes interwoven in flexible membranes of the neutralising product of 6-amino-1-hexanol and commercial poly(acrylic acid) (PAA) is developed. The APA/CNT is expected to form a robust conductive and elastic network by means of the esterification reinforced electrostatic/H-bonded self-assembly, in which the continuous conductive skeletons can segregate and grasp active nanoparticles by an efficient and robust “sheet-to-point” bonding mode, endowing the as-fabricated electrode with extraordinary structural and interfacial stability, and enhanced electronic conductivity. Remarkably, this new binder is applicable to both anode and cathode. The as-constructed Si/C anode exhibits superior cycling stability and high rate performance even under a high mass loading of 15 mg cm−2, and achieves a high areal capacity of 7.79 mA h cm−2, far exceeding those of other binder-based Si/C anodes. Moreover, the as-fabricated full cell also displays enhanced electrochemical behaviour and cycling durability. This simple and easy strategy should give a new valuable way to design cost-effective, universal yet environmentally friendly binder with steady and good adhesiveness for high-performance devices. |
Introduction
Lithium-ion batteries (LIBs), with the merits of sustainability, high voltage, high energy density and long cycling life, were regarded as one of the most progressive energy technologies to meet the ever-increasing demands of portable electronic products and electric vehicles.1–3 Unfortunately, the huge mechanical stress induced from the dramatic volumetric change of Si resulted in a thick and unstable solid electrolyte interphase (SEI) layer, thus leading to the poor circulating life of the Si anode.4,5 To address this issue, various fabrication approaches for the state-of-the-art Si-based material have been developed.6–10 Among them, the Si/C composites had made remarkable progress in enhancing the conductivity and alleviating the volumetric change of the Si-based anodes.11,12 However, this problem still remained unsettled, especially at increased energy densities, leading to the electrochemical performance of these Si/C anodes being significantly lower than the industrial standard.13In addition to the active materials, binders also had significant effect on maintaining the structural stability/integrity and prolonging the cyclic life.14,15 In the case of traditional poly(acrylic acid) (PAA), its abundant carboxyl groups (–COOH) can form H-bonds with the O-containing groups (i.e., –OH and –COOH) on the surface of the current collector to retain high adhesion and restrain cracks/delamination during the cycling. Nevertheless, some of the –COOH in the PAA polymer chain tended to form intermolecular H-bonds, and the resultant self-agglomeration inevitably resulted in an impaired adhesive force and elasticity.16,17 So far as is known, although a large number of linear binders enhanced the cycling behaviour of the Si anode because of their high modulus and high adhesive strength, they were prone to exfoliate with the high mechanical stress because the stress was concentrated on a single chain.18 Moreover, the deficient elasticity of these binders was still unable to satisfy the demand to accommodate the dramatic volumetric change upon the charge/discharge processes.
Introducing crosslinkers into these linear polymers to form continuous and robust 2D- or 3D-crosslinked binders can distribute the concentrated mechanical strain to the branched-chains via the interconnected points.19,20 For example, a variety of crosslinking systems based on sodium alginate/Cu+, amorphous CaCO3/PAA, citric acid (CA)/PAA, gelatin/boric acid, phytic acid/copper(II) phthalocyanine tetrasulfonate/PPy/Fe3O4, poly(ethylene oxide) (PEO)/poly(ethylenimine) (PEI)/PEDOT:PSS, and Fe3+-(tris) catechol/PDBP polymer, and so on had been adopted to enhance the mechanical stability/integrity of the electrodes.21–27 Furthermore, the 2D- or 3D-crosslinked binders formed can segregate and grasp active nanoparticles by efficient “sheet-to-point” bonding, enabling the as-fabricated electrodes to obtain extraordinary structural and interfacial stability.28,29
Moreover, neglecting the electronic conduction in the binder design always led to the demand of a higher binder content, because the conductive additives must be introduced to provide enhanced electrical contact. This situation tended to be serious with electrodes loaded with a highly active material, which usually faced dynamic degradation and external deformation. Thus, the achievement of stable cyclability and high areal capacity of the high mass-loaded electrodes still remained a major challenge.
Here, a new water-soluble conductive binder (APA/CNT) composed of carboxylic carbon nanotubes (CNTs) interwoven in flexible membranes of the neutralising product of 6-amino-1-hexanol (AH) and commercial PAA is developed. The –COOH groups in the PAA were transformed into the charged state via the deprotonation by AH. Due to the electrostatic repulsion of the adjacent electriferous (–COO−) groups, the self-associated H-bonds were depleted, and therefore lead to the more stretched PAA chains in the complex of PAA and AH. In the meantime, the robust reticular architecture was constructed from the emerged acceptors and donors of the H-bonds (i.e., –COO−, –OH), with the assistance of the electrostatic binding of –NH3+ and –COO−. The APA/CNT binder possessedd a porous reticular nanosheet construction, which originated from the interweaving of the CNTs onto the nanosheet-shaped neutralising product of AH and PAA via esterification reinforced electrostatic/H-bonded self-assembly. The sub-units (nanosheets) of the binder firmly wrapped and interconnected with the well-dispersed active nanoparticles to construct a continuously conductive yet robust network within the electrode, whereby the resulting electrodes can establish extraordinary structural and interfacial stability and enhanced electronic conductivity via the “sheet-to-point” bonding mode, even for thick (high mass loaded) electrodes. Consequently, the APA/CNT-based Si/C anode exhibits a superior cycling stability and rate performance even under a high mass loading of 15 mg cm−2, and achieves a high areal capacity of 7.79 mA h cm−2, far exceeding those of other binder-based Si/C anodes. Moreover, the APA/CNT-based LiFePO4/Si/C full cell also displays enhanced electrochemical behaviour and cycling durability.
