Open Access Article
Open Access ArticleGlobal softening to manipulate sound velocity for reliable high-performance MgAgSb thermoelectrics†
Airan
Li‡
 a,
Longquan
Wang‡
ab,
Jiankang
Li
ab and
Takao
Mori
a,
Longquan
Wang‡
ab,
Jiankang
Li
ab and
Takao
Mori
 *ab
*ab
aResearch Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, 305-0044, Japan. E-mail: MORI.Takao@nims.go.jp
bGraduate School of Pure and Applied Sciences, University of Tsukuba, Tennodai 1-1-1, Tsukuba, 305-8671, Japan
First published on 18th October 2024
Abstract
High-performance thermoelectric materials at room temperature are eagerly pursued due to their promising applications in the Internet of Things for sustainable power supply. Reducing sound velocity by softening chemical bonds is considered an effective approach to lowering thermal conductivity and enhancing thermoelectric performance. Here, different from softening chemical bonds at the atomic scale, we introduce a global softening strategy, which macroscopically softens the overall material to manipulate its sound velocity. This is demonstrated in MgAgSb, one of the most promising p-type thermoelectric materials at room temperature to replace (Bi,Sb)2Te3, that the addition of inherently soft organic compounds can easily lower its sound velocity, leading to an obvious reduction in lattice thermal conductivity. Despite a simultaneous reduction of the power factor, the overall thermoelectric quality factor B is enhanced, enabling softened MgAgSb by C18H36O2 addition to achieve a figure of merit zT value of ∼0.88 at 300 K and a peak zT value of ∼1.30. Consequently, an impressive average zT of ∼1.17 over a wide temperature range has been realized. Moreover, this high-performance MgAgSb is verified to be highly repeatable and stable. With this MgAgSb, a decent conversion efficiency of 8.6% for a single thermoelectric leg and ∼7% for a two-pair module have been achieved under a temperature difference of ∼276 K, indicating its great potential for low-grade heat harvesting. This work will not only advance MgAgSb for low-grade power generation, but also inspire the development of high-performance thermoelectrics with global softening in the future.
Broader contextThermoelectrics can harvest waste heat from the environment to power numerous sensors in the Internet of Things, aiding the realization of a carbon-neutral society. Efficient thermal-electricity transfer requires thermoelectric materials to exhibit not only an excellent power factor but also an extremely low thermal conductivity. Traditionally, reducing sound velocity by softening chemical bonds at atomic scale is effective to decrease thermal conductivity and enhance thermoelectric performance, but this approach often involves trial and error to find suitable doping or alloying elements. In this work, we adopt a more macroscopic method by incorporating soft organic compounds, which could globally soften the materials and intentionally reduce sound velocity of material. We demonstrate that in MgAgSb, a promising p-type thermoelectric material with intrinsically low thermal conductivity at room temperature, adding soft organic compounds like fatty acid C18H36O2 can effectively reduce its sound velocity, significantly lowering its lattice thermal conductivity further and improving its thermoelectric performance. Importantly, the addition of C18H36O2 also enhances the reproducibility of high-performance MgAgSb. Based on this reliable MgAgSb, high conversion efficiency can be achieved, showcasing its potential for practical application in the Internet of Things in the future. |
Introduction
Thermoelectric (TE) technology, which enables the mutual conversion of heat and electricity, holds great promise for power generation by harvesting waste heat.1,2 With the rapid development of the Internet of Things (IoTs) recently, there arises a growing demand for high-performance TE materials near room temperature to sustainably power the numerous sensors.3 However, the limited kinds of high-performance TE materials at room temperature hinder their widespread application. Generally, the TE performance of a material is judged by the figure of merit, zT = S2σT/κ, where S is the Seebeck coefficient, σ is electrical conductivity, T is the absolute temperature and κ is thermal conductivity. According to this formula, outstanding TE materials necessitate not only a superior power factor PF = S2σ but also an ultralow κ.In the past, various strategies have been employed to enhance zT by improving the PF.4 These strategies include, but are not limited to, band engineering,5,6 carrier scattering manipulation7–9 and carrier concentration optimization,10,11 which have led to the advancement of numerous high-performance TE materials since the last century.12 Besides enhancing PF, decreasing κ is also a viable approach to boosting TE performance.13–15 For most bulk materials, κ is composed of electronic thermal conductivity κe and lattice thermal conductivity κL, where κe depends on the transport of carriers (electrons and holes), and κL depends on the transport of phonons. According to the Wiedmann–Franz law, κe = LσT (L is the Lorenz number), κe has a strong positive relationship with σ. Therefore, κL is considered an independent TE parameter beyond S, σ and κe. Due to the complex relationship between S, σ and κe, the strategy targeting a decrease in κL is very attractive.
In bulk materials, κL is governed by the phonon transport and can be described by the “phonon gas” model, which gives rise to the formula = 1/3 × cvvg2τ, where cv, vg, and τ represent specific heat, phonon group velocity, and phonon relaxation time, respectively. This suggests that decreasing κL or finding materials with inherently low κL requires materials to possess low cv, vg and τ. Typically, materials with liquid-like ions can exhibit a reduction of cv,16 and it is challenging to manipulate the cv, because the energy carried by atoms approaches the Dulong–Petit limit of 3kBT per atom in bulk materials at high temperatures. Therefore, decreasing vg and/or τ become the main focus to obtain low κL material. Phonon relaxation time τ reflects the scattering process of phonons, including both intrinsic and extrinsic contributions. Intrinsic phonon scattering (Umklapp scattering, U) requires materials to possess large anharmonicity, which is an important characteristic of many high-performance TE materials, such as PbTe,17 SnSe18 and Mg3Sb2.19,20 Besides intrinsic U scattering, extrinsic phonon scattering by point defects, dislocations, grain boundaries, nanoporous and nanoprecipitate has been frequently introduced into the material to decrease κL, leading to significant advancements in TE materials to achieve high zT values.21–24
It is worth noting that strengthening phonon scattering can also influence vg.25 Generally, phonons in materials can be categorized as acoustic phonons and optical phonons. Since the velocity of optical phonons approaches 0, the main contribution to vg comes from acoustic phonons. Usually, the velocity of acoustic phonons can be approximated by the sound velocity vs due to their equivalence in the long wavelength limit. At the microscopic atomic scale level, vs is influenced by the chemical bonds.26,27 Low force constants between atoms and large atomic mass are beneficial for achieving low vs.28 Doping to soften chemical bonds has been demonstrated to effectively decrease κL and enhance the performance of TE materials.15 On the other hand, from a more macroscopic perspective, vs is determined by the strength of the overall sound transport medium, meaning the softer the medium, the lower the vs. This suggests that if the material can be globally softened, a decrease in κL can also be expected, which however, remains to be verified.
Traditionally, methods such as alloying, work hardening, grain refinement, and second-phase strengthening are commonly used to increase materials’ strength. The rule of mixtures serves as a guideline when adding a second phase into the matrix to form composites, predicting the composite's strength by averaging the strengths of the matrix and second phase, weighted by their respective proportions.29 Incorporating strong, hard second phases can significantly enhance materials' strength, as seen when adding strong SiC to Bi2Te3-based materials can improve their mechanical properties.30,31 Conversely, adding a soft second phase can reduce overall material strength, potentially lowering vs and leading to low κL and high zT. MgAgSb, regarded as one of the most promising p-type TE materials for replacing (Bi,Sb)2Te3 at room temperature, has garnered significant attention in the past.32–34 Due to its high band degeneracy and inherently low κL, MgAgSb can achieve a zT value of ∼0.7 at room temperature and a peak zT value of ∼1.2.35–39 Factors such as hierarchical weak chemical bonds, atomic disorder, local structural distortion and crystal-liquid duality have been identified as important contributors to its intrinsically low κL.28,40–43 Recently, efforts to further lower κL have been realized by nanostructuring and introducing nanopores into MgAgSb, which strengthen phonon scattering and result in an enhanced zT value in the temperature range from 300 K to 473 K.44 However, this ultrafine-grained and nanoporous MgAgSb faces the risk of grain growth at high temperatures, making it suitable for cooling applications at low temperatures rather than power generation at relatively high temperatures. Nevertheless, decreasing κL could be an effective way to improve the TE performance of MgAgSb.
In this work, we introduce and adopt a global softening strategy to decrease vs of MgAgSb, aiming to reduce its κL and enhance its TE performance. Firstly, by adding inherently soft organic compounds, we demonstrate that the vs of MgAgSb can be efficiently decreased. Then, focusing on the addition of C18H36O2, we find that the vs of MgAgSb can be gradually manipulated when adjusting the C18H36O2 content. Despite a decreased PF, the overall TE quality factor B gets enhanced due to the much-decreased κL, resulting in a high zT of ∼0.88 at 300 K and a peak zT of ∼1.30. Further, we reveal that this high-performance MgAgSb is highly repeatable and stable as a result of C18H36O2 addition. Finally, using this high-performance MgAgSb, we achieve a high conversion efficiency η of ∼8.6% and ∼7% for a single TE leg and a two-pair module, respectively, showing great potential for harvesting low-grade heat. This reliable high-performance MgAgSb paves the way for its practical usage for power generations in the future, and the proposed global softening strategy can also stimulate performance enhancement in other TE systems.
Results and discussions
Reduced sound velocity for high TE performance
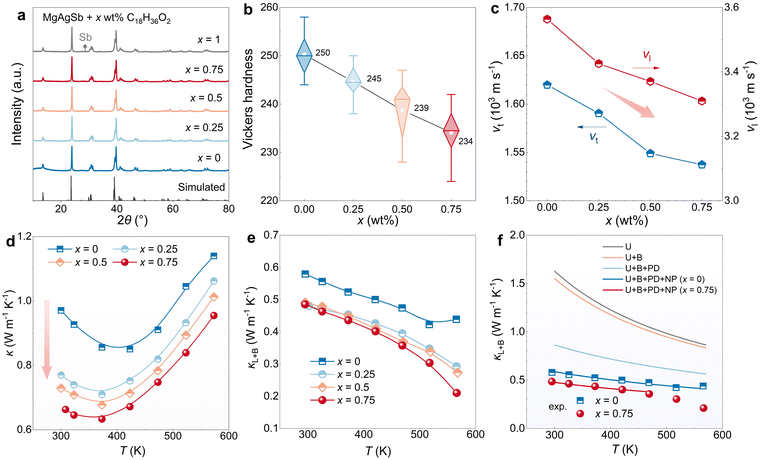
Due to the close relationship between phonon group velocity and vs, κL is closely related to vs. As shown in Fig. 1a, the curve of vsversus κL for most semiconductors reveals a positive proportion of κL to vs,28,45,46 indicating that materials with low vs tend to possess low κL and have the potential to be high-performance TE materials. MgAgSb is one such material with an intrinsically low vs of ∼1921 m s−1 (or 1844 m s−1), depending on the synthesis condition and composition.28,36 This low vs gives rise to the low κL of MgAgSb (∼0.6 W m−1 K−1). In this work, one-step ball milling has been used to synthesize MgAgSb, resulting in its vs of 1826 m s−1 and κL of 0.58 W m−1 K−1, similar to the literature.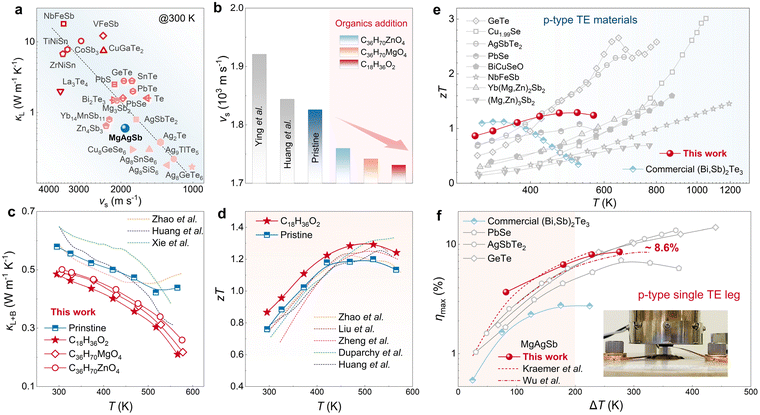 | ||
| Fig. 1 Global softening for high-performance MgAgSb. (a) vsversus κL at 300 K for various compounds;28,45,46 (b) vs of MgAgSb in literature28,36 and this work; (c) T dependence of κL of MgAgSb without addition and with organic addition, and their comparison to literature;33,36,44 (d) T dependence of zT of MgAgSb without addition and with C18H36O2 addition, and their comparison to literature;33,36,47–49 (e) T dependence of zT MgAgSb with C18H36O2 addition and its comparison to other state-of-art p-type TE materials;50–58 (f) applied ΔT dependence of maximum conversion efficiency ηmax of MgAgSb single-leg and its comparison to other state-of-art p-type single-leg.50,52,53,59–61 | ||
As mentioned above, vs is strongly dependent on sound transport medium. Therefore, globally softening the materials holds great promise for reducing vs of material, consequently leading to a reduction in κL. Organic compounds, which are normally softer than inorganic compounds, are ideal as possible additives for the softening of overall materials. Zinc stearate (C36H70ZnO4), magnesium stearate (C36H70MgO4) and stearic acid (C18H36O2), known for their universe usage in powder metallurgy,62,63 have been chosen to be added to MgAgSb. As shown in Fig. 1b, the vs of MgAgSb was successfully reduced from its original 1826 m s−1 to 1760 m s−1, 1742 m s−1, and 1731 m s−1 with C36H70ZnO4, C36H70MgO4 and C18H36O2 addition, respectively. As a result, the reduced vs leads to an obvious drop in κL of MgAgSb in the whole temperature range, as shown in Fig. 1c, where κL reaches 0.48 W m−1 K−1 at room temperature and 0.21 W m−1 K−1 at 573 K by C18H36O2 addition, which further brings about the enhancement of zT in MgAgSb. As shown in Fig. 1d, MgAgSb by C18H36O2 addition can achieve a high zT value of ∼0.88 at 300 K and a peak zT value of ∼1.30, which is excellent in the temperature range from 300 K to 573 K compared to the previous reports.33,36,47–49 Moreover, this high zT is very competitive among state-of-art p-type TE materials (Fig. 1e).50–58 With this high-performance MgAgSb, its single TE leg can achieve 3.6% and 8.6% maximum conversion efficiency under the temperature difference ΔT of about 80 K and 280 K, respectively, standing out as one of the best p-type single TE legs compared to PbSe, AgSbTe2 and GeTe.50,52,53,59–61
Manipulating sound velocity by adjusting C18H36O2 content
To delve deeper into the global softening strategy aimed at reducing vs, varying amounts (0–1 wt%) of C18H36O2 have been introduced into the matrix of MgAgSb. As shown in the X-ray diffraction (XRD) patterns in Fig. 2a, the addition of C18H36O2 (0.25–0.75 wt%) does not alter the phase of MgAgSb, while when 1 wt% C18H36O2 is added, a noticeable Sb secondary phase appears, which has been excluded from further discussion. Typically, C18H36O2 is stable below 573 K but decomposes at around 633 K. The presence of C18H36O2 in the MgAgSb can be evidenced by the increase in the vacuum of SPS chamber when the sintering temperature exceeds 700 K (Fig. S1, ESI†). Additionally, as shown in Fig. S2 (ESI†), the gradually increasing endothermic peak around 650 K in differential scanning calorimetry (DSC) curves of these C18H36O2-added MgAgSb further confirms the successful addition of C18H36O2 in MgAgSb.Due to the addition of C18H36O2, an expected softening effect on MgAgSb is observed, as indicated by the decreased Vickers hardness of MgAgSb shown in Fig. 2b. It should be noted that Vickers hardness is also influenced by changes in microstructure. Scanning electron microscopy (SEM) was used to observe the fracture morphology of pure MgAgSb and MgAgSb with 0.75 wt% C18H36O2. As shown in Fig. S3 (ESI†), the grain size decreases in the sample with C18H36O2 addition. However, despite the smaller grain size, the Vickers hardness of MgAgSb still decreases with C18H36O2 addition. This is surprising, as according to the Hall–Petch relationship, smaller grain sizes usually result in increased strength or hardness. The decreased Vickers hardness suggests a more pronounced softening effect with C18H36O2 addition.
The gradual softening of MgAgSb further leads to a corresponding gradual decrease in vs. As displayed in Fig. 2c, both the longitudinal sound velocity vl and transverse sound velocity vt decrease with the increasing C18H36O2 content. Consequently, the derived vs of MgAgSb also gradually decreases. This suggests that vs can be easily manipulated and tailored when adjusting the material's overall softness by different C18H36O2 contents. Due to the deceased vs, it correspondingly results in the decreased κ of MgAgSb. As shown in Fig. 2d, the κ of MgAgSb with 0.75 wt% C18H36O2 addition decreases to 0.65 W m−1 K−1 at room temperature, nearly a 30% reduction compared to 0.95 W m−1 K−1 of MgAgSb without addition. Further, when subtracting κe, calculated based on the simplified single parabolic band (SPB) model (ESI† Note),64 the decreased κL is also evident (Fig. 2e), where κL is decreased from 0.58 W m−1 K−1 to 0.48 W m−1 K−1 at room temperature.
It is important to note that the decreased vs will also influence the scattering process of phonons. For example, both U scattering and nanoprecipitates (NP) scattering are inversely correlated with phonon group velocity, indicating that lower vs will lead to more extensive phonon scattering. As shown in Fig. 2f, employing the Debye–Callaway model65 and including the U scattering, boundary (B) scattering, point defect (PD) scattering and NP scattering36,44,66 (Note and Table S1, ESI†), the simulated κL of MgAgSb without addition closely matches the experimental results. When incorporating the variation in vs with 0.75 wt% C18H36O2 addition, the simulated κL also aligns with the experiments around room temperature, confirming the significant role of vs reduction in decreasing κL. Furthermore, it is worth noting that the minimum thermal conductivity κmin is also related to vs. The κmin models proposed by Cahill et al.67 Clarke et al.68 or Snyder et al.69 all exhibit a positive relationship with vs. This suggests that materials with low vs will also have a lower limit of thermal conductivity, offering more rooms and opportunities for further decreasing κ and achieving high TE performance.
Enhanced TE quality factor B and high average figure of merit
Generally, although κL is independent of other TE parameters (S, σ and κe), manipulating κL often affects electrical properties. While C18H36O2 addition could effectively lower κL of MgAgSb, it is also important that this addition has less detrimental impact on electrical performances to achieve high TE performance. As shown in Fig. 3a and b, it can be noticed that σ gradually decreases, whereas the S increases, which indicates a decrease in carrier concentration with increasing C18H36O2 content. The overall PF decreases with higher C18H36O2 content (Fig. 3c), indicating the deterioration of overall electrical transport performance. Intrinsically, based on the intrinsic electrical conductivity σ0 (ESI† Note),64 which evaluates the electrical transport performance potential, as well as weighted mobility μW70 and electronic thermoelectric quality factor BE,71 it can be seen in Fig. S4 (ESI†) that σ0, μW and BE decrease with increasing C18H36O2 content, confirm the intrinsic deterioration of electrical transport performance of MgAgSb with C18H36O2 addition. Moreover, as shown in Fig. S5 (ESI†), the T−1.5 relationship of μW in both pure MgAgSb and MgAgSb with C18H36O2 indicates that carrier scattering is dominated by acoustic phonons. This suggests that the variation in S and σ is due to the addition of C18H36O2 rather than the grain size reduction. Additionally, the calculated effective mass m* for both pure MgAgSb and MgAgSb with C18H36O2 addition is about 1.76 me, which matches well with the reported value,44 suggesting that the band structure of MgAgSb remains unchanged with the addition of C18H36O2.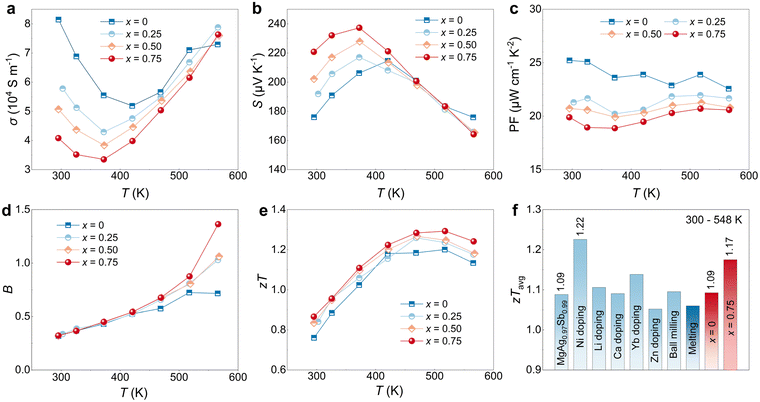 | ||
| Fig. 3 Electrical properties of MgAgSb with C18H36O2 addition. Temperature dependence of (a) σ, (b) S, (c) PF, (d) B factor, and (e) zT of MgAgSb with x wt% C18H36O2 addition (x = 0, 0.25, 0.5, 0.75); (f) the average zT of MgAgSb in literature33,36,47–49,72,73 and MgAgSb with no and 0.75 wt% C18H36O2 addition. | ||
However, the overall TE performance is not solely determined by electrical transport properties. Thermal transport properties also play a significant role. TE quality factor B = S02σ0T/κL is used to reflect the overall TE performance potential of the material, where S0 is a constant and 2S0 ≈ 173 μV K−1. As shown in Fig. 3d, despite the decrease in σ0, the decreased κL leads to an unchanged B factor at room temperature and even improved at high temperatures, suggesting the better TE potential for MgAgSb with C18H36O2 addition. Due to the enhanced B factor at high temperature, it results in a superior zT of MgAgSb with 0.75 wt% C18H36O2 addition, where zT value of 1.3 can be achieved at 523 K (Fig. 3e). Furthermore, despite the unchanged B factor at room temperature, the optimized S in MgAgSb with C18H36O2 addition enables it to exhibit zT ∼0.88 at 300 K. As a result, the zTavg value reaches 1.17 in the temperature of 300–548 K, making it one of the best values compared to state-of-art MgAgSb, as shown in Fig. 3f.33,36,47–49,72,73
Highly reliable MgAgSb by repeatable synthesis and test
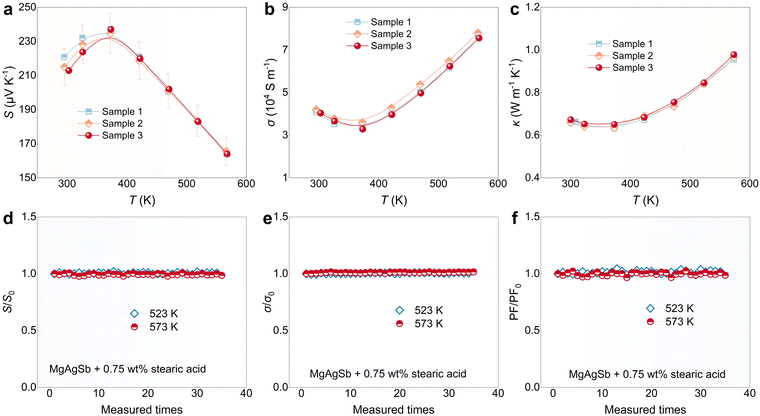
Besides the high TE performance, facile synthesis and excellent repeatability are additional benefits facilitated by the C18H36O2 addition. Due to the complex phase transition of MgAgSb,32 ball milling is usually preferred for synthesizing MgAgSb. However, powder adhesion to the jar walls during ball milling can pose a serious problem.49 This not only makes it difficult to retrieve the powder but also leads to an inhomogeneous composition. The TE performance of MgAgSb is reported to be highly sensitive to its composition,35,49 and powder adhesion is particularly problematic in its ball milling process.49 Typically, a two-step ball milling method has been adopted to synthesize MgAgSb.33,44 However, issues with repeatability of performance persist due to the uncontrollable powder adhesion during the synthesis of both the MgAg precursor and the final MgAgSb compounds.C18H36O2 is an effective process-control agent for addressing the problem of powder adhesion.63 In this work, the addition of C18H36O2 not only softens MgAgSb globally but also resolves the powder adhesion issue. As shown in Fig. S6 (ESI†), compared to MgAgSb without C18H36O2 addition, no power adhesives to the jar wall in MgAgSb with 0.75% C18H36O2 addition, making it easy to retrieve the alloyed MgAgSb powder. It is worth noting that MgAgSb tends to contain various second phases even when undetected by XRD.49 Energy-dispersive X-ray spectroscopy (EDS) mapping on both pure MgAgSb and MgAgSb with 0.75% C18H36O2 addition has been used to identify distribution of constituent elements. As shown in Fig. S7 (ESI†), both samples display uniform element distributions, though some Sb-rich second phases are observable in pure MgAgSb. By addressing the powder adhesion issue with C18H36O2, the second-phase problem can be alleviated. Another advantage of adding C18H36O2 is that it enables a one-step ball milling process (5 hours) for synthesizing the material, making it much more time- and energy-efficient compared to the two-step process, which usually requires 5 hours followed by an additional 10 hours.33,44 Importantly, due to the absence of powder adhesion, excellent repeatability of MgAgSb can be achieved. As shown in Fig. S8 (ESI†), three synthesized samples of MgAgSb from three separate ball milling jars exhibit very good phase purity. Furthermore, the TE transport properties of MgAgSb with C18H36O2 addition are also repeatable. As shown in Fig. 4a–c, the data for S, σ and κ of three separate samples match well and are within 5% measurement error of S and σ, and 3% measurement error of κ, respectively.
In addition to the repeatable synthesis of MgAgSb conducted three times, the stability of TE properties in MgAgSb with C18H36O2 addition warrants investigation, as C18H36O2 may enter a liquid phase around its melting point of 343 K. Fig. S9 (ESI†) shows an endothermic peak in MgAgSb with 0.75 wt% C18H36O2, likely due to its melting, but the peak is broad and weak because of its small content. Therefore, its impact on TE properties might be minimal. Further measurements of σ around 343 K show a smooth variation, indicating little influence from the potential melting of C18H36O2 (Fig. S9, ESI†). Additionally, the stability of the sample under prolonged high-temperature exposure should also be explored. As shown in Fig. 4d–f, the electrical transport properties (S, σ, and PF) at 523 K and 573 K exhibit minimal changes over 30 measurements. The ratios of Seebeck coefficient (S/S0), electrical conductivity (σ/σ0), and power factor (PF/PF0) for MgAgSb with 0.75 wt% C18H36O2 remain at 1, indicating excellent stability even at elevated temperatures. Here, S0, σ0 and PF0 represents the initial values of Seebeck coefficient, electrical conductivity and power factor, respectively. In all, as revealed above, despite being an organic compound, C18H36O2 has quite good stability below 573 K, highlighting its significant role in achieving reliable high TE performance in MgAgSb.
High conversion efficiency of MgAgSb single-leg and two-pair modules
Due to the enhanced average TE performance over a wide temperature range, MgAgSb with 0.75 wt% C18H36O2 addition is highly suitable for the low-grade heat harvesting. To access the materials’ heat conversion ability, a single TE leg has been fabricated, which is sandwiched by two layers of MgCuSb due to its low contact resistance and stability.74 As shown in Fig. 5a, it can be found that the contact resistance is very small, approximately 1 μΩ cm2. For measuring η, various ΔT are applied along the single TE leg.75 The largest hot-side temperature Th is limited to be 573 K considering the α-phase to β-phase transition of MgAgSb occurrs in 573–583 K.32 The measured output voltage V and output power P are displayed in Fig. 5b. The good linearity of the current-dependent V can be used to determine the internal resistance and open-circuit voltage of the single TE leg. A reduced slope indicates increased σ, which is consistent with the performance of our materials, and the increased open-circuit voltage is induced by the large ΔT. When Th is 573 K (ΔT is ∼276 K), the open-circuit voltage is 55 mV and the maximum output power Pmax is 60 mW. The decreased κ of MgAgSb helps to efficiently utilize the heat energy by suppressing heat flow to the cold side Q (Fig. 5c), which consequently results in a high maximum conversion efficiency ηmax of 3.6% under 82 K and 8.6% under 276 K (Fig. 5d). Furthermore, given the inherent stability of MgAgSb with C18H36O2 as revealed above, it is anticipated that leg performance will remain stable when aging, if the interface performance does not degrade.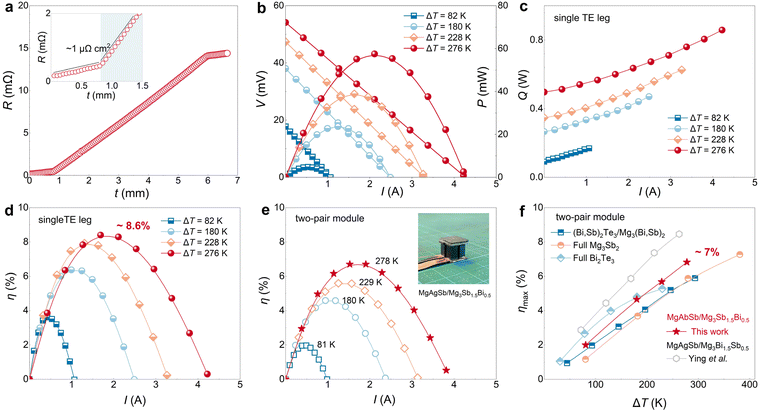 | ||
| Fig. 5 Conversion efficiency of MgAgSb single TE leg and two-pair module. (a) Contact resistance between MgAgSb and MgCuSb in the single TE leg; (b) I dependence of V and P, and (c) Q of MgAgSb single TE leg under different ΔT; (d) I dependence of η of MgAgSb single TE leg, and (e) MgAgSb/Mg3Sb1.5Bi0.5 two-pair module under different ΔT. The inset is the optical image of the two-pair module; (f) applied ΔT dependence of ηmax of MgAgSb/Mg3Sb1.5Bi0.5 and full Mg3Sb2-based, full Bi2Te3-based, (Bi,Sb)2Te3/Mg3(Bi,Sb)2-based and MgAgSb/Mg3Bi1.5Sb0.5-based two-pair modules in literature.76–79 | ||
In addition to the single TE leg, a two-pair TE module has also been demonstrated, with its optical image shown in the inset of Fig. 5e. The n-type TE legs are based on Sb-rich Mg3Sb1.5Bi0.5 due to its high chemical stability, as suggested by the recent study.80 The TE performance of n-type Mg3Sb1.5Bi0.5-based material is shown in Fig. S10 (ESI†), while Fig. S11 (ESI†) displays the measured V, P and Q under different applied ΔT and different applied current I. The Th is also limited to 573 K, considering the phase transition of MgAgSb. As shown in Fig. 5e, ηmax of this module can reach approximately 7% under ΔT of 278 K, comparable to recently reported Mg-based TE modules under ΔT of 300 K. It is noteworthy that this 7% conversion efficiency is based on n-type Sb-rich Mg3(Bi,Sb)2-based TE leg. Although its TE performance falls short of the Bi-rich Mg3(Bi,Sb)2-based material at room temperature,38,59,74,76,81 it could exhibit much higher chemical stability, making it much more promising for practical applications.
Furthermore, the achieved ηmax ∼ 7% in MgAgSb/Mg3Sb1.5Bi0.5-based module is quite impressive compared to full Mg3Sb2-based, full Bi2Te3-based and p-type (Bi,Sb)2Te3/n-type Mg3(Bi,Sb)2-based two-pair modules under the same ΔT.77–79 This highlights the superior potential of p-type MgAgSb for low-grade waste heat harvesting. Moreover, repeatable ηmax of both the single TE and the two-pair module have been achieved with two rounds of tests (Fig. S12, ESI†), demonstrating their good stability. If the TE performance of MgAgSb and Sb-rich Mg3Sb1.5Bi0.5 is further improved, greater enhancement of η can be expected in the future.
Conclusions
In this work, we introduce a global softening strategy to decrease vs in TE materials, thereby enhancing their TE performance. Unlike traditional methods by softening chemical bonds at the atomic scale to reduce vs, our strategy stems from a macroscopic view, by introducing inherently soft organic compounds into the material matrix to soften the overall material. Using MgAgSb, a promising p-type TE material, we demonstrate that adding various soft organics, such as zinc stearate (C36H70ZnO4), magnesium stearate (C36H70MgO4) and stearic acid (C18H36O2), effectively reduce vs, which brings about the reduction of κL and enhancement of TE performance.Specifically, it is found the vs of MgAgSb can be gradually tuned when adjusting C18H36O2 contents, and an ultralow κL ∼ 0.48 W m−1 K−1 can be achieved at room temperature in MgAgSb with 0.75 wt% C18H36O2 due to the reduced vs and increased phonon scattering, greatly lower than the 0.58 W m−1 K−1 in MgAgSb without addition. Despite a simultaneous decrease in PF by C18H36O2 addition, more reduction in κL results in an overall enhancement of the B factor. Consequently, a high zT value of ∼0.88 at 300 K and a peak zT value of ∼1.30 are achieved, which gives rise to an average zT of ∼1.17 in the temperature range of 300 K to 548 K, surpassing most state-of-art p-type TE materials. Moreover, the MgAgSb with C18H36O2 shows good repeatability and high stability, indicating high reliability. Using this high-performance MgAgSb compound, we achieve a high η of ∼8.6% and ∼7% in a single TE leg and a two-pair module under ΔT of ∼276 K, respectively, demonstrating great potential for harvesting low-grade heat. This work not only advances high-performance MgAgSb for low-grade power generation but also proposes a global softening strategy to manipulate the vs for performance enhancement in thermoelectrics.
Methods
Materials synthesis
MgAg0.97Sb0.99 (denoted as MgAgSb in the main text and below) with x wt% C18H36O2 (x = 0, 0.25, 0.5, 0.75, 1) were synthesized by using Mg turnings (99.95%), Ag powers (99.99%) Sb shots (99.999%), C36H70ZnO2 power (99.9%), C36H70MgO2 power (99.9%), and C18H36O2 power (99.9%). The raw materials were weighted stoichiometrically and then put into the ball milling jar with the inside of argon. Then, the jaw was mechanically alloyed for continuously 5 h (SPEX-8000D). The alloyed samples were scratched from the jaw and then compressed into the bulk by vacuum spark plasma sintering (SPS-322Lx, Dr Sintering) in the carbon die of 10 mm diameter under 573 K and 60 MPa for 5 min. The relative density of all samples reaches ∼97%. Mg3.2In0.005Sb1.5Bi0.49Te0.01 (denoted as Mg3Sb1.5Bi0.5 in the main text and below) was prepared by using Mg turnings (99.95%), Te shots (99.999%), Bi shots (99.999%), Sb shot (99.999%), and In powder (99.99%), which was weighted stoichiometrically and loaded into the ball milling jar with inside of argon, and then ball milled for 5 h (SPEX-8000D). The obtained powder was consolidated by SPS (SPS-1080 System, SPS SYNTEX INC) under 973 K and 60 MPa for 20 min. MgCuSb was prepared by using Mg powder (99.95%), Cu powder (99.999%) and Sb powder (99.999%), which was weighted stoichiometrically and loaded into the ball milling jar with the inside of argon, and then ball milled for 20 h (5 cycles, each cycle contains 4 hours running and 30 minutes rest) (SPEX-8000D).Characterization and measurements
The phases of obtained MgAgSb samples were characterized by using the X-ray diffractometer (SmartLab3, Rigaku) with Cu Kα radiation under 40 kV and 15 mA. The thermal analysis DSC was carried out by STA 449 (Netzsch) to check the existence of C18H36O2, the samples were loaded into an Al crucible and heated to 773 K with a heating rate of 10 K min−1. The fracture morphology of the sample were investigated by using scanning electron microscopy (FESEM, Hitachi SU8000), which is equipped with an energy dispersive spectrometer (EDS, XFlash FlatQUAD 5060![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F) to study the samples’ composition. The longitudinal (vl) and transverse (vt) sound velocity of obtained MgAgSb were measured by using a sing-around ultrasonic velocity measuring instrument (UVM-2, Ultrasonic Engineering Co., Ltd) with their sound velocity vs calculated according to vs−3 = (vl−3 + 2vt−3)/3. The Vickers hardness of MgAgSb was measured by a micro-Vickers hardness tester (HMV-G, Shimadzu), where 10 different spots in one sample were measured. The zT of MgAgSb samples was calculated by the formula: zT = S2σT/κ, in which the S and the σ were measured by ZEM-3 (Advance Riko, ± 5% uncertainty) under helium atmosphere, and the κ was calculated by formula: κ = DρCp, where the thermal diffusivity D was measured by LFA467 (Netzsch, ± 3% uncertainty), the sample density ρ was estimated by the Archimedes method, and heat capacity Cp was estimated by Dulong–Petit law. The Hall carrier concentration was measured by using a physical properties measuring systems, with an AC resistance option (PPMS, Quantum Design). The contact resistance of the MgAgSb/MgCuSb single TE leg was measured by a 2-axis resistance distribution measurement instrument (S1331, Mottainai energy).
F) to study the samples’ composition. The longitudinal (vl) and transverse (vt) sound velocity of obtained MgAgSb were measured by using a sing-around ultrasonic velocity measuring instrument (UVM-2, Ultrasonic Engineering Co., Ltd) with their sound velocity vs calculated according to vs−3 = (vl−3 + 2vt−3)/3. The Vickers hardness of MgAgSb was measured by a micro-Vickers hardness tester (HMV-G, Shimadzu), where 10 different spots in one sample were measured. The zT of MgAgSb samples was calculated by the formula: zT = S2σT/κ, in which the S and the σ were measured by ZEM-3 (Advance Riko, ± 5% uncertainty) under helium atmosphere, and the κ was calculated by formula: κ = DρCp, where the thermal diffusivity D was measured by LFA467 (Netzsch, ± 3% uncertainty), the sample density ρ was estimated by the Archimedes method, and heat capacity Cp was estimated by Dulong–Petit law. The Hall carrier concentration was measured by using a physical properties measuring systems, with an AC resistance option (PPMS, Quantum Design). The contact resistance of the MgAgSb/MgCuSb single TE leg was measured by a 2-axis resistance distribution measurement instrument (S1331, Mottainai energy).
Module fabrication and measurement
MgAgSb single TE leg was fabricated by sandwiching two layers of MgCuSb as the interface material and then sintered by SPS under 573 K and 60 MPa for 5 min. The obtained MgCuSb/MgAgSb/MgCuSb joints were cut into dice with dimensions of ∼3.8 × 3.8 × 6 mm3. The two-pair module was fabricated based on p-type MgCuSb/MgAgSb/MgCuSb TE legs and n-type 304 stainless steel/Mg3Sb1.5Bi0.5/304 stainless steel TE legs. The output power and conversion efficiency of the single TE leg and two-pair module were measured by Mini-PEM, (ADVANCE RIKO, Japan) with cold-side temperature maintained at 293 K and hot-side temperatures ranging from 373 K to 573 K in a vacuum condition.Author contributions
A. L., T. M. designed the project. A. L. prepared the samples and carried out the transport measurements and conversion efficiency measurement with the help of L. W. L. W. and J. L. provided the samples of Mg3(Sb,Bi)2 and MgCuSb, respectively. A. L. analyzed the data and wrote the original manuscript. T. M. supervised the whole project. All the authors reviewed and edited the manuscript.Data availability
All data generated or analyzed during this study are included in the published article and its ESI.† The data that support the findings of this study are available from the corresponding author upon request.Conflicts of interest
T. M. and A. L. have filed one Japanese patent application (2024-111372) on the work described here. The remaining authors declare no competing interests.Acknowledgements
This work was supported by JST Mirai Program (JPMJMI19A1).References
- J. He and T. M. Tritt, Science, 2017, 357, eaak9997 CrossRef PubMed.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed.
- I. Petsagkourakis, K. Tybrandt, X. Crispin, I. Ohkubo, N. Satoh and T. Mori, Sci. Technol. Adv. Mater., 2018, 19, 836–862 CrossRef CAS PubMed.
- T. Zhu, Y. Liu, C. Fu, J. P. Heremans, J. G. Snyder and X. Zhao, Adv. Mater., 2017, 29, 1605884 CrossRef PubMed.
- Y. Pei, H. Wang and G. J. Snyder, Adv. Mater., 2012, 24, 6125–6135 CrossRef CAS PubMed.
- A. Li, C. Hu, B. He, M. Yao, C. Fu, Y. Wang, X. Zhao, C. Felser and T. Zhu, Nat. Commun., 2021, 12, 5408 CrossRef CAS PubMed.
- D. Liu, D. Wang, T. Hong, Z. Wang, Y. Wang, Y. Qin, L. Su, T. Yang, X. Gao, Z. Ge, B. Qin and L.-D. Zhao, Science, 2023, 380, 841–846 CrossRef CAS PubMed.
- C. Hu, K. Xia, C. Fu, X. Zhao and T. Zhu, Energy Environ. Sci., 2022, 15, 1406–1422 RSC.
- T. Mori, Small, 2017, 13, 1702013 CrossRef PubMed.
- Y. Pei, Z. M. Gibbs, A. Gloskovskii, B. Balke, W. G. Zeier and G. J. Snyder, Adv. Energy Mater., 2014, 4, 1400486 CrossRef.
- M. Zhang, Z. Gao, Q. Lou, Q. Zhu, J. Wang, Z. Han, C. Fu and T. Zhu, Adv. Funct. Mater., 2024, 34, 2307864 CrossRef CAS.
- X.-L. Shi, J. Zou and Z.-G. Chen, Chem. Rev., 2020, 120, 7399–7515 CrossRef CAS PubMed.
- Z. Chen, X. Zhang and Y. Pei, Adv. Mater., 2018, 30, 1705617 CrossRef PubMed.
- M. K. Jana and K. Biswas, ACS Energy Lett., 2018, 3, 1315–1324 CrossRef CAS.
- A. R. Muchtar, B. Srinivasan, S. L. Tonquesse, S. Singh, N. Soelami, B. Yuliarto, D. Berthebaud and T. Mori, Adv. Energy Mater., 2021, 11, 2101122 CrossRef CAS.
- H. Liu, X. Shi, F. Xu, L. Zhang, W. Zhang, L. Chen, Q. Li, C. Uher, T. Day and G. J. Snyder, Nat. Mater., 2012, 11, 422–425 CrossRef CAS PubMed.
- O. Delaire, J. Ma, K. Marty, A. F. May, M. A. McGuire, M. H. Du, D. J. Singh, A. Podlesnyak, G. Ehlers, M. D. Lumsden and B. C. Sales, Nat. Mater., 2011, 10, 614–619 CrossRef CAS PubMed.
- L.-D. Zhao, S.-H. Lo, Y. Zhang, H. Sun, G. Tan, C. Uher, C. Wolverton, V. P. Dravid and M. G. Kanatzidis, Nature, 2014, 508, 373–377 CrossRef CAS PubMed.
- W. Peng, G. Petretto, G.-M. Rignanese, G. Hautier and A. Zevalkink, Joule, 2018, 2, 1879–1893 CrossRef CAS.
- J. Ding, T. Lanigan-Atkins, M. Calderón-Cueva, A. Banerjee, D. L. Abernathy, A. Said, A. Zevalkink and O. Delaire, Sci. Adv., 2021, 7, eabg1449 CrossRef CAS PubMed.
- J. Xin, H. Wu, X. Liu, T. Zhu, G. Yu and X. Zhao, Nano Energy, 2017, 34, 428–436 CrossRef CAS.
- A. U. Khan, K. Kobayashi, D.-M. Tang, Y. Yamauchi, K. Hasegawa, M. Mitome, Y. Xue, B. Jiang, K. Tsuchiya, D. Golberg, Y. Bando and T. Mori, Nano Energy, 2017, 31, 152–159 CrossRef CAS.
- R. Deng, X. Su, Z. Zheng, W. Liu, Y. Yan, Q. Zhang, V. P. Dravid, C. Uher, M. G. Kanatzidis and X. Tang, Sci. Adv., 2018, 4, eaar5606 CrossRef PubMed.
- R. He, T. Zhu, Y. Wang, U. Wolff, J.-C. Jaud, A. Sotnikov, P. Potapov, D. Wolf, P. Ying, M. Wood, Z. Liu, L. Feng, N. P. Rodriguez, G. J. Snyder, J. C. Grossman, K. Nielsch and G. Schierning, Energy Environ. Sci., 2020, 13, 5165–5176 RSC.
- S. Han, S. Dai, J. Ma, Q. Ren, C. Hu, Z. Gao, M. Duc Le, D. Sheptyakov, P. Miao, S. Torii, T. Kamiyama, C. Felser, J. Yang, C. Fu and T. Zhu, Nat. Phys., 2023, 19, 1649–1657 Search PubMed.
- S. Lee, K. Esfarjani, T. Luo, J. Zhou, Z. Tian and G. Chen, Nat. Commun., 2014, 5, 3525 Search PubMed.
- J. He, Y. Xia, W. Lin, K. Pal, Y. Zhu, M. G. Kanatzidis and C. Wolverton, Adv. Funct. Mater., 2022, 32, 2108532 CrossRef CAS.
- P. Ying, X. Li, Y. Wang, J. Yang, C. Fu, W. Zhang, X. Zhao and T. Zhu, Adv. Funct. Mater., 2017, 27, 1604145 CrossRef.
- H. S. Kim, Mater. Sci. Eng., A, 2000, 289, 30–33 CrossRef.
- J.-F. Li and J. Liu, Phys. Status Solidi A, 2006, 203, 3768–3773 CrossRef CAS.
- L.-D. Zhao, B.-P. Zhang, J.-F. Li, M. Zhou, W.-S. Liu and J. Liu, J. Alloys Compd., 2008, 455, 259–264 CrossRef CAS.
- M. J. Kirkham, A. M. dos Santos, C. J. Rawn, E. Lara-Curzio, J. W. Sharp and A. J. Thompson, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 144120 CrossRef.
- H. Zhao, J. Sui, Z. Tang, Y. Lan, Q. Jie, D. Kraemer, K. McEnaney, A. Guloy, G. Chen and Z. Ren, Nano Energy, 2014, 7, 97–103 CrossRef CAS.
- Z. Liu, J. Mao, J. Sui and Z. Ren, Energy Environ. Sci., 2018, 11, 23–44 RSC.
- P. Ying, X. Liu, C. Fu, X. Yue, H. Xie, X. Zhao, W. Zhang and T. Zhu, Chem. Mater., 2015, 27, 909–913 CrossRef CAS.
- Y. Huang, J. Lei, H. Chen, Z. Zhou, H. Dong, S. Yang, H. Gao, T.-R. Wei, K. Zhao and X. Shi, Acta Mater., 2023, 249, 118847 CrossRef CAS.
- X. Tan, L. Wang, H. Shao, S. Yue, J. Xu, G. Liu, H. Jiang and J. Jiang, Adv. Energy Mater., 2017, 7, 1700076 CrossRef.
- Z. Liu, W. Gao, H. Oshima, K. Nagase, C.-H. Lee and T. Mori, Nat. Commun., 2022, 13, 1120 CrossRef CAS PubMed.
- Z. Liu, N. Sato, W. Gao, K. Yubuta, N. Kawamoto, M. Mitome, K. Kurashima, Y. Owada, K. Nagase, C.-H. Lee, J. Yi, K. Tsuchiya and T. Mori, Joule, 2021, 5, 1196–1208 CrossRef CAS.
- D. Li, H. Zhao, S. Li, B. Wei, J. Shuai, C. Shi, X. Xi, P. Sun, S. Meng, L. Gu, Z. Ren and X. Chen, Adv. Funct. Mater., 2015, 25, 6478–6488 CrossRef CAS.
- J. Li, X. Li, Y. Zhang, J. Zhu, E. Zhao, M. Kofu, K. Nakajima, M. Avdeev, P.-F. Liu, J. Sui, H. Zhao, F. Wang and J. Zhang, Appl. Phys. Rev., 2024, 11, 011406 CAS.
- X. Li, P.-F. Liu, E. Zhao, Z. Zhang, T. Guidi, M. D. Le, M. Avdeev, K. Ikeda, T. Otomo, M. Kofu, K. Nakajima, J. Chen, L. He, Y. Ren, X.-L. Wang, B.-T. Wang, Z. Ren, H. Zhao and F. Wang, Nat. Commun., 2020, 11, 942 CrossRef CAS PubMed.
- J.-L. Mi, P.-J. Ying, M. Sist, H. Reardon, P. Zhang, T.-J. Zhu, X.-B. Zhao and B. B. Iversen, Chem. Mater., 2017, 29, 6378–6388 CrossRef CAS.
- L. Xie, J. Yang, Z. Liu, N. Qu, X. Dong, J. Zhu, W. Shi, H. Wu, G. Peng, F. Guo, Y. Zhang, W. Cai, H. Wu, H. Zhu, H. Zhao, Z. Liu and J. Sui, Mater. Today, 2023, 65, 5–13 CrossRef CAS.
- W. Li, S. Lin, B. Ge, J. Yang, W. Zhang and Y. Pei, Adv. Sci., 2016, 3, 1600196 CrossRef PubMed.
- M. Jin, S. Lin, W. Li, X. Zhang and Y. Pei, Mater. Today Phys., 2021, 21, 100508 CrossRef CAS.
- Z. Liu, Y. Wang, J. Mao, H. Geng, J. Shuai, Y. Wang, R. He, W. Cai, J. Sui and Z. Ren, Adv. Energy Mater., 2016, 6, 1502269 CrossRef.
- Y. Zheng, C. Liu, L. Miao, C. Li, R. Huang, J. Gao, X. Wang, J. Chen, Y. Zhou and E. Nishibori, Nano Energy, 2019, 59, 311–320 CrossRef CAS.
- A. Duparchy, L. Millerand, J. Camut, S. Tumminello, H. Kamila, R. Deshpande, A. Cowley, E. Mueller and J. de Boor, J. Mater. Chem. A, 2022, 10, 21716–21726 RSC.
- Z. Bu, X. Zhang, B. Shan, J. Tang, H. Liu, Z. Chen, S. Lin, W. Li and Y. Pei, Sci. Adv., 2021, 7, eabf2738 CrossRef CAS PubMed.
- H. Hu, Y. Ju, J. Yu, Z. Wang, J. Pei, H.-C. Thong, J.-W. Li, B. Cai, F. Liu, Z. Han, B. Su, H.-L. Zhuang, Y. Jiang, H. Li, Q. Li, H. Zhao, B.-P. Zhang, J. Zhu and J.-F. Li, Nat. Mater., 2024, 23, 527–534 CrossRef CAS PubMed.
- Y. Zhang, Z. Li, S. Singh, A. Nozariasbmarz, W. Li, A. Genç, Y. Xia, L. Zheng, S. H. Lee, S. K. Karan, G. K. Goyal, N. Liu, S. M. Mohan, Z. Mao, A. Cabot, C. Wolverton, B. Poudel and S. Priya, Adv. Mater., 2023, 35, 2208994 CrossRef CAS PubMed.
- S. Liu, Y. Wen, S. Bai, H. Shi, Y. Qin, B. Qin, D. Liu, Q. Cao, X. Gao, L. Su, C. Chang, X. Zhang and L.-D. Zhao, Adv. Mater., 2024, 2401828 CrossRef CAS PubMed.
- Y. Gu, X.-L. Shi, L. Pan, W.-D. Liu, Q. Sun, X. Tang, L.-Z. Kou, Q.-F. Liu, Y.-F. Wang and Z.-G. Chen, Adv. Funct. Mater., 2021, 31, 2101289 CrossRef CAS.
- C. Fu, S. Bai, Y. Liu, Y. Tang, L. Chen, X. Zhao and T. Zhu, Nat. Commun., 2015, 6, 8144 CrossRef PubMed.
- Z. Zhang, Y. Yan, X. Li, X. Wang, J. Li, C. Chen, F. Cao, J. Sui, X. Lin, X. Liu, G. Xie and Q. Zhang, Adv. Energy Mater., 2020, 10, 2001229 CrossRef CAS.
- Z. Ren, J. Shuai, J. Mao, Q. Zhu, S. Song, Y. Ni and S. Chen, Acta Mater., 2018, 143, 265–271 CrossRef CAS.
- K. Pang, L. Miao, Q. Zhang, Q. Pan, Y. Liu, H. Shi, J. Li, W. Zhou, Z. Zhang, Y. Zhang, G. Wu, X. Tan, J. G. Noudem, J. Wu, P. Sun, H. Hu, G.-Q. Liu and J. Jiang, Adv. Funct. Mater., 2024, 34, 2315591 CrossRef CAS.
- X. Wu, Y. Lin, C. Liu, Y. Wang, H. Li, B. Ge and W. Liu, Energy Environ. Sci., 2024, 17, 2879–2887 RSC.
- D. Kraemer, J. Sui, K. McEnaney, H. Zhao, Q. Jie, Z. F. Ren and G. Chen, Energy Environ. Sci., 2015, 8, 1299–1308 RSC.
- H.-L. Zhuang, H. Hu, J. Pei, B. Su, J.-W. Li, Y. Jiang, Z. Han and J.-F. Li, Energy Environ. Sci., 2022, 15, 2039–2048 RSC.
- L. Lu and Y. F. Zhang, J. Alloys Compd., 1999, 290, 279–283 CrossRef CAS.
- L. Shaw, J. Villegas, H. Luo, M. Zawrah and D. Miracle, Metall. Mater. Trans. A, 2003, 34, 159–170 CrossRef.
- C. Hu, Z. Gao, M. Zhang, S. Han, C. Fu and T. Zhu, Energy Environ. Sci., 2023, 16, 5381–5394 RSC.
- J. Callaway and H. C. von Baeyer, Phys. Rev., 1960, 120, 1149–1154 CrossRef CAS.
- W. Kim and A. Majumdar, J. Appl. Phys., 2006, 99, 084306 CrossRef.
- D. G. Cahill, S. K. Watson and R. O. Pohl, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6131–6140 CrossRef CAS PubMed.
- D. R. Clarke, Surf. Coat. Technol., 2003, 163–164, 67–74 CrossRef CAS.
- M. T. Agne, R. Hanus and G. J. Snyder, Energy Environ. Sci., 2018, 11, 609–616 RSC.
- G. J. Snyder, A. H. Snyder, M. Wood, R. Gurunathan, B. H. Snyder and C. Niu, Adv. Mater., 2020, 32, 2001537 CrossRef CAS PubMed.
- X. Zhang, Z. Bu, X. Shi, Z. Chen, S. Lin, B. Shan, M. Wood, A. H. Snyder, L. Chen, G. J. Snyder and Y. Pei, Sci. Adv., 2020, 6, eabc0726 CrossRef CAS PubMed.
- Z. Liu, Y. Wang, W. Gao, J. Mao, H. Geng, J. Shuai, W. Cai, J. Sui and Z. Ren, Nano Energy, 2017, 31, 194–200 CrossRef CAS.
- Z. Liu, Y. Zhang, J. Mao, W. Gao, Y. Wang, J. Shuai, W. Cai, J. Sui and Z. Ren, Acta Mater., 2017, 128, 227–234 CrossRef CAS.
- L. Xie, L. Yin, Y. Yu, G. Peng, S. Song, P. Ying, S. Cai, Y. Sun, W. Shi, H. Wu, N. Qu, F. Guo, W. Cai, H. Wu, Q. Zhang, K. Nielsch, Z. Ren, Z. Liu and J. Sui, Science, 2023, 382, 921–928 CrossRef CAS PubMed.
- R. Chetty, J. Babu and T. Mori, Joule, 2024, 8, 556–562 CrossRef.
- P. Ying, L. Wilkens, H. Reith, N. P. Rodriguez, X. Hong, Q. Lu, C. Hess, K. Nielsch and R. He, Energy Environ. Sci., 2022, 15, 2557–2566 RSC.
- M. Jiang, Y. Fu, Q. Zhang, Z. Hu, A. Huang, S. Wang, L. Wang and W. Jiang, Natl. Sci. Rev., 2023, 10, nwad095 CrossRef CAS PubMed.
- L. Yin, F. Yang, X. Bao, W. Xue, Z. Du, X. Wang, J. Cheng, H. Ji, J. Sui, X. Liu, Y. Wang, F. Cao, J. Mao, M. Li, Z. Ren and Q. Zhang, Nat. Energy, 2023, 8, 665–674 CrossRef CAS.
- X. Wu, Y. Lin, Z. Han, H. Li, C. Liu, Y. Wang, P. Zhang, K. Zhu, F. Jiang, J. Huang, H. Fan, F. Cheng, B. Ge and W. Liu, Adv. Energy Mater., 2022, 12, 2203039 CrossRef CAS.
- A. Li, P. Nan, Y. Wang, Z. Gao, S. Zhang, Z. Han, X. Zhao, B. Ge, C. Fu and T. Zhu, Acta Mater., 2022, 239, 118301 CrossRef CAS.
- P. Ying, R. He, J. Mao, Q. Zhang, H. Reith, J. Sui, Z. Ren, K. Nielsch and G. Schierning, Nat. Commun., 2021, 12, 1121 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee03521f |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |