Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Techno-economic insights and deployment prospects of permanent carbon dioxide sequestration in solid carbonates†
Andreas
Mühlbauer
 ab,
Dominik
Keiner
ab,
Dominik
Keiner
 *b and
Christian
Breyer
*b and
Christian
Breyer
 *b
*b
aDept. of Civil and Environmental Engineering, Stanford University, Stanford, CA 94305, USA
bSchool of Energy Systems, LUT University, Yliopistonkatu 34, 53850 Lappeenranta, Finland. E-mail: dominik.keiner@lut.fi; christian.breyer@lut.fi
First published on 17th October 2024
Abstract
While a rapid defossilisation of the energy-industry system is at the highest priority for climate change mitigation, additional post-fossil carbon dioxide removal (CDR) for net-negative emissions will likely be necessary to ensure a safe future. An in-depth techno-economic analysis of differentiated sequestration options for carbon dioxide (CO2) in solid carbonates is not yet available, as direct air capture-based mineralisation is usually aggregated in direct air capture and carbon sequestration. This research gap is closed by studying mineralisation as a key CDR option to sequester atmospheric CO2 permanently, based on available literature. The most frequently discussed routes for mineralisation, i.e., in situ, ex situ mineralisation, and enhanced rock weathering, are examined. The deployment potentials of these options are determined globally for nine major regions. Results indicate that costs for all mineralisation options can be kept below 100 € per tCO2 from 2050. From 2030 onwards, in situ mineralisation, with low energy-intensity, can be realised at cost of ≤131 € per tCO2, ex situ mineralisation at ≤189 € per tCO2, and enhanced weathering at ≤88 € per tCO2. Final energy demand for CO2 sequestration via in situ mineralisation is ≤1.8 MWh per tCO2, via ex situ mineralisation ≤3.7 MWh per tCO2, and via enhanced weathering ≤1.1 MWh per tCO2 from 2030. Large-scale deployment of mineralisation options supporting 60% of projected CDR demand is assessed to require up to 0.06% and 0.21% in global gross domestic product and up to 2.5% and 8.6% additional primary energy demand in 2070 for a 1.5 °C and 1.0 °C climate target, respectively. Implications, permanence of sequestration, and limitations are discussed, and a research outlook is provided.
Broader contextThe fight against climate change requires different actions. Beneath the adaption to climate change, mitigating climate change through the deployment of renewable energy is of upmost importance. However, even for the 1.5 °C climate target, an entirely renewable energy system is not sufficient anymore. An expected 500 GtCO2 must be removed to balance delayed action in climate change mitigation. In addition, climate restoration may be required to reach safe planetary boundaries at 350 ppm CO2 in the atmosphere, or a 1.0 °C climate target. This requires an expected 1750 GtCO2 to be removed from the atmosphere within the 21st century. For such large amounts of CO2 to be sequestered, many factors play a role in assessing suitable options for carbon sequestration, such as energy demand, costs, area demand, technology readiness level, or permanence. The latter is an important point of discussion for gaseous or geological sequestration of CO2 commonly associated with direct air carbon capture and sequestration. However, possible leakage, earthquakes, or well failures increase the risk of large-scale geological sequestration. This draws light on carbon mineralisation, where CO2 is fixed in solid carbonates not to be released for at least thousands of years. |
1. Introduction
The rising atmospheric greenhouse gas concentration leads to increasing global warming, putting human civilisation at risk.1 The trade-offs of intensive fossil fuel combustion enabling the industrial revolution and further economic growth2 are becoming increasingly severe with rising mean air temperatures and more frequent extreme weather events.3 To re-balance the climate system and to limit global warming to a sustainable level, rapid defossilisation and electrification of all industry sectors are necessary.4,5 However, the latest findings of the Intergovernmental Panel on Climate Change (IPCC) imply the requirement for negative carbon dioxide (CO2) emissions through carbon dioxide removal (CDR) employing negative emission technologies (NET) and natural climate solutions (NCS).6 While CDR can reduce CO2 levels in the atmosphere, the management of other greenhouse gases such as methane or nitrogen dioxide is subject to current research.7 CDR is partially required to offset residual emissions; however, the definition of residual emissions is subject to vested interests and often debatable.8 Post-fossil CDR is also essential to achieve a net-negative energy-industry system.4,9 To limit global warming to 1.5 °C by 2100, total negative emissions (TNE) of about 500 GtCO2 from the atmosphere must be realised.10,11 To reach even more ambitious yet safe and just targets of limiting global warming to 1.0 °C, TNE of up to 1750 GtCO2 may be required.10–12 The 1.5 °C and 1.0 °C climate targets will require annual CO2 removal rates of up to 10 GtCO2 per a and 40 GtCO2 per a, respectively, when fully ramped if the climate shall be rebalanced in a timely manner.10–12 The ambitious climate target of limiting global warming to 1.0 °C might be significantly more energy- and cost-intensive; however, it can help to achieve a sustainable and just future for civilisation12,13 by avoiding major tipping points and potential cascades thereof.14–16 An unprecedented reduction in cost of renewable electricity, which is currently observed, can enable such ambitious climate targets to return to the Holocene.17 It is expected that the mineralisation of CO2 can play a major role, with an expected potential of up to 10 GtCO2 per a by 2050, sequestering atmospheric CO2 safely for geological time scales.18,19 Sandalow et al.19 projected that, given adequate political incentives and measures, about 1 GtCO2 per a and 10 GtCO2 per a can be mineralised by 2035 and 2050, respectively. However, most integrated assessment models (IAM) that are considered as scientific basis for IPCC reports include either no mineralisation option or only enhanced weathering (EW) in their studies, making these options underrepresented in current climate change mitigation research.11NCS can sequester atmospheric CO2 and have co-benefits on the environment, but the long-term effectiveness and storage duration as well as sustainable scalability are potential bottlenecks.20,21 The CDR potential of NCS has been discussed intensively in literature.9,22 NETs including direct air carbon capture and sequestration (DACCS),23–25 bioenergy with carbon capture and sequestration (BECCS),26–29 EW,30–33 or biochar production34 must be considered and potentially deployed on large-scale,11,35 though research has indicated that diverse portfolios of CDR are preferable.11,36,37 Bio-geo-chemical options such as EW, biochar production, or afforestation combine the CO2 capture and storage step by sequestering atmospheric CO2 in carbonates or biogenic materials.11,31,38 In contrast, DACCS and BECCS are realised via concentrated CO2 that can either be used as feedstock for e-fuels and e-chemicals to defossilise hard-to-abate sectors39–43 or can be safely sequestered as negative emissions.11,44 While most IAMs as of today model DACCS and BECCS without further specifying CO2 sequestration modalities,11 the production of carbon-bearing and electricity-based solid materials is introduced as alternative option.45–49 The anticipated low-cost renewable electricity5,50 can be utilised to enable novel NETs with relatively high energy demand following the overall trend towards a Power-to-X Economy51 and higher sequestration security compared to geological underground CO2 sequestration.11,52 Solid carbon-bearing carbonates can be produced using ex situ mineralisation (MINEX)53,54 while simultaneously acting as valuable building material.55 Also, CO2 can be sequestered safely in suitable underground formations allowing for the in situ mineralisation (MININ) of CO2 to form solid carbonates within a few years.44,56,57 MININ can help mitigate concerns and potential flaws of other underground sequestration options such as leakage through previously impermeable caprocks58,59 or in deep ocean storage options.60 The sequestration of atmospheric CO2 in stable solid materials can enable permanent CDR21 and ensure effective negative emissions to aim for more ambitious climate targets.20 CO2 mineralisation may be a key technology to achieve such effective long-term stable atmospheric CO2 sequestration.
An overview of the most frequently discussed CO2 mineralisation options, i.e., EW, MINEX, and MININ is depicted in Fig. 1. While MINEX and EW can both use suitable mafic and ultramafic rock from open pit mines to produce solid carbonates as a main product, MININ dissolves CO2 in water to inject it into suitable deep formations of basaltic rocks or peridotites.44 MINEX can also use industrial solid wastes bearing magnesium (Mg) or calcium (Ca).61 While this is also reported for EW,62 this option is not considered within this work due to possible sustainability bottlenecks when applying waste material to large open areas.
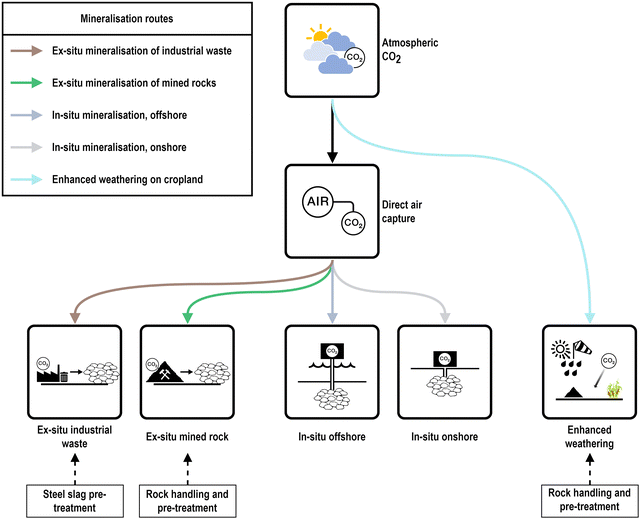 | ||
| Fig. 1 Schematic overview on CO2 mineralisation options adapted from Mühlbauer et al.11 | ||
The aim of this study is to provide a techno-economic assessment of options to sequester atmospheric CO2 in minerals by different processes, closing research gaps of lacking literature on comprehensive CO2 mineralisation options and a dedicated techno-economic assessment of respective NETs. A novelty of this study is the assessment of global-local potentials as well as economics of CO2 mineralisation to provide the basis for future research in the energy-industry-CDR nexus.4 Therefore, the novelties of this study include:
• Global-local potentials for CO2 mineralisation enabling dedicated energy-industry-CDR system transition studies considering different CO2 mineralisation options.
• Techno-economic parameters for CO2 mineralisation options for further use in future energy-industry-CDR studies, including final energy demand and primary energy demand per tonne of sequestered CO2.
• Technology readiness level (TRL) of all mineralisation options to assess maturity.
• Global implications for the cost and primary energy demand of large-scale CO2 sequestration with major shares of mineralisation.
By providing a novel basis for further research in implementing CO2 mineralisation in energy-industry-CDR system transition simulations, this study aims to support the discussion about CO2 mineralisation options for permanent CDR. Early investigations of future cost, energy demand, and global regional potential are required to pave a way to a safe future. This study aims to provide a step towards in-depth understanding of CO2 mineralisation in the context of energy-industry-CDR systems to simulate pathways to such respective safe futures.
2. Literature review
Geochemical NETs have been comprehensively reviewed19,63–67 and current research gaps, i.e., the identification of regional potentials or advanced understanding of biological influences on CO2 mineralisation rates and other performance factors, have been identified.57,66 Geochemical NETs have also recently been studied on a regional level as for the case of Spain,68 South Africa,69 the United Kingdom,70–72 Austria,73 or Japan.74 Also, field trials have been carried out75 to verify modelled CDR rates.76Minerals containing alkaline-earth metal oxides such as calcium oxide (CaO) or magnesium oxide (MgO) react with CO2 in an exothermic mineralisation reaction.77 The natural weathering of rocks, induced by intense tectonism, is a significant part of the Earth's carbon cycle,78 removing about 300 MtC per a from the atmosphere.19,79 Rock types containing significant shares of suitable metal oxides include olivine, brucite, pyroxene, serpentine, wollastonite, and dunite among others.64 Also, certain industrial wastes such as steelmaking slag can be used in MINEX to obtain useful products.80,81 Such dual use enables CO2 capture, utilisation, and sequestration (CCUS) via mineralisation82–84 or the production of renewable electricity-based and carbon-bearing solid materials.45–47 As an example, Pan et al.85 elaborate on the cases of electric arc furnace steelmaking, which also occur in a defossilised industry, and Portland cement manufacturing for a waste-to-resource supply chain by producing useful construction material from steelmaking slags.
The subsurface reaction of CO2 with suitable rock to carbonates is usually referred to as in situ mineralisation (MININ) of CO219,44,63,64,86 and is one out of four possible trapping mechanisms for CO2 underground sequestration.60 Suitable sequestration sites are basaltic rock formations or peridotites.57 Rapid mineralisation removes the need for a long-term stable caprock.44,87,88 MINEX refers to engineered processes to mineralise CO2 with suitable feedstock in reactors as first proposed by Lackner et al.53 as early as 1995.86,89 The reverse reaction of exothermal mineralisation, i.e., calcination, is favoured at high temperatures (>900 °C for CaCO3 and >300 °C for MgCO3 at 1 bar CO2 partial pressure), whereas mineralisation is favoured at relatively low temperatures.90 Various reactor setups and the impact of different process parameters such as retention time have been studied54,91 and lab-scale demonstration reactors are being operated.92 MINEX can be characterised either as direct or indirect mineralisation.77,90 Both direct and indirect routes can be conducted in an aqueous or gas–solid environment,77,90 whereas, in indirect mineralisation, the reactive oxide (of Mg or Ca) or hydroxide is extracted from the feedstock.93–95
Enhancing the natural surface weathering of rocks is commonly referred to as EW.31–33 Strefler et al.32 investigated in detail the effect of the weathering efficiency of three different rock types, the influence of temperature and soil pH, and the optimal grain size of rocks for a study of the global CDR potential and the cost of EW in high spatial resolution. Best suited locations are therein characterised as warm and humid,32 which was also confirmed in field experiments.96 Beerling et al.31 employ a one-dimensional vertical reactive transport model for basalt weathering with a steady-state flow to conduct their techno-economic and potential assessment for EW on a global scale in 2050. In contrast to Strefler et al.,32 Beerling et al.31 account for soil pH and varying grain size by considering a log-normal grain size distribution. Also, Beerling et al.31 apply the fractal dimension to account for uncertainties in grain topography and porosity and consider annual rock application over a 10-year time horizon. Goll et al.33 studied EW and the implications of fertility enhancement by basalt application to global hinterland. Enhancing the soil fertility by basalt application can improve the ecosystem's carbon uptake, further increasing the CDR potential.33 Cipolla et al.97 study the impact of rainfall, vegetation, and soil type on the efficacy of EW of olivine, calculated with a formerly introduced model98,99 for three case studies. Results indicate a major impact from annual rainfall distribution and the authors concluded that irrigation can substantially increase weathering rates.97 The grain size of the applied rock also significantly impacts the EW rate.73 Eufrasio et al.100 conducted a thorough life cycle assessment (LCA) of EW and found that, in order to maintain a high carbon efficiency, renewable electricity is required to satisfy the comminution electricity demand. Eufrasio et al.100 also build on the results provided by Beerling et al.31 and compare EW's impact in terms of energy demand, land requirement, and water requirement to other NET options. Vakilifard et al.101 studied the impact of EW, modelled by the approach introduced by Beerling et al.,31 co-deployment on the Earth system. The authors conclude that the additional CDR provided can increase of the likelihood of limiting global warming in 2100 to 1.5 °C and the ocean alkalinity to benefit marine ecosystems.101 EW using different suitable industrial waste as feedstock is studied frequently.31,62,102,103 Further literature findings on the cost and energy demand for EW are aggregated in Note 4 in the Supplementary material 1 (ESI†).
As stated by Sandalow et al.,19 the global potential for different CO2 mineralisation options in high spatial resolution is a major research gap that must be addressed. Additionally, Wei et al.104 find that current assessments should be harmonised using a hierarchical framework that they proposed. The global potential of mine tailings for CDR was studied by Bullock et al.105 on a global to regional scale. Kremer et al.106 mapped and categorised potential input material for CO2 mineralisation in Europe; however, comprehensive data in high spatial resolution on availability and accessibility of sequestration sites is still largely missing.19,57 This study aims to close the abovementioned research gaps by providing a comprehensive techno-economic overview on CO2 mineralisation options and respective global-local sequestration potentials.
3. Methods and data
Within this study, techno-economic parameters for different CO2 mineralisation options are elaborated to provide the basis for future energy-industry-CDR system analyses. The following sections are structured as follows. In subsection 3.1, assumptions for process configurations are described. In subsection 3.2, the assessed processes of this study are further specified, followed by a global-local potential investigation for these processes in subsection 3.3. Techno-economic data are aggregated from various sources in literature and presented in Table 1 for traceability. Considering the state of deployment of mineralisation options, those techno-economic assumptions are subject to uncertainty. All costs are corrected for inflation to the year 2020 and cost that are given in USD are adjusted to € with a long-term exchange rate of 1.2 USD per € applied.| Technology | Parameter | Unit | Value | Ref. |
|---|---|---|---|---|
| a Opexvar represents the cost including energy of feedstock preparation normalised to 1 tonne CO2 sequestered. b The electricity demand for rock spreading is assumed to be already included in rock transportation. | ||||
| Open-pit mining | CAPEX | € per (tOre/a) | 6.0 | 31 |
| OPEXfix | € per tOre | 4.6 | ||
| OPEXvar | € per tOre | 0.0 | ||
| Lifetime | years | 10 | ||
| Electricity input | kWhel per tOre | 5.2 | 32,89 | |
| Heat input | kWhth per tOre | 0.0 | ||
| Heat output | kWhth per tOre | 0.0 | ||
| Rock transportation | Cost | € per (tRock·100 km) | 4.4 | 32 |
| Lifetime | Years | — | ||
| Electricity input | kWhel per (tOre·100 km) | 1.4 | ||
| Heat input | kWhth per tOre | 0.0 | ||
| Heat output | kWhth per tOre | 0.0 | ||
| Rock comminution | CAPEX | € per (tOre/a) | — | |
| OPEXfix | € per tOre | — | ||
| OPEXvar | € per tOre | — | ||
| Lifetime | Years | — | ||
| Electricity input | kWhel per tOre | 127.8 | ||
| Heat input | kWhth per tOre | 0.0 | ||
| Heat output | kWhth per tOre | 0.0 | ||
| Rock spreading | CAPEX | € per (tCO2/a) | 208.4 | |
| OPEXfix | € per tCO2 | — | ||
| OPEXvara | € per tCO2 | 13.8 | ||
| Lifetime | Years | 50 | Own assumption | |
| Electricity inputb | kWhel per tCO2 | 0.0 | ||
| Heat input | kWhth per tCO2 | 0.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
| Direct aqueous mineralization – serpentine | CAPEX | € per (tCO2/a) | 208.4 | 55,107 |
| OPEXfix | € per tCO2 | 6.3 | ||
| OPEXvara | € per tCO2 | 13.8 | ||
| Lifetime | Years | 50 | 89 | |
| Electricity input | kWhel per tCO2 | 455.0 | 108 | |
| Heat input | kWhth per tCO2 | 452.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
| Direct aqueous mineralization – olivine | CAPEX | € per (tCO2/a) | 208.4 | 55,107 |
| OPEXfix | € per tCO2 | 6.3 | ||
| OPEXvara | € per tCO2 | 13.8 | ||
| Lifetime | years | 50 | 89 | |
| Electricity input | kWhel per tCO2 | 689.0 | 108 | |
| Heat input | kWhth per tCO2 | 103.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
| Direct aqueous mineralization – steel slag | CAPEX | € per (tCO2/a) | 208.4 | 55,107 |
| OPEXfix | € per tCO2 | 6.3 | ||
| OPEXvar | € per tCO2 | 13.8 | ||
| Lifetime | Years | 50 | 89 | |
| Electricity input | kWhel per tCO2 | 592.0 | 108 | |
| Heat input | kWhth per tCO2 | 407.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
| CO2 underground injection – onshore | CAPEX | € per (tCO2/a) | 35.0 | 109 |
| OPEXfix | € per tCO2 | 1.5 | ||
| OPEXvar | € per tCO2 | 0.0 | ||
| Lifetime | Years | 40 | ||
| Electricity input | kWhel per tCO2 | 70.0 | 44 | |
| Heat input | kWhth per tCO2 | 0.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
| CO2 underground injection – offshore | CAPEX | € per (tCO2/a) | 99.5 | 109 |
| OPEXfix | € per tCO2 | 3.5 | ||
| OPEXvar | € per tCO2 | 0.0 | ||
| Lifetime | Years | 40 | ||
| Electricity input | kWhel per tCO2 | 70.0 | 44 | |
| Heat input | kWhth per tCO2 | 0.0 | ||
| Heat output | kWhth per tCO2 | 0.0 | ||
3.1. Mineralisation routes
Carbonates, the final products of CO2 mineralisation, are highly stable solid products showing low Gibb's free enthalpy compared to CO2 and is, therefore, expected to remain stable and sequestering atmospheric CO2 safely.110 The low Gibb's free enthalpy of carbonates compared to gaseous CO2 is reflected in the high thermal inertia and stability of carbonates and results in reported CO2 sequestration times of geological timescales.44 Further fundamentals of the mineralisation reaction are elaborated in Note 1 in the Supplementary material 1 (ESI†).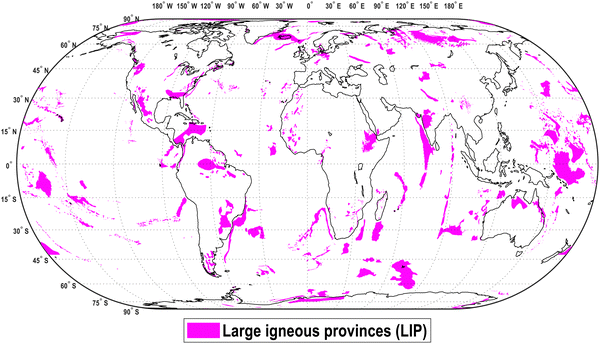 | ||
| Fig. 2 Global overview on large igneous provinces (LIP) and flood basalt showing potential MININ sequestration sites44 which are characterised by mafic and ultramafic basalt.44,57 Data source.117 | ||
The CO2 mineralisation reaction of Wollastonite (calcium silicate, CaSiO3) or Enstatite (magnesium silicate, MgSiO3) present in ultramafic rocks to magnesium carbonate (MgCO3) or calcium carbonate (CaCO3) and silicon dioxide (SiO2) can be summarised by eqn (1):67
| (Mg,Ca)SiO3(s) + CO2 → (Mg,Ca)CO3(s) + SiO2(s) | (1) |
For the mineralisation reaction, gaseous CO2 is generally dissolved in water. Further information on the MININ process is available in Supplementary material 1, Note 1 (ESI†).
The reaction of mined rocks is similar to eqn (1). The mineralisation of industrial waste, more specifically the containing minerals magnesium oxide (MgO), calcium oxide (CaO), magnesium hydroxide (Mg(OH)2), or calcium hydroxide (Ca(OH)2) to MgCO3 or CaCO3 and water (H2O) is described by eqn (2) and (3):67
| (Mg,Ca)O(s) + CO2 → (Mg,Ca)CO3(s) | (2) |
| (Mg,Ca)(OH)2(s) + CO2 → (Mg,Ca)CO3(s) + H2O(l) | (3) |
Gaseous CO2 may be in aqueous solution when reacting to solid carbonates, however, dry reactions are also possible, thus the state is not further specified in eqn (2) and (3).The ranges of metal oxide share in different rock types and industrial waste vary by a multitude of parameters and influence both the total weathering potential (WP) and reaction kinetics.63 Metal oxides contributing to mineralisation of industrial waste also encompass Al, Fe, Na, and K.122 The WPs of different rock types have been reviewed in several studies and elaborated in experiments.123 The dissolution rate of rock feedstock is crucial to understand reaction kinetics and research in creating comprehensive databases is advancing.124 The energy and material demand for the respective MINEX configurations are also adapted from Ostovari et al.89,108 (cf. Note 3 in the Supplementary material 1 and in the Supplementary material 2, ESI†).
For EW, as Strefler et al.32 describe, the annual CDR rate for a specific land area rCDRCO2 is determined by the total amount of rock spread m, the dissolution rate d, and the CO2 sequestration potential p of the applied rock type. The dissolution rate d is depending on the specific surface area a, the weathering rate w, and the molar mass M. The constant ϑ is used to investigate annual weathering using a conversion rate of 3.155 × 107 s a−1. The grain size x is applied in μm. Therefore, the amount of CO2 removed annually is calculated as described in eqn (4)–(6) as proposed by Strefler et al.32 All input parameters assumed for further calculations can be found in Supplementary material 2 (ESI†).
| rCDRCO2 = m·d(x)·p | (4) |
| d(x) = a(x)·w·M·ϑ | (5) |
| a(x) = 69.18·x−1.24 | (6) |
This study relies on geochemical modelling shown in previous studies31,32 and does not largely focus on physical processes of EW. Instead, this study focuses on the overall energy demand and economic parameters derived from previous studies.
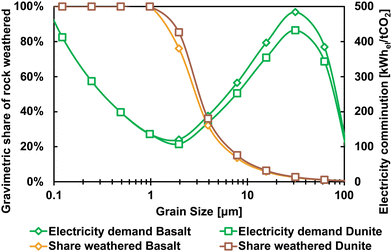 | ||
| Fig. 3 The share of rock weathered (left vertical axis) after one year at 20 °C based on formulas given by Strefler et al.32 and the resulting electricity demand for comminution per tonne of CO2 removed (right vertical axis) based on different grain size for Basalt and Dunite. | ||
As can be seen in Fig. 3, the electricity demand for comminution is optimal at a grain size of about 2 μm from a feed size of 100 μm at an electricity demand of about 120 kWhel per tCO2 (cf. Supplementary material 2 for details, ESI†). At this grain size, about 80% of the rock particles are weathered within one year minimising the specific electricity demand for rock comminution relative to the mass of CO2 mineralised. However, a grain size below 10 μm is anticipated to potentially bear health risks for humans.32 A grain size of ground feedstock material of 10 μm is assumed in this study corresponding to an electricity demand of 127.8 kWhel per tRock (cf. Note 3 in the Supplementary material 1, ESI†) which would correspond to an electricity demand of about 322 kWh per tCO2 and 288 kWh per tCO2 when using basalt and dunite, respectively. This assumption is made for EW as well as for MINEX feedstock preparation. For rock mining and crushing, an electricity demand of 50 kWhel per tRock is assumed (cf. Note 3 in the Supplementary material 1, ESI†). As rock mining and crushing does not affect the final grain size and, therefore, the rate of weathering, the electricity demand is equivalent to 165 kWh per tCO2 and 40 kWh per tCO2 when using basalt and dunite, respectively, when assuming the weathering efficiencies provided by Strefler et al.32
3.2. Techno-economics and process specifications
In this subsection, all techno-economic input data and assumed process configurations, which are also displayed in schematics shown in Fig. 1, are presented. Within this work all energy- and mass balances and cost are normalised to the functional unit of 1 tCO2 permanently removed and sequestered.In this study, it is assumed that industrial solid waste suitable for mineralisation can be acquired for no additional cost and without additional energy demand, i.e., it is available as waste product that is otherwise disposed. For DAC, techno-economic specifications from Fasihi et al.23 and as adapted in Mühlbauer et al.11 are assumed. Details are elaborated in the Supplementary material 1 (ESI†). Rock crushing, grinding, and comminution is estimated to be 8.3 € per t (10 USD per t) for full life-cycle cost including energy.125 Within this study, the estimation by Strefler et al.32 for electricity demand of 127.8 kWh t−1 is applied which leads to cost of 5.3 € per t in 2020. Technology learning is a well-established approach to quantify the future cost reduction of technologies.107,126,127 While rock comminution, rock mining and handling, as well as CO2 injection are assumed to have no further substantial learning due to the widespread maturity and high historic installed capacity, capital expenditure (CAPEX) learning of MINEX is assumed based on estimates by Faber et al.107 Thus, CAPEX reduction calculated with a learning rate of 10.55% and a deployment projection reaching 3.46 GtCO2 per a cumulative installed capacity for MINEX is assumed (cf. Supplementary material 2, ESI†). Cost reductions for DAC are adapted from Mühlbauer et al.11 which are based on Fasihi et al.23
The initial techno-economic input parameters used in this study are listed in Table 1. Assumptions regarding the future cost development are elaborated in more detail in the Notes 2–4 of Supplementary material 1 and in the Supplementary material 2 (ESI†).
CO2 mineralisation generally produces solid carbonates and other by-products. The potential of these by-products in the cement industry has been investigated in several studies.55,128–130 The energy- and CO2-intensive cement production is challenging to decarbonise,131 therefore, synergies with CO2 mineralisation can be a valuable option. For example, substituting up to 5% of Portland cement in mortars with feed and by-products of mineral carbonation was found to maintain compressive strength while reducing CO2 emissions and cost of waste disposal.128 Also, other industries such as paper or rubber production have been identified as potential customers for by-products of CO2 mineralisation.83
All process chains presented within this study are evaluated regarding their final energy (FE) demand and levelised cost of CDR. The FE demand of each process chain for CO2 mineralisation EFE,NET is calculated according to eqn (7).
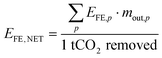 | (7) |
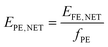 | (8) |
For each CO2 mineralisation process chain, the levelised cost of CDR LCOCDR is calculated according to eqn (9).
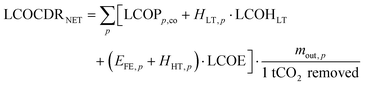 | (9) |
The LCOPp,co is calculated according to eqn (10)–(12) wherein the CAPEX, fixed operational expenditures OPEXfix, the variable operational expenditures OPEXvar, the weighted average cost of capital WACC and lifetime N, and the availability τ of each process p are used.
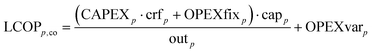 | (10) |
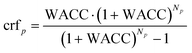 | (11) |
| outp = capp·τp | (12) |
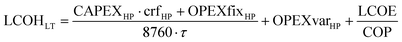 | (13) |
3.3. Applied scenarios for total cost and primary energy demand estimation
The primary energy demand and cost of large-scale mineralisation is assessed for the exemplary years 2050 and 2070. The analysis is conducted by assuming that 60% of the CDR demand for the 1.5 °C and 1.0 °C climate targets10,12 is covered with mineralisation options. The basic primary energy demand is estimated using LUT-DEMAND, applying the LUT-delayed economic equality scenario (LUT-DEES) for GDP per capita and medium population projection of the United Nations (UN) as macro-economic basis.10 Of these 60%, the contribution is assumed to be 20% each onshore and offshore MININ, 10% EW, and 3.3% for each MINEX using serpentine, olivine, and industrial waste. Details on the calculation can be found in the Supplementary material 2 (ESI†), where alternative assumptions can be applied.3.4. Assessment of sequestration potentials
Literature findings on the cumulative and annual CO2 mineralisation potential on a regional spatial resolution are allocated to the nine major regions as of the LUT Energy System Transition Model (LUT-ESTM), which are Europe, Eurasia, Middle East and North Africa (MENA), sub-Saharan Africa (SSA), South Asian Association for Regional Cooperation (SAARC), Northeast Asia, Southeast Asia, North America, and South America.50For MININ, a summary on global-regional total potential by Oelkers et al.57 is used. To identify economically feasible sequestration capacities, 10% of the capacity reported by Oelkers et al.57 is assumed to be economic potential and sequestration sites are assigned to the above-mentioned major regions. This approach bears significant uncertainty and should be further challenged as a major point on the research agenda for MININ proposed by Oelkers et al.57 To derive the annual injection and sequestration potential of MININ locations in MtCO2 per a, the surface area, as communicated by Oelkers et al.,57 is multiplied by the area specific injection rate as proposed by Wijaya et al.133 Several methods for sequestration capacity estimation of CO2 underground sequestration sites have been established.60 Nevertheless, thorough MININ potential analysis in high spatial resolution is a current research gap.57 Vishal et al.134 estimate a total MININ potential for India of 97–316 GtCO2 as a conservative theoretical estimate. They apply methods proposed by McGrail et al.135 and Snæbjörnsdóttir et al.136 to assess the sequestration potential of Indian basaltic formations.
Myers and Nakagaki74 conducted a regional study on MINEX and concluded that Japan alone can achieve CDR at the rate of up to 7.6 GtCO2 per a. Slag-based MINEX alone is expected to enable cumulative MINEX of 26.4–41.9 GtCO2 between 2020 and 2100.137 Steel slag's high CaO and MgO content of about 37%wt and 9.1%wt, respectively, and the resulting WP of around 384.7 kgCO2 per t of slag, make it a valuable feedstock for MINEX with an expected global potential of 320–870 MtCO2 per a in 2100.122 Renforth122 notes that about 185 t of blast furnace slag and 117 t of steel slag are produced per tonne of crude steel produced. Through the decarbonisation of steel production, blast furnaces will be phased out and blast furnace slag is therefore not further considered in this work. Production of one tonne of aluminium produces 3.45 t of bauxite residues, that can neutralise 44–66 kgCO2 per t of bauxite residues.122 About 115 kg of cement kiln dust are produced per tonne of cement clinker.122 All these industrial solid wastes may be used for CO2 mineralisation. In this study, only industrial solid waste is assumed as feedstock to MINEX and additional potential of mined rock, e.g., serpentine or olivine is neglected. Pan et al.61 also emphasise the potential for additional indirectly avoided CO2 emissions by utilising carbonates as filler material in concrete blocks or cement mortars.
EW potential on agricultural land for the nine major regions considered in this work is determined as follows. The available agricultural land of each country138 is multiplied by a basalt application rate of 40 t per (ha·a) and a lower as well as higher estimate for the EW efficiency of 0.5 tCO2 per tRock and 0.67 tCO2 per tRock, respectively.
4. Results
In subsection 4.1, the global potential for MININ, MINEX, and EW is presented for the nine major regions of LUT-ESTM.10 Subsection 4.2 elaborates on the techno-economic findings for CO2 mineralisation, and subsection 4.3 condenses key findings on the technology readiness levels of the mineralisation options. Results are calculated in five-year steps from 2020–2100, though presented for 2030, 2050, and 2070, as the key years. These years provide concise information for times steps decisive for ramping CDR to reach a safe future.12 Results for all years can be found in Supplementary material 2 (ESI†) where all calculations can be traced.4.1. Global-regional mineralisation potential
The global-regional potential for MININ is depicted in Fig. 4. The global calculated annual injection potential equals about 0.7% per a of the total sequestration capacity. This stems from a combination of area and sequestration potential estimates in Oelkers et al.57 and the assumed area demand for injection of 28 km2 per (MtCO2/a).133 Some MININ sequestration locations can only achieve 0.2% per a injection rates due to different geologic characteristics. In Fig. 4, the secondary (right) vertical axis presenting the annual MININ potential is therefore normalised to 0.7% per a of the maximum total MININ potential presented on the primary (left) vertical axis. Hence, discrepancies in length of the coloured and black bar, shows discrepancies in the ratio of annual to total sequestration potential.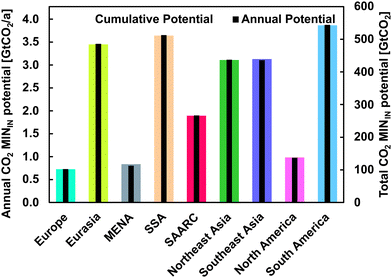 | ||
| Fig. 4 Global-regional CO2in situ mineralisation potential. Coloured bars indicate the total sequestration potential and the black bars indicate the annual injection and sequestration potential in each region. The left vertical axis is normalised to 0.7% per a of total potential as derived from the global total and annual sequestration potential. The total sequestration potential is represented by the right vertical axis. Divergences of this average value can be seen in the figure. Further information can be found in the Supplementary material 2 (ESI†). | ||
As can be seen in Fig. 4, all major regions except for Europe, MENA and North America are assumed to have Gt-scale annual injection and MININ potential. South America shows the highest total and annual potential for MININ at 543.0 GtCO2 and 3.9 GtCO2 per a, respectively. Only Europe and the MENA region show annual potential considerably below 1 GtCO2 per a. The SAARC region can sequester about 2 GtCO2 per a. The secondary vertical axis in Fig. 4 indicates that some potential MININ sites have different estimated height (volume-to-area ratio) which results in a slight divergence in the annual injection rate calculated when using the area demand approximation for CO2 underground injection provided by Wijaya et al.133
The annual and cumulative potential for MINEX using industrial solid wastes, i.e., steel slag, cement kiln dust, and red mud, are depicted in Fig. 5. Steelmaking slag occurs also in defossilised processes utilising hydrogen and electric arc furnaces139 and is, therefore, expected to be available despite the transition to green steel.
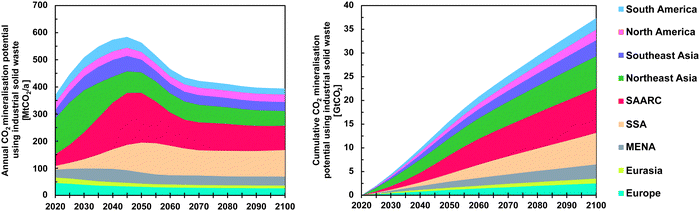 | ||
| Fig. 5 Global-regional CO2ex situ mineralisation potential using steel slag, cement kiln dust, and red mud for the nine major regions used in LUT-ESTM.10 The left panel shows the annual CO2 mineralisation potential, and the right panel shows the cumulative CO2 mineralisation potential in the 21st century. | ||
SAARC is expected to produce most of the global industrial waste output that can be utilised for CO2 MINEX by the mid of the 21st century. The global MINEX potential using industrial waste is projected to peak in 2045 at about 584 MtCO2 per a before declining to 394 MtCO2 per a by 2100. These results confirm findings of Pan et al.61 who found a global total direct MINEX potential using alkaline solid wastes of about 310 MtCO2 per a without considering some countries, especially in South America and Africa. Northeast Asia, especially China, is a major contributor both in results provided by Pan et al.61 and in this study; however results in this study indicate that SAARC and SSA will overtake Northeast Asia in terms of CO2 mineralisation using alkaline solid wastes. By the end of the 21st century, SSA is projected to be the major producer of industrial waste enabling substantial MINEX at an annual rate of 98 MtCO2 per a. A total of 37.4 GtCO2 can be permanently sequestered in carbonates using industrial solid waste as input for MINEX, which confirms the findings by Myers et al.137 It can be seen in Fig. 5 that the potential for MINEX,IW is lowest in Europe, Eurasia, MENA, North America, and South America. The potential projected for Southeast Asia is significantly lower compared to Northeast Asia, SAARC, and SAA where significant economic growth is projected in the LUT-DEES macro-economic scenario.10
The technical potential for EW in the nine major regions of LUT-ESTM is depicted in Fig. 6. The EW potential is directly related to the available cropland in each region. Whether the full technical potential of EW on croplands can be realised is uncertain and must be assessed in future studies.
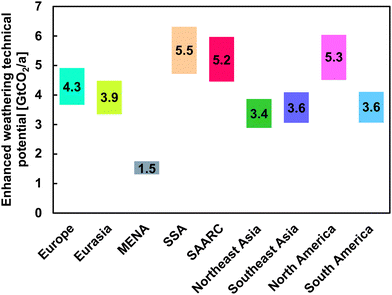 | ||
| Fig. 6 Global-regional potential for EW using basalt on cropland for the nine LUT-ESTM major regions.50 The position of the numeric values indicates the mean of the available technical potential range. There is uncertainty in the weathering efficiency when considering soil acidity140 as weathering rates differ with soil pH. As the average soil pH across the major regions ranges from about 5.7–7.5 (cf. Supplementary material 1 note 3, ESI†), this effect is not further considered. | ||
SSA has the highest theoretical EW potential with about 5.5 GtCO2 per a. North America and SAARC follow with a theoretical EW potential of about 5.3 GtCO2 per a and 5.2 GtCO2 per a, respectively. MENA has a theoretical EW potential of about 1.5 GtCO2 per a, and South America, Southeast Asia, Northeast Asia, Eurasia, and Europe all have average theoretical EW potential in the range of 3.4–4.3 GtCO2 per a. As EW performance is linked to precipitation, arid regions will show lower sequestration kinetics than humid regions, potentially constraining the annual CDR potential.
4.2. Techno-economic process parameters and cost of carbon sequestration
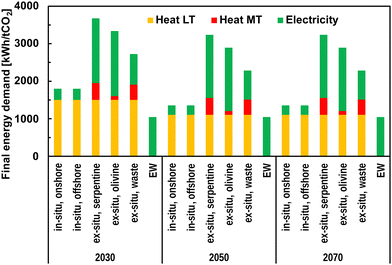
Subsection 4.2.1 presents findings on the PE demand of different CO2 mineralisation options. Subsection 4.2.2 then shows the resulting future projected cost of CO2 mineralisation to make these CO2 mineralisation options suitable for energy-industry-CDR system integration.EW is the only mineralisation option that solely relies on electricity. All other options require also heat for DAC and the mineralisation process. Mature technologies for rock mining, transportation, and comminution are assumed to show no reduction in final energy. EW requires the least total FE of about 1.0 MWh per tCO2. The total FE demand for MINEX is 3.7 MWh per tCO2, 3.3 MWh per tCO2, and 2.7 MWh per tCO2 if serpentine, olivine, and industrial solid waste is used as a feedstock in 2030. The total FE is expected to decrease in the future mainly due to advances in DAC technologies. In 2070, the total FE demand using MINEX is expected to decrease to 3.2 MWh per tCO2, 2.9 MWh per tCO2, and 2.3 MWh per tCO2 when using serpentine, olivine, and industrial solid waste, respectively. Using olivine for MINEX results in the lowest mid-temperature heat demand but shows the highest total electricity demand. MININ requires a total FE supply of about 1.8 MWh per tCO2 and 1.4 MWh per tCO2 in 2030 and from 2050 onwards, respectively. While EW shows the lowest total FE demand of all mineralisation options examined in this study, the electricity demand is sensitive to the rock transportation distance. Increasing the transportation distance from the base assumption of 200 km to 400 km can increase the total FE demand from 1.0 MWh per tCO2 to 1.9 MWh per tCO2. For MINEX options, the longer transportation distance increases the total FE to 4.4 MWh per tCO2 and 3.9 MWh per tCO2 when using serpentine and olivine, respectively, in 2030.
The PE demand for CDR with CO2 mineralisation for long-term stable sequestration is presented in Fig. 8.
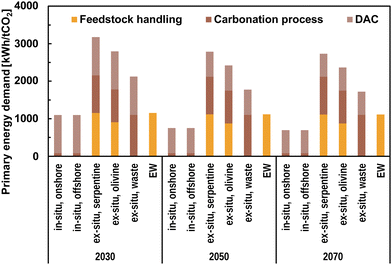 | ||
| Fig. 8 Primary energy demand for CO2 mineralisation options in 2030, 2050, and 2070. The energy demand is distinguished between feedstock handling, which includes open-pit mining, transportation, and comminution, energy demand directly related to the sequestration process, such as reactor operation for MINEX, and additional energy demand for DAC. DAC assumptions are taken from Fasihi et al.23 and adapted as in Mühlbauer et al.11 The rock transportation distance is assumed to be 200 km for EW and MINEX. | ||
The PE demand of MINEX using industrial solid waste is the highest at about 2.1 MWh per tCO2 in 2030 which declines to 1.8 MWh per tCO2 and 1.7 MWh per tCO2, in 2050 and 2070, respectively. Using serpentine and olivine as feedstock for MINEX, increases the PE demand of mineralisation to 3.2 MWh per tCO2 and 2.8 MWh per tCO2, respectively, in 2030. In 2070, MINEX using mined rocks requires about 2.7 MWh per tCO2 and 2.4 MWh per tCO2 for serpentine and olivine, respectively. MININ does not require feedstock handling and has significantly lower PE demand related to the sequestration process, which results in comparably lower total PE demand of 1.1 MWh per tCO2, 0.8 MWh per tCO2, and 0.7 MWh per tCO2 in 2030, 2050, and 2070, respectively. EW has the lowest PE demand at about 1.2 MWh per tCO2 in 2030; however, the demand increases with the distance of rock transportation. Increased transportation distance from the base assumption of 200 km to 400 km results in a PE demand of 1.9 MWh per tCO2 in 2030.
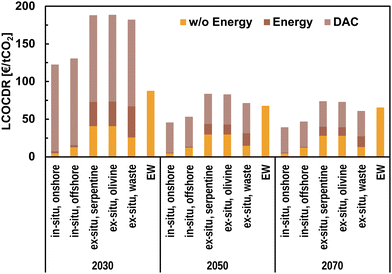 | ||
| Fig. 9 Future estimations of the levelised cost of CDR (LCOCDR) with secure CO2 sequestration via mineralisation. Projected DAC cost are adapted from Mühlbauer et al.11 | ||
The high initial cost related to DAC makes MININ and MINEX options relatively expensive compared to EW in the short term. MININ options cost about 123 € per tCO2 and 131 € per tCO2 if CO2 is injected onshore and offshore, respectively, in 2030. The cost is expected to decline to 40 € per tCO2 and 47 € per tCO2 for onshore and offshore MININ, respectively, until 2070. EW costs about 88 € per tCO2, 68 € per tCO2, and 66 € per tCO2 in 2030, 2050, and 2070, respectively. Therefore, anticipated future cost decline for DAC makes MININ cost-competitive with EW starting in 2050. For MINEX, using industrial waste is economically preferable over mined rocks due to the avoided cost for feedstock mining and handling. The cost of MINEX using serpentine, olivine, and industrial solid waste for mineralisation is estimated to be 188 € per tCO2, 189 € per tCO2, and 182 € per tCO2, respectively, in 2030. Until 2070, the costs for those MINEX options are expected to decrease to 74 € per tCO2, 73 € per tCO2, and 61 € per tCO2, respectively. Generally, the LCOCRs of all mineralisation options’ are in the range of 50–100 € per tCO2 from 2050 onwards, making mineralisation an economically viable option for safe and long-term CO2 sequestration. Future cost projections are uncertain, and those findings will need re-evaluation once technologies such as DAC become more mature and robust cost estimates are publicly available. Technology learning is only assumed for MINEX, as both MININ and EW can build fully on well-established and mature technologies used in mining and fossil fuel extraction.
4.3. Technology readiness
TRL is an important measure for energy system modelling to project future technology development. As Young et al.141 find, low-TRL technologies often experience cost escalations before becoming mature (high TRL) technologies, which show technology learning reducing the cost.127 Rock mining and handling is a well-established industry with mature technology. Furthermore, EW relies solely on relatively simple technology requiring feedstock mining, handling, transportation, and spreading.11,31,32 The TRL of EW can therefore be assessed at high level of 8–9. MINEX, in contrast, requires a specialised reactor, and no large-scale plants have been reported yet. Consequently, the TRL is lower compared to EW at a level of 5–6. MININ generally builds on well-established technologies such as deep drilling and fluid injection that are used for geothermal energy, fossil fuel extraction or enhanced oil and gas recovery. While the subsurface reservoir is different, there are already several relatively large-scale operations proofing the concept on a TRL of 8–9.1424.4. Cost and primary energy demand of large-scale mineralisation
The marginal energy demand and cost of CDR options with long-term CO2 sequestration via mineralisation are presented in subsections 4.2.1 and 4.2.2, respectively. Also, mineralisation is evaluated to be ready to satisfy a major share of future CDR demand to achieve ambitious climate targets (cf. subsection 4.3). The results for the assumptions mentioned above are presented in Table 2.| Unit | 1.5 °C targeta | 1.0 °C targeta | ||||
|---|---|---|---|---|---|---|
| 2050 | 2070 | 2050 | 2070 | |||
| a The CDR demand is adapted from Keiner et al.10 and Breyer et al.12 for a climate target at the end of this century, limiting global warming to 1.5 °C and 1.0 °C. The 1.5 °C target assumes a total CDR demand of 500 GtCO2, the 1.0 °C target 1750 GtCO2. | ||||||
| Cost | Total GDP (LUT-DEES) | b€ | 340,827 | 566,658 | 340,827 | 566,658 |
| Total annualised cost mineralisation | b€ | 69.1 | 333.4 | 241.7 | 1166.9 | |
| Share in projected total GDP | % | 0.02 | 0.06 | 0.07 | 0.21 | |
| Energy | Primary energy demand energy-industry system | PWh | 192.4 | 271.7 | 192.4 | 271.7 |
| Primary energy demand mineralisation | PWh | 1.3 | 6.7 | 4.5 | 23.4 | |
| Share in projected total primary energy demand | % | 0.67% | 2.46% | 2.34% | 8.60% | |
The cost of large-scale mineralisation deployment for long-term CO2 sequestration is about 69.1 b€ in 2050 and increases to 333.4 b€ in 2070 due to a fully ramped CDR sector in a trajectory compliant with limiting long-term global warming to 1.5 °C. For more ambitious targets, i.e., limiting global warming to 1.0 °C, the cost increase to 241.7 b€ and 1166.9 b€ in 2050 and 2070, respectively. If these results are put into perspective with the projected total GDP, the cost of sequestering 60% of projected CDR demand long-term in stable materials is estimated at 0.06% and 0.21% of the GDP for the 1.5 °C and 1.0 °C trajectories, respectively.
Primary energy demand for CO2 mineralisation in the discussed case amounts to about 1.3 PWh and 6.7 PWh in 2050 and 2070, respectively, for the 1.5 °C climate target. For the 1.0 °C target, the primary energy demand increases to about 4.5 PWh in 2050 and 23.4 PWh in 2070. This increase implies additional primary energy demand compared to the basic energy-industry system of 2.46% and 8.6% in 2070 for a 1.5 °C and 1.0 °C climate target, respectively.
5. Discussion
The discussion section focuses on the implications drawn from the presented results (subsection 5.1), the key issue of permanence of CDR (subsection 5.2), and the limitations and the research outlook (subsection 5.3) of this study.5.1. Implications from the results
As concluded in a recent study,11 CO2 mineralisation shows the highest permanence and safety, i.e., permanent CO2 sequestration, at a cost-competitive level compared to other CDR options. While there is still significant uncertainty in the global potentials of the options, the presented results indicate that all CDR from NETs based on captured dilute atmospheric CO2 and concentrated gaseous CO2 as intermediate step can be realised with the safe sequestration of CO2 in carbonates via mineralisation.The required energy for CO2 mineralisation would lead to gross emissions in the fossil fuel-dominated energy system.63 Resulting low carbon efficiencies would make net CDR significantly more expensive and emphasises the priority of a rapid defossilisation of the current energy-industry system and future energy-industry-CDR system before large-scale CDR with DAC and CO2 mineralisation can be implemented effectively.12,17 The need for raw material seems negligible for MININ but is significant for MINEX, with mined rocks, and EW. Both technologies can at least partially build on existing infrastructures; however, the satisfaction of raw material demand should be questioned carefully on the background of substantial pressure on material availability.143
As shown in previous work, CO2-to-solid processes can play a major role in future CDR endeavours if the security of CO2 sequestration is prioritised.11 Safe and long-term sequestration of atmospheric CO2 will be key to achieve ambitious climate targets to enable a safe future within planetary boundaries.12,13
CO2 mineralisation options in general, and MINEX in particular, show higher cost and energy demand compared to geological underground sequestration of CO2 or afforestation.11 However, non-permanent CDR such as afforestation and potentially geological underground CO2 sequestration come with several challenges including complicated and challenging accounting and subsequent monetary compensation and the risk of potential leakage, respectively.21,144 As stated by Vielstädte et al.,52 former hydrocarbon extraction sites with multiple injection wells may not always be suitable for geologic CO2 sequestration due to potential leakage.
Alternative pathways to produce solid materials from atmospheric CO2 have been presented in previous works with electricity-based silicon carbide and carbon fibres as promising options.45,46 The business-case of selling by-products from MINEX and potential synergies with the cement industry to reduce the cost of CO2 sequestration has been elaborated;55,130 however, MINEX is less dependent on a market and demand for by-products compared to electricity-based silicon carbide and carbon fibres, which are considerably more energy- and cost-intensive.45,46 Nevertheless, MINEX is largely constrained by the availability of industrial waste because mined rocks for carbonation make the approach significantly more expensive.
Further technologies relying on CO2 mineralisation for removal and sequestration of atmospheric CO2 have been proposed. Repeated ambient weathering of MgO for DAC was studied63,141,145 and is found to be a cost-competitive alternative to other DAC technologies.
Due to the high potential demand for minerals, concerns such as mineral poverty should be considered throughout the discussion of geochemical measures for CDR.143 However, in most literature, a large potential for CO2 mineralisation is presented (cf. section 2) and even a fraction of that could be sufficient to enable the safe sequestration of CO2 at the scale required for a 1.5 °C or 1.0 °C climate target. Future LCAs will be needed to thoroughly determine the impacts of different mineralisation options on the environment. The insights of such studies can be utilised to enhance the implications on future CDR portfolios for different societal preferences.11
While the additional primary energy demand is significant, renewable and clean energy sources, i.e., wind and especially solar photovoltaic, are on a promising trajectory to supply abundant low-cost electricity. It is not expected that large-scale CDR for ambitious climate targets is adversely affected by a possible limitation due to the availability of renewable electricity.146 As the additional demand in 2050 for electricity is limited to about 0.67% and 2.34% of projected total PE demand for a 1.5 °C target and a 1.0 °C target, respectively, the deployment of CDR, especially mineralisation, is not expected to significantly hamper the energy transition of other sectors such as power, transport, or industry through competition for renewable electricity.
CO2 mineralisation faces several current challenges. MININ or direct aqueous MINEX are currently operated with potable freshwater.44,119 This may severely compromise the sustainable potential of these operation considering the rising challenges in the global freshwater scarcity. MININ may be operated with seawater instead of freshwater in the foreseeable future44 and MINEX shows a high water recovery rate119 which should further be improved to solve water demand issues. EW has some sustainability issues considering potential health threats through dispersion of ultrafine particles32 or particles which are potentially contaminated, which calls for stringent management of operations and use of non-hazardous waste only102 in the future.
5.2. Permanence and timing of mineralisation
Mineralisation produces solid materials from gaseous atmospheric CO2. The permanence of CO2 sequestration in carbonates is well documented.20,65 While EW and MINEX can deliver permanent CDR as well as desirable co-benefits, MININ shows relatively low cost and energy demand and can be an attractive alternative to geologic sequestration as commonly proposed and often ‘over used’ in IAM scenarios.147 Geologic sequestration comes with several challenges. Fossil fuel producers that now own depleted reservoirs will benefit from underground sequestration of CO2 in their sites. This starkly contradicts the polluters-pay approach that is often proposed in the context of CO2 emissions148 by providing an attractive business case for fossil fuel producers for both polluting and restoring the atmosphere.One public concern regarding geologic CO2 sequestration is the risk of potential leakage87,149 that imposes significant risk to the environment and of morbidity.150,151 This risk requires significant efforts to actively monitor sequestration sites.152,153 For example, similar to nuclear power plants in Germany, there are currently no options for insuring geologic sequestration projects.154 Although there are no major leakage events reported so far, the risk is acknowledged and more investigation and research on the topic are needed.155 Non-permanent CDR options are difficult to properly account21,144 and, in light of the above-mentioned uncertainties regarding geologic sequestration, CO2 mineralisation can be a key technology in sustainable large-scale CDR.
5.3. Limitations and research outlook
Even though the CO2 mineralisation potentials are elaborated on a global and regional basis, further improving the spatial resolution is necessary to provide effective guidance to decision makers. However, since the required data is still not sufficient,57 providing potential estimations for all CO2 mineralisation options that are currently discussed in a comprehensive manner will advance the discussion about CO2 mineralisation for permanent CDR. Precisely mapping technical, economic, and sustainable potentials is yet to be conducted in regional level assessments. Missing data indicates the requirement of field trials and further endeavours to precisely estimate the potential of underground MININ sites globally in high spatial resolution. The CO2 mineralisation potential projection for the 21st century in conjunction with techno-economic parameters will enable future energy-industry-CDR system transition analyses.A static techno-economic framework is chosen to evaluate the cost and energy demand for large-scale CO2 mineralisation enabling permanent CDR on the large scale. Therefore, results of this study are intended to advance the consideration of CO2 mineralisation in energy-industry-CDR systems by providing the required data, but the results should be validated in comprehensive system transition optimisation studies and LCAs. Further developments of CO2 mineralisation options in energy demand are omitted and should be further studied in specialised assessments. This also relates to the consideration of local parameters such as soil pH on the weathering rate for EW mineralisation. Future research with a high spatial resolution will be required to assess mineralisation options in full global-local detail.
6. Conclusions
This work provides a techno-economic assessment as well as estimations for the global-regional potential of the CO2 mineralisation options with high probability to shape the future energy-industry-carbon dioxide removal system if a safe and long-term sequestration of atmospheric CO2 is desired. Three main pillars of CO2 mineralisation can play a significant role in future energy-industry-carbon dioxide removal systems: enhanced weathering, in situ mineralisation, and ex situ mineralisation. Enhanced weathering can be implemented on agricultural crop land or marginal hinterland as a potentially no-regret option for carbon dioxide removal. in situ mineralisation is similar to geologic underground sequestration, but it requires different sequestration sites that enable the rapid mineralisation and therefore safe long-term sequestration at elevated temperature and pressure of CO2 in aqueous solution. These suitable sequestration sites are composed of basaltic rocks or peridotite, are distributed globally, and show sufficient CO2 mineralisation potential. At similar cost and energy demand to geological underground sequestration, in situ mineralisation of CO2 provides an attractive alternative. ex situ mineralisation is the option investigated with highest cost and energy demand. Mined rocks must be transported, crushed, grinded, eventually pre-treated, and then mineralised in special reactors. Alternatively, industrial wastes of steelmaking, among others, can be used for ex situ mineralisation.The in situ mineralisation adds a moderate extra energy demand and cost required to direct air capture of CO2 enabling safe and long-term atmospheric CO2 sequestration. The cost of all mineralisation options for permanent CDR is expected to fall below 100 € per tCO2 by 2050 while enhanced weathering will likely reach that threshold by 2030. The primary energy demand for CDR via CO2 mineralisation is lowest for enhanced weathering, which does not require any direct air CO2 capture operations or powering a reactor as it only requires feedstock mining and spreading. At about 1150 kWh per tCO2, enhanced weathering would add moderate additional primary energy demand on the future projected demand. in situ mineralisation requires similar primary energy at about 1100 kWh per tCO2 in 2030, which is expected to decrease mainly due to improvements in direct air CO2 capture in the long term. While direct air CO2 capture constitutes about 50% of the total primary energy demand, ex situ mineralisation for carbon dioxide removal requires about 2100–3200 kWh per tCO2 in 2030, which reduces to about 1700–2700 kWh per tCO2 in 2070. The primary energy demand of mineralisation options must be considered for the determination of future carbon dioxide removal portfolios, but the sharply declining cost of renewable energy makes other indicators, which must be studied in dedicated life cycle assessments, potentially more important. in situ mineralisation is already conducted commercially on industrial scale and is considered to be on an advanced technology readiness level of 8 or 9. While enhanced weathering and ex situ mineralisation are not yet deployed on significant scale, both options rely on established technology with the exception of aqueous carbonation reactors.
Large-scale deployment of mineralisation to contribute a 60% share of total carbon dioxide removal endeavours necessary to achieve ambitious climate targets is shown to be manageable at a share of 0.06% and 0.21% in projected gross domestic product until 2070 for a 1.5 °C and 1.0 °C temperature target, respectively. The total energy demand for mineralisation in this case requires about 2.5% and 8.6% additional primary energy demand compared to the total primary energy demand for all other sectors. While this demand is substantial, current development of cost and deployment of clean, renewable sources such as wind power and solar photovoltaics can support additional primary energy demand for safe CO2 sequestration. Also, due to the limited additional demand in 2050, i.e., 0.67% and 2.34% for the 1.5 °C and 1.0 °C temperature target, respectively, additional primary energy demand for CO2 mineralisation is not expected to substantially hinder the defossilisation of all other sectors until 2050. Possible synergies of by-products are discussed.
With this work, a first step for implementation of CO2 mineralisation for permanent carbon dioxide removal in modelling frameworks for investigating the characteristics of future energy-industry-carbon dioxide removal systems is taken. Future iterations of techno-economic and potential assumptions for the presented technology options will further reduce uncertainty that exists at this early stage of development in CO2 mineralisation and carbon dioxide removal in general. Permanent carbon dioxide removal via CO2 mineralisation can be a most valuable endeavour to reach ambitious climate targets that should be considered for the transition phase, which include large-scale carbon dioxide removal in a 100% renewable post-fossil energy-industry-carbon dioxide removal system.
Nomenclature
| BECCS | Bioenergy with carbon capture and sequestration |
| Ca | Calcium |
| CAPEX | Capital expenditures |
| CDR | Carbon dioxide removal |
| CO2 | Carbon dioxide |
| DAC | Direct air capture |
| DACCS | Direct air carbon capture and sequestration |
| EW | Enhanced weathering |
| FE | Final energy |
| IAM | Integrated assessment model |
| IPCC | Intergovernmental Panel on Climate Change |
| LCA | Life cycle assessment |
| LCOCDR | Levelised cost of carbon dioxide removal |
| MENA | Middle East and North Africa |
| Mg | Magnesium |
| MINEX | Ex situ mineralisation |
| MININ | In situ mineralisation |
| NCS | Natural climate solutions |
| NET | Negative emission technology |
| OPEX | Operational expenditures |
| OPEXfix | Fixed operational expenditures |
| OPEXvar | Variable operational expenditures |
| PE | Primary energy demand |
| SAARC | South Asian Association for Regional Cooperation |
| SSA | Sub-Saharan Africa |
| TNE | Total negative emissions |
| TRL | Technology readiness level |
| UN | United Nations |
| WP | Weathering potential |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully acknowledge the public financing of the Research Council of Finland for the ‘Industrial Emissions & CDR’ project under the number 343053 which partly funded this research. Dominik Keiner would like to thank the Jenny and Antti Wihuri Foundation for the valuable grant. The authors would like to thank Gabriel Lopez for proofreading.References
- [IPCC] - Intergovernmental Panel on Climate Change, Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel on Climate Change (IPCC), Geneva, Switzerland, First., 2023.
- B. H. Kreps, Am. J. Econ. Sociol., 2020, 79, 695–717, DOI:10.1111/ajes.12336.
- B. Clarke, F. Otto, R. Stuart-Smith and L. Harrington, Environ. Res. Clim., 2022, 1, 012001, DOI:10.1088/2752-5295/ac6e7d.
- C. Breyer, S. Khalili, D. Bogdanov, M. Ram, A. S. Oyewo, A. Aghahosseini, A. Gulagi, A. A. Solomon, D. Keiner, G. Lopez, P. A. Ostergaard, H. Lund, B. V. Mathiesen, M. Z. Jacobson, M. Victoria, S. Teske, T. Pregger, V. Fthenakis, M. Raugei, H. Holttinen, U. Bardi, A. Hoekstra and B. K. Sovacool, IEEE Access, 2022, 10, 78176–78218, DOI:10.1109/ACCESS.2022.3193402.
- G. Luderer, S. Madeddu, L. Merfort, F. Ueckerdt, M. Pehl, R. Pietzcker, M. Rottoli, F. Schreyer, N. Bauer, L. Baumstark, C. Bertram, A. Dirnaichner, F. Humpenöder, A. Levesque, A. Popp, R. Rodrigues, J. Strefler and E. Kriegler, Nat. Energy, 2021, 7, 32–42, DOI:10.1038/s41560-021-00937-z.
- [IPCC] - Intergovernmental Panel on Climate Change, Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group lll to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022.
- M. W. Jones, G. P. Peters, T. Gasser, R. M. Andrew, C. Schwingshackl, J. Gütschow, R. A. Houghton, P. Friedlingstein, J. Pongratz and C. L. Quéré, Sci. Data, 2023, 10, 155, DOI:10.1038/s41597-023-02041-1.
- H. J. Buck, W. Carton, J. F. Lund and N. Markusson, Nat. Clim. Change, 2023, 13, 351–358, DOI:10.1038/s41558-022-01592-2.
- S. Smith, O. Geden, G. F. Nemet, M. Gidden, W. F. Lamb, C. Powis, R. Bellamy, M. Callaghan, A. Cowie, E. Cox, S. Fuss, T. Gasser, G. Grassi, J. Greene, S. Lück, A. Mohan, F. Muller-Hansen, G. Peters, Y. Pratama, T. Repke, K. Riahi, F. Schenuit, J. Steinhauser, J. Strefler, J. M. Valenzula and J. C. Minx, The State of Carbon Dioxide Removal - 1st Edition, 2023.
- D. Keiner, A. Gulagi and C. Breyer, Energy, 2023, 272, 127199, DOI:10.1016/j.energy.2023.127199.
- A. Mühlbauer, D. Keiner, C. Gerhards, M. Sterner and C. Breyer, Submitted.
- C. Breyer, D. Keiner, B. W. Abbott, J. L. Bamber, F. Creutzig, C. Gerhards, A. Mühlbauer, G. F. Nemet and Ö. Terli, Plos Clim., 2023, 2, e0000234, DOI:10.1371/journal.pclm.0000234.
- J. Rockström, J. Gupta, D. Qin, S. J. Lade, J. F. Abrams, L. S. Andersen, D. I. Armstrong McKay, X. Bai, G. Bala, S. E. Bunn, D. Ciobanu, F. DeClerck, K. Ebi, L. Gifford, C. Gordon, S. Hasan, N. Kanie, T. M. Lenton, S. Loriani, D. M. Liverman, A. Mohamed, N. Nakicenovic, D. Obura, D. Ospina, K. Prodani, C. Rammelt, B. Sakschewski, J. Scholtens, B. Stewart-Koster, T. Tharammal, D. Van Vuuren, P. H. Verburg, R. Winkelmann, C. Zimm, E. M. Bennett, S. Bringezu, W. Broadgate, P. A. Green, L. Huang, L. Jacobson, C. Ndehedehe, S. Pedde, J. Rocha, M. Scheffer, L. Schulte-Uebbing, W. De Vries, C. Xiao, C. Xu, X. Xu, N. Zafra-Calvo and X. Zhang, Nature, 2023, 619, 102–111, DOI:10.1038/s41586-023-06083-8.
- J. Hansen, M. Sato, P. Kharecha, K. von Schuckmann, D. J. Beerling, J. Cao, S. Marcott, V. Masson-Delmotte, M. J. Prather, E. J. Rohling, J. Shakun, P. Smith, A. Lacis, G. Russell and R. Ruedy, Earth Syst. Dyn., 2017, 8, 577–616, DOI:10.5194/esd-8-577-2017.
- D. I. Armstrong McKay, A. Staal, J. F. Abrams, R. Winkelmann, B. Sakschewski, S. Loriani, I. Fetzer, S. E. Cornell, J. Rockström and T. M. Lenton, Science, 2022, 377, eabn7950, DOI:10.1126/science.abn7950.
- N. Wunderling, R. Winkelmann, J. Rockström, S. Loriani, D. I. Armstrong McKay, P. D. L. Ritchie, B. Sakschewski and J. F. Donges, Nat. Clim. Change, 2023, 13, 75–82, DOI:10.1038/s41558-022-01545-9.
- B. W. Abbott, C. Abrahamian, N. Newbold, P. Smith, M. Merritt, S. S. Sayedi, J. Bekker, M. Greenhalgh, S. Gilbert, M. King, G. Lopez, N. Zimmermann and C. Breyer, Earths Future, 2023, 11, e2023EF003639, DOI:10.1029/2023EF003639.
- [IPCC] - Intergovernmental Panel on Climate Change, Mineral carbonation and industrial uses of carbon dioxide, IPCC, 2018.
- D. Sandalow, R. Aines, J. Friedmann, P. Kelemen, C. McCormick, I. M. Power, B. Schmidt and W. Siobhan, Carbon Mineralization Roadmap, ICEF Innovation Roadmap Project, 2021.
- S. Chiquier, P. Patrizio, M. Bui, N. Sunny and N. Mac Dowell, Energy Environ. Sci., 2022, 15, 4389–4403, 10.1039/D2EE01021F.
- A. Prado and N. Mac Dowell, Joule, 2023, 7, 700–712, DOI:10.1016/j.joule.2023.03.006.
- B. W. Griscom, J. Adams, P. W. Ellis, R. A. Houghton, G. Lomax, D. A. Miteva, W. H. Schlesinger, D. Shoch, J. V. Siikamäki, P. Smith, P. Woodbury, C. Zganjar, A. Blackman, J. Campari, R. T. Conant, C. Delgado, P. Elias, T. Gopalakrishna, M. R. Hamsik, M. Herrero, J. Kiesecker, E. Landis, L. Laestadius, S. M. Leavitt, S. Minnemeyer, S. Polasky, P. Potapov, F. E. Putz, J. Sanderman, M. Silvius, E. Wollenberg and J. Fargione, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 11645–11650, DOI:10.1073/pnas.1710465114.
- M. Fasihi, O. Efimova and C. Breyer, J. Cleaner Prod., 2019, 224, 957–980, DOI:10.1016/j.jclepro.2019.03.086.
- C. Breyer, M. Fasihi, C. Bajamundi and F. Creutzig, Joule, 2019, 3, 2053–2057, DOI:10.1016/j.joule.2019.08.010.
- M. Sendi, M. Bui, N. Mac Dowell and P. Fennell, One Earth, 2022, 5, 1153–1164, DOI:10.1016/j.oneear.2022.09.003.
- S. V. Hanssen, V. Daioglou, Z. J. N. Steinmann, J. C. Doelman, D. P. Van Vuuren and M. A. J. Huijbregts, Nat. Clim. Change, 2020, 10, 1023–1029, DOI:10.1038/s41558-020-0885-y.
- S. V. Hanssen, Z. J. N. Steinmann, V. Daioglou, M. Čengić, D. P. V. Vuuren and M. A. J. Huijbregts, GCB Bioenergy, 2022, 14, 307–321, DOI:10.1111/gcbb.12911.
- Z. Ai, N. Hanasaki, V. Heck, T. Hasegawa and S. Fujimori, Nat. Sustainability, 2021, 4, 884–891, DOI:10.1038/s41893-021-00740-4.
- J. S. Næss, O. Cavalett and F. Cherubini, Nat. Sustainability, 2021, 4, 525–536 CrossRef.
- D. J. Beerling, J. R. Leake, S. P. Long, J. D. Scholes, J. Ton, P. N. Nelson, M. Bird, E. Kantzas, L. L. Taylor, B. Sarkar, M. Kelland, E. DeLucia, I. Kantola, C. Müller, G. Rau and J. Hansen, Nat. Plants, 2018, 4, 138–147, DOI:10.1038/s41477-018-0108-y.
- D. J. Beerling, E. P. Kantzas, M. R. Lomas, P. Wade, R. M. Eufrasio, P. Renforth, B. Sarkar, M. G. Andrews, R. H. James, C. R. Pearce, J.-F. Mercure, H. Pollitt, P. B. Holden, N. R. Edwards, M. Khanna, L. Koh, S. Quegan, N. F. Pidgeon, I. A. Janssens, J. Hansen and S. A. Banwart, Nature, 2020, 583, 242–248, DOI:10.1038/s41586-020-2448-9.
- J. Strefler, T. Amann, N. Bauer, E. Kriegler and J. Hartmann, Environ. Res. Lett., 2018, 13, 034010, DOI:10.1088/1748-9326/aaa9c4.
- D. S. Goll, P. Ciais, T. Amann, W. Buermann, J. Chang, S. Eker, J. Hartmann, I. Janssens, W. Li, M. Obersteiner, J. Penuelas, K. Tanaka and S. Vicca, Nat. Geosci., 2021, 14, 545–549, DOI:10.1038/s41561-021-00798-x.
- J. Lehmann, A. Cowie, C. A. Masiello, C. Kammann, D. Woolf, J. E. Amonette, M. L. Cayuela, M. Camps-Arbestain and T. Whitman, Nat. Geosci., 2021, 14, 883–892, DOI:10.1038/s41561-021-00852-8.
- J. Strefler, N. Bauer, F. Humpenöder, D. Klein, A. Popp and E. Kriegler, Environ. Res. Lett., 2021, 16, 074021, DOI:10.1088/1748-9326/ac0a11.
- S. Fuss, J. G. Canadell, P. Ciais, R. B. Jackson, C. D. Jones, A. Lyngfelt, G. P. Peters and D. P. V. Vuuren, One Earth, 2020, 3, 145–149, DOI:10.1016/j.oneear.2020.08.002.
- J. Fuhrman, C. Bergero, M. Weber, S. Monteith, F. M. Wang, A. F. Clarens, S. C. Doney, W. Shobe and H. McJeon, Nat. Clim. Change, 2023, 13, 341–350, DOI:10.1038/s41558-023-01604-9.
- S. Sri Shalini, K. Palanivelu, A. Ramachandran and R. Vijaya, Biomass Convers. Biorefinery, 2021, 11, 2247–2267, DOI:10.1007/s13399-020-00604-5.
- J. Mertens, C. Breyer, K. Arning, A. Bardow, R. Belmans, A. Dibenedetto, S. Erkman, J. Gripekoven, G. Léonard, S. Nizou, D. Pant, A. S. Reis-Machado, P. Styring, J. Vente, M. Webber and C. J. Sapart, Joule, 2023, 7, 442–449, DOI:10.1016/j.joule.2023.01.005.
- T. Galimova, M. Ram, D. Bogdanov, M. Fasihi, S. Khalili, A. Gulagi, H. Karjunen, T. N. O. Mensah and C. Breyer, J. Cleaner Prod., 2022, 373, 133920, DOI:10.1016/j.jclepro.2022.133920.
- G. Lopez, D. Keiner, M. Fasihi, T. Koiranen and C. Breyer, Energy Environ. Sci., 2023, 16, 2879–2909, 10.1039/D3EE00478C.
- P. Gabrielli, M. Gazzani and M. Mazzotti, Ind. Eng. Chem. Res., 2020, 59, 7033–7045, DOI:10.1021/acs.iecr.9b06579.
- R. Sacchi, V. Becattini, P. Gabrielli, B. Cox, A. Dirnaichner, C. Bauer and M. Mazzotti, Nat. Commun., 2023, 14, 3989, DOI:10.1038/s41467-023-39749-y.
- S. Ó. Snæbjörnsdóttir, B. Sigfússon, C. Marieni, D. Goldberg, S. R. Gislason and E. H. Oelkers, Nat. Rev. Earth Environ., 2020, 1, 90–102, DOI:10.1038/s43017-019-0011-8.
- A. Mühlbauer, D. Keiner, T. Galimova and C. Breyer, Mitig. Adapt. Strateg. Glob. Change, 2024, 29, 4, DOI:10.1007/s11027-023-10100-6.
- D. Keiner, A. Mühlbauer, G. Lopez, T. Koiranen and C. Breyer, Mitig. Adapt. Strateg. Glob. Change, 2023, 28, 52, DOI:10.1007/s11027-023-10090-5.
- X. Liu, X. Wang, G. Licht and S. Licht, J. CO2 Util., 2020, 36, 288–294, DOI:10.1016/j.jcou.2019.11.019.
- J. H. Lee, J. H. Lee, I. K. Park and C. H. Lee, J. CO2 Util., 2018, 26, 522–536, DOI:10.1016/j.jcou.2018.06.007.
- C. Molina-Jirón, M. R. Chellali, C. N. S. Kumar, C. Kübel, L. Velasco, H. Hahn, E. Moreno-Pineda and M. Ruben, ChemSusChem, 2019, 12, 3509–3514, DOI:10.1002/cssc.201901404.
- D. Bogdanov, M. Ram, A. Aghahosseini, A. Gulagi, A. S. Oyewo, M. Child, U. Caldera, K. Sadovskaia, J. Farfan, L. De Souza Noel Simas Barbosa, M. Fasihi, S. Khalili, T. Traber and C. Breyer, Energy, 2021, 227, 120467, DOI:10.1016/j.energy.2021.120467.
- C. Breyer, G. Lopez, D. Bogdanov and P. Laaksonen, Int. J. Hydrogen Energy, 2024, 49, 351–359, DOI:10.1016/j.ijhydene.2023.08.170.
- L. Vielstädte, P. Linke, M. Schmidt, S. Sommer, M. Haeckel, M. Braack and K. Wallmann, Int. J. Greenhouse Gas Control, 2019, 84, 190–203, DOI:10.1016/j.ijggc.2019.03.012.
- K. S. Lackner, C. H. Wendt, D. P. Butt, E. L. Joyce and D. H. Sharp, Energy, 1995, 20, 1153–1170, DOI:10.1016/0360-5442(95)00071-N.
- D. Kremer, C. Dertmann, S. Etzold, R. Telle, B. Friedrich and H. Wotruba, J. CO2 Util., 2022, 58, 101928, DOI:10.1016/j.jcou.2022.101928.
- T. Strunge, P. Renforth and M. Van der Spek, Commun. Earth Environ., 2022, 3, 59, DOI:10.1038/s43247-022-00390-0.
- S. R. Gislason, D. Wolff-Boenisch, A. Stefansson, E. H. Oelkers, E. Gunnlaugsson, H. Sigurdardottir, B. Sigfusson, W. S. Broecker, J. M. Matter and M. Stute, Int. J. Greenhouse Gas Control, 2010, 4, 537–545, DOI:10.1016/j.ijggc.2009.11.013.
- E. H. Oelkers, S. R. Gislason and P. B. Kelemen, Carbon Capture Sci. Technol., 2023, 6, 100098, DOI:10.1016/j.ccst.2023.100098.
- A. Lyngfelt, D. J. A. Johansson and E. Lindeberg, Int. J. Greenhouse Gas Control, 2019, 87, 27–33, DOI:10.1016/j.ijggc.2019.04.022.
- J. Duer, SPE Annual Technical Conference and Exhibition, San Antonio, Texas, USA, 2017 DOI:10.2118/187100-MS.
- M. D. Aminu, S. A. Nabavi, C. A. Rochelle and V. Manovic, Appl. Energy, 2017, 208, 1389–1419, DOI:10.1016/j.apenergy.2017.09.015.
- S.-Y. Pan, Y.-H. Chen, L.-S. Fan, H. Kim, X. Gao, T.-C. Ling, P.-C. Chiang, S.-L. Pei and G. Gu, Nat. Sustainability, 2020, 3, 399–405, DOI:10.1038/s41893-020-0486-9.
- R. Yoshioka, K. Nakamura, R. Sekiai, J. Wang and N. Watanabe, Front. Environ. Sci., 2022, 10, 1068656, DOI:10.3389/fenvs.2022.1068656.
- P. B. Kelemen, N. McQueen, J. Wilcox, P. Renforth, G. Dipple and A. P. Vankeuren, Chem. Geol., 2020, 550, 119628, DOI:10.1016/j.chemgeo.2020.119628.
- P. Kelemen, S. M. Benson, H. Pilorgé, P. Psarras and J. Wilcox, Front. Clim., 2019, 1, 9, DOI:10.3389/fclim.2019.00009.
- J. S. Campbell, S. Foteinis, V. Furey, O. Hawrot, D. Pike, S. Aeschlimann, C. N. Maesano, P. L. Reginato, D. R. Goodwin, L. L. Looger, E. S. Boyden and P. Renforth, Front. Clim., 2022, 4, 879133, DOI:10.3389/fclim.2022.879133.
- C. N. Maesano, J. S. Campbell, S. Foteinis, V. Furey, O. Hawrot, D. Pike, S. Aeschlimann, P. L. Reginato, D. R. Goodwin, L. L. Looger, E. S. Boyden and P. Renforth, Front. Clim., 2022, 4, 945332, DOI:10.3389/fclim.2022.945332.
- C. D. Hills, N. Tripathi and P. J. Carey, Front. Energy Res., 2020, 8, 142, DOI:10.3389/fenrg.2020.00142.
- L. A. Bullock, J. Alcalde, F. Tornos and J.-L. Fernandez-Turiel, Sci. Total Environ, 2023, 867, 161287, DOI:10.1016/j.scitotenv.2022.161287.
- L. A. Bullock, Z. Nkosi, M. Vele and M. Amponsah-Dacosta, Int. J. Greenhouse Gas Control, 2023, 124, 103844, DOI:10.1016/j.ijggc.2023.103844.
- F. L. Buckingham, G. M. Henderson, P. Holdship and P. Renforth, Appl. Geochem., 2022, 147, 105482, DOI:10.1016/j.apgeochem.2022.105482.
- L. J. West, S. A. Banwart, M. V. Martin, E. Kantzas and D. J. Beerling, Appl. Geochem., 2023, 151, 105591, DOI:10.1016/j.apgeochem.2023.105591.
- E. P. Kantzas, M. Val Martin, M. R. Lomas, R. M. Eufrasio, P. Renforth, A. L. Lewis, L. L. Taylor, J.-F. Mecure, H. Pollitt, P. V. Vercoulen, N. Vakilifard, P. B. Holden, N. R. Edwards, L. Koh, N. F. Pidgeon, S. A. Banwart and D. J. Beerling, Nat. Geosci., 2022, 15, 382–389, DOI:10.1038/s41561-022-00925-2.
- T. Rinder and C. von Hagke, J. Cleaner Prod., 2021, 315, 128178, DOI:10.1016/j.jclepro.2021.128178.
- C. Myers and T. Nakagaki, Environ. Res. Lett., 2020, 15, 124018, DOI:10.1088/1748-9326/abc217.
- A. R. Stubbs, C. Paulo, I. M. Power, B. Wang, N. Zeyen and S. A. Wilson, Int. J. Greenhouse Gas Control, 2022, 113, 103554, DOI:10.1016/j.ijggc.2021.103554.
- C. S. Larkin, M. G. Andrews, C. R. Pearce, K. L. Yeong, D. J. Beerling, J. Bellamy, S. Benedick, R. P. Freckleton, H. Goring-Harford, S. Sadekar and R. H. James, Front. Clim., 2022, 4, 959229, DOI:10.3389/fclim.2022.959229.
- Neeraj and S. Yadav, Mater. Sci. Energy Technol., 2020, 3, 494–500, DOI:10.1016/j.mset.2020.03.005.
- M. E. Raymo and W. F. Ruddiman, Nature, 1992, 359, 117–122, DOI:10.1038/359117a0.
- E. Beaulieu, Y. Goddéris, Y. Donnadieu, D. Labat and C. Roelandt, Nat. Clim. Change, 2012, 2, 346–349, DOI:10.1038/nclimate1419.
- S.-Y. Pan, E. E. Chang and P.-C. Chiang, Aerosol Air Qual. Res., 2012, 12, 770–791, DOI:10.4209/aaqr.2012.06.0149.
- M. Ibrahim, M. El-Naas, A. Benamor, S. Al-Sobhi and Z. Zhang, Processes, 2019, 7, 115, DOI:10.3390/pr7020115.
- R. Baciocchi and G. Costa, Front. Energy Res., 2021, 9, 592600, DOI:10.3389/fenrg.2021.592600.
- D. Kremer, T. Strunge, J. Skocek, S. Schabel, M. Kostka, C. Hopmann and H. Wotruba, J. CO2 Util., 2022, 62, 102067, DOI:10.1016/j.jcou.2022.102067.
- S. Teir, T. Auvinen, A. Said, T. Kotiranta and H. Peltola, Front. Energy Res., 2016, 4(6) DOI:10.3389/fenrg.2016.00006.
- S.-Y. Pan, T.-C. Chung, C.-C. Ho, C.-J. Hou, Y.-H. Chen and P.-C. Chiang, Sci. Rep., 2017, 7, 17227, DOI:10.1038/s41598-017-17648-9.
- V. Romanov, Y. Soong, C. Carney, G. E. Rush, B. Nielsen and W. O’Connor, ChemBioEng Rev., 2015, 2, 231–256, DOI:10.1002/cben.201500002.
- R. F. Suleman Kali, D. Fan, N. Hazel and A. Striolo, Environ. Sci. Adv., 2022, 1, 138–155, 10.1039/D1VA00036E.
- R. Zevenhoven, J. Fagerlund and J. K. Songok, Greenhouse Gases Sci. Technol., 2011, 1, 48–57, DOI:10.1002/ghg3.7.
- H. Ostovari, A. Sternberg and A. Bardow, Sustain. Energy Fuels, 2020, 4, 4482–4496, 10.1039/D0SE00190B.
- A. A. Olajire, J. Pet. Sci. Eng., 2013, 109, 364–392, DOI:10.1016/j.petrol.2013.03.013.
- M. S. Ncongwane, J. L. Broadhurst and J. Petersen, Int. J. Greenhouse Gas Control, 2018, 77, 70–81, DOI:10.1016/j.ijggc.2018.07.019.
- R. M. Santos, P. C. M. Knops, K. L. Rijnsburger and Y. W. Chiang, Front. Energy Res., 2016, 4, 5, DOI:10.3389/fenrg.2016.00005.
- A. Sanna, M. Uibu, G. Caramanna, R. Kuusik and M. M. Maroto-Valer, Chem. Soc. Rev., 2014, 43, 8049–8080, 10.1039/C4CS00035H.
- J. Fagerlund, E. Nduagu, I. Romao and R. Zevenhoven, Energy, 2012, 41(1), 184–191, DOI:10.1016/j.energy.2011.08.032.
- A. Scott, C. Oze, V. Shah, N. Yang, B. Shanks, C. Cheeseman, A. Marshall and M. Watson, Commun. Earth Environ., 2021, 2, 25, DOI:10.1038/s43247-021-00099-6.
- P. A. E. Pogge von Strandmann, C. Tooley, J. J. P. A. Mulders and P. Renforth, Front. Clim., 2022, 4, 827698, DOI:10.3389/fclim.2022.827698.
- G. Cipolla, S. Calabrese, A. Porporato and L. V. Noto, Biogeosciences, 2022, 19, 3877–3896, DOI:10.5194/bg-19-3877-2022.
- G. Cipolla, S. Calabrese, L. V. Noto and A. Porporato, Adv. Water Resour., 2021, 154, 103934, DOI:10.1016/j.advwatres.2021.103934.
- G. Cipolla, S. Calabrese, L. V. Noto and A. Porporato, Adv. Water Resour., 2021, 154, 103949, DOI:10.1016/j.advwatres.2021.103949.
- R. M. Eufrasio, E. P. Kantzas, N. R. Edwards, P. B. Holden, H. Pollitt, J.-F. Mercure, S. C. L. Koh and D. J. Beerling, Commun. Earth Environ., 2022, 3, 106, DOI:10.1038/s43247-022-00436-3.
- N. Vakilifard, E. P. Kantzas, N. R. Edwards, P. B. Holden and D. J. Beerling, Environ. Res. Lett., 2021, 16, 094005, DOI:10.1088/1748-9326/ac1818.
- X. Jia, Z. Zhang, F. Wang, Z. Li, Y. Wang, K. B. Aviso, D. Y. C. Foo, P. N. S. B. Nair, R. R. Tan and F. Wang, Resour., Conserv. Recycl., 2022, 176, 105910, DOI:10.1016/j.resconrec.2021.105910.
- K. B. Aviso, J.-Y. Lee, A. T. Ubando and R. R. Tan, Clean Technol. Environ. Policy, 2022, 24, 21–37, DOI:10.1007/s10098-021-02053-8.
- N. Wei, X. Li, Z. Jiao, P. H. Stauffer, S. Liu, K. Ellett and R. S. Middleton, Front. Earth Sci., 2022, 9, 777323, DOI:10.3389/feart.2021.777323.
- L. A. Bullock, A. Yang and R. C. Darton, Sci. Total Environ, 2022, 808, 152111, DOI:10.1016/j.scitotenv.2021.152111.
- D. Kremer, S. Etzold, J. Boldt, P. Blaum, K. M. Hahn, H. Wotruba and R. Telle, Minerals, 2019, 9, 485, DOI:10.3390/min9080485.
- G. Faber, A. Ruttinger, T. Strunge, T. Langhorst, A. Zimmermann, M. van der Hulst, F. Bensebaa, S. Moni and L. Tao, Front. Clim., 2022, 4, 820261, DOI:10.3389/fclim.2022.820261.
- H. Ostovari, L. Müller, F. Mayer and A. Bardow, J. Cleaner Prod., 2022, 360, 131750, DOI:10.1016/j.jclepro.2022.131750.
- [IEAGHG] - International Energy Agency Greenhouse Gas R&D Programme, The Costs of CO2 storage, IEA GHG, 2013.
- S. Teir, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Energy Convers. Manag., 2006, 47, 3059–3068, DOI:10.1016/j.enconman.2006.03.021.
- S. R. Gislason and E. H. Oelkers, Science, 2014, 344, 373–374, DOI:10.1126/science.1250828.
- S. Ó. Snæbjörnsdóttir, C. Marieni, M. Voigt and B. Sigfússon, Comprehensive Renewable Energy, Elsevier, 2022, pp. 315–330, DOI:10.1016/B978-0-12-819727-1.00108-4.
- D. S. Goldberg, T. Takahashi and A. L. Slagle, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9920–9925, DOI:10.1073/pnas.0804397105.
- H. Kristjánsdóttir and S. Kristjánsdóttir, Balt. J. Econ. Stud., 2021, 7, 1–9, DOI:10.30525/2256-0742/2021-7-1-1-9.
- M. Voigt, C. Marieni, A. Baldermann, I. M. Galeczka, D. Wolff-Boenisch, E. H. Oelkers and S. R. Gislason, Geochim. Cosmochim. Acta, 2021, 308, 21–41, DOI:10.1016/j.gca.2021.05.056.
- S. Self, M. F. Coffin, M. R. Rampino and J. A. Wolff, The Encyclopedia of Volcanoes, Elsevier, 2015, pp. 441–455, DOI:10.1016/B978-0-12-385938-9.00024-9.
- L. Johansson, S. Zahirovic and R. D. Müller, Geophys. Res. Lett., 2018, 45, 5380–5389, DOI:10.1029/2017GL076691.
- M. Dri, A. Sanna and M. M. Maroto-Valer, Energy Procedia, 2014, 63, 6544–6547, DOI:10.1016/j.egypro.2014.11.690.
- H. Geerlings and R. Zevenhoven, Annu. Rev. Chem. Biomol. Eng., 2013, 4, 103–117, DOI:10.1146/annurev-chembioeng-062011-080951.
- C. Arpagaus, F. Bless, M. Uhlmann, J. Schiffmann and S. S. Bertsch, Energy, 2018, 152, 985–1010, DOI:10.1016/j.energy.2018.03.166.
- S. J. Gerdemann, W. K. O’Connor, D. C. Dahlin, L. R. Penner and H. Rush, Environ. Sci. Technol., 2007, 41, 2587–2593, DOI:10.1021/es0619253.
- P. Renforth, Nat. Commun., 2019, 10, 1401, DOI:10.1038/s41467-019-09475-5.
- E. E. E. M. te Pas, M. Hagens and R. N. J. Comans, Front. Clim., 2023, 4, 954064, DOI:10.3389/fclim.2022.954064.
- M. Heřmanská, M. J. Voigt, C. Marieni, J. Declercq and E. H. Oelkers, Chem. Geol., 2022, 597, 120807, DOI:10.1016/j.chemgeo.2022.120807.
- Thunder Said Energy, Mining: crushing, grinding and comminution costs?, https://thundersaidenergy.com/downloads/mining-crushing-grinding-and-communition-costs/, (accessed 14 July 2024).
- G. Thomassen, S. V. Passel and J. Dewulf, Renew. Sustain. Energy Rev., 2020, 130, 109937, DOI:10.1016/j.rser.2020.109937.
- J. I. Lewis and G. F. Nemet, WIREs Clim. Change, 2021, 12(5), e730, DOI:10.1002/wcc.730.
- E. Benhelal, M. I. Rashid, C. Holt, M. S. Rayson, G. Brent, J. M. Hook, M. Stockenhuber and E. M. Kennedy, J. Cleaner Prod., 2018, 186, 499–513, DOI:10.1016/j.jclepro.2018.03.076.
- H. Ostovari, L. Muller, J. Skocek and A. Bardow, Environ. Sci. Technol., 2021, 55, 5212–5223, DOI:10.1021/acs.est.0c07599.
- A. M. Bremen, T. Strunge, H. Ostovari, H. Spütz, A. Mhamdi, P. Renforth, M. van der Spek, A. Bardow and A. Mitsos, Ind. Eng. Chem. Res., 2022, 61, 13177–13190, DOI:10.1021/acs.iecr.2c00984.
- P. Fennell, J. Driver, C. Bataille and S. J. Davis, Nature, 2022, 603, 574–577, DOI:10.1038/d41586-022-00758-4.
- D. Keiner, A. Gulagi and C. Breyer, LUT-DEMAND (version 1.1), Zenodo, 2023 DOI:10.5281/zenodo.7189337.
- N. Wijaya, D. Vikara, D. Morgan, T. Grant and D. Remson, in Day 1 Tue, April 26, 2022, SPE, Bakersfield, California, USA, 2022, D011S005R004.
- V. Vishal, Y. Verma, D. Chandra and D. Ashok, Int. J. Greenhouse Gas Control, 2021, 111, 103458, DOI:10.1016/j.ijggc.2021.103458.
- B. P. McGrail, H. T. Schaef, A. M. Ho, Y.-J. Chien, J. J. Dooley and C. L. Davidson, J. Geophys. Res. Solid Earth, 2006, 111, B12201, DOI:10.1029/2005JB004169.
- S. Ó. Snæbjörnsdóttir, F. Wiese, T. Fridriksson, H. Ármansson, G. M. Einarsson and S. R. Gislason, Energy Procedia, 2014, 63, 4585–4600, DOI:10.1016/j.egypro.2014.11.491.
- C. A. Myers, T. Nakagaki and K. Akutsu, Int. J. Greenhouse Gas Control, 2019, 87, 100–111, DOI:10.1016/j.ijggc.2019.05.021.
- Worldometer, Cropland Area by Country, https://www.worldometers.info/food-agriculture/cropland-by-country/, (accessed 1 July 2024).
- V. Vogl, M. Åhman and L. J. Nilsson, J. Cleaner Prod., 2018, 203, 736–745, DOI:10.1016/j.jclepro.2018.08.279.
- W. Wieder, Regridded Harmonized World Soil Database v1.2, 2014 DOI:10.3334/ORNLDAAC/1247.
- J. Young, N. McQueen, C. Charalambous, S. Foteinis, O. Hawrot, M. Ojeda, H. Pilorgé, J. Andresen, P. Psarras, P. Renforth, S. Garcia and M. Van Der Spek, One Earth, 2023, 6, 899–917, DOI:10.1016/j.oneear.2023.06.004.
- Global CCS Institute, GLOBAL STATUS OF CCS 2022, Global CCS Institute, 2022.
- D. M. Franks, J. Keenan and D. Hailu, Nat. Sustainability, 2022, 6, 21–27, DOI:10.1038/s41893-022-00967-9.
- O. Edenhofer, M. Franks, M. Kalkuhl and A. Runge-Metzger, On the Governance of Carbon Dioxide Removal - A Economics Perspective, Munich, 2023.
- N. McQueen, P. Kelemen, G. Dipple, P. Renforth and J. Wilcox, Nat. Commun., 2020, 11, 3299, DOI:10.1038/s41467-020-16510-3.
- N. M. Haegel, P. Verlinden, M. Victoria, P. Altermatt, H. Atwater, T. Barnes, C. Breyer, C. Case, S. De Wolf, C. Deline, M. Dharmrin, B. Dimmler, M. Gloeckler, J. C. Goldschmidt, B. Hallam, S. Haussener, B. Holder, U. Jaeger, A. Jaeger-Waldau, I. Kaizuka, H. Kikusato, B. Kroposki, S. Kurtz, K. Matsubara, S. Nowak, K. Ogimoto, C. Peter, I. M. Peters, S. Philipps, M. Powalla, U. Rau, T. Reindl, M. Roumpani, K. Sakurai, C. Schorn, P. Schossig, R. Schlatmann, R. Sinton, A. Slaoui, B. L. Smith, P. Schneidewind, B. Stanbery, M. Topic, W. Tumas, J. Vasi, M. Vetter, E. Weber, A. W. Weeber, A. Weidlich, D. Weiss and A. W. Bett, Science, 2023, 380, 39–42, DOI:10.1126/science.adf6957.
- L. Warszawski, E. Kriegler, T. M. Lenton, O. Gaffney, D. Jacob, D. Klingenfeld, R. Koide, M. M. Costa, D. Messner, N. Nakicenovic, H. J. Schellnhuber, P. Schlosser, K. Takeuchi, S. Van Der Leeuw, G. Whiteman and J. Rockström, Environ. Res. Lett., 2021, 16, 064037, DOI:10.1088/1748-9326/abfeec.
- M. Honegger, Nat. Commun., 2023, 14, 534, DOI:10.1038/s41467-023-36199-4.
- R. Gholami, A. Raza and S. Iglauer, Earth Sci. Rev., 2021, 223, 103849, DOI:10.1016/j.earscirev.2021.103849.
- M. Molari, K. Guilini, C. Lott, M. Weber, D. De Beer, S. Meyer, A. Ramette, G. Wegener, F. Wenzhöfer, D. Martin, T. Cibic, C. De Vittor, A. Vanreusel and A. Boetius, Sci. Adv., 2018, 4, eaao2040, DOI:10.1126/sciadv.aao2040.
- A. Vinca, J. Emmerling and M. Tavoni, Front. Energy Res., 2018, 6, 40, DOI:10.3389/fenrg.2018.00040.
- T. Ajayi, J. S. Gomes and A. Bera, Pet. Sci., 2019, 16, 1028–1063, DOI:10.1007/s12182-019-0340-8.
- T. Dixon and K. D. Romanak, Int. J. Greenhouse Gas Control, 2015, 41, 29–40, DOI:10.1016/j.ijggc.2015.05.029.
- [BMWi] - Bundesministerium für Wirtschaft und Energie, 2020.
- P. S. Ringrose, A. S. Mathieson, I. W. Wright, F. Selama, O. Hansen, R. Bissell, N. Saoula and J. Midgley, Energy Procedia, 2013, 37, 6226–6236, DOI:10.1016/j.egypro.2013.06.551.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee03166k |
| This journal is © The Royal Society of Chemistry 2024 |