Experimental section
Preparation of the APA/CNT binder
The AH (75 mg) and PAA (100 mg) were dissolved in 50 mL of absolute ethyl alcohol. After stirring for 3 h under room temperature, the solution was left to stand for another 1 h. The AH-PAA complex (labelled as APA) was obtained after centrifugation, and then dried.The APA (90 mg) was dissolved in 100 mL of deionised water at ambient temperature. Then, 60 mg carboxylic CNT and 2 mg of 4-dimethylaminopyridine were added to the APA solution, and sonicated for 2 h. The APA/CNT binder was obtained after the freeze-drying processing. For comparison, a series of APA/CNT binders with a variable mass ratio of APA to CNT from 1.0 to 2.0 were prepared.
Preparation of the electrode and cell
The APA/CNT and PAA were used separately to fabricate the Si/C anode and the LiFePO4 cathode. For the APA/CNT-based electrode, the composition was active material: APA/CNT = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 by weight. For PAA-based electrode, the composition was active material
20 by weight. For PAA-based electrode, the composition was active material![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNTs
CNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA = 80
PAA = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 by weight. Both of APA/CNT- and PAA-based systems used deionised water as the solvent. The slurries of APA/CNT and PAA were then separately casted onto the current collector and dried in a vacuum oven for 12 h at 120 °C.
10 by weight. Both of APA/CNT- and PAA-based systems used deionised water as the solvent. The slurries of APA/CNT and PAA were then separately casted onto the current collector and dried in a vacuum oven for 12 h at 120 °C.
Electrochemical measurements
The APA/CNT- or PAA-based electrodes were assembled in the CR2032-type half cells with a poly(propylene) separator (Celgard 2400) and a lithium metal counter electrode. The loading of the active material for the electrode obtained was 1–15 mg cm−2. The 1.0 M LiPF6 in diethyl carbonate/ethyl carbonate (DEC/EC) = 1/1 (v/v) served as the electrolyte. The charge/discharge cycling and rate performances were examined by using a battery test system (Neware, CT-4008). The cyclic voltammetry (CV) measurements and EIS were executed on an electrochemical workstation (CH Instruments, CHI760e). The relevant details and correlative formulae are described in the ESI.†Results and discussion
Preparation and characterisation of the APA/CNT binder
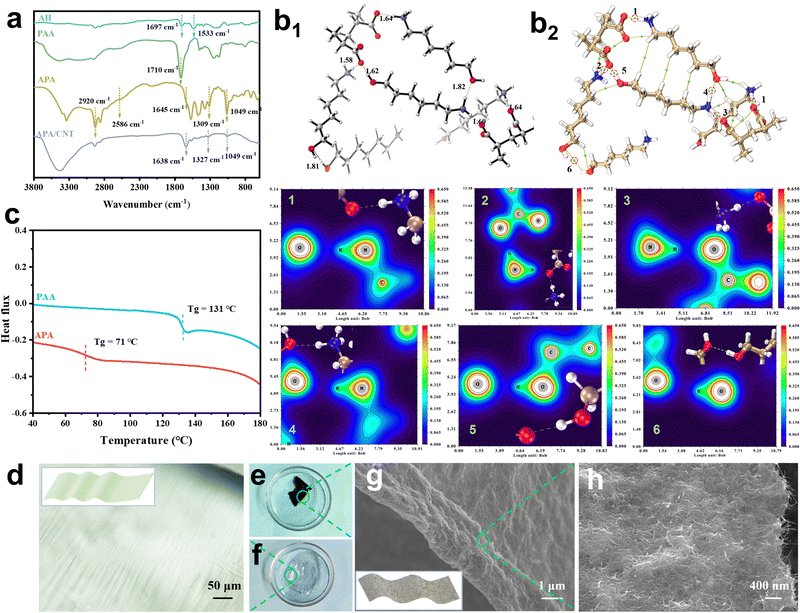
The APA/CNT binder was prepared via the simple acid–base neutralisation reaction of AH and PAA, followed by interweaving the as-obtained APA and the carboxylic CNTs (Fig. 1). The FTIR spectra was obtained to verify the deprotonation of the PAA by AH and the esterification with the CNTs. As shown in Fig. 2a, in the spectra of PAA, a peak at 1710 cm−1 corresponded to the –COOH group of the carboxylic acid dimer (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HO–). The peaks at 1697 cm−1 and 1533 cm−1 assigned to the asymmetric bending of NH2 were observed for AH.30 The spectra of APA featured ammonium (NH3+) stretching vibration bands located at about 2920 and 2586 cm−1, and the antisymmetrical bending band of the NH3+ appeared at 1645 cm−1. For APA/CNT, the appearance of the new peaks at 1049, 1327 and 1638 cm−1 were attributable to the symmetric stretching of the formed –C–O–C– and –C
O⋯HO–). The peaks at 1697 cm−1 and 1533 cm−1 assigned to the asymmetric bending of NH2 were observed for AH.30 The spectra of APA featured ammonium (NH3+) stretching vibration bands located at about 2920 and 2586 cm−1, and the antisymmetrical bending band of the NH3+ appeared at 1645 cm−1. For APA/CNT, the appearance of the new peaks at 1049, 1327 and 1638 cm−1 were attributable to the symmetric stretching of the formed –C–O–C– and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, respectively (Fig. 2a).31 The degree of deprotonation of the COOH groups in PAA was investigated by 1H-NMR spectra via an established method,32,33 and the value was found to be 63.5% (Fig. S1 and Table S1, ESI†). The glass transition temperature of APA, obtained from the inflexion point of the differential scanning calorimetry (DSC) thermogram, was about 71 °C, which was well below that of PAA (131 °C), and indicating the better chain flexibility (Fig. 2c). In a typical preparation, the as-obtained APA transparent membranes (Fig. 2d and f) and carboxylic CNTs were uniformly dispersed in water by ultrasonic mixing. After a freezing-thawing procedure, this new water-soluble and conductive APA/CNT binder was constructed (Fig. 2e). The APA/CNT binder (Fig. 2g and h) showed a 2D-reticular porous structure, which was constructed by the tight entanglement of APA membranes and CNTs via a hydrogen bonding interaction and a covalent bonding interaction (ester linkages).
O group, respectively (Fig. 2a).31 The degree of deprotonation of the COOH groups in PAA was investigated by 1H-NMR spectra via an established method,32,33 and the value was found to be 63.5% (Fig. S1 and Table S1, ESI†). The glass transition temperature of APA, obtained from the inflexion point of the differential scanning calorimetry (DSC) thermogram, was about 71 °C, which was well below that of PAA (131 °C), and indicating the better chain flexibility (Fig. 2c). In a typical preparation, the as-obtained APA transparent membranes (Fig. 2d and f) and carboxylic CNTs were uniformly dispersed in water by ultrasonic mixing. After a freezing-thawing procedure, this new water-soluble and conductive APA/CNT binder was constructed (Fig. 2e). The APA/CNT binder (Fig. 2g and h) showed a 2D-reticular porous structure, which was constructed by the tight entanglement of APA membranes and CNTs via a hydrogen bonding interaction and a covalent bonding interaction (ester linkages).
The density functional theory (DFT) calculation was introduced to explore the AH-induced enhancement of the mechanical stability of PAA. Fig. 2b1 revealed that the amine moieties of AH were protonated by the carboxylic acid groups in PAA, which suggested the existence of an electrostatic interaction, which agreed with the FTIR results. Simulation results also confirmed the presence of a variety of H-bonds in APA, such as –NH3+⋯HO–, –OH⋯OH–, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HO–, instead of the self-associated H-bonds (–COOH⋯HOOC–) in PAA. Using the atoms in molecules theory based on electron density, the bond strength of those H-bonds were arranged in following order: –NH3+⋯HO– > –C
O⋯HO–, instead of the self-associated H-bonds (–COOH⋯HOOC–) in PAA. Using the atoms in molecules theory based on electron density, the bond strength of those H-bonds were arranged in following order: –NH3+⋯HO– > –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HO– > –COOH⋯HOOC– > –OH⋯OH– (Fig. 2b2 and Table S2, ESI†). Multiple H-bonds with higher energies in APA enabled more and stronger linkages on the polymer skeletons, and the improved entanglement between the adjacent polymer chains could dissipate stress via the dynamic non-covalent connection points. In comparison, the presence of sole, less intensive H-bonds (–COOH⋯HOOC–) accounted for the poor adhesiveness of PAA (Fig. S2 and Table S2, ESI†).
O⋯HO– > –COOH⋯HOOC– > –OH⋯OH– (Fig. 2b2 and Table S2, ESI†). Multiple H-bonds with higher energies in APA enabled more and stronger linkages on the polymer skeletons, and the improved entanglement between the adjacent polymer chains could dissipate stress via the dynamic non-covalent connection points. In comparison, the presence of sole, less intensive H-bonds (–COOH⋯HOOC–) accounted for the poor adhesiveness of PAA (Fig. S2 and Table S2, ESI†).
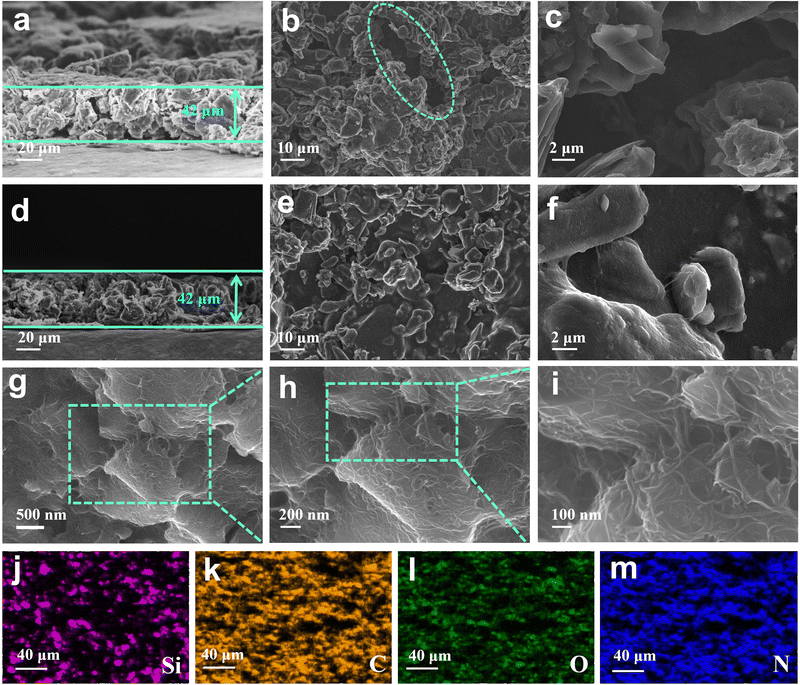
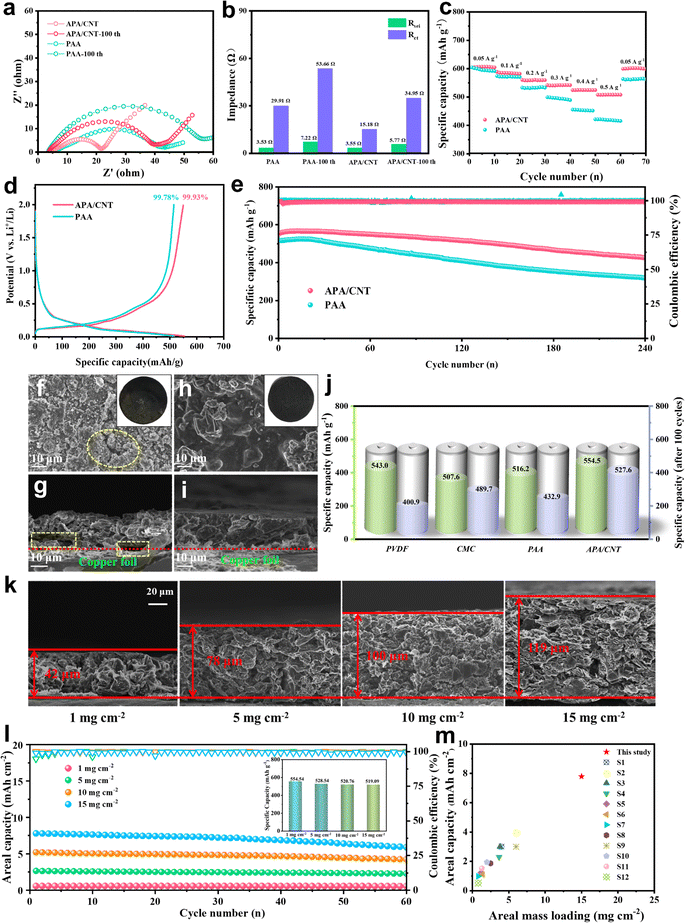
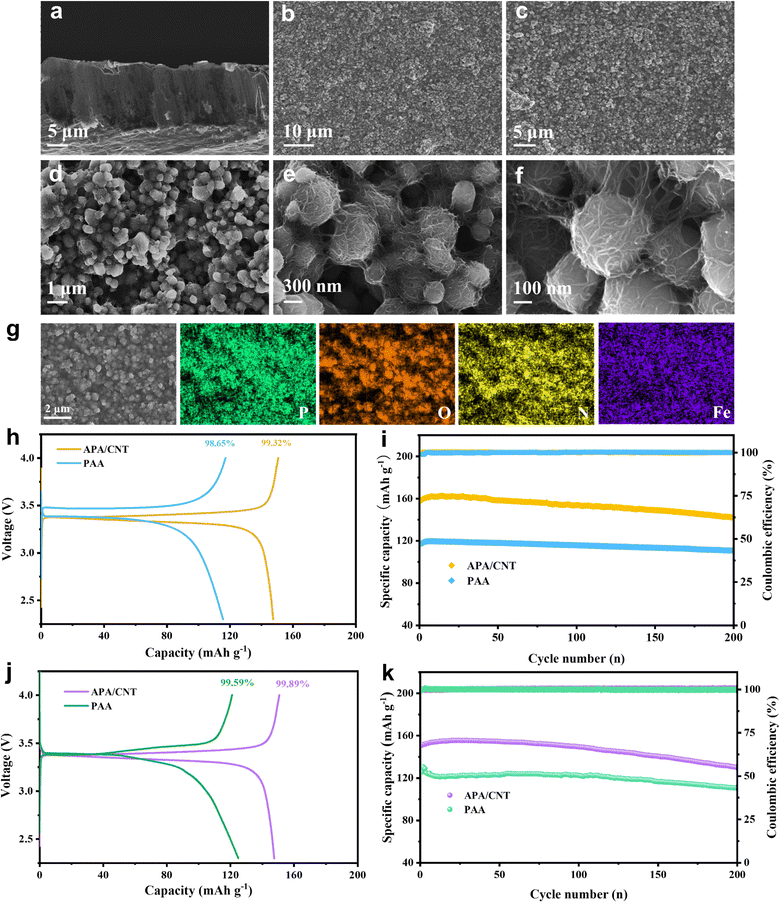
The crystallinity of APA/CNT and PAA was investigated by powder X-ray diffraction (PXRD). All the APA, APA/CNT and PAA binders presented broad peaks at 15–30°, revealing their amorphous state (Fig. S3, ESI†), which was conducive to maintaining the uniform distribution of the composite nanoparticles and the mechanical integrity of the electrodes during the drying process. The surface morphologies of PAA, APA, and APA/CNT were investigated using optical microscopy. As shown in Fig. S4 (ESI†), the PAA was an amorphous powder, whereas the APA and APA/CNT exhibited uniform and thin films, which were beneficial to their coverage on the current collector. The overall structure of the Si/C anodes fabricated with APA/CNT and PAA were investigated using SEM images. The APA/CNT-based anodes displayed a dense, flat and smooth surface (Fig. 3d–f), whereas large holes or cracks appeared at the PAA-based anodes because of its relatively poor adhesion and film forming ability (Fig. 3a–c), which lead to the inferior cycling stability.34 Moreover, the inhomogeneous distribution of the conductive additives (CNTs) could be clearly observed for the PAA-based anodes (Fig. 3c). The robust conductive architecture of APA/CNT tightly wrapped and interconnected the Si/C particles, endowing the as-fabricated anodes with a rapid charge distribution and good mechanical performance (Fig. 3g–i). Therefore, the APA/CNT-based anode was expected to deliver enhanced cycling behaviour and rate performance. The energy dispersive spectroscopy (EDS) mapping confirmed a uniform component dispersion of the APA/CNT-based anode (Fig. 3j–m).
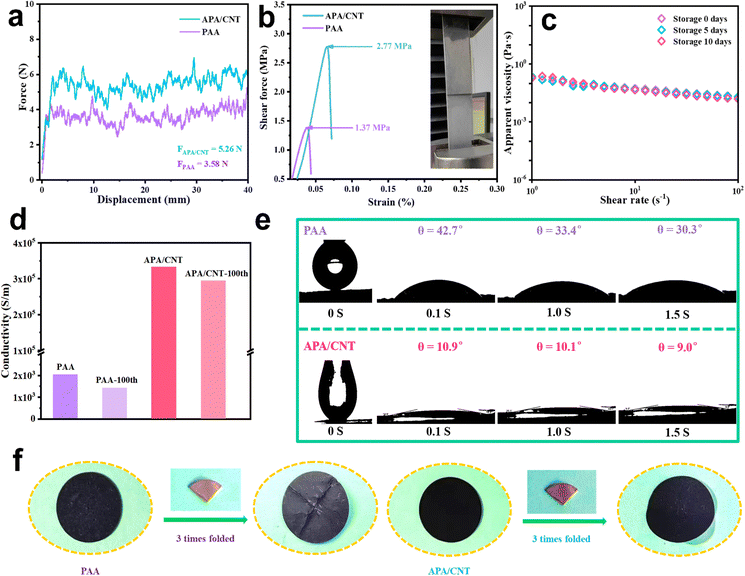
The mechanical property is one of the most important evaluation parameters of the binders.35 The force-displacement curves of APA/CNT and PAA are shown in Fig. 4a. Due to the abundant hydrogen- and covalent-bonding interactions, together with the efficient “sheet-to-point” bonding mode, the adhesion strength of APA/CNT (5.26 N) was obviously enhanced when compared to that of PAA (3.58 N). Fig. 4b shows the stress–strain responses of APA/CNT and PAA. The maximum stress of APA/CNT was 2.77 MPa, which was much larger than that of PAA (1.37 MPa), once again confirming the enhanced adhesive strength of APA/CNT. Furthermore, the rich polarity groups endowed APA/CNT with good hydrophilicity and the apparent viscosity. The APA/CNT-based ink remained almost unchanged after different storage times, confirming its outstanding storage stability (Fig. 4c).
The outstanding mechanical properties of the APA/CNT-based electrode could be largely attributed to the highly integrated reticular architecture and stable physicochemical features, which was expected to deliver high durability during the cycling process. Additionally, the electrical conductivity, another key parameter that impacted the battery performance, was studied and the results are shown in Fig. 4d. The conductivity before and after 100 cycles of APA/CNT-based electrode were found to be 3.3 × 105 S m−1 and 2.9 × 105 S m−1, far surpassing that of the PAA-based electrode (2.0 × 103 S m−1 and 1.4 × 103 S m−1), which demonstrated that the stable electrical contacts had been built by the continuous conductive skeleton. The wettability was also critical to the battery performance of the as-fabricated electrode, which mainly related to the surface chemical properties of the binder.36 The initial contact angle (θ) of the APA/CNT-based electrode was found to be 10.9° and quickly evolved into 9.0° after 1.5 s. In contrast, the penetration rate of the electrolyte on the PAA-based electrode was much slower, indicating the superior wettability of the APA/CNT-based electrode (Fig. 4e). In addition, the mechanical stability of the PAA- and APA/CNT-based electrodes were evaluated by using folding tests (Fig. 4f). After folding the electrodes with the same angle three times, some cracks appeared on the surface of the PAA-based electrode, but the APA/CNT-based electrode remained almost unchanged, demonstrating its superior adhesive strength and elasticity.
Electrochemical performance of the APA/CNT-based electrode
Electrochemical tests were first conducted to evaluate the application of APA/CNT as a binder in the Si/C anode. The CV curves of the APA/CNT-based electrodes are shown in Fig. S5 (ESI†). The peak at about 0.23 V belonged to the conversion from Si to the LixSiy phase, whereas the peaks appearing at 0.25 V and 0.28 V were related to the dealloying process of the LixSiy alloys to Si.37,38 From the charge–discharge curves of the APA/CNT-based electrode at 0.2 A g−1 (Fig. S6, ESI†), the profile of the 5th cycle almost coincided with that of 50th, which indicating its high cycling stability. As is already known, the dramatic volumetric change of Si upon repeated lithiation/delithiation processes resulted in the fragmentation of the electrode, and thus lead to the increase of the impedance,39 so the EIS measurements were performed to confirm the improved Li+ ion diffusion of the APA/CNT binder (Fig. 5a). After cycling for 100 cycles, the charge-transfer resistance (Rct) of the APA/CNT-based electrode presented a slightly increase (Fig. 5b), which indicating that a continuous conductive matrix was formed by the APA/CNT, which preserved the electrically connected integrity and ensured a fast electron transfer.40 The rate capabilities of the APA/CNT- and PAA-based electrodes were compared at the range of 0.05–0.50 A g−1 (Fig. 5c). In particular, the APA/CNT-based electrode could still maintain a high capacity of 507.9 mA h g−1 even at 0.5 A g−1, far surpassing that of the PAA-based electrode (415.5 mA h g−1).Fig. 5e and Fig. S7 (ESI†) reflected the long-term cycling performance of the APA/CNT-, APA- and PAA-based electrodes at 0.2 A g−1. After 100 charge/discharge cycles, the APA/CNT- and APA-based electrodes showed nearly no capacity fading, and still delivered a capacity of 527.6 mA h g−1 (95.1% of its initial capacity) and 512.8 mA h g−1 (87.7% of its initial capacity), respectively. Furthermore, the coulombic efficiency after initial charge–discharge (CE) was found to be 99.93% (Fig. 5d), and the capacity value of the APA/CNT-based electrode could reach as high as 426.2 mA h g−1 after 240 cycles. The superior CE and capacity retention rate could be attributed to the abundant hydrogen- and covalent-bonding interactions, the efficient “sheet-to-point” bonding mode, together with the continuous conductive framework, which was beneficial for building good contact of the active nanoparticles, and therefore forming a more stable SEI layer.41 The morphology variation of the APA-, APA/CNT- and PAA-based electrodes after cycling was investigated. After the cycling test, the APA- and APA/CNT-based electrode could basically maintain a uniform surface (Fig. 5h, i and Fig. S8, ESI†), whereas the PAA-based electrode exhibited severe cracks with widths of several microns (Fig. 5f and g). The obvious differences may be attributable to the enhanced adhesion and continuous conductive skeleton of the APA/CNT, which can tightly grasp the composite nanoparticles and preserves the stable pore structure of the electrode. Moreover, the other two commercial binders, poly(vinylidene fluoride) (PVDF) and carboxymethyl cellulose (CMC), were introduced to assess the superiority of this aqueous conductive adhesive (Fig. S9, ESI†). All of these commercial binders exhibited a lower specific capacity and inferior cycling performance (Fig. 5j).
To further reveal the reinforcement of the APA/CNT for thick electrodes, the electrochemical behaviours of the APA/CNT-based electrodes with Si/C loadings ranging from 5 to 15 mg cm−2 were evaluated. Fig. 5k shows the relationship between the cross-sectional morphologies and thickness of the APA/CNT-based Si/C anodes. The dense and smooth surface together with the uniform dispersion state, was observed over the whole of the APA/CNT anodes. In contrast, the PAA failed to fabricate anodes with a high mass loading (≥5 mg cm−2) (Fig. S10, ESI†). As shown in Fig. 5l and Fig. S11 (ESI†), the areal discharge capacities of the APA/CNT anodes increased with the active mass loading, whereas their specific capacity remained nearly unchanged (inset of Fig. 5l), demonstrating that the APA/CNT anode facilitated to fully realize the theoretical specific capacity of Si/C over the entire loading range of 1–15 mg cm−2. Furthermore, the APA/CNT anode with higher mass loadings (5 mg cm−2, 10 mg cm−2, and 15 mg cm−2) displayed stable cyclic performances (Fig. 5l), again confirming the efficient yet stable adhesiveness of the APA/CNT. Notably, the high areal capacity of 7.79 mA h cm−2 accompanied by a good specific capacity (519.1 mA h g−1) enabled by the APA/CNT, far exceeded those of other binders previously reported (Fig. 5m).
The electrochemical performance of the APA/CNT-based LiFePO4 cathode was studied to assess the universality of this new adhesive. The cross-sectional view showed that well-packed layers with active nanoparticles, and the porosity of the cathode were retained (Fig. 6a). The top-view SEM images (Fig. 6b and c) showed that the as-fabricated cathode possessed a uniform and compact surface without any cracking. High-magnification imaged revealed that the APA/CNT network can interconnect with active nanoparticles via an efficient and robust “sheet-to-point” bonding mode (Fig. 6d–f). Most notably, the flexible and conductive skeletons extended “tentacles” to tightly grasp the active nanoparticles, allowing the fabricated electrodes to possess a steady electrical connection, and the electrodes showed a remarkable structural integrity (Fig. 6f). The EDS mapping confirmed the uniform component dispersion of the APA/CNT-based cathode (Fig. 6g). The charge–discharge profiles of the cathode are shown in Fig. S12 (ESI†). The APA/CNT-based cathode displayed a high specific capacity of 158.2 mA h g−1 and a high CE of 99.32% (Fig. 6h) at 0.5 C. After 200 cycles, the APA/CNT-based cathode could still deliver a capacity of 141.7 mA h g−1, which corresponded to a 90.0% retention (Fig. 6i). The outstanding performance revealed the satisfactory universality and the reinforcing effect of the APA/CNT to active materials, which could not be matched by commercial PAA and traditional water system adhesives. Moreover, the full cell was assembled by using an APA/CNT-based Si/C anode and an APA/CNT-based LiFePO4 cathode with a mass loading of 0.3 mg cm−2. The APA/CNT-based LiFePO4/Si/C full cell delivered a high initial charge capacity (calculation based on the weight of the cathode) of 150.7 mA h g−1 and a high CE of 99.89% (Fig. 6j) at 0.4 C, and maintained capacity as high as 130.0 mA h g−1 after 200 cycles, which was 86.2% of the initial capacity (Fig. 6k and Fig. S13, ESI†).
The satisfactory electrochemical behaviour of APA/CNT can be attributable to the cooperative effect of the unique 2D-reticular framework and the high “inherent” conductivity. Firstly, the flexible 2D-reticular nanosheet structure could firmly grasp active materials via efficient “sheet-to-point” bonding, endowing the resulting electrodes with extraordinary structural and interfacial stability. Secondly, the continuous adhesive networks and conductive skeletons played a leading role to achieve the “two-in-one” effect, without the need to add conductive agents anymore, and thus enhancing the bonding strength and electrical conductivity. In addition, this water-soluble binder revealed advantages of simplicity, low cost, universality, scalability and environmental friendliness when compared to the traditional solvent-based binders.
Conclusions
In conclusion, a universal aqueous conductive binder for LIBs was designed via simple neutralisation of the commercially supplied PAA binder. The original self-associated carboxylic acid dimers were expected to be decoupled by the deprotonation of PAA by AH, thus forming the robust 2D-reticular architecture by means of the abundant electrostatic binding and hydrogen-bond interaction between –COO, –NH3+ and –OH. Compared to the parent PAA, the as-constructed APA/CNT binder displayed a significantly enhanced adhesion strength, electrical conductivity, and wettability. With these advantages, the APA/CNT-based electrodes exhibited higher capacities, superior cycling stability and rate performance when compared to the PAA-based Si/C anode and LiFePO4 cathode. Furthermore, a high areal capacity of 7.79 mA h cm−2 accompanied with a good specific capacity (519.1 mA h g−1) enabled by the APA/CNT far exceeded those of other binders reported thus far. Moreover, the APA/CNT-based LiFePO4/Si/C full cell also exhibited an enhanced electrochemical behaviour and cycling durability. This simple and easy strategy should give a new valuable way to design a cost-effective, universal yet environmentally friendly binder with dependable adhesiveness for the high-performance devices.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 52202307), the Science and Technology Innovation Program of Hunan Province (Grant No. 2020RC1009, 2023JJ20036), the Hunan Provincial Education Office Foundation of China (Grant No. 21B0146), and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515110347).Notes and references
- H. Q. Pham, G. Kim, H. M. Jung and S.-W. Song, Adv. Funct. Mater., 2018, 28, 1704690 CrossRef.
- S. Jiang, B. Hu, Z. Shi, W. Chen, Z. Zhang and L. Zhang, Adv. Funct. Mater., 2020, 30, 1908558 CrossRef CAS.
- X. Jiao, J. Yin, X. Xu, J. Wang, Y. Liu, S. Xiong, Q. Zhang and J. Song, Adv. Funct. Mater., 2021, 31, 2005699 CrossRef CAS.
- B. Jin, D. Wang, J. Zhu, H. Guo, Y. Hou, X. Gao, J. Lu, X. Zhan, X. He and Q. Zhang, Adv. Funct. Mater., 2021, 31, 2104433 CrossRef CAS.
- Y. Su, X. Feng, R. Zheng, Y. Lv, Z. Wang, Y. Zhao, L. Shi and S. Yuan, ACS Nano, 2021, 15, 14570 CrossRef CAS PubMed.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS PubMed.
- W. An, B. Gao, S. Mei, B. Xiang, J. Fu, L. Wang, Q. Zhang, P. K. Chu and K. Huo, Nat. Commun., 2019, 10, 1447 CrossRef PubMed.
- H. Jia, X. Li, J. Song, X. Zhang, L. Luo, Y. He, B. Li, Y. Cai, S. Hu, X. Xiao, C. Wang, K. M. Rosso, R. Yi, R. Patel and J.-G. Zhang, Nat. Commun., 2020, 11, 1474 CrossRef CAS PubMed.
- N. Fukata, M. Mitome, Y. Bando, W. Wu and Z. L. Wang, Nano Energy, 2016, 26, 37 CrossRef CAS.
- M.-H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim, S. Ahn, Y. Cui and J. Cho, Nano Lett., 2009, 9, 3844 CrossRef CAS PubMed.
- L. Hu, M. Jin, Z. Zhang, H. Chen, F. B. Ajdari and J. Song, Adv. Funct. Mater., 2022, 32, 2111560 CrossRef CAS.
- P. Li, J. Y. Hwang and Y. K. Sun, ACS Nano, 2019, 13, 2624 CAS.
- L. Zhang, C. Wang, Y. Dou, N. Cheng, D. Cui, Y. Du, P. Liu, M. Al-Mamun, S. Zhang and H. Zhao, Angew. Chem., Int. Ed., 2019, 58, 8824 CrossRef CAS.
- S. Choi, T. W. Kwon, A. Coskun and J. W. Choi, Science, 2017, 357, 279 CrossRef CAS PubMed.
- F. Zhang, H. Xia, T. Wei, B. Liu, H. Li, Z. Lu and M. Yang, Adv. Funct. Mater., 2023, 2303339 CrossRef CAS.
- M. Li, J. Zhang, Y. Gao, X. Wang, Y. Zhang and S. Zhang, J. Mater. Chem. A, 2021, 9, 2375 RSC.
- Z.-J. Han, K. Yamagiwa, N. Yabuuchi, J.-Y. Son, Y.-T. Cui, H. Oji, A. Kogure, T. Harada, S. Ishikawa, Y. Aokid and S. Komaba, Phys. Chem. Chem. Phys., 2015, 17, 3783 RSC.
- J. Feng, D. Wang, Q. Zhang, J. Liu, Y. Wu and L. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 44312 CrossRef CAS PubMed.
- F. Zou and A. Manthiram, Adv. Energy Mater., 2020, 10, 2002508 CrossRef CAS.
- S. Huo, D. Necas, F. Zhu, D. Chen, J. An, N. Zhou, W. Liu, L. Wang, Y. Cheng, Y. Liu and R. Ruan, Chem. Eng. J., 2021, 406, 127160 CrossRef CAS.
- J. Xia, Z. Wang, N. D. Rodrig, B. Nan, J. Zhang, W. Zhang, B. L. Lucht, C. Yang and C. Wang, Adv. Mater., 2022, 34, 2205229 CrossRef CAS PubMed.
- M. Tian, X. Chen, S. Sun, D. Yang and P. Wu, Nano Res., 2019, 12, 1121 CrossRef CAS.
- Y. Wang, H. Xua, X. Chen, H. Jin and J. Wang, Energy Storage Mater., 2021, 38, 121 CrossRef.
- R. Sun, J. Hu, X. Shi, J. Wang, X. Zheng, Y. Zhang, B. Han, K. Xia, Q. Gao, C. Zhou and L. Mai, Adv. Funct. Mater., 2021, 31, 2104858 CrossRef CAS.
- Y. Shi, J. Zhang, A. M. Bruck, Y. Zhang, J. Li, E. A. Stach, K. J. Takeuchi, A. C. Marschilok, E. S. Takeuchi and G. Yu, Adv. Mater., 2017, 29, 1603922 CrossRef.
- W. Zeng, L. Wang, X. Peng, T. Liu, Y. Jiang, F. Qin, L. Hu, P. K. Chu, K. Huo and Y. Zhou, Adv. Energy Mater., 2018, 8, 1702314 CrossRef.
- Y. K. Jeong and J. W. Choi, ACS Nano, 2019, 13, 8364 CrossRef CAS PubMed.
- H. Wang, J. Fu, C. Wang, R. Zhang, Y. Yang, Y. Li, C. Li, Q. Sun, H. Li and T. Zhai, Adv. Funct. Mater., 2021, 31, 2102284 CrossRef CAS.
- L. Li, Z. Zuo, H. Shang, F. Wang and Y. Li, Nano Energy, 2018, 53, 135 CrossRef CAS.
- P. Jackson, K. Robinson, G. Puxty and M. Attalla, Energy Procedia, 2009, 1, 985 CrossRef CAS.
- Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Ceram. Soc., 2012, 134, 11852 CrossRef CAS PubMed.
- P. Guo, H. Zhang, X. Liu and J. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 2105 CrossRef CAS PubMed.
- K. Chen, Y. Wang, J. Yao and H. Li, J. Phys. Chem. B, 2018, 122, 309 CrossRef CAS PubMed.
- L. Deng, Y. Zheng, X. Zheng, T. Or, Q. Ma, L. Qian, Y. Deng, A. Yu, J. Li and Z. Chen, Adv. Energy Mater., 2022, 12, 2200850 CrossRef CAS.
- Y. Su, X. Feng, R. Zheng, Y. Lv, Z. Wang, Y. Zhao, L. Shi and S. Yuan, ACS Nano, 2021, 15, 14570 CrossRef CAS PubMed.
- P. Liu, Y. Wang, H. Hao, S. Basu, X. Feng, Y. Xu, J. A. Boscoboinik, J. Nanda, J. Watt and D. Mitlin, Adv. Mater., 2020, 32, 2002908 CrossRef CAS PubMed.
- S. Batool, M. Idrees, J. Kong, J. Zhang, S. Kong, M. Dong, H. Hou, J. Fan, H. Wei and Z. Guo, J. Alloys Compd., 2020, 832, 154644 CrossRef CAS.
- Z. Lu, N. Liu, H.-W. Lee, J. Zhao, W. Li, Y. Li and Y. Cui, ACS Nano, 2015, 9, 2540 CrossRef CAS PubMed.
- X. Shen, Z. Tian, R. Fan, L. Shao, D. Zhang, G. Cao, L. Kou and Y. Bai, J. Energy Chem., 2018, 27, 1067 CrossRef.
- B. Yang, F. Liu, Y. Liu, J. Dong, M. Liu, S. Wang and L. Zhang, J. Power Sources, 2023, 553, 232274 CrossRef CAS.
- G. Zhang, Y. Yang, Y. Chen, J. Huang, T. Zhang, H. Zeng, C. Wang, G. Liu and Y. Deng, Small, 2018, 14, 18011 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee02377j |
| ‡ Farong Zhang and Hongyu Xia contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |