Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Circular battery design: investing in sustainability and profitability
Andreas
Wolf
 ab,
Felix
Nagler
ab,
Felix
Nagler
 a,
Philip
Daubinger
a,
Philip
Daubinger
 a,
Christoph
Neef
a,
Christoph
Neef
 c,
Karl
Mandel
c,
Karl
Mandel
 ab,
Andreas
Flegler
a and
Guinevere A.
Giffin
ab,
Andreas
Flegler
a and
Guinevere A.
Giffin
 *ad
*ad
aFraunhofer Institute for Silicate Research ISC, Neunerplatz 2, 97082, Würzburg, Germany. E-mail: Guinevere.giffin@isc.fraunhofer.de
bDepartment of Chemistry and Pharmacy, Inorganic Chemistry, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstraße 1, D-91058 Erlangen, Germany
cFraunhofer Institute for Systems and Inovation Research ISI, Breslauer Straße 48, 76139 Karlsruhe, Germany
dJulius-Maximilians-University Würzburg (JMU), Chair of Chemical Technology of Materials Synthesis, Röntgenring 11, 97070 Würzburg, Germany
First published on 14th October 2024
Abstract
Sustainability along the battery value chain is a much talked about goal but currently comes third after cost and performance. Historically, improved sustainability comes with a penalty in terms of cost and performance. This interplay will certainly evolve in the coming years. Ecological and social aspects driven by legislative frameworks guarantee recycling of lithium-ion batteries (LIBs) to prevent hazardous waste in landfills. The trend in the electric vehicle (EV) sector towards low-cost chemistries like lithium iron phosphate (LFP) represents a double-edged sword, as the recycling profitability of such materials is extremely low for the established recycling methods. Extending battery lifetime and enabling direct recycling, where anode and cathode materials maintain their structure and functionality, are key strategies to increase sustainability and profitability. However, their implementation necessitates a shift in LIB design priorities. This Perspective highlights design for circularity as an enabler for improved battery longevity and direct recycling and represents a key tipping element for reducing cost and increasing sustainability in LIB production and disposition concurrently. We outline challenges and opportunities in battery production with special focus on the European EV sector and define actions required from various stakeholders along the value chain to overcome the mindset of linear economies.
Broader contextThe rapid growth of lithium-ion batteries (LIBs) has transformed the electric vehicle (EV) industry by offering efficient energy storage for sustainable transportation. As EV demand grows to cut carbon emissions and fossil fuel dependence, LIB production has surged. The use of critical raw materials and energy during LIB production represents limitations with regard to advances in sustainability. A more circular LIB ecosystem would further increase sustainability along the LIB supply chain and prevent environmental hazards. However, LIB production follows the traditional dynamics of linear economies in which production cost and performance during the first life are optimized. Historically, an investment in sustainability is considered to come with a penalty regarding performance and/or costs. Based on increasing end-of-life cost, changing LIB chemistries and geopolitical constraints, this interplay could change in the coming decades, especially for the European LIB sector. In this work, we derive design for circularity as key strategy for extending LIB lifetimes and enabling direct recycling from the R9 framework for circular economies and identify the actions required from research and stakeholders along the value chain to overcome the mindset of linear economies. This creates the opportunity to concurrently reduce overall cost and increase sustainability for LIBs. |
1. Introduction
In 2024, the proliferation of the lithium-ion battery (LIB) within the automotive sector is irrefutable and cannot be impeded. The electrical vehicle (EV) market is growing rapidly with an expected 140 million EVs on the roads worldwide in 2030.1 The amount of decommissioned EV batteries that reach end-of-life (EoL) is increasing correspondingly. By 2040, the annual return rate of EV batteries is projected to amount to 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons.2,3 These spent LIBs represent both hazardous waste and a valuable source of scarce raw materials in concentrated form. Thus, both the necessity and the opportunities for LIB recycling are clear.
000 metric tons.2,3 These spent LIBs represent both hazardous waste and a valuable source of scarce raw materials in concentrated form. Thus, both the necessity and the opportunities for LIB recycling are clear.
A wide variety of different recycling approaches exist. The most common are pyro- and hydrometallurgical recycling, but direct recycling is drawing more attention and is prominent in various European development roadmaps.4,5 Pyrometallurgical recycling is based on a high temperature smelting furnace, where only the most valuable metals are recycled.6 Although this process is notable for its simplicity and technological readiness, drawbacks include high energy consumption and high dependency on the presence of high-value metals like cobalt and nickel regarding economic profitability.7 For hydrometallurgical recycling, battery modules are mostly shredded and sorted into the material fractions, such as plastics, metal substrates/casing and the black-mass, which is primarily a mixture of the anode and cathode active materials. Graphite, as the most common anode material, is valuable, but the material fraction of most interest and highest value is the cathode active material (CAM). Hydrometallurgical treatment is intended to extract the valuable elements from the CAM via leaching and selective extraction.7 While this process needs less energy than pyrometallurgy and shows medium to high technological readiness, it requires large amounts of solvents and other chemicals and downgrades the CAM into its elemental precursors. Direct recycling aims to overcome this downgrading by preserving the CAM structure and thus saving the costs for CAM re-synthesis. However, direct recycling currently implies a significant process complexity and cannot offer a high technological readiness level (TRL > 5) in the short-term future. Some companies and start-ups have decided to take on the challenging journey of direct recycling, but most efforts at the pilot-scale only cover initial shredding or dismantling processes leading to the black mass. The regeneration of CAM to a high-quality state-of-the art active material to fully close the loop has only been shown on the laboratory scale.8 The benefits and merits of pyro- and hydrometallurgical, as well as direct recycling routes have been discussed in detail in a plethora of articles in a more quantitative way.9–14 The overarching consensus is that, on the one hand, it is necessary to add further value to the more established pyro- and hydrometallurgical routes in the short- and midterm future and increase the capacity to handle the growing quantities of discarded LIBs within the next decade. On the other hand, direct recycling holds the greatest theoretical potential for enhancing the profitability of recycling processes and implementing a more sustainable circular battery economy over the long run.9,15,16
The battery chemistry, and more specifically the CAM, is one of the main parameters that determine which recycling pathway is best-suited. The early and current generations of EV batteries mostly contain nickel manganese cobalt oxide (NMC) with varying stoichiometries. The recycling profitability of such batteries mainly stems from the scarcity and high value of cobalt and nickel.17 However, the renaissance of lithium iron phosphate (LFP) as CAM in the EV market is ongoing and with predictions as high as ≈47% market share by 2026.18 The large-scale adoption of LFP is mainly motivated by less expensive raw materials and improved battery safety during operational use.19,20 Although the trend towards LFP means lower criticality of raw materials, it represents a double-edged sword regarding circularity. Without the presence of scarce and high-value elements like nickel and cobalt, LIB recycling becomes less profitable. In particular, pyro- and hydrometallurgical recycling routes, which focus on the recovery of the most valuable elements, are not profitable for LFP-based LIBs.11,13 Hence, the focus currently lies on maximizing the lifetime of LFP-based batteries in first and 2nd-life applications. While this might delay their EoL, it is inevitable that in the long-term future a significant portion of discarded LIBs contain a high share of elements that are considered low-cost in this context.
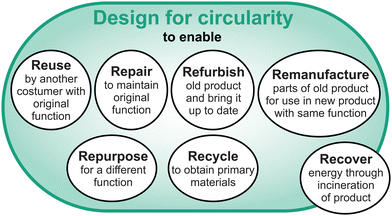
As landfills for batteries can be considered an environmental disaster and need to be avoided, it is crucial to make recycling of LFP and other future low-cost battery systems as profitable as possible. Furthermore, a more holistic perspective that considers geopolitical tensions, global market volatilities and logistical issues regarding global shipping routes, relativizes the term “low-cost”, especially for the European Union (EU).21 Aside from the lack of resources for CAM raw materials in the EU, the energy intensive production/synthesis of CAMs is also centered in China.22 As the EU will not be able to compete on this front in the near future, the CAMs imported into the EU in form of batteries need to be seen as a valuable raw material and ideally should be conserved in their high value state as CAM. Consequently, it is crucial for the EU to incentivize direct recycling of LIBs to create circular value chains. This holds true in a similar manner for the United States (USA) due to a lack of primary resources. The benefits of circular-designed batteries and direct recycling also exist for China. However, the urgency to move towards such LIB design priorities is less pronounced due to the easier access to primary resources, as well as lower energy and labor costs (the latter ones are increasing but have not yet reached the level of the EU). In contrast, the technological solutions for individual processes along the direct recycling pathway for EoL LIBs currently lack technological readiness to be upscaled from lab to pilot in the near future due to processing complexity and the growing variety of battery types on the market. While advances in sorting, dismantling and regeneration of CAMs are helpful, design for recycling must be considered as key enabler to make direct recycling profitable. The potential of direct recycling can only be harvested with a holistic approach intended to design a battery as circular product, which is not only optimized for its operational life, but considers disassembly and remanufacturing, ideally aiming for a zero-waste value chain. However, it remains challenging to predict the point in time when techno-economic feasibility of direct recycling will be achieved for EoL batteries. In the short- and mid-term future, direct recycling will be limited to production scrap.23,24 A focus on increasing the longevity of LIBs will be just as important as recycling to increase circularity. In this perspective, we extend the concept of design for recycling to the idea of design for circularity. Unlike design for recycling, design for circularity also encompasses strategies like reuse, repair, refurbish and remanufacture, as well as efficient repurposing for 2nd-life applications (Fig. 1).
The near-term implementation of such circular design features may necessitate standardization and performance or cost trade-offs. The acceptance of battery manufacturers and OEMs to compromise on performance and/or cost is still extremely low in consideration of benefits that can only be harvested in the long-term future. Understandably, this originates from the inherent competitiveness of the LIB market, influenced by the conventional mindset of linear economies, partially low consumer awareness and the necessity to appease corporate stakeholders within short timeframes, i.e., years rather than decades. However, as long as LIB recycling stays an EoL topic, which receives attention whenever the present battery generation is already on the market, the term circular economy will not outgrow the stage of a “green-washing buzzword” in the battery sector. Hence, the research community and policy makers need to provide technological advances and legislative frameworks that incentivize the industry sufficiently to make long-term investments into circular battery designs.
With this work, we intend to illuminate a route to overcome the obsolete framing of linear economies that views LIB recycling only as EoL concern. Based on the R9 framework for circular economies,25 we elaborate the importance of design for circularity as the key enabler for profitable LIB recycling to eventually maximize sustainability and concurrently reduce costs. We discuss how the relationship between design to performance and cost versus design for sustainability, which is currently a trade-off, will evolve over time by differentiating between a scenario that solely focuses on performance and cost and the alternative approach involving early investment in design for circularity. As the commitment to circular battery design can and should be a profitable long-term investment, we detail the necessary contributions for each research phase to implement design for circularity. Finally, we identify the industrial stakeholders that need to be incentivized to enhance the technological readiness of direct LIB recycling, with a particular emphasis on the European EV battery sector. While the general effect of more circular battery designs on the future battery ecosystem described in this perspective holds true for the global battery ecosystem, it is particularly pronounced for the European ecosystem due to the low availability of primary resources and high energy costs.
2. Design for circularity as key enabler for circular battery economies
The definition of sustainability is most commonly based on social, environmental and economic pillars.26 Herein, we mainly consider the environmental dimension of sustainability, which can partially be quantified in form of the global warming potential (GWP in CO2-equivalents).27–29 However, further aspects of the battery ecosystem like resource availability, private mobility and public transport are irrefutably also connected to social and economic facets of sustainability. Throughout the last decades, the implementation of circular economies has been declared as one of the main instruments to create more sustainable societies and to reduce the pressure on earth's ecosphere with its limited resources.30,31 However, the definition of circular economy is under constant debate and while there is a correlation between sustainability and circularity, there is no causality.32 Circularity does not automatically imply sustainability and instead, can come at the cost of sustainability in specific cases. This is particularly true when circularity is understood as the circularity rate of material streams (measured in percent). More recent definitions of a circular economy provide a broader perspective on resource efficiency and include narrowing (using less material and energy), slowing (using products and components longer) and closing (post-use circulation of materials) strategies.33 The circularity rate is a relevant metric to evaluate closing. However, an overall stronger correlation of circularity and sustainability exists only when narrowing and slowing aspects like reduction of critical raw materials and energy consumption, as well as longevity of products, are considered.In contrast to fossil fuels, LIBs are energy storage devices that are theoretically suited to be circular products. From an ecological perspective, it is evident that transforming the LIB market from a linear to a (more) circular economy would help to avoid environmental damage caused by hazardous waste. However, the global LIB market follows the traditional dynamics of linear economies, where manufacturing cost and performance during operational life are optimized with very little room to compromise for other aspects and where recycling remains predominately an EoL concern.
The R9 framework for circular economies provides ten (R0–R9) strategies to increase the circularity of a product (in this case, batteries).25 In Fig. 2, these R-strategies are aligned with the course of a battery lifetime. This lifetime is considered to be 10–15 years in the EV sector, although there will be exceptions in both directions. The graph in Fig. 2 displays the potential of the individual R-strategies to increase the circularity of LIBs (left y-axis) in relation to the accumulated ecological footprint (right y-axis) over the course of a battery lifetime. The strategies R0 (Refuse), R1 (Rethink) and R2 (Reduce) correspond to the previously mentioned narrowing (using less material and energy). The strategies R3 (Reuse), R4 (Repair), R5 (Refurbish), R6 (Remanufacture) and R7 (Repurpose) can be attributed to slowing (using products and components longer) and R8 (Recycle) and R9 (Recover) are part of closing (post-use circulation of materials). The previously-mentioned recycling routes (pyro-, hydro- and direct recycling) can correspond to more than just R8 (Recycle) within the R9 framework. For instance, pyrometallurgical is a combination of R8 and R9, as the metals are recycled, but all organic components are incinerated and only recovered in from of energy. In contrast, the recovery of the CAM within direct recycling can rather be considered as R5 (Refurbish). Additional actions during operational use, aligning with R3–R6 to extend and enhance battery life, may involve battery management systems (BMS) that detect cells with degraded health, enabling their individual replacement within modules, or sensing combined with advanced self-healing mechanisms within the cells, as proposed in the Battery 2030+ Roadmap.15 While R8 (Recycle) and R9 (Recover) are required to close the loop (closing), i.e. to increase circularity rates and to avoid hazardous waste in landfills and ecological disasters, they are considered to have less impact on circularity within the R9 framework than R-strategies implemented during operational life or manufacturing.
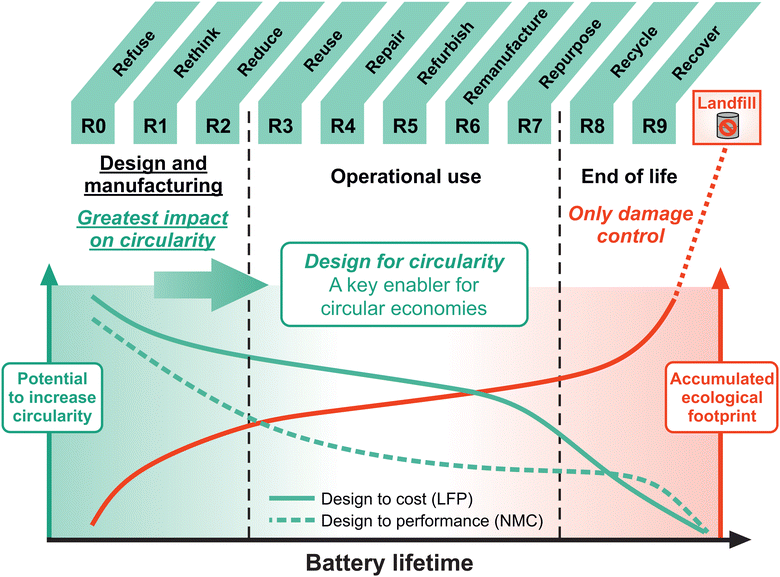 | ||
| Fig. 2 The R9 Framework25 applied for circular battery economies. The R9 framework includes ten (R0–R9) strategies to increase the circularity of a product (in this case, batteries). The design and manufacturing phase of a product (R0–R2) possesses the greatest potential to increase circularity (left y-axis), whereas R8 (Recycle) and R9 (Recover) at the EoL can only be considered as damage control. Applying the R9 framework to LIBs shows that the potential to increase the overall circularity does not scale proportionally with the accumulated ecological footprint (right y-axis). In fact, design for circularity at the beginning of a battery lifetime is the key enabler for the implementation of all further R-strategies and thus, makes the greatest impact on increased circularity. For design to cost materials, increasing longevity is even more crucial to improve circularity than for design to performance materials. LFP and NMC are chosen as representative materials for these battery chemistries based on today's state of the art. | ||
It is the design and manufacturing phase of a battery (R0–R2), which determines how easily further R-strategies (R3–R9) can be implemented during operational use or at EoL. The design and manufacturing phase include R0 (Refuse: make product redundant by abandoning its function), R1 (Rethink: make product use more effective and suited for multiple lifecycles) and R2 (Reduce: increase efficiency in product manufacturing or use by consuming fewer natural resources). Design for circularity mainly stems from R1 to rethink the operational use and to consider recycling from the start. Furthermore, design for circularity is the key enabler to prolong 1st- and 2nd-life and to harvest the potential of direct recycling. For example, in contrast to state-of-the-art shredding of LIBs at their EoL, controlled disassembly offers greater efficiency for subsequent material processing. However, due to the absence of design considerations for LIB disassembly, the process remains unnecessarily difficult and labor intensive.34,35
In Fig. 2, the potential to increase circularity of the individual R-strategies is specified for two different categories of battery materials, namely design to cost materials such as LFP and design to performance such as NMC. For the sake of clarity, LFP and NMC have been chosen as representative materials for these categories based on today's state of the art. However, there are many other materials, including the various stoichiometries of NMC, which could be included within these categories. R-strategies to extend the operational use during 1st- and 2nd-life applications have an even larger potential to increase circularity for design to cost materials compared to design to performance materials. Longevity becomes more important as the techno-economic hurdles for recycling become higher. Design for circularity is crucial to enable extended battery lifetimes and efficient recycling for all battery chemistries. However, the benefits of design for circularity are more pronounced for design to cost battery chemistries.
3. The changing interplay of performance, cost and sustainability regarding battery design over time
To promote circular economies in the long term necessitates consideration of circular aspects not only in research, politics and industry, but also in secondary level education.36 However, for short- and mid-term transformation, policy makers need to incentivize the industry with legislative frameworks that create financial benefits for more circular battery designs. In China, more than ten nationwide and local regulations and policies concerning various aspects on LIB recycling have been enacted since 1995.12,16,37 In the United States (USA), the LIB recycling is legislatively controlled primarily on the state level and in 2022 only four states had regulations on LIB collecting and recycling.12 Recently, the European Parliament signed the amending battery regulation,38 repealing previous regulations and the Directive 2006/66/EC on (waste) batteries and accumulators.39 The new regulation sets ambitious minimum levels of materials recovered from waste batteries by 2027 (lithium 50%, cobalt 90%, nickel 90%, and lead 90%) and 2031 (lithium 80%, cobalt 95%, nickel 95%, and lead 95%). Additionally, minimum recycled content levels in new batteries are specified, with targets to be met by 2031 (lithium 6%, cobalt 16%, nickel 6%, and lead 85%) and 2036 (lithium 12%, cobalt 26%, nickel 15%, and lead 85%). The regulation also includes a possible reevaluation of these values in 2028. One of the main concepts within this regulation is the extended producer responsibility (EPR), which makes the battery producers responsible for the entire lifecycle, including eventual disposition and recycling. In combination with the Directive 2009/125/EC for Eco-design requirements for energy-related products,40 the EPR should incentivize more circular battery designs. The EPR also includes the logistical effort of collecting spent LIBs at EoL, the physical recycling process, reporting and compliance issues, as well as the financial accountability for these domains. Consequently, the facet of linear economies, where costs are only generated during product manufacturing and distribution, is obsolete in the context of more complex and long-term generation of costs in circular product streams. This emphasis on circularity leads to a different calculation for LIB cost as EoL aspects must be included.The LIB landscape in 2024 still follows the dynamics of a linear economy and the total product cost includes the raw materials, active material synthesis, cell manufacturing, system integration and safety features. In general, the goal is to minimize cost and maximize performance concurrently. The search for the best compromise between these two aspects is causing the large-scale adoption of LFP and its preference over NMC for much of the new LIB manufacturing capacity that is currently being build or in planning. Although LFP possesses a lower theoretical energy density on the cell level, leaner system designs due to higher inherent safety of LFP, as well as lower raw material costs overcome this drawback from the industrial perspective.20 Generally, LFP is considered to be a design to cost material and NMC is typically design to performance.4,41 Given the wide range of NMC compositions (especially if other dopant elements are considered), this material can be tailored for safety, rate capability, power or energy density, where the design priorities depend on the application. This perspective aims to outline an overarching trend for EV batteries, which is why for simplicity reasons these aspects are combined within the term design to performance.
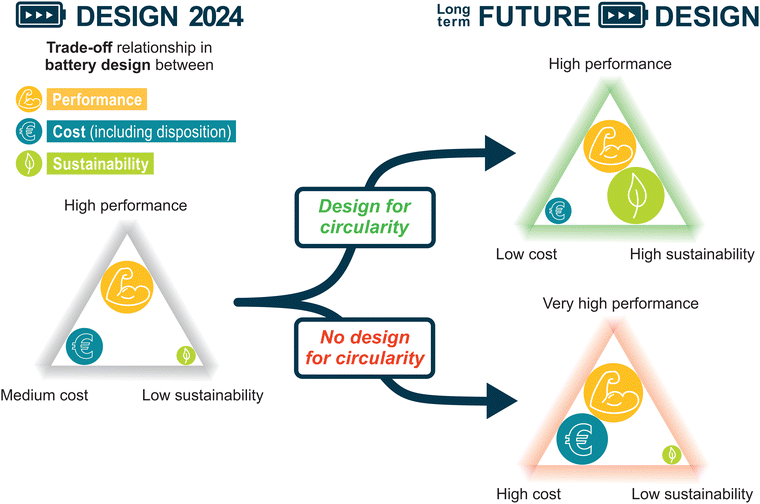
Herein, we introduce sustainability as a pivotal third dimension, alongside cost and performance, and assess how current and future LIB designs influence these three aspects. By focusing on the EV market, which accounts for a substantial and rapidly growing number of LIBs with similar application demands, we can come to a more precise conclusion for both the industry and the consumers.42 In Fig. 3, the trade-off relationship between performance, cost (manufacturing and disposition) and sustainability is schematically displayed. The current LIB design is considered to achieve high performance at medium cost, while sustainability is neglected.
In the short-term future, the historical trend of improving performance and simultaneously decreasing cost is likely to continue.43 Pyro- and hydrometallurgical recycling facilities will be a profitable business with suited capacities for the European market until 2030 and have the potential for further capacity increases in the following years.44 However, this profitability depends largely on factors such as the chemical composition of the cathode materials17 and the price of raw materials. Due to the increasing volumes of EoL batteries and, in particular, production waste, several analysts have started to set up price indices for recycling materials (black mass) and EoL LIB.45–48 These indices are intended to improve the tradability of recycling materials and allow an analysis of costs and earnings potential. The price indicators take into account the battery chemistry (e.g. NMC type) as well as the quality (e.g. black mass of anode and cathode or cathode only) and are based on achievable spot market prices for Ni, Co and partly also for Li. The price indicators may be subject to regional fluctuations, e.g. differences between the European and Chinese markets are to be expected.
For June 2024, the analyst SMM48 calls for a price of around 5.5 USD per kg for LCO and just over 1 USD per kg for LFP EoL batteries in the Chinese market. Depending on their composition, used NCM batteries are around 4 USD per kg. Even higher prices can be achieved for processed black mass, as the proportion of Co, Ni and Li is higher. The indicators for black mass suggest achievable prices of around 70% of the Co, Ni and Li spot market prices, measured by the proportion of elements in the black mass (as of June 2023, Chinese market). The price indicator for LFP black mass is therefore only slightly more than a quarter of the indicator for NCM. Converted according to current Li prices,49 this Li-based value for LFP cathode black mass is around 1.7 USD per kg.
This contrasts with recycling costs, which in the case of specialized facilities are also likely to fluctuate slightly with chemistry, but are generally quite similar for all battery types and could be in the range below or around 2 USD per kg with current processes.50,51 The potential profit margin for recycling EoL LFP batteries is therefore currently extremely narrow or non-existent. In Europe, recycling LFP batteries could even cost money. It should be noted that this is an effect of the currently extremely favorable Li–carbonate and hydroxide prices. Assuming average spot market prices for Li from 2022 to mid-2023, the price for LFP cathode black mass calculated according to the same scheme rises to 5–10 USD per kg. Whether these price levels for lithium will ever be reached again is completely unclear. Hence, long-term future cost/revenue predictions are extremely challenging particularly for low cost chemistries, but also due to changing supply and demand of raw materials, geopolitical volatilities and the possibility of technological breakthroughs (e.g. solid-state batteries).52 Nonetheless, it is likely that the interplay of performance, cost and sustainability will evolve over time.
Direct recycling will not be technologically feasible without design for circularity. Without design for circularity, pyro- and hydrometallurgical processes, which may continue to lack of profitability for LFP, will have to be used for recycling and recovery.17,53 Considering the aforementioned EPR, the expense of waste management will increase the overall LIB cost17 (No design for circularity scenario in Fig. 3). On top of the increasing cost due to inefficient recycling, the geopolitical dependencies are exacerbated in this scenario. The EU can hardly compete with China regarding the energy intensive CAM synthesis.17,54 Hence, keeping cathode materials in their high value form as CAM within the EU, could decrease geopolitical dependencies and increase the robustness of the European LIB ecosystem against rising raw material and energy cost, as well as volatilities of global supply chains.22,55,56 However, the direct recycling approach, which is required to avoid downscaling of CAMs into elemental precursors, can only be established on a large scale within the EU through a holistic design for circularity approach, in which all of the aforementioned R-strategies are addressed (design for circularity scenario in Fig. 3).
With design for circularity as the key tipping element, the relationship between sustainability and cost might be reversed in the long run, assuming growing consumer awareness and a continuous legislative effort to promote a circular battery economy. Whereas historically, neglecting sustainability issues made it easier to maximize performance and minimize cost, circular battery designs might be able to reduce cost (including both the cost of manufacturing and disposition) and increase sustainability at the same time with a minimal compromise regarding performance (design for circularity scenario in Fig. 3). Although a more circular battery design does not necessarily amount to a slightly lower performance, we assume a minor compromise is the more realistic scenario in the short- and mid-term future, especially considering the current trend of cell-to-pack designs with irreversible bonding of individual cells. Through a dedicated effort to design LIBs as circular product that supports direct recycling, the financial break-even point can be surpassed also for low-cost chemistries like LFP and recycling turned into a profitable endeavor.17,53
In traditional recycling business models, the revenue side from achievable prices for recyclates, e.g. for battery-grade Co- and Ni-sulphate or Li-carbonate precursors, is countered by the costs of the recycling materials (EoL batteries or scrap) and the costs of the recycling process. The achievable prices for battery precursors today are essentially determined by the extraction costs and demand for primary raw materials and can be regarded as fixed from a recycler's perspective. The situation is different for business models in direct recycling. The revenue opportunities are not given by precursor prices but by achievable prices for (recycled) active materials.
In a TCO comparison (total cost of acquisition and operation), as is suggested by the new EU battery regulation and its recycling obligations, the costs of direct recycling plus possible additional costs for an enabling design for recycling therefore compete with the costs of traditional recycling plus the costs of precursor CAM (pCAM) and CAM production. CAM are 20–40% more expensive than their battery grade precursors.57–60 This not only leaves a large scope for the costs and design of direct recycling processes (currently around 5 to 7 USD per kg for the pCAM and CAM process costs (NMC) and 1.5 to 2 USD per kg (LFP) without raw materials in battery quality57,59 or more than 10 USD per kWh translated to the cell level), but also holds the potential to reduce the TCO of LIB compared to the current recycling of materials down to the metal level. Furthermore, a holistic design for circularity approach not only enables direct recycling of LFP as CAM, but also the remanufacturing of other LIB components such as anode active materials like graphite, carbon black, plastics and the electrolyte, which is necessary to move towards net zero-waste economies and which would also further improve the revenue side of direct recycling.
Focusing on the EU LIB ecosystem, another positive impact within the design for circularity scenario is the decreased volatility towards increasing raw material prices, as higher shares of CAMs will come from recycling streams ideally within the EU. Although the large-scale adoption of LFP into the LIB manufacturing sector lowers the dependencies on cobalt and nickel, the market for lithium and copper is also far from being stable and short-term imbalances in supply and demand can lead to additional cost.54,61
Implementing new technologies and enhancing manufacturing efficiency will certainly be the biggest lever to decrease the carbon-footprint of LIBs in the short- and mid-term future.62–64 Addressing circular design aspects likely comes in form of a compromise regarding manufacturing efficiency. However, focusing on the long term, a minor compromise during manufacturing might enable a more circular battery economy, create higher recycling profitability of “low-cost” chemistries, decrease overall cost considering the EPR, strengthen the European LIB economy and prevent ecological disasters in form of landfills for batteries. However, as long-term predictions for the highly volatile battery market are difficult, the assessment of the scenarios illustrated in Fig. 3 is mainly meant create awareness for the potential of circular battery designs to increase sustainability and lower costs concurrently, a relationship currently neglected by most stakeholders along the battery value chain. The challenge is to define the contributions required from various research areas and global stakeholders to implement circular battery designs with the optimal compromise between additional manufacturing efforts and positive future impacts.
4. The steps towards circular-designed batteries: contributions required from research and stakeholders along the value chain
The main challenge is not to make a circular battery, but to keep the penalty in terms of performance and manufacturing cost as small as possible while doing so. It is primarily the task of researchers to develop the technology required for this goal. Industry then needs to implement this technology and – equally important – needs to apply corresponding marketing strategies to create consumer awareness about circularity and the acceptance for small compromises regarding performance. This consumer awareness towards the socio-ecological benefits of circular batteries can only be established via shifts in educational and cultural priorities. These shifts are expected to take several decades and will likely not happen concurrently on the globe. Herein, the discussion is restricted to the technological, economic and political aspects that are required to create a business model for circular batteries.The design features of an EV LIB that would allow the aforementioned R-strategies to be addressed are outlined in the following section starting at the LIB system/pack (meter-scale) and leading down to active material particles (micro- to nano-scale). Before starting the recycling process, a LIB system should be designed to maximize LIB lifetime and efficiency in 1st- and 2nd-life. A competition between repurposing for 2nd-life application and recycling may arise at EoL of the 1st-life. In general, repurposing for 2nd-life applications is likely less economically favorable as long as recycling is highly profitable and vice versa. As long as techno-economical hurdles for direct recycling are too high, maximizing the lifetime of design to cost battery types remains the more feasible and relevant strategy. Thus, in the short- and mid-term future, design for reuse, repair, refurbish, remanufacture and repurpose are favored and design for direct recycling of batteries at EoL will become more relevant in the long-term future.
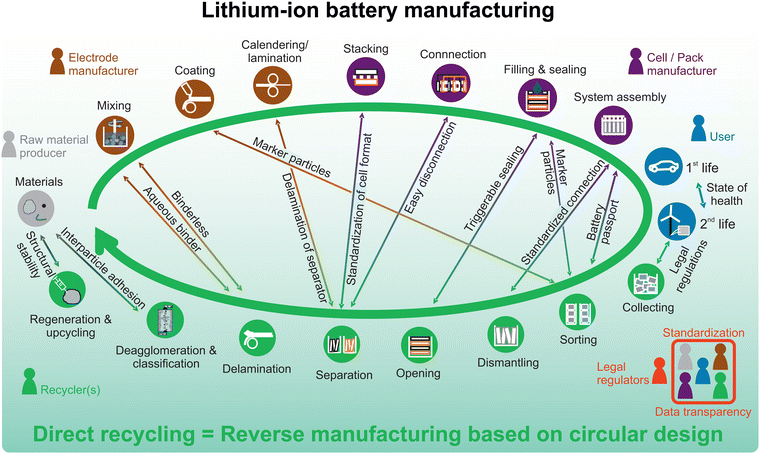
Continuous improvements in sensors, methods for detection, and battery management systems (BMS) are crucial to provide data about the state of health (SOH) of individual cells. Based on this data, smarter decisions can be made, e.g., whether individual cells should be replaced to prolong the 1st life application or if the time for 2nd life has come and which 2nd life application would be best-suited. For these decisions the LIB data, including information about manufacturing and lifetime, needs to be stored and easily accessible for all stakeholders along the value chain. Furthermore, a battery passport is necessary to ensure identification and traceability of LIBs packs and to tackle the logistical challenge of transport to the best-suited 2nd life or recycling facility. Once a LIB reaches a recycler, the information about exact cell chemistry and state of health is again crucial. An ideal recycling process would reverse the production process, ensuring that all components are received in high purity and in a similar physical state to their beginning of life (Fig. 4). For this, the battery pack design should be as modular as possible, which would allow for individual repair, refurbish, remanufacturing or recycling of different components. To do so, design for circularity must be integrated at all hierarchy levels, from the battery pack to the active materials, requiring actions from all stakeholders along the value chain. LIBs that are not specifically designed for circularity, such as those with glued individual cells that make them difficult to open, require a relatively straightforward recycling method involving shredding, followed by an energy-intensive pyro- or hydrometallurgical process.
Fig. 4 illustrates the interaction and dependency between manufacturing and direct recycling processes throughout the entire LIB value chain, assuming an established circular LIB design and direct recycling process. To begin the recycling process, the most important step is to collect the battery at EoL after 1st or 2nd life from the user. Legal regulations or financial rewards for the user might be required to ensure this handover. According to Eurostat, the collection rate for portable batteries in the EU in 2021 was 48%.65 Based on the calculation methodology and market growth in recent years, the real collection rate is likely to have been significantly higher. There are no figures available for EV batteries yet and the collection rate in Europe is likely to remain difficult to determine in the future as a high proportion of used cars are traditionally exported from Europe to other regions. However, some studies assume high collection rates for batteries that reach their end-of-life in Europe.66 In the much more developed Chinese market, initial estimates are already possible: the volume of recycled EoL power batteries in China in 2022 is estimated to have been in the range of 100 kilotons,67 which is about half the amount of traction LIBs placed on the Chinese market in 2015 (the year in which sales of LIBs in China skyrocketed).68 In the years before 2015, the LIB market in China was almost ten times smaller. Even assuming a very short lifespan of 7 years (2015 to 2022), a large proportion of the LIBs placed on the market at that time would appear to be ending up in recycling today. Reliable data on battery collection rates is also lacking for the USA. The same applies at the global level, highlighting the need to improve traceability in order to better monitor and to achieve higher collection rates.
After collecting the batteries at EoL, the cells/packs have to be sorted according to their cell chemistry and, ideally, also their state of health (SOH) to enable a tailored regeneration step at the end of recycling. A battery passport should make this information easily accessible to the recycler.69 In addition to conventional labeling methods like bar-codes and RFID chips, marker materials could be integrated into the cell to avoid counterfeiting. For example, magnetic micrometer-sized marker particles might be suited to carry such information and could be implemented on multiple material hierarchy levels within the LIB (e.g. in the seal or even within the electrodes).70 Sophisticated identification technologies in combination with LIB data ontologies could enable the pairing of LIB packs or even individual cells with their digital twins, thereby gaining access to essential information, such as the cell chemistry, specification (and origin) of raw materials used, state of health and state of charge, information about stakeholders involved in the manufacturing and possibly further aspects, required for efficient recycling.
After sorting the battery packs or modules according to their chemistry, design for disassembly becomes crucial to enable automated separation of packs and modules into cells.71 Otherwise manual disassembly alone, especially in regions with high labor costs like Europe, would consume most of the potential revenue from recycling, making recycling economically unviable.6,34–36 Achieving this level of automation might require standardization with regard to the type and placement of battery pack and module connections.37,38 Additionally, cell-to-pack designs should be implemented not only for the benefit in energy density, but should also to address design for disassembly features to simplify the recycling process by eliminating the need for manual disassembly.
At the cell level, design for separation and sorting features should be integrated to obtain high purity material streams, i.e. consisting of difference cell components. The separation of the anode and cathode electrodes is particularly important. To do so, an externally-triggerable cell opening mechanism might be needed, which could be activated by magnetic or electric fields, temperature changes, or pH variations.72 However, these features must not compromise safety during the 1st and 2nd life. Subsequently, the stacks or jelly rolls need to be disassembled into anodes, cathodes, and separators. Automizing this separation requires a standardization of cell formats, since the current variety of cell formats necessitates manual disassembly.73
Direct recycling of LIBs shifts the sole focus for recovery from the metal containing components, i.e., the CAM and the current collectors, to include the other valuable materials to reduce waste and prevent dissipation. These include the anode (currently, primarily graphite, but increased silicon contents are expected in the future), the electrolyte, conductive carbon and further metal and plastic components can be addressed. The number of sophisticated approaches to tackle these material streams is growing rapidly. For further details in the current state of the art, the reader is referred to the literature concerning the direct recycling of graphite,74,75 separators,76,77 electrolyte78–80 or lithium recovery from the process water.81–83 The unifying premise for these technologies is that they can only be realized through circular LIB designs that enable the recycling process steps displayed in Fig. 4.
The electrodes consist of several components (i.e., CAM, conductive additive and binder) that, during manufacturing, are mixed together into an electrode composite and coated on a current collector. To facilitate separation, a design that allows for easy delamination of the electrode composite from the current collector is essential. This can be achieved through the dissolution or thermal decomposition of the binder responsible for adhesion to the current collector and the connection of different materials in the electrode composite. The use of water-soluble binders for dissolution would be advantageous allowing an aqueous delamination process.84 Consequently, the initial electrode processing should also be water-based. For water sensitive CAMs like Ni-rich layered oxides, additional protection with surface coating or suitable process conditions might be necessary.85,86 Alternatively, binder-less electrodes can be considered for easier separation.87 Dismantling, opening and delamination processes have to be fine-tuned in a way such that contamination of electrode materials with foreign metal debris from casing or current collectors is avoided. Electrode manufacturers cannot allow risks in terms of performance and safety caused by remaining metal contaminants from recycling. Thus, the quality requirements for electrode materials are high. Direct recycling can only become a scalable and competitive alternative to the established metallurgical routes, if the amount of microscopic contaminants is minimized and the detection of such contaminants is established on the scale necessary to certify the purity of recycled electrode materials. However, for state-of-the-art cell designs and using shredding as the dismantling method of choice, both of which leads to a high share of contamination, this will likely not be feasible. Only LIB cells that are designed for the purpose of being opened and delaminated in a way which prevents microscopic metals debris can lead to high purity material streams.
Following the delamination process, the separation of materials within the electrode composite becomes imperative. Generally, the cohesion between particles of the same material kind should exceed the adhesion between components made of different materials to facilitate separation and to obtain material fractions with high purities.88 This would enable the straightforward separation of distinct materials, potentially through methods like centrifugal fractionation,89 froth flotation53,90,91 and/or in combination with heavy liquids.92 Such separation techniques might also be capable of removing the aforementioned foreign metal debris. However, as this further increases the number of required process steps, it is considered favorable to avoid contamination in the first place.
In the final stages of the recycling process, particular attention must be paid to regenerating the CAM. This implies repairing degradation which occurred during cycling or during the recycling process, including, but not limited to the loss of lithium inventory. An enhanced structural stability of the CAM would be beneficial for regeneration, guaranteeing a good lithium diffusivity into the material. Several promising strategies, such as the application of surface coatings,85,86 the utilization of single crystal93–95 or textured particles,94,96 and the implementation of gradient composition,88,97 have emerged as effective means of preserving the structural integrity of active materials and thus can facilitate the regeneration process.
For the successful regeneration of CAMs and subsequent reuse in electrodes, not only is elemental purity and the absence of foreign debris crucial, but also control of the material properties such as particle size/morphology, surface area/chemistry and tap density. There is a large variety of CAMs, which are nominally the same, e.g. LFP, that differ in terms of performance characteristics as a result of the material properties. Since electrode manufacturers rely on a lengthy qualification process of CAMs to guarantee and maintain product quality, regenerated CAMs from direct recycling will also need to have a narrow range of such material properties. Ideally, only the CAM from one supplier with the same characteristics would be processed in a single direct recycling batch. Technologically, this can be realized via advanced battery passports and data transparency, i.e., that the recycler has access to all the necessary information about the battery properties including the data related to the CAM. However, this certainly adds logistical complexity regarding the material input stream of recycling plants and limits continuity in some direct recycling processes, as batch purity needs to be guaranteed and certified.
A further economical challenge for direct recycling which possibly reduces overall profitability is the relevance of active materials at EoL. CAMs (or anode materials) that were manufactured 15–20 years earlier are not likely compatible with state-of-the-art materials. Hence, in addition or in parallel to regeneration, upcycling of active materials that were manufactured e.g. 20 years ago is required. A plethora of articles report upcycling routes for various CAMs,98 including changing elemental compositions (e.g. from NMC111 to NMC62299,100 or from LFP to LMFP101,102). Although technologically feasible in most scenarios, upcycling adds another intricate process step, which potentially reduces overall profitability of direct recycling. This specific challenge can only partially be addressed via design for circularity, as there is a high uncertainty about the precise nature and material properties of future active materials. Strategies to enable and/or improve regeneration (e.g. surface coatings, textured particles, gradient compositions, etc.) might also be beneficial for upcycling, which is why design for circularity is not irrelevant for addressing the techno-economic challenge of upcycling.
Direct recycling will likely not be able to refurbish 100% of the LIB materials and some material fractions will be downcycled due to purity standards meaning remanufacturing or repurposing for different applications. Downcycling or recycling is expected to be most relevant for the electrolyte. According to SMM103 current electrolyte prices for LFP or NMC batteries are between 2 and 5 USD per kg, whereas the solvents (e.g. EC, DMC) are traded for less than 1 USD per kg.104,105 The conductive salts, additional additives and the complex purification procedures are what makes electrolytes expensive. The current commodity value of lithium contained in 1 kg of electrolyte is about 0.3 USD (LP30 electrolyte). This is the value that theoretically could be achieved through advanced pyrometallurgical or hydrometallurgical recycling. Direct recycling methods could certainly maximize the recycled materials, e.g. by regaining the solvents of the electrolyte. However, it is currently not known how high the residual value of the solvent mixture would be. The removal of trace metals from that solvent mixture would be required to reformulate a new electrolyte from them. All in all, the technological challenges for direct recycling of the electrolyte seem unbalanced to the low revenue margin, which is why downcycling of some of the electrolyte components is more likely to be achieved in an economic manner.
It is crucial to define standards and purity requirements for all material streams and components, so that optimal decisions can be made, whether a material fraction should be refurbished, remanufactured, repurposed or recycled. In this context, the recycling process, and thus the material output, would depend on the condition of the cell at EoL. The definition and compliance to standards requires close cooperation between all stakeholders along the value chain as well as legal regulators. Standardization also includes the availability of all of the relevant data (i.e., data transparency) from manufacturing, lifetime (BMS) and recycling, organized with a consistent semantic information structure and accessible via the battery passport.106,107
At EoL different recycling routes will be in competition with each other in terms of the most profitable and sustainable way to deal with decommissioned batteries. As such, we expect that several different recycling routes will coexist in the future. Furthermore, a competition between recycling and repurposing (2nd life applications) may also arise. The data available from the BMS and the battery passport including information about cell chemistry, state of health, module and pack design as well as logistical and regulatory aspects like point of collection and geographical distance to recycling facilities will influence the decision about which recycling route or 2nd life is best-suited. Design for circularity will not lead to a one-size-fits-all scenario, regarding the best possible way how to deal with batteries at EoL. Instead, design for circularity creates the possibility to implement R-strategies providing multiple options at EoL, leading to overall optimized material streams regarding sustainability and profitability.
5. The impact of future “low-cost” chemistry battery types on circularity and direct recycling
The large-scale market entry of sodium-ion batteries (SIBs) in the EV sector is foreseeable.108 Thus, the trend towards design to cost battery materials in the EV sector will not stop with LFP, but will gain further momentum. A substantial market share of SIBs, which do not contain cobalt, lithium and copper, will likely have an even more drastic impact on the recycling profitability by the traditional recycling processes.109–111 Most roadmaps for future battery materials also predict breakthroughs for metal-ion, metal–sulfur and metal–air batteries, whereby magnesium, calcium, zinc or aluminum are metal candidates of interest with high abundancy.108 Such batteries will not penetrate the EV market in the short and midterm future. Nonetheless, the development of such battery chemistries leads to the following question: Can design to cost batteries become so cheap in the future that even direct recycling will be unprofitable and design for circularity becomes less relevant to achieve increased sustainability and profitability?First, the impact of extending the cycle life of such “extreme low-cost” batteries becomes even greater and thus, implementing R3–R7 design for circularity strategies during the use phase are more relevant. Second, batteries based on abundant materials will not be considered as a valuable raw material source and thus will pose challenges for waste management. In this scenario, direct recycling provides a solution to a waste problem by collecting, sorting, dismantling, opening and separating the materials in these battery cells. Furthermore, this scenario assumes that these batteries consist only of highly abundant materials, which will not be achieved within the next decades. Although the share of abundant raw materials will likely increase, batteries will remain complex systems in the mid-term future, i.e., will be composed of mixtures of functional materials based on valuable and/or abundant raw materials. While the direct recycling of all material fractions might not be profitable in and of itself, it has the potential to reduce EoL costs by purifying waste and recycling streams. In consideration of legislative regulations like the EPR, which contributes to the importance of EoL costs, direct recycling might still be able to increase overall profit and certainly affects the ecological impact of waste streams. However, this potential depends on the future weight of ecological aspects in comparison to techno-economic viability. As a result, a quantitative prediction is not possible at this time.
6. Conclusions
The next decades will show whether the battery sector is capable and willing to overcome some of the traditional market facets of linear economies, in which generation of waste is tolerated, since sustainability places third, far behind cost and performance. While the increased market share of low-cost chemistries like LFP poses challenges for the recycling industry due to low profitability, it also creates the opportunity to focus on circularity in the battery market. Profitability of low-cost chemistry batteries, considering not only manufacturing cost and profit from the sale, but also cost and profit from disposition or recycling, can only be achieved via the extension of battery lifetimes and/or direct recycling, requiring a holistic design for circularity approach. As such battery designs might require a compromise in terms of performance and/or cost during manufacturing, legislative frameworks need to further incentivize circular battery designs, since industry, which traditionally follows linear economy dynamics, is likely not willing to make this compromise for the sake of sustainability. Understandably, this compromise cannot jeopardize competitiveness. If circular battery designs lead to significant penalties in performance and concurrently do not result in sufficient overall cost saving, this strategy cannot be pursued on the global LIB market. With a rising global awareness for sustainability aspects in the energy sector and the implementation of legislative regulations worldwide, this risk can be manageable. In fact, considering the high energy- and salary costs, high safety standards, complex logistics, as well as resource availability, Europe will likely not be able to compete with China in terms of raw material processing, CAM synthesis or pyrometallurgical recycling. Instead, a rather long-term investment into circular battery designs and concurrently into the upscaling of direct recycling capacities represents an opportunity to establish a strengthened industrial and geopolitical position in the automotive and energy sector.The increased urgency for a higher technological readiness level of direct recycling processes, as well as building and upscaling of direct recycling capacities also enhances the growth potential of the secondary industrial sector that provides the required equipment. The European and, in particular, the German plant engineering industry possesses extensive expertise in industrial recycling technologies across various recyclable material sectors. The EU should build on that expertise to promote pilot-scale research and upscaling of recycling facilities with special focus on direct recycling approaches.112 Furthermore, design for circularity standards for LIBs need to be established and implemented. Close cooperation of industrial players in the automotive and battery sector needs to be incentivized to balance the inevitable merits and drawbacks of standardization.113 By implementing a design for circularity strategy, the EV battery industry could become a role-model for a gradual transition from a linear into a circular economy. Realizing this transformation requires strong support from politics, research and society as a whole.
Author contributions
A. Wolf wrote the original draft and designed the figures with support of F. Nagler, C. Neef and G. A. Giffin. All authors contributed to the conceptualization of the article. Administration and funding were done by K. Mandel, A. Flegler and G. A. Giffin. All authors were involved in the review and editing of the article.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this perspective. [Circular Battery Designs: Investing in Sustainability and Profitability].Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding of the German Federal Ministry of Education and Research within the projects AdRecBat (grant ID 03XP0518B), HydroLIBRec (grant ID 03XP0339A), IDCycLIB (grand ID 03XP0303C), REWIND (grand ID 03XP0596) and BETSY (grand ID 03XP0540B). Furthermore, the Cusanuswerk e. V. is acknowledged for the doctoral scholarship funding of A. Wolf. The authors thank Dr Michael Hofmann for fruitful discussions and building expertise within the department over the recent years. The authors acknowledge Prof. Dr Tobias Kraus for contributing to the conceptualization of design for LIB dismantling ideas within the AdRecBat project. Furthermore, the authors thank Dr Victor Trapp for providing insights into the recent legislative regulations in the field.References
- M. Jacoby, It's time to get serious about recycling lithium-ion batteries. A projected surge in electric-vehicle sales means that researchers must think about conserving natural resources and addressing battery end-of-life issues, available at: https://cen.acs.org/materials/energy-storage/time-serious-recycling-lithium/97/i28, accessed 1 September 2023.
- Y. Bai, N. Muralidharan, Y.-K. Sun, S. Passerini, M. Stanley Whittingham and I. Belharouak, Mater. Today, 2020, 41, 304–315 CrossRef CAS.
- K. Richa, C. W. Babbitt, G. Gaustad and X. Wang, Resour., Conserv. Recycl., 2014, 83, 63–76 CrossRef.
- Batteries Europe, Batteries Europe Reserach and Innovation Roadmap 2023. https://batterieseurope.eu/wp-content/uploads/2023/09/Batteries-Europe_Research-and-Innovation-Roadmap-2023_.pdf, 2023.
- F. Aguesse, J. Amici, P. Asinari, E. Ayerbe, P. Barboux, C. Battaglia, M. Berecibar, A. Bhowmik, J. Carrasco, M. C. Cabanas, I. E. Castelli, I. Cekic-Laskovic, C. Chanson, S. Clark, E. Crespo, K. B. Dermenci, G. Domann, R. Dominko, K. Edström, M. Fichtner, E. Flores, A. Franco, I. Gandiaga, G. Giffin, K. Hermansson, A. Hutter, P. Jacques, A. Latz, S. Lyonnard, D. Mayer, M. Meeus, S. Perraud, M. Priour, C. Punckt, O. Raccurt, E. Regårdh, J. Rupp, M. Reynaud, R. Schmuch, H. Stein, J.-M. Tarascon, V. Trapp, T. Vegge, P. Veit, M. Vilkman, M. Weil and W. Wenzel, Battery 2030+ Roadmap. Inventing the sustainable batteries of the future, 2023 Search PubMed.
- B. Makuza, Q. Tian, X. Guo, K. Chattopadhyay and D. Yu, J. Power Sources, 2021, 491, 229622 CrossRef CAS.
- J. C.-Y. Jung, P.-C. Sui and J. Zhang, J. Energy Storage, 2021, 35, 102217 CrossRef.
- H. Gao, D. Tran and Z. Chen, Curr. Opin. Electrochem., 2022, 31, 100875 CrossRef CAS.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- J. Mao, C. Ye, S. Zhang, F. Xie, R. Zeng, K. Davey, Z. Guo and S. Qiao, Energy Environ. Sci., 2022, 15, 2732–2752 RSC.
- P. Xu, Q. Dai, H. Gao, H. Liu, M. Zhang, M. Li, Y. Chen, K. An, Y. S. Meng, P. Liu, Y. Li, J. S. Spangenberger, L. Gaines, J. Lu and Z. Chen, Joule, 2020, 4, 2609–2626 CrossRef CAS.
- J. Neumann, M. Petranikova, M. Meeus, J. D. Gamarra, R. Younesi, M. Winter and S. Nowak, Adv. Energy Mater., 2022, 12 Search PubMed.
- R. E. Ciez and J. F. Whitacre, Nat. Sustain., 2019, 2, 148–156 CrossRef.
- P. Xu, D. H. S. Tan and Z. Chen, Trends Chem., 2021, 3, 620–630 CrossRef CAS.
- J. Amici, P. Asinari, E. Ayerbe, P. Barboux, P. Bayle-Guillemaud, R. J. Behm, M. Berecibar, E. Berg, A. Bhowmik, S. Bodoardo, I. E. Castelli, I. Cekic-Laskovic, R. Christensen, S. Clark, R. Diehm, R. Dominko, M. Fichtner, A. A. Franco, A. Grimaud, N. Guillet, M. Hahlin, S. Hartmann, V. Heiries, K. Hermansson, A. Heuer, S. Jana, L. Jabbour, J. Kallo, A. Latz, H. Lorrmann, O. M. Løvvik, S. Lyonnard, M. Meeus, E. Paillard, S. Perraud, T. Placke, C. Punckt, O. Raccurt, J. Ruhland, E. Sheridan, H. Stein, J.-M. Tarascon, V. Trapp, T. Vegge, M. Weil, W. Wenzel, M. Winter, A. Wolf and K. Edström, Adv. Energy Mater., 2022, 12 Search PubMed.
- X. Yu, W. Li, V. Gupta, H. Gao, D. Tran, S. Sarwar and Z. Chen, Global Chall., 2022, 6, 2200099 CrossRef PubMed.
- L. Lander, T. Cleaver, M. A. Rajaeifar, V. Nguyen-Tien, R. J. R. Elliott, O. Heidrich, E. Kendrick, J. S. Edge and G. Offer, iScience, 2021, 24, 102787 CrossRef PubMed.
- S. Korus, ARK Invest, 2022, 2022, https://ark-invest.com/articles/analyst-research/lithium-iron-phosphate-batteries/.
- T. Yang, D. Luo, A. Yu and Z. Chen, Adv. Mater., 2023, e2203218 CrossRef.
- H. Walvekar, H. Beltran, S. Sripad and M. Pecht, IEEE Access, 2022, 10, 63834–63843 Search PubMed.
- J. Dunn, M. Slattery, A. Kendall, H. Ambrose and S. Shen, Environ. Sci. Technol., 2021, 55, 5189–5198 CrossRef CAS PubMed.
- A. L. Cheng, E. R. H. Fuchs, V. J. Karplus and J. J. Michalek, Nat. Commun., 2024, 15, 2143 CrossRef CAS.
- M. Ahuis, A. Aluzoun, M. Keppeler, S. Melzig and A. Kwade, J. Power Sources, 2024, 593, 233995 CrossRef CAS.
- N. Hayagan, I. Gaalich, P. Loubet, L. Croguennec, C. Aymonier, G. Philippot and J. Olchowka, Batteries Supercaps, 2024, 7 Search PubMed.
- J. Potting, M. Hekkert, E. Worrell and A. Hanemaaijeer, Circular economy: Measuring innovation in the product chain, 2017 Search PubMed.
- B. Purvis, Y. Mao and D. Robinson, Sustain. Sci., 2019, 14, 681–695 CrossRef.
- A. Reisinger, M. Meinshausen and M. Manning, Environ. Res. Lett., 2011, 6, 24020 CrossRef.
- S. C. Neubauer and J. P. Megonigal, Ecosystems, 2015, 18, 1000–1013 CrossRef.
- M. Gutsch and J. Leker, J. Energy Storage, 2022, 52, 105030 CrossRef.
- J. Kirchherr, D. Reike and M. Hekkert, Resour., Conserv. Recycl., 2017, 127, 221–232 CrossRef.
- J.-P. Schöggl, L. Stumpf and R. J. Baumgartner, Resour., Conserv. Recycl., 2020, 163, 105073 CrossRef.
- J. Brändström and M. Saidani, J. Cleaner Prod., 2022, 371, 133537 CrossRef.
- N. M. P. Bocken, I. de Pauw, C. Bakker and B. van der Grinten, J. Ind. Prod. Eng., 2016, 33, 308–320 Search PubMed.
- D. Thompson, C. Hyde, J. M. Hartley, A. P. Abbott, P. A. Anderson and G. D. J. Harper, Resour., Conserv. Recycl., 2021, 175, 105741 CrossRef CAS.
- S. Glöser-Chahoud, S. Huster, S. Rosenberg, S. Baazouzi, S. Kiemel, S. Singh, C. Schneider, M. Weeber, R. Miehe and F. Schultmann, Resour., Conserv. Recycl., 2021, 174, 105735 CrossRef.
- J. Kirchherr and L. Piscicelli, Resour., Conserv. Recycl., 2019, 150, 104406 CrossRef.
- S. Sun, C. Jin, W. He, G. Li, H. Zhu and J. Huang, Sci. Total Environ., 2021, 776, 145913 CrossRef CAS PubMed.
- Amending Battery Regulation TA-9-2023-0237, 2023.
- Directive 2006/66/EC. OJEU 2006 L 266/1, 2006.
- Directive 2009/125/EC. OJEU 2009 L 285/10, 2009.
- T. Hettesheimer, C. Neef, I. Rosellón Inclán, S. Link, T. Schmaltz, F. Schuckert, A. Stephan, M. Stephan, A. Thielmann, L. Weymann and T. Wicke, Lithium-Ion Battery Roadmap – Industrialization Perspectives toward 2030, 2023.
- Y. Ding, Z. P. Cano, A. Yu, J. Lu and Z. Chen, Electrochem. Energ. Rev., 2019, 2, 1–28 CrossRef CAS.
- Bloomberg NEF, Race to Net Zero: The Pressures of the Battery Boom in Five Charts, 2022.
- J. Neuhausen, P. Rose, J.-H. Bomke, F. Ferk, A. Kampker, C. Offermanns, M. Frank and T. Elliger, EU recycling market. The EU recycling market – a viable and sustainable business, Strategy& RWTH Aachen, 2023.
- Fastmarkets, Launch of europe additional asia blac mass payable assessments, available at: https://www.fastmarkets.com/insights/launch-of-europe-additional-asia-black-mass-payable-assessments/, accessed 22 July 2024.
- Benchmark, Price assessments black mass, available at: https://www.benchmarkminerals.com/price-assessments/black-mass/?tab=table, accessed 22 July 2024.
- Fastmarkets, Black mass prices, available at: https://www.fastmarkets.com/metals-and-mining/black-mass-prices/.
- SMM – Shanghai Metal Markets, Used Lithium ion Battery, available at: https://www.metal.com/price/New-Energy/Used-Lithium-ion-Battery, accessed 22 July 2024.
- Fastmarkets, China lithium spot prices inch down, available at: https://www.fastmarkets.com/insights/china-lithium-spot-prices-inch-down/, accessed 22 July 2024.
- J. Dunn, A. Kendall and M. Slattery, Resour., Conserv. Recycl., 2022, 185, 106488 CrossRef CAS.
- M. Gutsch and J. Leker, Appl. Energy, 2024, 353, 122132 CrossRef CAS.
- H. Bajolle, M. Lagadic and N. Louvet, Energy Res. Soc. Sci., 2022, 93, 102850 CrossRef.
- G. Wei, Y. Liu, B. Jiao, N. Chang, M. Wu, G. Liu, X. Lin, X. Weng, J. Chen, L. Zhang, C. Zhu, G. Wang, P. Xu, J. Di and Q. Li, iScience, 2023, 26, 107676 CrossRef CAS PubMed.
- International Energy Agency IEA, Critical Minerals Market Review, 2023.
- A. Mayyas, D. Steward and M. Mann, Sustainable Mater. Technol., 2019, 19, e00087 CrossRef CAS.
- X. Sun, H. Hao, P. Hartmann, Z. Liu and F. Zhao, Mater. Today Energy, 2019, 14, 100347 CrossRef.
- SMM – Shanghai Metal Markets, Lithium prices, available at: https://www.metal.com/price/New-Energy/Lithium, accessed 22 July 2024.
- S. Ahmed, P. A. Nelson, K. G. Gallagher, N. Susarla and D. W. Dees, J. Power Sources, 2017, 342, 733–740 CrossRef CAS.
- SMM – Shanghai Metal Markets, Lithium Battery Cathode Precursor and Material prices, available at: https://www.metal.com/price/New%20Energy/Lithium%20Battery%20Cathode%20Precursor%20and%20Material, accessed 22 July 2024.
- G. Zang, J. Zhang, S. Xu and Y. Xing, Energy, 2021, 218, 119504 CrossRef CAS.
- H. Wang, K. Feng, P. Wang, Y. Yang, L. Sun, F. Yang, W.-Q. Chen, Y. Zhang and J. Li, Nat. Commun., 2023, 14, 1246 CrossRef CAS PubMed.
- M. Mohr, J. F. Peters, M. Baumann and M. Weil, J. Ind. Ecol., 2020, 24, 1310–1322 CrossRef CAS.
- J. Šimaitis, S. Allen and C. Vagg, J. Ind. Ecol., 2023, 27, 1291–1303 CrossRef.
- F. Degen, M. Winter, D. Bendig and J. Tübke, Nat. Energy, 2023, 27, 101023 CrossRef.
- Eurostat Waste Statistics, Recycling of batteries and accumulators, available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators, accessed 13 September 2024.
- Nationale Plattform Zukunft der Mobilität – Arbeitsgruppe 4, Sicherung des Mobilitäts- und Produktionsstandortes, Batteriezellproduktion, Rohstoffe und Recycling, Bildung und Qualifizierung, available at: https://www.plattform-zukunft-mobilitaet.de/wp-content/uploads/2021/10/NPM_AG4_Batterierecycling.pdf, accessed 13 September 2024.
- Phate Zhang, China recycles more waste power batteries in first 5 months than all of last year, available at: https://cnevpost.com/2023/07/19/china-recycles-more-waste-batteries-jan-may-than-2022/, accessed 13 September 2024.
- Avicenne Energy June 2022, Battery market for Hybrid, Plug-in & Electric Vehicles, available at: https://www.avicenne.com/pdf/Presentation_Avicenne_Energy_June_2022.pdf, accessed 13 September 2024.
- K. Berger, J.-P. Schöggl and R. J. Baumgartner, J. Cleaner Prod., 2022, 353, 131492 CrossRef.
- S. Müssig, J. Reichstein, J. Prieschl, S. Wintzheimer and K. Mandel, Small, 2021, 17, e2101588 CrossRef PubMed.
- L. Lander, C. Tagnon, V. Nguyen-Tien, E. Kendrick, R. J. R. Elliott, A. P. Abbott, J. S. Edge and G. J. Offer, Appl. Energy, 2023, 331, 120437 CrossRef.
- K. R. Mulcahy, A. F. R. Kilpatrick, G. D. J. Harper, A. Walton and A. P. Abbott, Green Chem., 2022, 24, 36–61 RSC.
- H. Ali, H. A. Khan and M. Pecht, Renewable Sustainable Energy Rev., 2022, 168, 112809 CrossRef CAS.
- W. Chen, R. V. Salvatierra, J. T. Li, C. Kittrell, J. L. Beckham, K. M. Wyss, N. La, P. E. Savas, C. Ge, P. A. Advincula, P. Scotland, L. Eddy, B. Deng, Z. Yuan and J. M. Tour, Adv. Mater., 2023, 35, e2207303 CrossRef PubMed.
- Q. Cheng, B. Marchetti, X. Chen, S. Xu and X.-D. Zhou, J. Environ. Chem. Eng., 2022, 10, 107312 CrossRef CAS.
- M. Bhar, U. Bhattacharjee, K. Yalamanchili and S. K. Martha, J. Power Sources, 2023, 580, 233403 CrossRef CAS.
- S. Natarajan, K. Krishnamoorthy, A. Sathyaseelan, V. K. Mariappan, P. Pazhamalai, S. Manoharan and S.-J. Kim, Nano Energy, 2022, 101, 107595 CrossRef CAS.
- R. Zhang, X. Shi, O. C. Esan and L. An, Global Chall., 2022, 6, 2200050 CrossRef PubMed.
- B. Niu, Z. Xu, J. Xiao and Y. Qin, Chem. Rev., 2023, 123, 8718–8735 CrossRef CAS PubMed.
- F. Arshad, L. Li, K. Amin, E. Fan, N. Manurkar, A. Ahmad, J. Yang, F. Wu and R. Chen, ACS Sustainable Chem. Eng., 2020, 8, 13527–13554 CrossRef CAS.
- A. Battistel, M. S. Palagonia, D. Brogioli, F. La Mantia and R. Trócoli, Adv. Mater., 2020, 32, e1905440 CrossRef PubMed.
- S. Kim, J. Kim, S. Kim, J. Lee and J. Yoon, Environ. Sci.: Water Res. Technol., 2018, 4, 175–182 RSC.
- L. Prasakti, A. Prasetya, R. M. S. D. Suryohendrasworo and S. N. S. H. Puteri, IOP Conf. Ser.: Earth Environ. Sci., 2021, 882, 12069 CrossRef.
- G. D. J. Harper, E. Kendrick, P. A. Anderson, W. Mrozik, P. Christensen, S. Lambert, D. Greenwood, P. K. Das, M. Ahmeid, Z. Milojevic, W. Du, D. J. L. Brett, P. R. Shearing, A. Rastegarpanah, R. Stolkin, R. Sommerville, A. Zorin, J. L. Durham, A. P. Abbott, D. Thompson, N. D. Browning, B. L. Mehdi, M. Bahri, F. Schanider-Tontini, D. Nicholls, C. Stallmeister, B. Friedrich, M. Sommerfeld, L. L. Driscoll, A. Jarvis, E. C. Giles, P. R. Slater, V. Echavarri-Bravo, G. Maddalena, L. E. Horsfall, L. Gaines, Q. Dai, S. J. Jethwa, A. L. Lipson, G. A. Leeke, T. Cowell, J. G. Farthing, G. Mariani, A. Smith, Z. Iqbal, R. Golmohammadzadeh, L. Sweeney, V. Goodship, Z. Li, J. Edge, L. Lander, V. T. Nguyen, R. J. R. Elliot, O. Heidrich, M. Slattery, D. Reed, J. Ahuja, A. Cavoski, R. Lee, E. Driscoll, J. Baker, P. Littlewood, I. Styles, S. Mahanty and F. Boons, J. Phys. Energy, 2023, 5, 21501 CrossRef.
- M. Hofmann, F. Nagler, U. Guntow, G. Sextl and G. A. Giffin, J. Electrochem. Soc., 2021, 168, 60511 CrossRef CAS.
- M. Hofmann, F. Nagler, M. Kapuschinski, U. Guntow and G. A. Giffin, ChemSusChem, 2020, 13, 5962–5971 CrossRef CAS PubMed.
- L. Gaines, One Earth, 2019, 1, 413–415 CrossRef.
- L. Li, P. Zheng, T. Yang, R. Sturges, M. W. Ellis and Z. Li, JOM, 2019, 71, 4457–4464 CrossRef CAS.
- A. Wolf, A. Flegler, J. Prieschl, T. Stuebinger, W. Witt, F. Seiser, T. Vinnay, T. Sinn, M. Gleiß, H. Nirschl and K. Mandel, Chem. Eng. Process., 2021, 160, 108310 CrossRef CAS.
- T.-O. Folayan, A. L. Lipson, J. L. Durham, H. Pinegar, D. Liu and L. Pan, Energy Technol., 2021, 9, 259 Search PubMed.
- R. Zhan, Z. Yang, I. Bloom and L. Pan, ACS Sustainable Chem. Eng., 2021, 9, 531–540 CrossRef CAS.
- H. Al-Shammari and S. Farhad, Resour., Conserv. Recycl., 2021, 174, 105749 CrossRef CAS.
- Y. Guo, X. Liao, P. Huang, P. Lou, Y. Su, X. Hong, Q. Han, R. Yu, Y.-C. Cao and S. Chen, Energy Storage Mater., 2021, 43, 348–357 CrossRef.
- J. Tan, Q. Wang, S. Chen, Z. Li, J. Sun, W. Liu, W. Yang, X. Xiang, X. Sun and X. Duan, Energy Storage Mater., 2021, 41, 380–394 CrossRef.
- X. Ma, P. Vanaphuti, J. Fu, J. Hou, Y. Liu, R. Zhang, S. Bong, Z. Yao, Z. Yang and Y. Wang, Nano Energy, 2021, 87, 106194 CrossRef CAS.
- Y. Zou, G. Chang, S. Chen, T. Liu, Y. Xia, C. Chen and D. Yang, J. Chem. Eng., 2018, 351, 340–347 CrossRef CAS.
- K. Jia, J. Ma, J. Wang, Z. Liang, G. Ji, Z. Piao, R. Gao, Y. Zhu, Z. Zhuang, G. Zhou and H.-M. Cheng, Adv. Mater., 2023, 35, e2208034 CrossRef PubMed.
- X. Xiao, L. Wang, Y. Wu, Y. Song, Z. Chen and X. He, Energy Environ. Sci., 2023, 16, 2856–2868 RSC.
- T. Wang, H. Luo, J. Fan, B. P. Thapaliya, Y. Bai, I. Belharouak and S. Dai, iScience, 2022, 25, 103801 CrossRef CAS PubMed.
- K. Davis and G. P. Demopoulos, Next Energy, 2024, 4, 100122 CrossRef.
- Y. Zhang, Z. Gao and Z. Su, Energy Technol., 2024, 41, 5068 Search PubMed.
- J. Zhou, C. Xing, J. Huang, Y. Zhang, G. Li, L. Chen, S. Tao, Z. Yang, G. Wang and L. Fei, Adv. Energy Mater., 2024, 14, 8702 Search PubMed.
- SMM – Shanghai Metal Markets, Electrolyte prices, available at: https://www.metal.com/price/New-Energy/Electrolyte, accessed 13 September 2024.
- SMM - Shanghai Metal Markets, Ethylene Carbonate EC (Ex-factory price), available at: https://www.metal.com/Electrolyte/202005210012.
- SMM – Shanghai Metal Markets, Dimethyl Carbonate DMC (Ex-factory price), accessed 13 September 2024.
- E. Ribeiro da Silva, J. Lohmer, M. Rohla and J. Angelis, Resour., Conserv. Recycl., 2023, 193, 106969 CrossRef.
- F. M. Zanotto, D. Z. Dominguez, E. Ayerbe, I. Boyano, C. Burmeister, M. Duquesnoy, M. Eisentraeger, J. F. Montaño, A. Gallo-Bueno, L. Gold, F. Hall, N. Kaden, B. Muerkens, L. Otaegui, Y. Reynier, S. Stier, M. Thomitzek, A. Turetskyy, N. Vallin, J. Wessel, X. Xu, J. Abbasov and A. A. Franco, Batteries Supercaps, 2022, 5, 12 Search PubMed.
- A. Stephan, T. Hettesheimer, C. Neef, T. Schmaltz, S. Link, M. Stephan, L. Heizmann and A. Thielmann, Alternativ Battery Technologies Roadmap 2030+, Fraunhofer-Institut für System- und Innovationsforschung ISI, 2023.
- W. Guo, T. Feng, W. Li, L. Hua, Z. Meng and K. Li, J. Energy Storage, 2023, 72, 108589 CrossRef.
- T. Liu, Y. Zhang, C. Chen, Z. Lin, S. Zhang and J. Lu, Nat. Commun., 2019, 10, 1965 CrossRef PubMed.
- Y. Zhao, Y. Kang, J. Wozny, J. Lu, H. Du, C. Li, T. Li, F. Kang, N. Tavajohi and B. Li, Nat. Rev. Mater., 2023, 451, 652 Search PubMed.
- C. Neef, T. Schmaltz and A. Thielmann, Recycling of Lithium-Ion Batteries: Opportunities and Challenges for Mechanical and Plant Engineering, Fraunhofer – Institut für System – und Innovationsforschung ISI, 2021.
- G. Bridge and E. Faigen, Energy Res. Soc. Sci., 2022, 89, 102659 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |