Regulating the electron spin orbital by sulfur doping of Ti vacancies to manipulate spin flip for enhancing PEC water splitting performance†
Abstract
Titanium dioxide (TiO2) as a semiconductor photocatalyst has been broadly investigated due to its high chemical stability, nontoxicity and low cost. Nevertheless, TiO2 still suffers from low visible-light response and rapid photogenerated charge carrier recombination. Herein, to overcome the limitations of TiO2, different sulfur sources were incorporated in Ti vacancy defects by a vacancy capture strategy. Compared to oxygen vacancies, the Ti vacancies (VTi) on TiO2 can induce the transfer of electrons from the electron spin-down orbital (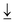 ) in the valence band to the electron spin-up orbital (
) in the valence band to the electron spin-up orbital (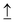 ) in the conduction band. The excited electrons are forbidden by the spin-down orbital in the valence band due to the spin pinning effect, which inhibits recombination of electron–hole pairs for enhancing PEC water splitting performance. More significantly, the sulfur dopants can manipulate electron spin flip to regulate the electron spin direction and increase the number of charge carriers. This can solve the key problem of the common doping strategy, which is a high recombination rate of electron–hole pairs due to the reduced band gap through doping the p state above the valence band. This work provides an efficient pathway to improve PEC activity by manipulating spin flip through cationic vacancy modification technology.
) in the conduction band. The excited electrons are forbidden by the spin-down orbital in the valence band due to the spin pinning effect, which inhibits recombination of electron–hole pairs for enhancing PEC water splitting performance. More significantly, the sulfur dopants can manipulate electron spin flip to regulate the electron spin direction and increase the number of charge carriers. This can solve the key problem of the common doping strategy, which is a high recombination rate of electron–hole pairs due to the reduced band gap through doping the p state above the valence band. This work provides an efficient pathway to improve PEC activity by manipulating spin flip through cationic vacancy modification technology.



 Please wait while we load your content...
Please wait while we load your content...